Introduction
Bladder cancer is one of the most common cancer
types of the urological tract, with increasing incidence in recent
years (1–3). Despite systemic therapy for bladder
cancer, the clinical outcome is modest, mainly due to metastasis,
recurrence and drug resistance (4).
During the development and progression of bladder cancer, numerous
tumour suppressors and oncogenes, including certain microRNAs
(miRNAs/miRs) and long non-coding RNAs (lncRNAs), have been
determined to be deregulated (1,5,6). Gaining insight into the molecular
mechanisms of bladder cancer is beneficial for developing novel
biomarkers and therapeutic targets for its diagnosis and treatment
(7).
lncRNAs are a class of non-protein-coding
transcripts with lengths of >200 base pairs and are involved in
the pathological process of numerous human diseases through
regulating processes including cell proliferation, apoptosis,
migration and angiogenesis (8–12).
Accumulating evidence has indicated that numerous lncRNAs have
either oncogenic or tumour-suppressive roles in bladder cancer
(13,14). For instance, the lncRNA activated by
transforming growth factor-β (TGF-β) is upregulated in bladder
cancer and promotes the proliferation, migration and invasion of
bladder cancer cells by inhibiting the expression of miR-126
(15). Wang et al (16) determined a negative correlation
between growth arrest specific 5 (GAS5) levels and clinical stage
in bladder cancer, and overexpression of GAS5 reduced bladder
cancer cell viability and induced apoptosis.
X inactivation is an early developmental process in
mammalian females that transcriptionally silences one of the pairs
of X chromosomes (17). The lncRNA X
inactive specific transcript (XIST) is required for X inactivation
during development (18). Recently,
XIST has been reported to be frequently upregulated in various
human cancer types and to function as an oncogene (19,20). For
instance, it may accelerate cervical cancer progression via
upregulating Fus through competitively binding with miR-200a
(20). In addition, XIST may promote
gastric cancer progression through TGF-β1 via targeting miR-185
(19).
The oncogenic role of XIST in bladder cancer has
also been reported (21,22). For instance, Xu et al
(21) suggested that the lncRNA XIST
inhibits the stemness properties and tumourigenicity of bladder
cancer cells through inhibiting the expression of miR-200c. In
addition, XIST promotes cell growth and metastasis through
regulating the miR-139-5p-mediated Wnt/β-catenin signalling pathway
in bladder cancer (22). Recently,
Wei et al (23) reported that
XIST promoted pancreatic cancer cell proliferation through
regulating the expression of miR-133a and epidermal growth factor
receptor (EGFR). However, whether miR-133a is also involved in
XIST-mediated bladder cancer has remained elusive.
In the present study, the clinical significance of
XIST in bladder cancer was assessed and in addition, the regulatory
mechanism of XIST in bladder cancer cell proliferation and
migration were explored.
Materials and methods
Clinical tissue samples
Bladder cancer tissues and adjacent tissues were
collected from 52 primary bladder cancer patients at the Second
Xiangya Hospital (Changsha, China) between March 2011 and April
2013. The clinical characteristics of the patients are summarized
in Table I and included 32 male and
20 female patients, aged between 42–75 years. Prior to surgery,
none of the cancer patients had been treated by chemotherapy or
radiotherapy. The tissues were rapidly frozen in liquid nitrogen
and stored until use.
 | Table I.Association between XIST expression
and clinicopathological characteristics in bladder cancer. |
Table I.
Association between XIST expression
and clinicopathological characteristics in bladder cancer.
|
|
| XIST
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Cases (n=52) | High (n=19) | Low (n=33) | P-value |
|---|
| Age (years) |
|
|
| 0.546 |
|
<55 | 18 | 8 | 10 |
|
|
≥55 | 34 | 11 | 23 |
|
| Sex |
|
|
| 0.558 |
|
Male | 32 | 13 | 19 |
|
|
Female | 20 | 6 | 14 |
|
| Degree of
differentiation |
|
|
| 0.095 |
|
Well/moderate | 40 | 12 | 28 |
|
|
Poor | 12 | 7 | 5 |
|
| Lymph node
metastasis |
|
|
| 0.001 |
| No | 33 | 6 | 27 |
|
|
Yes | 19 | 13 | 6 |
|
| Distant
metastasis |
|
|
| 0.001 |
| No | 46 | 13 | 33 |
|
|
Yes | 6 | 6 | 0 |
|
| TNM stage |
|
|
| 0.001 |
|
I/II | 28 | 4 | 24 |
|
|
III/IV | 24 | 15 | 9 |
|
Cell culture and transfection
The SV-HUC-1 normal urinary tract epithelial cell
line and four common bladder cancer cell lines, T24, 253J, RT112
and HT-1376, were obtained from the Cell Bank of the Chinese
Academy of Sciences. These cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.)
supplemented with 10% foetal bovine serum (Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. For cell
transfection, 253J and RT112 cells were transfected with 100 nM
XIST small interfering (si)RNA1 (5′-GCAAAUGAAAGCUACCAAU-3′; Thermo
Fisher Scientific, Inc.), XIST siRNA2 (5′-GCACAAUAUCUUUGAACUA-3′;
Thermo Fisher Scientific, Inc.) or 100 nM negative control (NC)
siRNA (cat. no. 4459405; Thermo Fisher Scientific, Inc.) or
co-transfected with 100 nM NC siRNA and 100 nM NC inhibitor (cat.
no. 4464076; Thermo Fisher Scientific, Inc.), or 100 nM NC siRNA
and 100 nM miR-133a inhibitor (cat. no. 4464084; Thermo Fisher
Scientific, Inc.), or 100 nM XIST siRNA and 100 nM miR-133a
inhibitor, or 100 nM XIST siRNA and 100 nM NC inhibitor using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. At 48 h after transfection, the cells
were harvested and used for the subsequent assays.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from tissues and cell lines
using TRIzol reagent (Thermo Fisher Scientific, Inc.) and RT was
performed by processing 500 ng RNA with the First-Stand cDNA
Synthesis Kit (Tiangen Biotech Co., Ltd) according to the
manufacturer's protocols. qPCR analysis was performed using SYBR
Green PCR Master Mix (Takara Bio, Inc.) according to the
manufacturer's protocols. The reaction conditions were 95°C for 3
min followed by 40 cycles of 95°C for 15 sec and 60°C for 15 sec.
Relative gene expression was calculated using the 2−ΔΔCq
method (24). GAPDH and U6 were used
as internal references. The primers utilized in PCR were as
follows: XIST forward, 5′-ACGCTGCATGTGTCCTTAG-3′ and reverse,
5′-GAGCCTCTTATAGCTGTTTG-3′; GAPDH forward,
5′-CTGGGCTACACTGAGCACC-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAATG-3′;
miR-133a forward, 5′-TTTGGTCCCCTTCAACCAGCTG-3′ and reverse,
5′-TAAACCAAGGTAAAATGGTCGA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Cell Counting Kit-8 (CCK-8) assay
At 48 h after transfection, 253J and RT112 cells
were seeded onto 96-well plates at 5,000 cells per well. After
incubation at 37°C with 5% CO2 for 0, 24, 48 and 72 h,
cell proliferation was measured using the CCK-8 assay (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
Absorbance was detected at an optical density of 450 nm by a
spectrophotometer (BioRad Laboratories, Inc.).
Wound healing assay
To study cell migration, transfected 253J and RT112
cells were seeded into 6-well plates. After culturing at 37°C with
5% CO2 for 24 h, a line was scraped into the monolayer
with a 200-µl micropipette tip. The cells were washed twice with
PBS and cultured in serum-free DMEM for 24 h. Images of the wounded
area at 0 and 24 h were captured under a light microscope (CX22;
Olympus Corporation).
Bioinformatics analysis and luciferase
reporter gene assay
The potential targeting miRs of the lncRNA XIST were
predicted using RNAhybrid 2.12 (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
(25). XIST sequences containing the
wild-type (WT) or mutant-type (MT) miR-133a binding sites were
subcloned into the pmiR-GLo luciferase reporter vector (Promega
Corp.). Lipofectamine 2000 was then used to transfect 253J and
RT112 cells with miR-NC or miR-133a mimics together with the WT- or
MT-XIST reporter plasmid according to the manufacturer's protocols.
At 48 h after transfection, a Dual Luciferase Reporter Assay System
(Promega Corp.) was used to determine luciferase activities
according to the manufacturer's protocols.
Statistical analysis
Values are expressed as the mean ± standard
deviation. SPSS 20.0 software (IBM Corp.) was used for statistical
analysis. An unpaired two-tailed Student's t-test was used to
compare the differences between two groups. One-way analysis of
variance followed by Tukey's post-hoc test was used for multiple
comparisons. The association between XIST expression and the
clinical features of bladder cancer patients was analysed using the
chi-square test. For survival analysis, Kaplan-Meier survival
curves were drawn and the log-rank test was applied. The Spearman
rank correlation was used to analyse the correlation between the
expression of XIST and miR-133a in bladder cancer tissues.
P<0.05 was considered to indicate statistical significance.
Results
Upregulation of XIST is associated
with tumour progression and poor prognosis in bladder cancer
To explore the function of XIST in bladder cancer,
its expression in the normal urinary tract epithelial cell line
SV-HUC-1 and four common bladder cancer cell lines, T24, 253J,
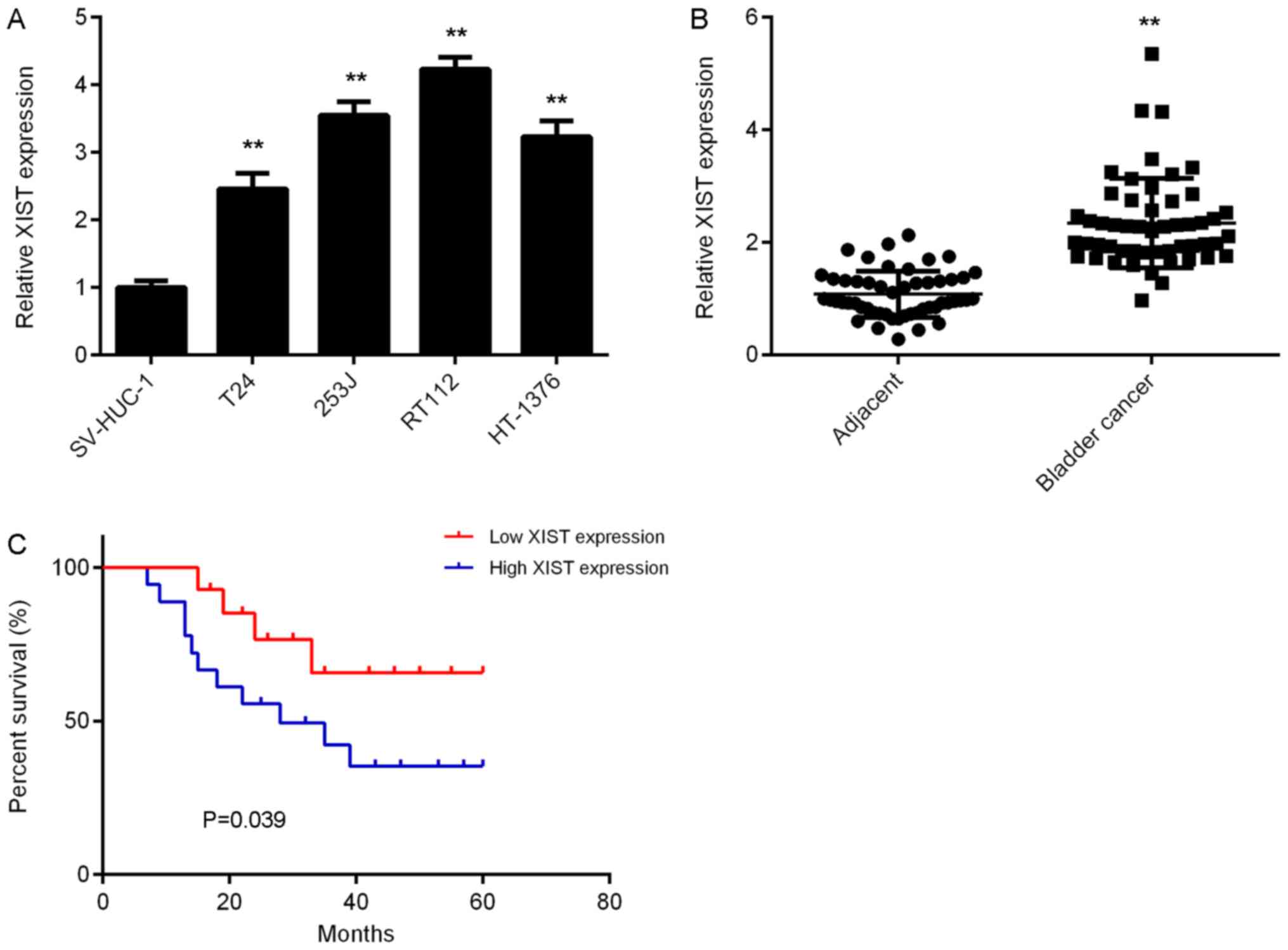
RT112 and HT-1376, was first examined. The results of the RT-qPCR
analysis suggested that the expression levels of XIST were
significantly increased in bladder cancer cells compared with those
in SV-HUC-1 cells (Fig. 1A). To
confirm these results, 52 paired bladder cancer tissues and
adjacent normal tissues were collected and assessed for expression
of XIST. The results revealed that XIST was also significantly
upregulated in bladder cancer tissues vs. adjacent normal tissues
(Fig. 1B). Furthermore, high XIST
expression was significantly associated with the presence of
metastasis, advanced tumour-nodes-metastasis (TNM) stage and a
shorter survival time of bladder cancer patients (Table I, Fig.
1C). It was therefore indicated that upregulation of XIST is
associated with tumour progression and poor prognosis in bladder
cancer.
Inhibition of XIST suppresses bladder
cancer cell proliferation and migration
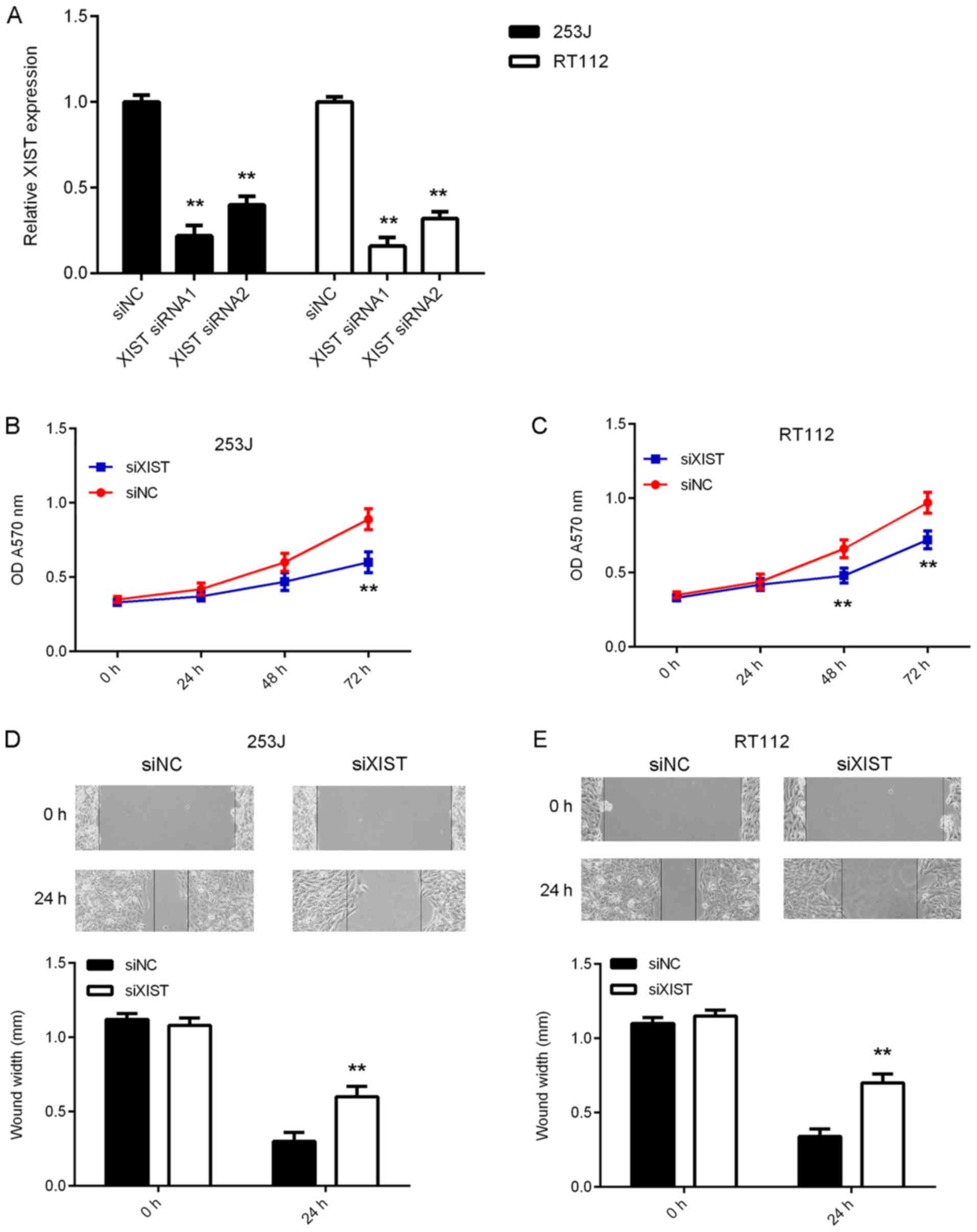
In the present study, 253J and RT112 cells were
subjected to in vitro loss-of-function experiments to
further reveal the function of XIST in bladder cancer. 253J and
RT112 cells were transfected with NC siRNA, or XIST siRNA1 or
siRNA2. After transfection, the expression levels of XIST were
significantly reduced in the XIST siRNA1 and siRNA2 groups compared
with those in the siNC group (Fig.
2A). As XIST siRNA1 exhibited a better suppressive effect on
XIST expression, it was used in the subsequent experiments. The
results of the CCK-8 assay indicated that silencing of XIST
expression caused a reduction in bladder cancer cell proliferation
(Fig. 2B and C). Furthermore, as
indicated in Fig. 2D and E, the
migration of 253J and RT112 cells was also inhibited after
knockdown of XIST. Taken together, these results suggest that the
inhibition of XIST suppresses bladder cancer growth and
metastasis.
XIST directly targets miR-133a in
bladder cancer cells
Next, a Bioinformatics analysis was performed to
predict potential XIST-miR interactions. The results indicated that
miR-133a had a potential binding site in XIST (Fig. 3A). Furthermore, knockdown of XIST
expression significantly promoted miR-133a expression in bladder
cancer cells (Fig. 3B). To verify
the predicted direct binding interaction, a luciferase reporter
gene assay was then performed. As indicated in Fig. 3C and D, miR-133a mimics significantly
inhibited the luciferase activity of the XIST-WT luciferase
reporter gene plasmid but had no effect on that of the XIST-MT
plasmid in bladder cancer cells, suggesting that XIST directly
targets miR-133a in bladder cancer cells.
 | Figure 3.XIST directly targets miR-133a in
bladder cancer cells. (A) Bioinformatics analysis suggested that
miR-133a has putative XIST binding sites, and luciferase reporter
plasmids containing the WT and MT miR-133a binding sites in XIST
were generated. (B) Knockdown of XIST significantly promoted
miR-133a expression in 253J and RT112 cells. **P<0.01 vs. siNC.
(C and D) Luciferase reporter gene assay suggested that
transfection with miR-133a mimics decreased the luciferase activity
of WT XIST reporter plasmid, while not affecting the luciferase
activity of MT XIST reporter plasmid in 253J and RT112 cells.
**P<0.01 vs. miR-NC. XIST, X inactive specific transcript; miR,
microRNA; miR-NC, miR mimics negative control; WT, wild type; MT,
mutant type; siRNA, small interfering RNA; siNC, negative control
siRNA; siXIST, siRNA targeting XIST. |
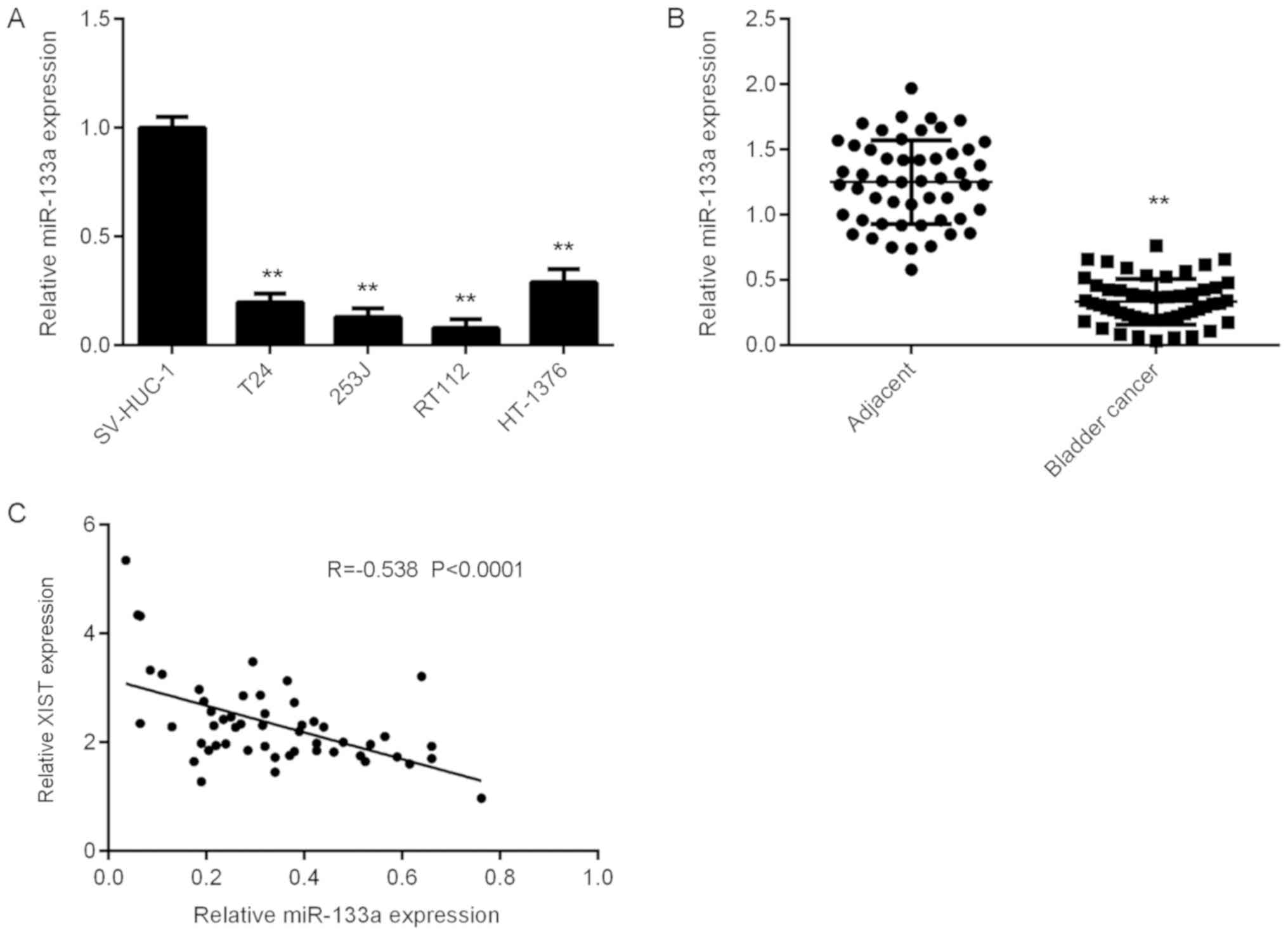
Downregulation of miR-133a in bladder
cancer
The expression levels of miR-133a in bladder cancer
were then assessed. As presented in Fig.
4A, miR-133a was significantly downregulated in bladder cancer
cells compared with that in SV-HUC-1 cells. Furthermore, the
expression levels of miR-133a were significantly downregulated in
bladder cancer tissues compared with those in adjacent normal
tissues (Fig. 4B). Further
investigation revealed an inverse correlation between miR-133a and
XIST expression in bladder cancer tissues (Fig. 4C), suggesting that the reduced
expression of miR-133a may be due to the increased expression of
XIST in bladder cancer.
miR-133a acts as a downstream effector
in XIST-mediated bladder cancer cell proliferation and
migration
Next, it was assessed whether miR-133a acts as a
downstream effector in XIST-mediated bladder cancer cell
proliferation and migration. 253J and RT112 cells were
co-transfected with NC siRNA and NC inhibitor (siNC+anti-NC), NC
siRNA and miR-133a inhibitor (si-NC+anti-miR-133a), XIST siRNA and
NC inhibitor (siXIST+anti-NC), or XIST siRNA and miR-133a inhibitor
(siXIST+anti-miR-133a). After transfection, the miR-133a levels
were significantly reduced in the si-NC+anti-miR-133a group, but
significantly increased in the siXIST+anti-NC group, when compared
with those in the siNC+anti-NC group (Fig. 5A and B). However, no significant
difference in miR-133a expression was observed between the
siNC+anti-NC and siXIST+anti-miR-133a groups. CCK-8 and wound
healing assays were then performed to assess the effects of the
above treatments on cell proliferation and migration. As indicated
in Fig. 5C-F, the proliferation and
migration of bladder cancer cells were significantly increased in
the siNC+anti-miR-133a group, but significantly decreased in the
siXIST+anti-NC group, when compared with those in the siNC+anti-NC
group. In addition, no significant difference was identified
between the siNC+anti-NC and siXIST+anti-miR-133a groups (Fig. 5C-F). These results indicate that
inhibition of miR-133a impairs the suppressive effects of XIST
downregulation on the proliferation and migration of bladder cancer
cells. Therefore, miR-133a acts as a downstream effector in
XIST-mediated bladder cancer cell proliferation and migration.
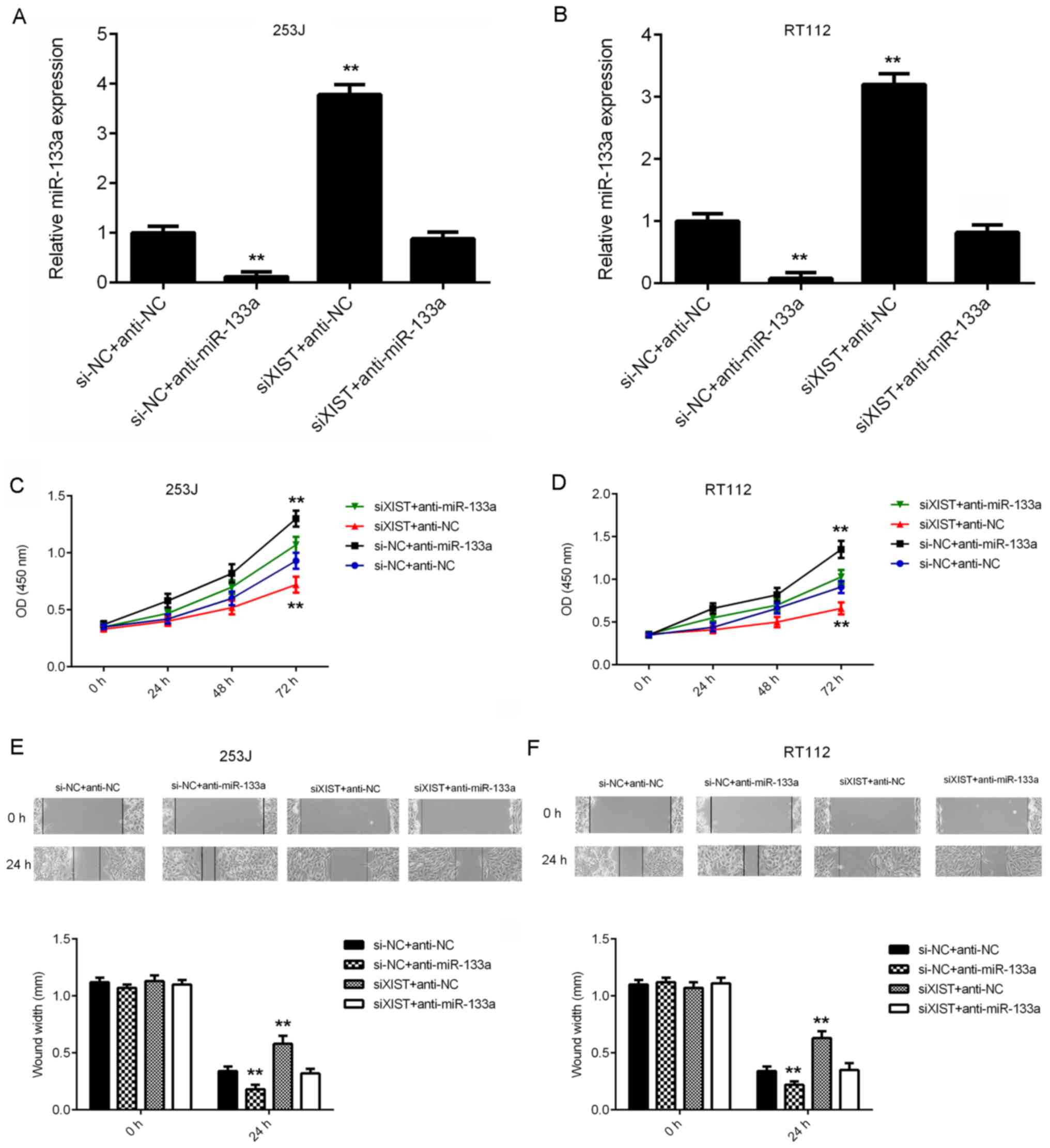 | Figure 5.miR-133a acts as a downstream
effector in XIST-mediated bladder cancer cell proliferation and
migration. 253J and RT112 cells were co-transfected with NC siRNA
and NC inhibitor, NC siRNA and miR-133a inhibitor, XIST siRNA and
NC inhibitor or XIST siRNA and miR-133a inhibitor, respectively. (A
and B) The expression of XIST was examined using
reverse-transcription quantitative PCR. (C and D) A Cell Counting
Kit-8 assay and (E and F) a wound healing assay (magnification,
×40) were performed to assess cell proliferation and migration,
respectively. **P<0.01 vs. si-NC+anti-NC. XIST, X inactive
specific transcript; miR, microRNA; anti-miR-133a, miR-133a
inhibitor; anti-NC, miR inhibitor control, siRNA, small interfering
RNA; siNC, negative control siRNA; siXIST, siRNA targeting XIST;
OD, optical density. |
Discussion
It has been demonstrated that lncRNAs affect the
expression of a large number of genes and miRNAs at the
transcriptional and post-transcriptional levels, through which they
regulate various cellular biological processes, including cell
proliferation, cell cycle progression, apoptosis, migration and
tumourigenesis (10,11,21,26,27).
However, the regulatory mechanisms of the lncRNA XIST during
bladder cancer progression have remained to be fully elucidated. In
the present study, it was indicated that XIST was significantly
upregulated in bladder cancer tissues vs. adjacent normal tissues.
Furthermore, its expression was also reduced in several common
bladder cancer cell lines. High expression of XIST was
significantly associated with tumour progression and poor prognosis
of patients with bladder cancer. The in vitro experiments
suggested that knockdown of XIST significantly reduced the
proliferation and migration of bladder cancer cells. Furthermore, a
luciferase assay confirmed that XIST directly binds with miR-133a
at the predicted binding site. It was also determined that miR-133a
was significantly downregulated in bladder cancer, and its
expression levels were inversely correlated with XIST expression
levels in bladder cancer tissues. In addition, loss- and
gain-of-function experiments suggested that miR-133a acts as a
downstream effector in XIST-mediated bladder cancer cell
proliferation and migration.
In recent years, the lncRNA XIST has been reported
to be frequently upregulated in certain common types of cancer, and
its upregulation is associated with tumour progression and poor
prognosis of cancer patients (28–30). For
instance, Yang et al (31)
indicated that the expression of XIST was significantly increased
in osteosarcoma tissues and cell lines, and negatively correlated
with clinical prognosis. Sun et al (32) reported that XIST was markedly
upregulated in pancreatic cancer, and its overexpression
significantly promoted cancer cell proliferation, migration and
invasion. In the present study, increased expression of XIST in
bladder cancer tissues and cell lines was detected and high
expression of XIST was associated with positive metastasis,
advanced TNM stage and shorter survival time of bladder cancer
patients. Similarly, Xiong et al (33) also reported that XIST was upregulated
in bladder cancer tissues and that higher XIST expression was
associated with advanced TNM stage of bladder cancer. In addition,
Hu et al (22) reported that
XIST was significantly upregulated in bladder cancer tissues and
cell lines, and correlated with poor prognosis of bladder cancer
patients. Taken together, the results of previous and the present
study suggest that the lncRNA XIST has a key role during bladder
cancer progression and may thus serve as a potential therapeutic
target.
To further study the function of XIST in tumour
growth and metastasis, bladder cancer cells were transfected with
XIST siRNA to knock down its expression, and CCK-8 and wound
healing assays suggested that the downregulation of XIST caused a
significant reduction in bladder cancer cell proliferation and
migration. These results suggest that the lncRNA XIST may have a
promoting role during bladder cancer growth and metastasis.
Consistent with these results, Xiong et al (33) also reported that XIST knockdown
inhibited the proliferation, invasion and migration of bladder
cancer cells. In addition, Hu et al (22) indicated that silencing of XIST
expression significantly reduced bladder cancer cell growth and
metastasis in vitro and tumour growth in vivo.
However, the molecular mechanisms of the effect of XIST on bladder
cancer progression have remained to be fully elucidated.
The present study focused on downstream miRNAs of
XIST, as numerous miRNAs have been reported to act as tumour
suppressors and oncogenes in bladder cancer (5,34).
Bioinformatics analysis predicted that XIST and miR-133a contain
mutual binding sites. In fact, several previous studies have
reported that miR-133a functions as a tumour suppressor in bladder
cancer. For instance, miR-133a was demonstrated to induce bladder
cancer cell apoptosis through directly targeting glutathione
S-transferase pi 1 (35). Zhou et
al (36) reported that miR-133
inhibited the proliferation, migration and invasion of bladder
cancer cells by targeting EGFR and its downstream effector
proteins. In the present study, it was demonstrated that reduced
expression of miR-133a was inversely correlated with increased
expression of XIST in bladder cancer tissues and that knockdown of
XIST caused an upregulation of miR-133a in bladder cancer cells,
suggesting that the increased expression of XIST may contribute to
the decreased expression of miR-133a in bladder cancer.
Furthermore, it was observed that silencing of miR-133a impaired
the inhibitory effects of XIST knockdown on the proliferation and
migration of bladder cancer cells, indicating that miR-133a is
indeed involved in XIST-mediated bladder cancer. In addition to
miR-133a, several other miRNAs, including miR-124 (33), miR-139 (22) and miR-200 (21), have also been identified as target
miRNAs of XIST in bladder cancer cells. Xiong et al
(33) reported that XIST directly
interacts with miR-124 and thus promotes the expression of androgen
receptor in bladder cancer cells. Hu et al (22) indicated that XIST promoted bladder
cancer cell growth and metastasis through interacting with
miR-139-5p and thus affected the activity of the Wnt/β-catenin
signalling pathway. The present study enhances the current
understanding of the function of the XIST/miRNA axis in bladder
cancer cells. Future studies should focus on identifying the
downstream protein targets of miR-133a in bladder cancer.
In conclusion, the present study demonstrated for
the first time that XIST promotes bladder cancer cell proliferation
and migration via modulation of miR-133a and thus suggests that
XIST may be used as a potential therapeutic target for bladder
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
KZ and JY designed the study and wrote the
manuscript. WC collected clinical tissues. KZ, XL and WC performed
all experiments and performed the statistical analysis.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Second Xiangya Hospital (Changsha, China). Written informed
consent was obtained from all patients involved in this study.
Patient consent for publication
All patients provided written informed consent for
their data to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sathe A and Nawroth R: Targeting the
PI3K/AKT/mTOR Pathway in Bladder Cancer. Methods Mol Biol.
1655:335–350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X and Zhang Y: Bladder cancer and
genetic mutations. Cell Biochem Biophys. 73:65–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao XW, Xiao JQ, Li ZY, Zheng YC and Zhang
N: Effects of microRNA-135a on the epithelial-mesenchymal
transition, migration and invasion of bladder cancer cells by
targeting GSK3β through the Wnt/β-catenin signaling pathway. Exp
Mol Med. 50:e4292018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie H, Liao X, Chen Z, Fang Y, He A, Zhong
Y, Gao Q, Xiao H, Li J, Huang W and Liu Y: LncRNA MALAT1 inhibits
apoptosis and promotes invasion by antagonizing miR-125b in bladder
cancer cells. J Cancer. 8:3803–3811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitra AP: Molecular substratification of
bladder cancer: Moving towards individualized patient management.
Ther Adv Urol. 8:215–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smolle MA and Pichler M: The role of long
non-coding RNAs in osteosarcoma. Noncoding RNA. 4(pii):
E72018.PubMed/NCBI
|
|
9
|
Peng Z, Liu C and Wu M: New insights into
long noncoding RNAs and their roles in glioma. Mol Cancer.
17:612018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong W, Huang C, Deng H, Jian C, Zen C,
Ye K, Zhong Z, Zhao X and Zhu L: Oncogenic non-coding RNA NEAT1
promotes the prostate cancer cell growth through the SRC3/IGF1R/AKT
pathway. Int J Biochem Cell Biol. 94:125–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cen C, Li J, Liu J, Yang M, Zhang T, Zuo
Y, Lin C and Li X: Long noncoding RNA LINC01510 promotes the growth
of colorectal cancer cells by modulating MET expression. Cancer
Cell Int. 18:452018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Cui Y, Liu LF, Ren WB, Li QQ, Zhou
X, Li YL, Li Y, Bai XY and Zu XB: High expression of long noncoding
RNA MALAT1 indicates a poor prognosis and promotes clinical
progression and metastasis in bladder cancer. Clin Genitourin
Cancer. 15:570–576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo J, Chen J, Li H, Yang Y, Yun H, Yang S
and Mao X: LncRNA UCA1 promotes the invasion and EMT of bladder
cancer cells by regulating the miR-143/HMGB1 pathway. Oncol Lett.
14:5556–5562. 2017.PubMed/NCBI
|
|
14
|
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X,
Wang M, Huang C, Wang L, Zeng F and Jiang G: LncRNA SPRY4-IT1
sponges miR-101-3p to promote proliferation and metastasis of
bladder cancer cells through up-regulating EZH2. Cancer Lett.
388:281–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhai X and Xu W: Long noncoding RNA ATB
promotes proliferation, migration and invasion in bladder cancer by
suppressing microRNA-126. Oncol Res. 26:1063–1072. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang M, Guo C, Wang L, Luo G, Huang C, Li
Y, Liu D, Zeng F, Jiang G and Xiao X: Long noncoding RNA GAS5
promotes bladder cancer cells apoptosis through inhibiting EZH2
transcription. Cell Death Dis. 9:2382018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Migeon BR: Choosing the Active X: The
human version of X inactivation. Trends Genet. 33:899–909. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shenoda BB, Tian Y, Alexander GM,
Aradillas-Lopez E, Schwartzman RJ and Ajit SK: miR-34a-mediated
regulation of XIST in female cells under inflammation. J Pain Res.
11:935–945. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Chen B, Liu P and Yang J: XIST
promotes gastric cancer (GC) progression through TGF-β1 via
targeting miR-185. J Cell Biochem. 119:2787–2796. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu H, Zheng T, Yu J, Zhou L and Wang L:
LncRNA XIST accelerates cervical cancer progression via
upregulating Fus through competitively binding with miR-200a.
Biomed Pharmacother. 105:789–797. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu R, Zhu X, Chen F, Huang C, Ai K, Wu H,
Zhang L and Zhao X: LncRNA XIST/miR-200c regulates the stemness
properties and tumourigenicity of human bladder cancer stem
cell-like cells. Cancer Cell Int. 18:412018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Deng C, Zhang H, Zhang J, Peng B and
Hu C: Long non-coding RNA XIST promotes cell growth and metastasis
through regulating miR-139-5p mediated Wnt/β-catenin signaling
pathway in bladder cancer. Oncotarget. 8:94554–94568. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei W, Liu Y, Lu Y, Yang B and Tang L:
LncRNA XIST promotes pancreatic cancer proliferation through
miR-133a/EGFR. J Cell Biochem. 118:3349–3358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kruger J and Rehmsmeier M: RNAhybrid:
microRNA target prediction easy, fast and flexible. Nucleic Acids
Res. 34:W451–W454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao Z, Qu Z, Chen Z, Fang Z, Zhou K,
Huang Z, Guo X and Zhang Y: LncRNA HOTAIR is a prognostic biomarker
for the proliferation and chemoresistance of colorectal cancer via
MiR-203a-3p-mediated Wnt/ß-catenin signaling pathway. Cell Physiol
Biochem. 46:1275–1285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu K, Yao H, Wen Y, Zhao H, Zhou N, Lei S
and Xiong L: Functional role of a long non-coding RNA
LIFR-AS1/miR-29a/TNFAIP3 axis in colorectal cancer resistance to
pohotodynamic therapy. Biochim Biophys Acta Mol Basis Dis.
1864:2871–2880. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Wan L, Liu Z, Xu G, Wang S, Su Z,
Zhang Y, Zhang C, Liu X, Lei Z and Zhang HT: Long non-coding RNA
XIST promotes TGF-β-induced epithelial-mesenchymal transition by
regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer.
Cancer Lett. 418:185–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang H, Zhang H, Hu X and Li W: Knockdown
of long non-coding RNA XIST inhibits cell viability and invasion by
regulating miR-137/PXN axis in non-small cell lung cancer. Int J
Biol Macromol. 111:623–631. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Z, Xu J, Lu H, Lin B, Cai S, Guo J,
Zang F and Chen R: LARP1 is regulated by the XIST/miR-374a axis and
functions as an oncogene in non-small cell lung carcinoma. Oncol
Rep. 38:3659–3667. 2017.PubMed/NCBI
|
|
31
|
Yang C, Wu K, Wang S and Wei G: Long
non-coding RNA XIST promotes osteosarcoma progression by targeting
YAP via miR-195-5p. J Cell Biochem. 119:5646–5656. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Z, Zhang B and Cui T: Long non-coding
RNA XIST exerts oncogenic functions in pancreatic cancer via
miR-34a-5p. Oncol Rep. 39:1591–1600. 2018.PubMed/NCBI
|
|
33
|
Xiong Y, Wang L, Li Y, Chen M, He W and Qi
L: The long non-coding RNA XIST interacted with MiR-124 to modulate
bladder cancer growth, invasion and migration by targeting androgen
receptor (AR). Cell Physiol Biochem. 43:405–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Xu J, Yang G, Li H and Guo X:
miR-202 inhibits cell proliferation, migration, and invasion by
targeting EGFR in human bladder cancer. Oncol Res. 26:949–957.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Uchida Y, Chiyomaru T, Enokida H, Kawakami
K, Tatarano S, Kawahara K, Nishiyama K, Seki N and Nakagawa M:
MiR-133a induces apoptosis through direct regulation of GSTP1 in
bladder cancer cell lines. Urol Oncol. 31:115–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou Y, Wu D, Tao J, Qu P, Zhou Z and Hou
J: MicroRNA-133 inhibits cell proliferation, migration and invasion
by targeting epidermal growth factor receptor and its downstream
effector proteins in bladder cancer. Scand J Urol. 47:423–432.
2013. View Article : Google Scholar : PubMed/NCBI
|