Introduction
Parkinson's disease (PD) is the second most common
neurological disorder worldwide and is caused by the degeneration
of midbrain dopamine (DA) neurons (1). The exposure to environmental toxins
during development, which impact DA precursor factors, may lead to
neurodegeneration and can increase the risk of developing PD
(2,3).
Wingless 1 (Wnt1) and Wingless 5a (Wnt5a), which are
two members of the Wnt family, are developmental factors that
regulate the proliferation and differentiation of DA precursors
(4). Wnt1 enhances mesencephalic DA
neuron differentiation (5), and
Wnt5a can regulate midbrain DA axon growth and guidance (6). Additionally, Wnt1 and Wnt5a cooperate
to regulate nuclear receptor-related factor 1 (NURR1) and tyrosine
hydroxylase (TH) expression (7,8). NURR1
is a brain-specific transcription factor located in DA neurons
(9) and induces the neurogenesis of
DA-phenotype neurons (10), while TH
is a rate-limiting enzyme located in DA neurons and is associated
with synthesizing DA (11). NURR1
can activate the promoter of TH gene in neural progenitor cells
increasing TH expression (12,13).
A previous study demonstrated that the developmental
exposure to the herbicide paraquat (PQ) and the fungicide maneb
(MB) in pregnant and lactating rats influenced the expression of
Wnt1, Wnt5a, NURR1 and TH in the midbrain DA neurons of offspring
(14). However, the mechanism of Wnt
signaling in PD is still yet to be determined.
PQ and MB are two common agricultural chemicals, and
their combined exposure has been used to model Parkinson-like motor
deficits in rodents and to investigate the mechanisms of
pathogenesis in this disease (15–18).
However, the combined effect of PQ and MB in vitro, and
their mechanism causing PD, is rarely reported. In the current
study, the human SH-SY5Y neuroblastoma cell line, which is used as
an in vitro cellular model of DA neurons, was exposed to PQ
and MB to explore the mechanisms of Wnt signaling in PD.
Materials and methods
Chemicals, reagents and
antibodies
PQ was purchased from J&K Technology Co. Ltd.
and MB was purchased from Sigma-Aldrich (Merck KGaA). Cell counting
kit-8 (CCK-8) assay was purchased from TransGen Biotech Co., Ltd.
Lipofectamine 2000® reagent was purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). Human Wnt1 gene small
interfering RNA (siRNA; cat. no. sc-36839) was purchased from Santa
Cruz Biotechnology, Inc.
Mouse monoclonal Wnt1 (cat. no. ab105740) and rabbit
polyclonal Wnt5a (cat. no. ab174963) antibodies were purchased from
Abcam. Rabbit polyclonal β-actin (cat. no. 20536-1-AP), rabbit
polyclonal β-catenin (cat. no. 17565-1-AP), rabbit polyclonal NURR1
(cat. no. 10975-2-AP) and mouse monoclonal TH (cat. no. 6634-1-Ig)
antibodies were purchased from Wuhan Sanying Biotechnology.
Horseradish peroxidase (HRP)-labeled goat anti-rabbit (cat. no.
ZB-2301) and goat anti-mouse (cat. no. ZB-2305) antibodies were
purchased from Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd. Alexa Fluor 647 labeled goat anti-rabbit (cat. no. ab150079),
Alexa Fluor 488 labeled goat anti-mouse (cat. no. ab150113) and
Alexa Fluor 488 labeled goat anti-rabbit (cat. no. ab150077)
antibodies were purchased from Abcam.
Cell culture
SH-SY5Y cells were gifted from the School of
Pathology in Harbin Medical University (Heilongjiang, China). Cells
were cultured in high-glucose Dulbecco's Modified Eagle Medium
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in an incubator, under 5% CO2 and 95% air. Cells were
induced to differentiate through the administration of 10 µM
retinoic acid (Shanghai Aladdin Biochemical Technology Co., Ltd.)
for 3–5 days in low serum medium, according to Kovalevich and
Langford (19). Then, differentiated
cells with a more pyramidal shaped body were used for the
subsequent experiments.
CCK-8 assay
SH-SY5Y cells were seeded in 96-well plates and
exposed to a range of doses of PQ, from 0–320 µM, and MB, from
0–12.8 µM for 24 h. The culture medium was subsequently removed.
Following the manufacturer's protocol, CCK-8 reagent was diluted in
culture medium and added to wells. Each 96-well plate was incubated
at 37°C for 2 h prior to the detection of absorbance at 450 nm.
Inhibition rates were calculated according to the manufacturer's
protocol.
Silencing Wnt1 in SH-SY5Y cells
A period of 1 day prior to transfection, cells were
seeded in 75 cm2 culture bottles or 24-well plates in
order to achieve 70% confluence for transfection. A specific siRNA
(cat. no. sc-36839) for the human Wnt1 gene (GenBank accession no.
NM_005430) was purchased from Santa Cruz Biotechnology, Inc. The
human SH-SY5Y cell line was transfected using a Lipofectamine
2000® reagent according to the manufacturer's protocol.
Cells in 75 cm2 culture bottles were transfected with
12.5 µl of 10 µmol/l Wnt1 siRNA, while cells in 24-well plates were
transfected with 4.5 µl of 10 µmol/l Wnt1 siRNA. A period of 4–6 h
following transfection, the media was replaced with fresh growth
media. Transfection efficiency was determined using western blot
analysis.
Western blot analysis
Cells were collected and lysed in lysis buffer
containing 1% protease inhibitor (both Beyotime Institute of
Biotechnology) for 1 h at 4°C. Samples were then centrifuged at
10,000 × g at 4°C for 15 min. Supernatants were collected and
protein concentration was determined using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). Equal
amounts (40 µg) of protein were separated by 10% SDS-PAGE and
electrotransferred onto a PVDF (Merck KGaA). Membranes were blocked
with 5% non-fat milk for 1 h at room temperature prior to
incubation overnight at 4°C in a solution of rabbit polyclonal
β-actin (1:1,000), mouse monoclonal Wnt1 (1:500), rabbit polyclonal
Wnt5a, rabbit polyclonal β-catenin, rabbit polyclonal NURR1 or
mouse monoclonal TH (all 1:1,000). The next day, membranes were
washed three times with tris buffered saline and incubated with
HRP-labeled goat anti-rabbit and anti-mouse (both 1:5,000), Alexa
Fluor 647-labeled goat anti-rabbit, and Alexa Fluor 488-labeled
goat anti-rabbit and anti-mouse (all 1:1,000) secondary antibodies
for 1 h at room temperature, and then washed three times with tris
buffered solution with 0.5% tween. Targeted proteins were
visualized using ECL reagent (Beyotime Institute of Biotechnology)
and exposed to a film. Densities of specific protein bands were
acquired using an Adobe Photoshop CS6 software (version 13.0; Adobe
Systems Software Corporation). The results were expressed as the
ratio of target protein to β-actin.
Immunofluorescence
Cell slides in the culture plate were washed with
PBS three times. Cells were then fixed with 4% paraformaldehyde for
15 min at room temperature, and washed with PBS three times. Next,
0.5% Triton X-100 was used to permeabilize cell membranes for 20
min at room temperature. After washing slides three times with PBS,
slides were dried with an absorbent paper. Non-specific antigens
were blocked using ready-to-use goat serum (Beyotime Institute of
Biotechnology) for 30 min at room temperature. Excess fluid was
subsequently removed using absorbent paper. Mouse monoclonal Wnt1
(1:200), rabbit polyclonal Wnt5a, rabbit polyclonal β-catenin,
rabbit polyclonal NURR1 or mouse monoclonal TH (all 1:500) primary
antibodies were then dropped onto slides, which were then incubated
in a wet box overnight at 4°C. The next day, slides were washed
three times with PBS containing 0.5% tween (PBST) and incubated
with Alexa Fluor 488-labeled goat anti-mouse or anti-rabbit, or
Alexa Fluor 647-labeled goat anti-rabbit or anti-mouse secondary
antibodies (all 1:1,000) in a wet box for 1 h at room temperature.
Slides were washed with PBST three times in the dark and then dried
with an absorbent paper, and sealed with a sealing liquid
containing anti-fluorescence quenching agent (Beyotime Institute of
Biotechnology). Slides were then observed and images were collected
using a Nikon Eclipse Ti fluorescence microscope (Nikon
Corporation) with a magnification of ×200.
Statistical analysis
All the data are presented as the mean ± SEM and
analyzed using SPSS 20.0 software (IBM Corp.). A one-way ANOVA was
performed followed by a Dunnett's T3 test to analyze differences of
Wnt pathway protein expression between the control and treatment
groups following PQ and MB exposure (n=3). A two sample Student's
t-test was used to analyze the differences of Wnt pathway protein
expression between normal SH-SY5Y cells and Wnt1-silenced SH-SY5Y
cells (n=3). P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibition of SH-SY5Y cell viability
by PQ-MB exposure
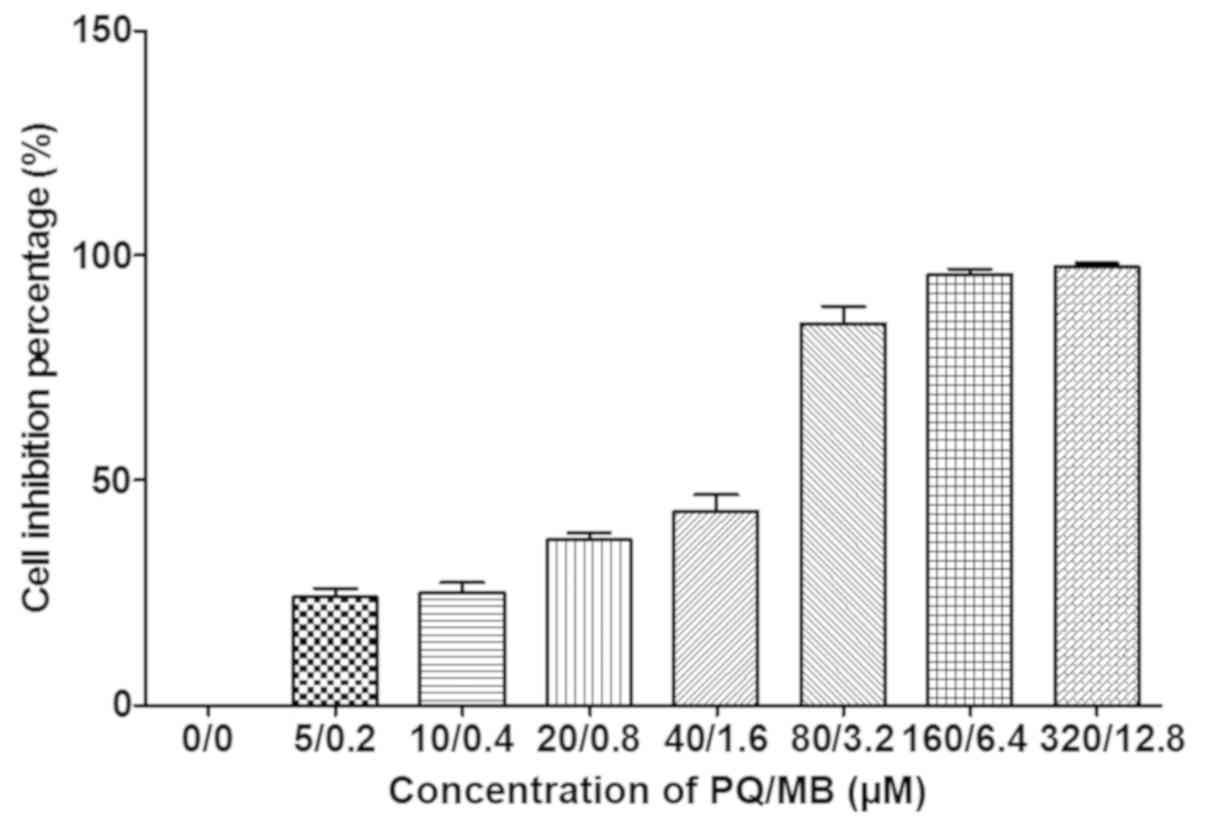
As presented in Fig.
1, average growth inhibition for 24 h in SH-SY5Y cells were 0,
17, 23, 35, 46, 78, 95 and 97% at PQ/MB doses of at 0/0, 5/0.2,
10/0.4, 20/0.8, 40/1.6, 80/3.2, 160/6.4 and 320/12.8 µM,
respectively.
Effects of combined exposure to PQ and
MB on Wnt signaling
The first four doses of PQ and MB (saline, 5/0.2,
10/0.4 and 20/0.8 µM) were used as control, low, middle and high
dosages of exposure for SH-SY5Y cells as the inhibitions were
<50%. Western blot analysis was used to measure the effects of
protein expression that was associated with Wnt signaling.
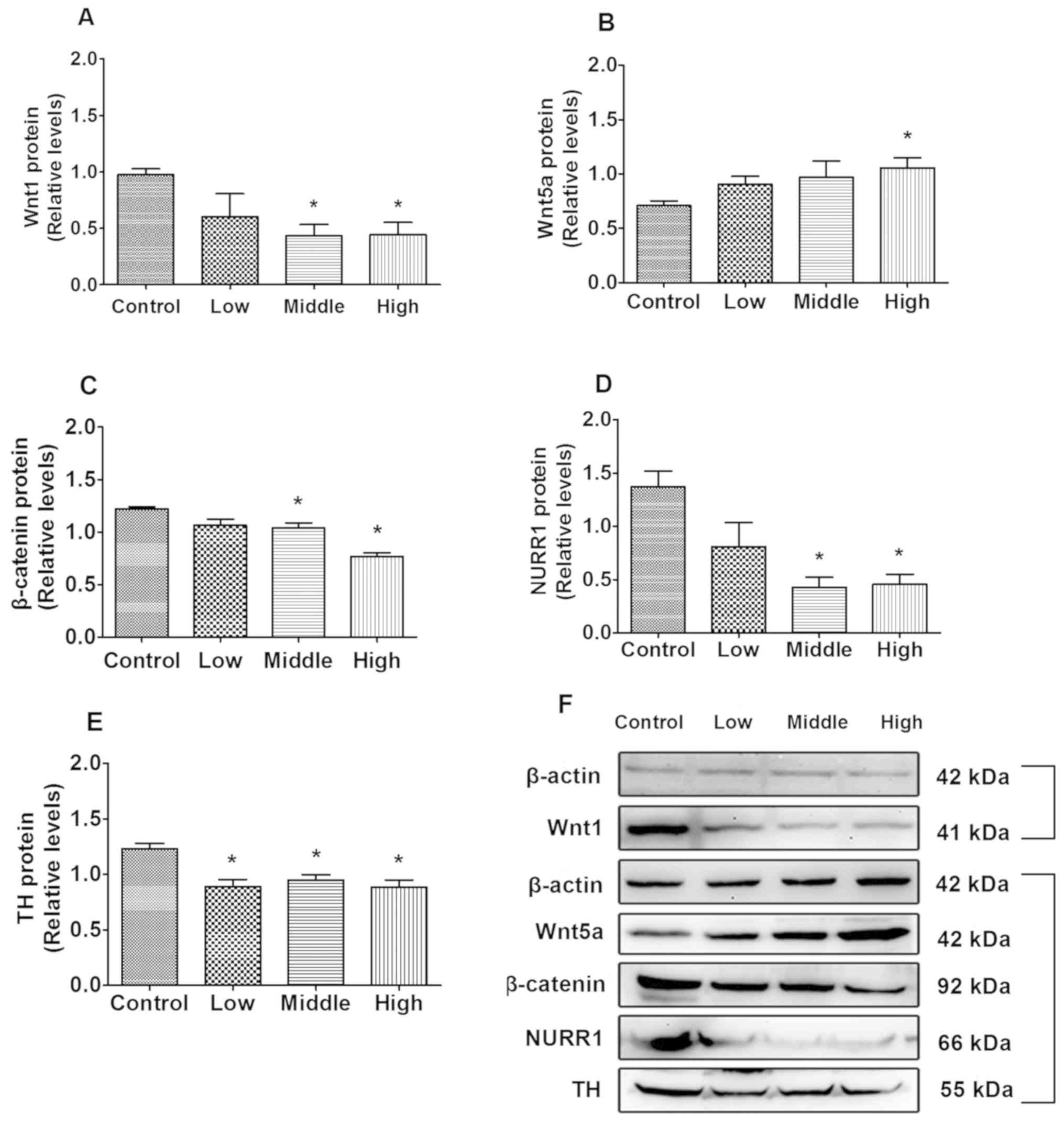
As indicated in Fig.
2A, compared with the control, Wnt1 protein expression
significantly decreased in the middle- and high-dose groups
(P=0.013 and P=0.019, respectively). Additionally, β-catenin and
NURR1 expression in the middle- (P=0.044 and P=0.001, respectively;
Fig. 2C and D) and high-dose groups
(P=0.007 and P=0.007, Fig. 2C and D,
respectively) were significantly decreased compared with the
control group, and TH expression in the low, middle and high dose
groups were significantly decreased compared with the control group
(P=0.010, P=0.009 and P=0.009, respectively; Fig. 2E). Wnt5a protein levels were
significantly increased in the high-dose group compared with the
control group (P=0.047; Fig.
2B).
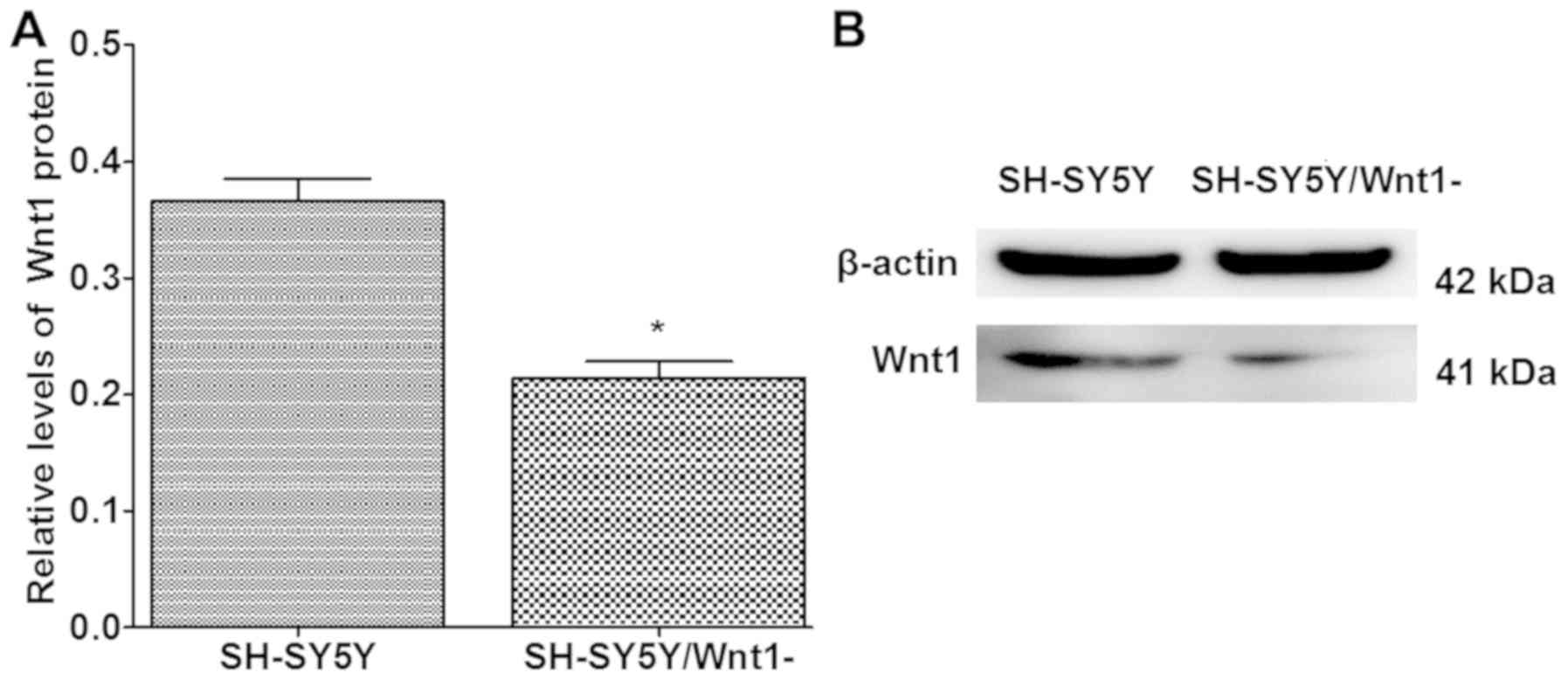
Silencing efficiency of Wnt1
Human-specific Wnt1 siRNA was used to silence Wnt1
expression in SH-SY5Y cells. The silencing efficiency of Wnt1 in
SH-SY5Y cells was ~50%, and the difference was significant when
compared with normal SH-SY5Y cells (P<0.001; Fig. 3).
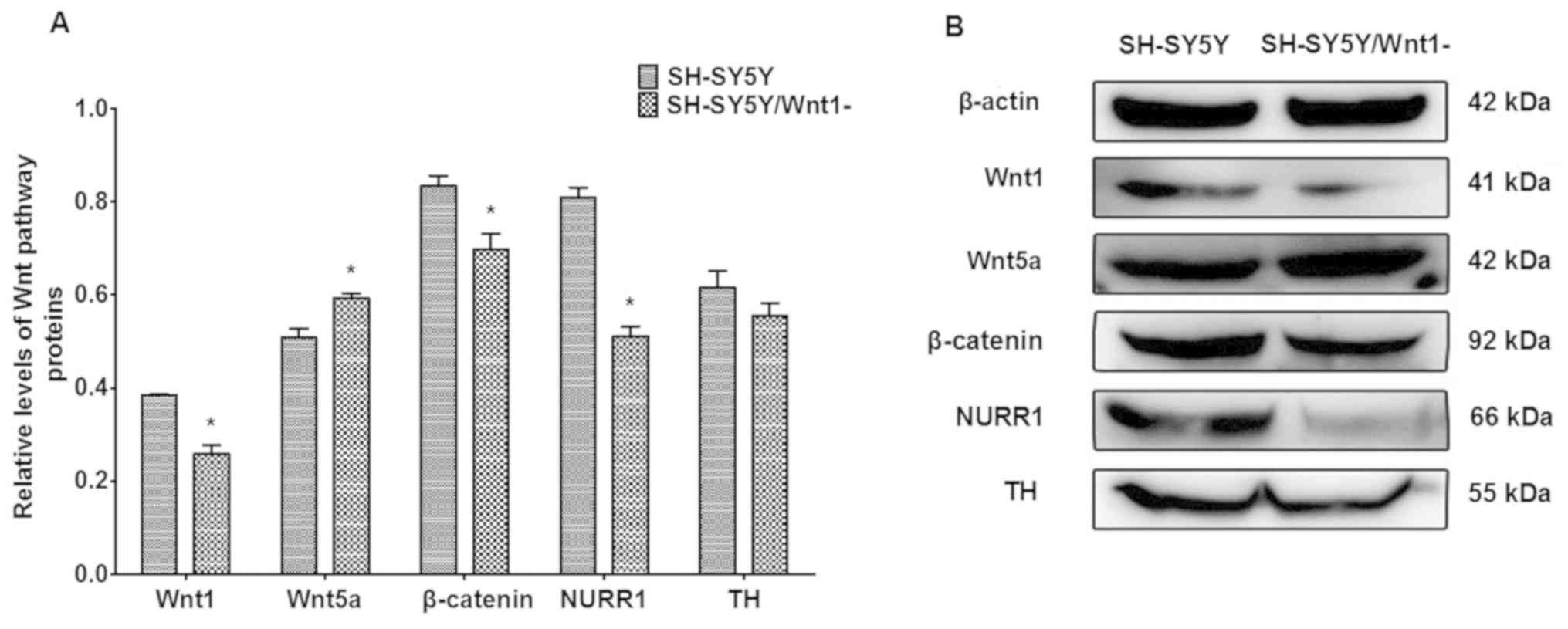
Effects of Wnt1 silencing on
dopaminergic factors
The expression of proteins that are associated with
Wnt signaling were investigated following Wnt1 silencing in SH-SY5Y
cells. As presented in Fig. 4, Wnt1,
β-catenin and NURR1 protein levels were significantly decreased
(P=0.164, P=0.024 and P=0.001, respectively), whereas the level of
Wnt5a protein significantly increased (P=0.022). TH expression also
decreased, although the difference was not significant. These
changes were similar to the effects of PQ- and MB-induced toxicity
on protein levels indicated in a previous in vivo study
(14).
Wnt1 silencing enhances toxicity
induced by combined exposure to PQ and MB
Normal SH-SY5Y and Wnt1-silencing SH-SY5Y cells were
exposed to PQ and MB (at doses of 20 and 0.8 µM, respectively).
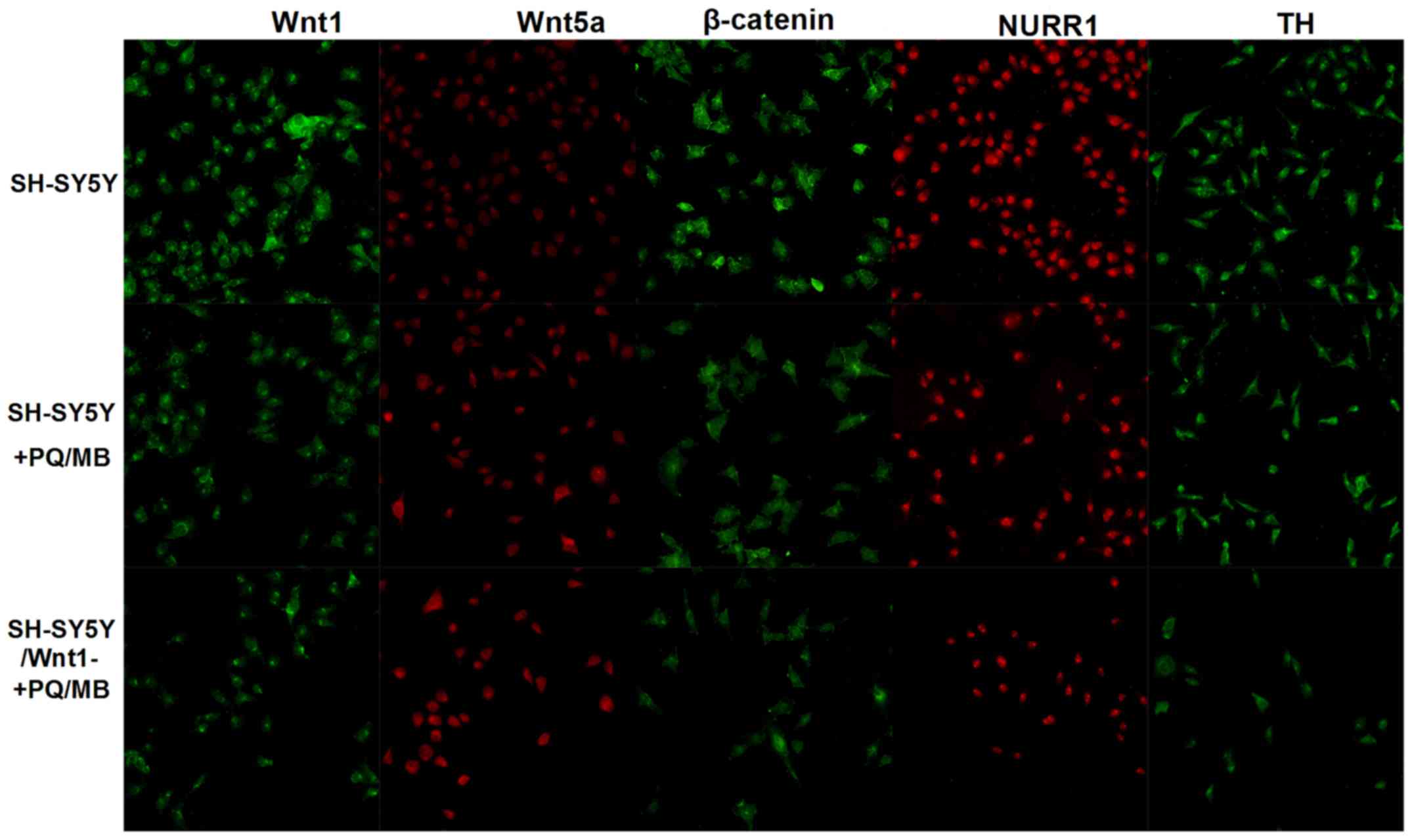
Immunofluorescence was used to visualize Wnt1, Wnt5a, β-catenin,
NURR1 and TH proteins. As shown in Fig.
5, the fluorescence of Wnt1, β-catenin, NURR1 and TH proteins
weakened in SH-SY5Y and SH-SY5Y/Wnt1-cells treated with PQ and MB
compared with untreated SH-SY5Y cells, whereas the fluorescence of
Wnt5a intensified.
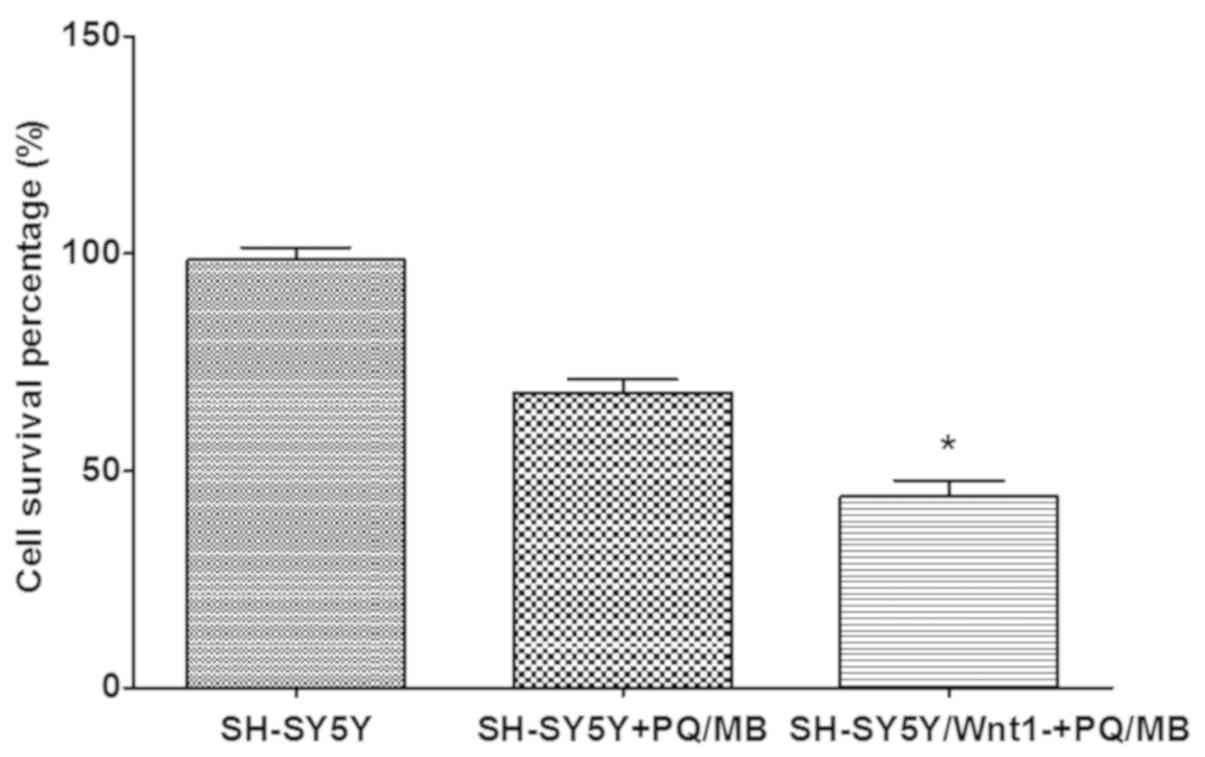
Cytotoxicity was performed using a CCK-8 assay. The
results presented in Fig. 6
indicated that the cell survival percentage in Wnt1-silencing cells
decreased more than normal SH-SY5Y cells after exposure to the same
doses of PQ and MB, which indicated that cell damage was more
severe. The differences were significant in the Wnt1 silenced and
the PQ/MB treatment groups when compared with the control group
(P<0.001).
Collectively, the hypothesized relationship between
Wnt1, Wnt5a, β-catenin, NURR1 and TH is summarized in Fig. 7. Wnt1 plays a positive role on
down-stream genes, β-catenin, NURR1 and TH, Wnt5a plays a negative
role. There is also an interaction between Wnt1 and Wnt5a.
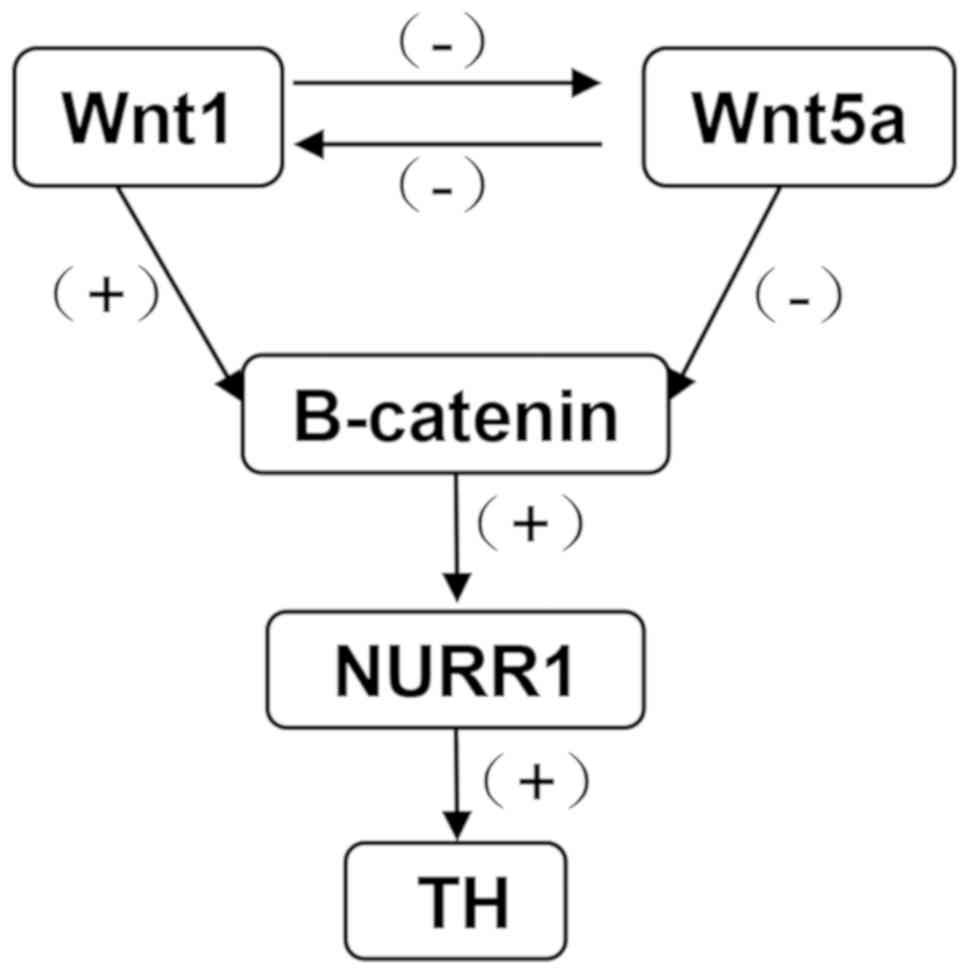 | Figure 7.Diagram presenting the interactions
between Wnt1, Wnt5a, β-catenin, NURR1 and TH following exposure to
PQ and MB. Wnt1 has a positive regulation on down-stream genes,
β-catenin, NURR1 and TH, and a negative regulation on Wnt5a. Wnt1,
wingless 1; Wnt5a, wingless 5a; NURR1, nuclear receptor-related
factor 1; TH, tyrosine hydroxylase; PQ, paraquat; MB, maneb; +,
positive regulation; -, negative regulation. |
Discussion
The SH-SY5Y cell line is frequently used as an in
vitro cellular model of DA neurons in neurotoxicity research
(20–23). However, according to Kovalevich and
Langford (19), the SH-SY5Y cell
line exhibits three morphologically distinct phenotypes during
development: A spindle shaped cell body, pyramidal shaped body and
epithetial-like cell body. The pyramidal shaped cell exhibits
increased neuronal functions. Therefore, spindle shaped cells were
induced to differentiate into pyramidal cells in a method that was
reported by Kovalevich and Langford (19). The PQ and MB dose used in the current
study was selected based on previous reports (24,25).
Inhibition in SH-SY5Y cells was acquired for 24 h under exposure to
PQ and MB. Doses with inhibition rates of 0, 17, 23, and 35% were
used as the control, low-, middle-, and high-exposure groups,
respectively.
In the current in vitro study, PQ and
MB-induced toxicity in SH-SY5Y cells decreased Wnt1, NURR1 and TH
expression, and increased Wnt5a expression. These results are
consistent with results from a previous in vivo study
(14). Additionally, the in
vitro expression of β-catenin was investigated in the current
study. β-Catenin is part of the canonical Wnt pathway, which is
essential for the neurogenesis of midbrain DA neurons (26–28).
Mice with a targeted deletion of β-catenin have been demonstrated
to exhibit deficits in motor learning and memory (29). The results of the current study
indicated that exposure to PQ and MB reduces β-catenin and Wnt1
expression levels in SH-SY5Y cells. This reduction may also
influence the survival rate of SH-SY5Y cells.
β-Catenin has also been demonstrated to protect PC12
cells against rotenone-induced neurotoxicity through the induction
of NURR1 expression (30). NURR1 is
a transcription factor that regulates the development of DA
precursors into mature DA neurons (31). In NURR1-deficient mice, DA
dysfunction occurs during ageing (32–34).
Therefore, NURR1 is considered to be a PD candidate gene (35). TH, which is a DA neuronal marker
expressed in mature DA neurons, has been indicated to be regulated
by NURR1 (36). In the current
study, NURR1, TH, Wnt1 and β-catenin were all decreased after
exposure to PQ and MB.
Wnt5a serves a role in the development of midbrain
DA neurons (37). However, in
contrast to Wnt1, β-Catenin, NURR1 and TH protein levels, Wnt5a
protein levels did not decrease in toxic SH-SY5Y cells induced by
PQ and MB, and conversely increased Wnt5a in the current study and
a previously reported in vivo study (14). This increased Wnt5a may be a
compensatory response to the induction of NURR1 and TH expression.
A previous study has also indicated that Wnt5a can inhibit the
canonical Wnt pathway and promote cardiac progenitor development
(38). Therefore, it can be
suggested that increased Wnt5a may also inhibit Wnt1 expression,
but this needs to be investigated.
In developing DA neurons, Wnt1 is expressed on
embryonic day 8 (E8) (39), prior to
the expression of Wnt5a on E9.5 (40), β-catenin on E9.5 (41), NURR1 on E10.5 (42) and TH on E11.5 (43). To explore the effect of Wnt1 on
β-catenin, Wnt5a, NURR1 and TH, Wnt expression was silenced in
SH-SY5Y cells. The results were similar to those of toxicity
induced by PQ and MB, which indicated that β-catenin, NURR1, and TH
were decreased while Wnt5a was increased. Therefore, the results of
the current study demonstrated that Wnt1 expression serves an
important role in maintaining DA neuron function.
After exposure of normal SH-SY5Y cells and
Wnt1-silenced SH-SY5Y cells to 20 µM PQ and 0.8 µM MB, a decreased
cell survival was observed in Wnt1-silencing cells compared with
normal SH-SY5Y cells. The results of the present study demonstrated
that Wnt1 silencing enhances the neurotoxicity that is induced by
PQ and MB. This finding is similar to that of a previous study,
which reported that exogenous Wnt1 protects SH-SY5Y cells against
6-hydroxydopamine toxicity (44). In
conclusion, the results of the current study revealed that Wnt1 may
be an effective candidate gene for the treatment of PD, but this
needs to be investigated further.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81402711).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BXL and YS designed the study. CH performed the
experiments and wrote the manuscript. JM analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Harbin Medical
University (Heilongjiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PD
|
Parkinson's disease
|
|
DA
|
dopamine
|
|
PQ
|
paraquat
|
|
MB
|
maneb
|
|
Wnt1
|
Wingless 1
|
|
Wnt5a
|
Wingless 5a
|
|
NURR1
|
nuclear receptor-related factor 1
|
|
TH
|
tyrosine hydroxylase
|
|
TBS
|
Tris-buffered saline
|
|
TBST
|
Tris-buffered saline with Tween
|
|
PBS
|
phosphate buffered saline
|
|
PBST
|
phosphate buffered saline with
Tween
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
References
|
1
|
Calabrese V, Santoro A, Monti D, Crupi R,
Di Paola R, Latteri S, Cuzzocrea S, Zappia M, Giordano J, Calabrese
EJ and Franceschi C: Aging and Parkinson's Disease: Inflammaging,
neuroinflammation and biological remodeling as key factors in
pathogenesis. Free Radic Biol Med. 115:80–91. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng P, Zhang X, Li D, Ji C, Yuan Z, Wang
R, Xue G, Li G and Hölscher C: Two novel dual GLP-1/GIP receptor
agonists are neuroprotective in the MPTP mouse model of Parkinson's
disease. Neuropharmacology. 133:385–394. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chinta SJ, Woods G, Demaria M, Rane A, Zou
Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J and
Andersen JK: Cellular senescence is induced by the environmental
neurotoxin paraquat and contributes to neuropathology linked to
Parkinson's disease. Cell Rep. 22:930–940. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Castelo-Branco G, Wagner J, Rodriguez FJ,
Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J and
Arenas E: Differential regulation of midbrain dopaminergic neuron
development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA.
100:12747–12752. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Gregorio R, Pulcrano S, De Sanctis C,
Volpicelli F, Guatteo E, von Oerthel L, Latagliata EC, Esposito R,
Piscitelli RM, Perrone-Capano C, et al: miR-34b/c regulates Wnt1
and enhances mesencephalic dopaminergic neuron differentiation.
Stem Cell Reports. 10:1237–1250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blakely BD, Bye CR, Fernando CV, Horne MK,
Macheda ML, Stacker SA, Arenas E and Parish CL: Wnt5a regulates
midbrain dopaminergic axon growth and guidance. PLoS One.
6:e183732011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitagawa H, Ray W, Glantschnig H,
Nantermet P, Yu Y, Leu CS, Kato S and Freedman L: A regulatory
circuit mediating convergence between Nurr1 transcriptional
regulation and Wnt signaling. Mol Cell Biol. 34:9172014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andersson ER, Saltó C, Villaescusa JC,
Cajanek L, Yang S, Bryjova L, Nagy II, Vainio SJ, Ramirez C, Bryja
V and Arenas E: Wnt5a cooperates with canonical Wnts to generate
midbrain dopaminergic neurons in vivo and in stem cells. Proc Natl
Acad Sci USA. 110:E602–E610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li HY, Liu F and Wang HR: Correlation
between Nurr1 expression and drug resistance in the brain of rats
with epilepsy. Eur Rev Med Pharmacol Sci. 22:1506–1513.
2018.PubMed/NCBI
|
|
10
|
Chen XX, Qian Y, Wang XP, Tang ZW, Xu JT,
Lin H, Yang ZY, Song XB, Lu D, Guo JZ, et al: Nurr1 promotes
neurogenesis of dopaminergic neuron and represses inflammatory
factors in the transwell coculture system of neural stem cells and
microglia. CNS Neurosci Ther. 24:790–800. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagatsu T and Nagatsu I: Tyrosine
hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other
catecholamine-related enzymes, and their human genes in relation to
the drug and gene therapies of Parkinson's disease (PD): Historical
overview and future prospects. J Neural Transm. 123:1255–1278.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim KS, Kim CH, Hwang DY, Seo H, Chung S,
Hong SJ, Lim JK, Anderson T and Isacson O: Orphan nuclear receptor
Nurr1 directly transactivates the promoter activity of the tyrosine
hydroxylase gene in a cell-specific manner. J Neurochem.
85:622–634. 2010. View Article : Google Scholar
|
|
13
|
Ding Y, Zhang Z, Ma J, Xia H, Wang Y, Liu
Y, Ma Q, Sun T and Liu J: Directed differentiation of postnatal
hippocampal neural stem cells generates nuclear receptor related-1
protein- and tyrosine hydroxylase-expressing cells. Mol Med Rep.
14:1993–1999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma J, Huang C, Ma K, Wu YP, Li BX and Sun
Y: Effect of Wnt1 and Wnt5a on the development of dopaminergic
neurons, and toxicity induced by combined exposure to paraquat and
maneb during gestation and lactation. Mol Med Rep. 16:9721–9728.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tinakoua A, Bouabid S, Faggiani E, De
Deurwaerdere P, Lakhdar-Ghazal N and Benazzouz A: The impact of
combined administration of paraquat and maneb on motor and
non-motor functions in the rat. Neuroscience. 311:118–129. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bastias-Candia S, Di Benedetto M,
D'Addario C, Candeletti S and Romualdi P: Combined exposure to
agriculture pesticides, paraquat and maneb, induces alterations in
the N/OFQ-NOPr and PDYN/KOPr systems in rats: Relevance to sporadic
Parkinson's disease. Environ Toxicol. 30:656–663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Desplats P, Patel P, Kosberg K, Mante M,
Patrick C, Rockenstein E, Fujita M, Hashimoto M and Masliah E:
Combined exposure to maneb and paraquat alters transcriptional
regulation of neurogenesis-related genes in mice models of
Parkinson's disease. Mol Neurodegener. 7:492012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta SP, Patel S, Yadav S, Singh AK,
Singh S and Singh MP: Involvement of nitric oxide in maneb- and
paraquat-induced Parkinson's disease phenotype in mouse: Is there
any link with lipid peroxidation? Neurochem Res. 35:1206–1213.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kovalevich J and Langford D:
Considerations for the use of SH-SY5Y neuroblastoma cells in
neurobiology. Methods Mol Biol. 1078:9–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu T, Niu C, Zhang X and Dong M:
β-Ecdysterone protects SH-SY5Y cells against β-amyloid-induced
apoptosis via c-Jun N-terminal kinase- and Akt-associated
complementary pathways. Lab Invest. 98:489–499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Y, Liu Y and Chen X: MiR-212
attenuates MPP+-induced neuronal damage by targeting
KLF4 in SH-SY5Y cells. Yonsei Med J. 59:416–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang CY, Sun ZN, Wang MX and Zhang C:
SIRT1 mediates salidroside-elicited protective effects against
MPP+-induced apoptosis and oxidative stress in SH-SY5Y
cells: Involvement in suppressing MAPK pathways. Cell Biol Int.
42:842018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Presgraves SP, Borwege S, Millan MJ and
Joyce JN: Involvement of dopamine D(2)/D(3) receptors and BDNF in
the neuroprotective effects of S32504 and pramipexole against
1-methyl-4-phenylpyridinium in terminally differentiated SH-SY5Y
cells. Exp Neurol. 190:157–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roede JR, Hansen JM, Go YM and Jones DP:
Maneb and paraquat-mediated neurotoxicity: Involvement of
peroxiredoxin/thioredoxin system. Toxicol Sci. 121:368–375. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Caputi FF, Carretta D, Lattanzio F,
Palmisano M, Candeletti S and Romualdi P: Proteasome subunit and
opioid receptor gene expression down-regulation induced by paraquat
and maneb in human neuroblastoma SH-SY5Y cells. Environ Toxicol
Pharmacol. 40:895–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang MZ and Huang EJ: β-Catenin controls
neurogenesis of midbrain dopamine neurons through cell adhesion and
junctional complex formation. Int J Dev Neurosci. 26:886. 2008.
View Article : Google Scholar
|
|
27
|
Colini Baldeschi A, Pittaluga E, Andreola
F, Rossi S, Cozzolino M, Nicotera G, Sferrazza G, Pierimarchi P and
Serafino A: Atrial natriuretic peptide acts as a neuroprotective
agent in in vitro models of Parkinson's disease via Up-regulation
of the Wnt/β-catenin pathway. Front Aging Neurosci. 10:202018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davis SW, Mortensen AH, Keisler JL,
Zacharias AL, Gage PJ, Yamamura K and Campe SA: β-catenin is
required in the neural crest and mesencephalon for pituitary gland
organogenesis. BMC Dev Biol. 16:162016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diaz-Ruiz O, Zhang Y, Shan L, Malik N,
Hoffman AF, Ladenheim B, Cadet JL, Lupica CR, Tagliaferro A, Brusco
A and Bäckman CM: Attenuated response to methamphetamine
sensitization and deficits in motor learning and memory after
selective deletion of β-catenin in dopamine neurons. Learn Mem.
19:341–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Luan C, Qu S, Lei W, Mo M, Feng
J, Sun C, Xiao Y, Qin L, Li S, et al: Enhancing beta-catenin
activity via GSK3beta inhibition protects PC12 cells against
rotenone toxicity through Nurr1 induction. PLoS One.
11:e01529312016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smits SM, Ponnio T, Conneely OM, Burbach
JP and Smidt MP: Involvement of Nurr1 in specifying the
neurotransmitter identity of ventral midbrain dopaminergic neurons.
Eur J Neurosci. 18:1731–1738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahn JH, Lee JS, Cho JH, Park JH, Lee TK,
Song M, Kim H, Kang SH, Won MH and Lee CH: Age-dependent decrease
of Nurr1 protein expression in the gerbil hippocampus. Biomed Rep.
8:517–522. 2018.PubMed/NCBI
|
|
33
|
Dong J, Wang Y, Liu XY and Le WD: Nurr1
deficiency-mediated inflammatory injury to nigral dopamine neurons
in Parkinson's disease. Parkinsonism Relat Disord. 46:e662018.
View Article : Google Scholar
|
|
34
|
Kummari E, Guo-Ross S and Eells JB: Region
specific effects of aging and the Nurr1-Null heterozygous genotype
on dopamine neurotransmission. Neurochem Neuropharmacol. 3(pii):
1142017.PubMed/NCBI
|
|
35
|
Le W, Pan T, Huang M, Xu P, Xie W, Zhu W,
Zhang X, Deng H and Jankovic J: Decreased NURR1 gene expression in
patients with Parkinson's disease. J Neurol Sci. 273:29–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sakurada K, Ohshima-Sakurada M, Palmer TD
and Gage FH: Nurr1, an orphan nuclear receptor, is a
transcriptional activator of endogenous tyrosine hydroxylase in
neural progenitor cells derived from the adult brain. Development.
126:4017–4026. 1999.PubMed/NCBI
|
|
37
|
Parish CL, Castelo-Branco G, Rawal N,
Tonnesen J, Sorensen AT, Salto C, Kokaia M, Lindvall O and Arenas
E: Wnt5a-treated midbrain neural stem cells improve dopamine cell
replacement therapy in parkinsonian mice. J Clin Invest.
118:149–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bisson JA, Mills B, Paul Helt JC, Zwaka TP
and Cohen ED: Wnt5a and Wnt11 inhibit the canonical Wnt pathway and
promote cardiac progenitor development via the Caspase-dependent
degradation of AKT. Dev Biol. 398:80–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Panhuysen M, Vogt Weisenhorn DM, Blanquet
V, Brodski C, Heinzmann U, Beisker W and Wurst W: Effects of Wnt1
signaling on proliferation in the developing mid-/hindbrain region.
Mol Cell Neurosci. 26:101–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andersson ER, Prakash N, Cajanek L, Minina
E, Bryja V, Bryjova L, Yamaguchi TP, Hall AC, Wurst W and Arenas E:
Wnt5a regulates ventral midbrain morphogenesis and the development
of A9-A10 dopaminergic cells in vivo. PLoS One. 3:e35172008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Joksimovic M and Awatramani R:
Wnt/β-catenin signaling in midbrain dopaminergic neuron
specification and neurogenesis. J Mol Cell Biol. 6:27–33. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prakash N and Wurst W: Development of
dopaminergic neurons in the mammalian brain. Cell Mol Life Sci.
63:187–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alves dos Santos MT and Smidt MP: En1 and
Wnt signaling in midbrain dopaminergic neuronal development. Neural
Dev. 6:232011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei L, Sun C, Lei M, Li G, Yi L, Luo F, Li
Y, Ding L, Liu Z, Li S and Xu P: Activation of Wnt/β-catenin
pathway by exogenous Wnt1 Protects SH-SY5Y cells against
6-hydroxydopamine toxicity. J Mol Neurosci. 49:105–115. 2013.
View Article : Google Scholar : PubMed/NCBI
|