Introduction
Large bone defects caused by acute injuries, trauma,
metabolic or genetic bone diseases, spinal degenerative diseases,
fall fractures in patients with osteoporosis, tumors and congenital
deformities are very common in clinical orthopedic cases (1,2).
Accumulating evidence has suggested that there are >3,000,000
patients with bone defects in China; in addition, the number of
bone defects is increasing 10% each year with increases in
population aging. Autogenous bone transplantation, which has the
advantages of biocompatibility and the lack of immunogenicity, or
alloplastic bone substitutes would be the gold standard for these
patients (3,4). Nevertheless, the clinical practice of
these therapies has been largely limited due to the lack of sources
for autogenous bone and by significant complications, including
infection, bleeding, pain and fracture. Therefore, it is of
critical importance to identify suitable substitutes or alternative
materials for bone transplantation.
In previous reports bone marrow stromal cells
(BMSCs) have been widely applied for the treatment of various
diseases, including graft-versus-host disease, osteogenesis
imperfecta and myocardial infarction (5,6). In
addition, multiple studies have clearly demonstrated that BMSCs
have great potency for promoting the regeneration of bone defects
in animal models and in humans, due to their high capacity for
self-renewal and multipotentiality for differentiation; therefore,
they are now being considered for use in a wide range of tissue
engineering applications, and in cell or gene therapy as an
alternative strategy and promising option (7,8).
Although it is accepted that the treatment of bone defects using
BMSCs or genetically modified BMSCs may effectively promote bone
regeneration in human and animal models, the size of the
regenerated bone has been a limiting factor for complete bone
repair, primarily due to a lack of vessels in the grafts, which
prevents sufficient nutritional support to the entire bone graft
(9). However, endothelial progenitor
cells (EPCs), a subpopulation of pluripotent hematopoietic stem
cells, may proliferate and migrate to sites of damaged endothelium
and differentiate into vascular endothelial cells (10). For example, exogenous EPCs were
implanted into various ischemic tissue models, including areas of
myocardial infarction and ischemic hindlimbs, to facilitate
neovascularization (11,12). Additionally, it has been demonstrated
that EPCs may contribute to new bone formation in fracture healing
(13,14). Therefore, EPCs may serve a critical
role in vessel regeneration and functional recovery following bone
injuries. With regard to the respective characteristics of BMSCs
and EPCs, the present study aimed to construct a cell sheet that
combined BMSCs and EPCs for the study of bone regeneration.
Bone morphogenetic proteins (BMPs) are potent
osteoinductive growth factors that induce ectopic bone formation
(15). Of these, BMP-2 is one of the
most potent osteoinductive cytokines and has been demonstrated to
initiate the differentiation of mesenchymal stem cells into
osteoblasts and chondrocytes in several animal models (16,17).
Recombinant human BMP-2 was first approved by the United States of
America Food and Drug Administration in 2002 to be used as a bone
graft substitute in the field of bone surgery, in procedures
including the fixation of open tibial fractures, spinal fusion
surgery, or oral and maxillofacial surgery (18). Therefore, the present study examined
whether BMP-2 exhibited a synergistic stimulatory effect on bone
formation in a co-culture of BMSCs and EPCs.
Materials and methods
BMSCs and EPC isolation and
culture
All experiments in the present study were performed
in compliance with the recommendations in the Guide for the Care
and Use of Laboratory Animals (19)
and approved by the Ethics Committee of The First Hospital of
Kunming Medical College (Kunming, China). Adult male Sprague-Dawley
(SD) rats (n=20; age, 5 weeks; weight, ~220 g) were
purchased from Charles River Laboratories. Rats were housed in
standard translucent ventilated laboratory rat cages, maintained in
climate-controlled rooms (21–24°C; 50–55% humidity) with diurnal
lighting (12-h light/dark cycle; lights on at 06:00). Bone marrow
collected from male SD rats aged 5 weeks was flushed out from the
femurs and tibias with Dulbecco's modified Eagle's medium (DMEM;
HyClone; GE Healthcare Life Sciences) containing 10% fetal bovine
serum (FBS; HyClone; GE Healthcare Life Sciences), 100 U/ml
penicillin and 100 g/ml streptomycin using a 1 ml syringe under
sterile conditions. To obtain the BMSCs, the bone marrow was placed
on top of Ficoll solution (cat. no. B340217P; Bioplorer; http://www.biomart.cn/infosupply/61288999.htm) and
centrifuged at 150 × g at 4°C for 25 min. The opaque white layer on
the surface of the Ficoll solution was carefully collected using
Pasteur pipettes and resuspended in DMEM. The collected cells were
analyzed by flow cytometry, and CD44 antigen (CD44)+ and
transferrin receptor protein 1 (CD71)+ cells were
isolated using fluorescence-assisted cell sorting (FACS), as
described subsequently. Then, the isolated cells were seeded into
tissue culture flasks at a final concentration of 1×106
cells/ml in DMEM. After 24 h incubation in a 37°C humidified
atmosphere with 5% CO2, non-adherent cells were removed,
and the adherent fraction was cultured in fresh medium. Cells used
for subsequent experiments were passaged ≤10 times.
The isolation of EPCs involved the use of Percoll,
rather than Ficoll, for density gradient centrifugation, 300 × g at
room temperature for 10 min. Similar to the BMSCs, the collected
EPCs were analyzed by flow cytometry, and platelet endothelial cell
adhesion molecule (CD31)+, prominin-1
(CD133)+ and vascular endothelial growth factor receptor
(VEGFR)+ cells were isolated using FACS, as described
subsequently. Isolated EPCs were washed twice with PBS and then
suspended at a density of 1×106 cells/ml in endothelial
cell growth medium (EGM) media (PromoCell GmbH) supplemented with
2% FBS and growth factors, including 50 ng/ml VEGF, 1 ng/ml basic
fibroblast growth factor and 2 ng/ml insulin-like growth factor 1.
Non-adherent cells were removed after 24 h incubation in 5%
CO2/95% air at 37°C in a humidified atmosphere, and
fresh EGM media was added to the culture dishes. After 5–7 days,
the adhered cells at 90% confluence were separated for subsequent
passages.
Flow cytometry analysis of cells
To analyze the expression of surface markers
characteristic of BMSCs and EPCs, FACS was performed using specific
fluorochrome-conjugated monoclonal antibodies corresponding to each
cell type. Briefly, BMSCs and EPCs were harvested at passage 3, and
1×106 cells were washed with 10% FBS/PBS and centrifuged
at 300 × g for 5 min at room temperature to pellet the cells. The
collected cells were blocked using Innovex Fc Receptor Blocker
(Newcomer Supply, Inc.) for 1 h at 4°C and washed using ice-cold
PBS and centrifuged at 300 × g at 4°C for 5 min. Subsequently,
primary antibodies purchased from BIOSS, including fluorescein
isothiocyanate/phycoerythrin (FITC/PE)-conjugated rat anti-CD44
(cat. no. bs-4916R-FITC), FITC/PE-conjugated rat anti-CD71 (cat.
no. bs-1782R-FITC) and FITC/PE-conjugated rat anti-receptor-type
tyrosine-protein phosphatase C (CD45) (cat. no. bs-10599R-FITC) at
a concentration of 2 mg/ml for the identification of BMSCs; and
FITC/PE-conjugated rat anti-CD31 (cat. no. bs-0195R-FITC),
FITC/PE-conjugated rat anti-CD133 (cat. no. bs-0395R-FITC) and
FITC/PE-conjugated rat anti-VEGFR (cat. no. bs-0170R-FITC) were
added to the cells at a concentration of 2 mg/ml and incubated with
anti-mouse compensation beads for 30 min in the dark for the
identification of EPCs. Subsequently, unbound antibody was removed
by washing with 2 ml of 10% FBS/PBS, and pellets were resuspended
in 500 µl PBS and examined by flow cytometry, with 10,000 events
recorded for each condition. Flow cytometry data was analyzed using
BD CellQuest™ Pro software Version 5.1 (BD Bioscience).
BMP2 gene transfer
BMP2 adenovirus (ad-BMP2) was purchased from Sangon
Biotech Co., Ltd. The optimal virus concentration for gene transfer
was evaluated by examining a range of multiplicities of infection
(MOI), according to the manufacturer's protocol. For the
transduction of BMSCs, ad-LacZ (control) or ad-BMP2 adenovirus was
added to the cells at a MOI of 100 in serum-free DMEM. After 4 h,
FBS was added to a final concentration of 2%, and cells were
cultured for an additional 24 h, using a protocol as described
previously (20).
Co-culture of BMSCs and EPCs
Following the third passage of BMSCs and 24 h after
adenoviral transduction, a mixture of EPCs and ad-lacZ or
ad-BMP-2-transduced BMSCs were co-cultured at EPC:BMSC ratio of
5:1, according to the optimized conditions as previously described
(9). This EPC: BMSC mixture was
seeded into 6-well plates at a density of 3.6×105
cells/well. When the cells grew to 80–90% confluence, the cell
culture medium was shifted to cell sheet-inducing medium (α-minimum
essential medium supplemented with 10% FBS and 1%
penicillin/streptomycin).
Proliferation assay
The proliferative capacity of the cells was measured
using a Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc.) according to the manufacturer's protocol.
Briefly, 2,000 cells/well were seeded into 96-well plates and
transfected with different adenoviral particles as aforementioned.
At the indicated time points (days 3 and 7), 10 µl CCK-8 reagent
was added to each well and cells were cultured for an additional 2
h, followed by measurement of the optical density (OD) at an
absorbance wavelength of 450 nm using an enzyme immunoassay
analyzer (Bio-Rad Laboratories, Inc.).
Assessment of BMP-2 release in
vitro
BMP-2 levels in BMSC: EPC-conditioned cell medium
were measured using a human-specific BMP-2 Quantikine ELISA kit
(cat. no. DBP2000; R&D Systems, Inc.) according to the
manufacturer's protocols.
Alkaline phosphatase (ALP)
activity
To determine ALP activity, cells from the different
treatment groups were lysed with 50 µl CelLytic M and 100 µl
substrate solution consisting of 3.33 mM MgCl2 (VWR
International, LLC) and 500 mM 2-amino-2-methyl-1-propanol
(Sigma-Aldrich; Merck KGaA) in distilled water with a pH 7.4, and
repeatedly frozen-thawed 3 times to disrupt the cell membranes.
Then, aliquots of the cell lysates were incubated with 0.5 µg/ml
p-nitrophenolphosphate (Sigma-Aldrich; Merck KGaA) for 30 min at
37°C, and the results were quantified at 405 nm using a microplate
reader (BioTek Instruments, Inc.). A standard curve for total ALP
activity was generated using increasing concentrations of the ALP
reaction product, 4-nitrophenol (0–1 nmol/µl; Sigma-Aldrich; Merck
KGaA).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
The expression levels of runt related transcription
factor 2 (Runx2), distal-less homeobox 5 (Dlx5), ALP and
integrin-binding sialoprotein (Ibsp) were analyzed by RT-qPCR in
cell cultures after 0, 1, 3, 7 and 14 days of growth. Total RNA was
isolated using RNeasy kit (Qiagen China Co., Ltd.), and its
quantity and purity were estimated using a NanoDrop™ 2000 (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). Only samples with an
A260/A280 nm ratio between 1.8 and 2.0 were
used. A total of 1 µg total RNA sample was used as a template for
conversion into cDNA using a SuperScript® First-Strand
Synthesis System kit (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. qPCR was performed using a
7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.); the reactions contained cDNA, primers (Table I) and SYBR® Premix Ex Taq™
(Takara Bio, Inc.). Post-PCR melting curves confirmed the
specificity of single-target amplification, and the fold change in
the gene of interest relative to GAPDH was determined. All
reactions were performed in triplicate. qPCR was performed with
SYBR® Premix Ex Taq™ in accordance with the
manufacturer's protocols (Takara Bio, Inc.) in a 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc). The
thermocycler conditions were as follows: Initial denaturation at
95°C for 10 sec, followed by 40 cycles of 95°C 10 for sec and 34
sec at 60°C. Each cDNA sequence was examined in triplicate, and a
dissociation melt curve protocol was conducted following each PCR
procedure, to determine the specificity of the products. The
relative amount of expressed mRNA was also calculated using the
2−ΔΔCq method (21).
 | Table I.Sequences of primers used in the
present study. |
Table I.
Sequences of primers used in the
present study.
| Gene | Primer
sequences |
|---|
| Runx2 | Forward,
5′-CGTCCACTCTGAATACCTGT-3′ |
|
| Reverse,
5′-TTGCCATTTTCAGTTTTGCAT-3′ |
| Dlx5 | Forward,
5′-CTCGGCTTCCTGGTACCCAA-3′ |
|
| Reverse,
5′-TCCATTGTTCAAACATCCCCGTA-3′ |
| ALP | Forward,
5′-TCAAAGCAGCATCTTACCAGT-3′ |
|
| Reverse,
5′-TGCCACAGTCAATACCGGAA-3′ |
| Ibsp | Forward,
5′-TCAACTCAGGAAGGTGCAAT-3′ |
|
| Reverse,
5′-CAGCCCTGATTTACGATGACC-3′ |
| GAPDH | Forward,
5′-CAAAGTGGACATTGTTGCCAT-3′ |
|
| Reverse,
5′-TCACCCCATTTGATGTTAGCG-3′ |
Western blot analysis
Total protein was extracted from all samples with
radioimmunoprecipitation assay lysis buffer (0.5 mM HEPES, pH 7.4,
5.0 M NaCl, 10% Triton X-100, 10% glycerol, 0.2 M
Na3VO4, 0.5 M NaF and 0.1 M NaPP) and
quantified using the Bicinchoninic Acid assay method (Promega
Corporation). A total of 30 µg protein per sample was
electrophoresed on an 8–10% denaturing SDS-PAGE gel and transferred
to a polyvinylidene difluoride membrane (EMD Millipore) at a
constant current of 200 mA for 60 min. The membrane was blocked
with 5% non-fat milk in TBS with 0.1% Tween-20 (TBST) for 2 h at
room temperature and incubated overnight with the following primary
antibodies: Rabbit anti-rat Runx2 (1:1,500; cat. no. ab92336;
Abcam); rabbit anti-rat Dlx (1:1,000; cat. no. ab109737; Abcam);
rabbit anti-rat ALP (1:500; cat. no. sc-365765; Santa Cruz
Biotechnology, Inc.); rabbit anti-rat Ibsp (1:1,000; cat. no.
ab52128; Abcam); rabbit anti-rat VEGF (1:1,000; cat. no. ab53465;
Abcam); rabbit anti-rat osteonectin (also known as SPARC; 1:2,000;
cat. no. ab245733; Abcam); rabbit anti-rat osteopontin (1:2,000;
cat. no. ab8448; Abcam); and rabbit anti-rat type I collagen
(1:1,500; cat. no. 84336; Cell Signaling Technology, Inc.) for 1.5
h at room temperature. Following extensive washing with TBST, the
membrane was exposed to the corresponding horseradish peroxidase
(HRP)-conjugated secondary antibodies (1:3,000; cat. no. ab97080;
Abcam) for 1 h at room temperature and detected using the
Phototope-HRP Western Detection kit (Thermo Fisher Scientific,
Inc). Expression levels of each protein were normalized to that of
GAPDH.
Scanning electron microscopy
(SEM)
SEM was used to examine the surface and
microstructure of the cell sheets. Briefly, the cells from
different groups were washed with PBS twice, fixed with 4%
glutaraldehyde solution at room temperature for 2 h and dehydrated
in increasing concentrations of ethanol (50, 70, 80, 90, 95 and
100%). Subsequently, the samples were freeze-dried, coated with a
gold layer using a sputter coater, and imaged by SEM.
Statistical analysis
All experimental values are presented as the mean ±
standard deviations, and all analyses were performed using the SPSS
v17.0 software (SPSS, Inc.) One-way ANOVA followed by Tukey's
post-hoc test was used to compare data between >2 groups, and
one-tailed t-test between two groups by using SPSS software
(version 19.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification and isolation of BMSCs
and EPCs
The present study assessed the phenotypes of BMSCs,
EPCs and cells isolated from BMSCs and EPCs by flow cytometric
analysis. Ficoll density gradient-separated BMSCs were identified
to express high levels of CD44 and CD71, but did not express CD45
(Fig. 1A). In addition, Percoll
density gradient-separated EPCs were identified to express high
levels of EPC surface markers, including CD31, CD133 and VEGFR
(Fig. 1B). CD44+ and
CD71+ cells were isolated from pure BMSCs and
CD31+, CD133+ and VEGFR+ cells
were isolated from pure EPCs using FACS. The isolated BMSCs and
EPCs were cultured for subsequent experiments. The results from the
flow cytometry analysis suggested that the isolated cells were
classic BMSCs and EPCs, respectively.
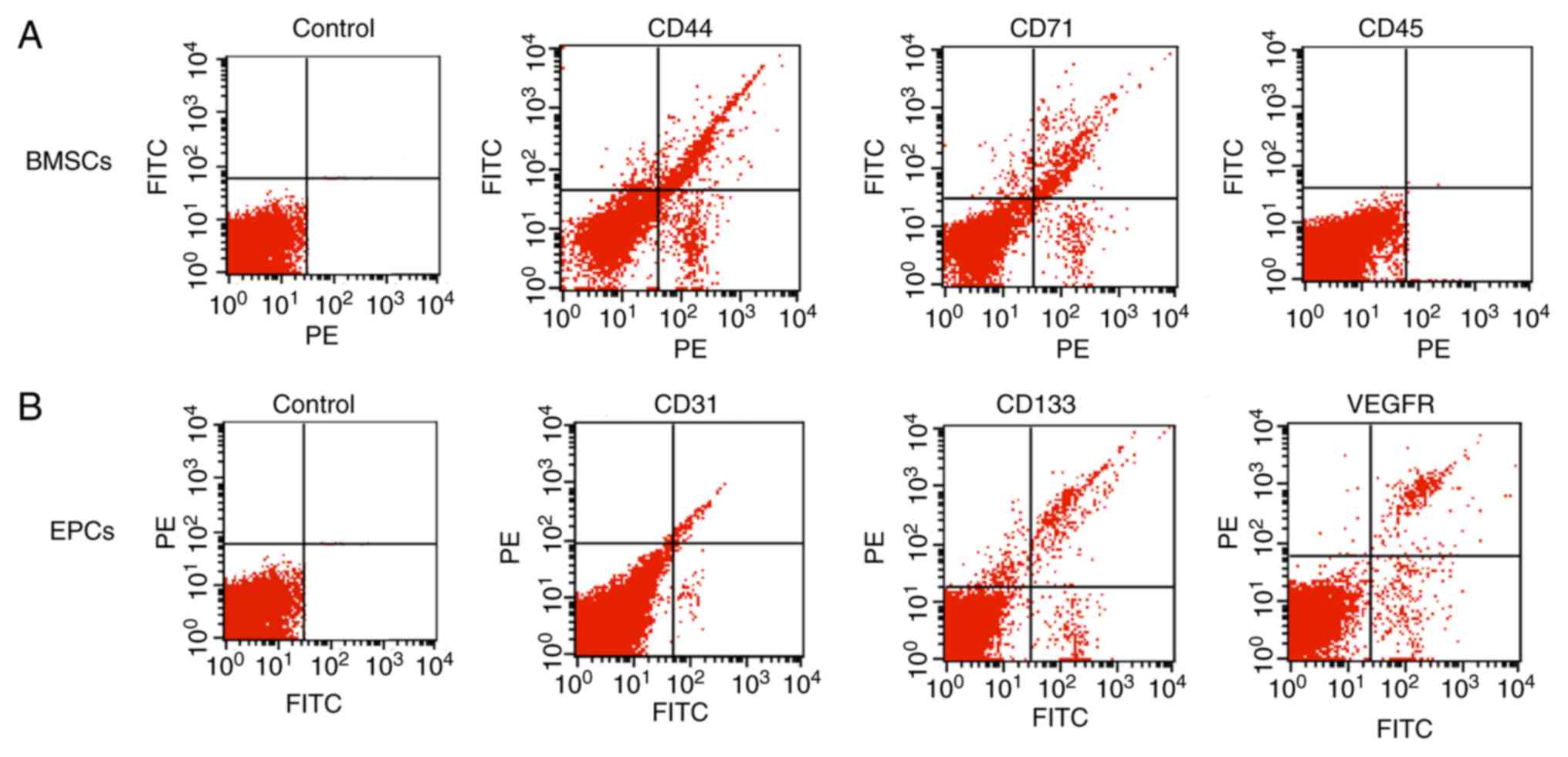 | Figure 1.Characterization of BMSCs and EPCs.
(A) Flow cytometry analysis of Ficoll-separated BMSCs surface
markers, including CD44, CD71 and CD45. (B) Flow cytometry analysis
of Percoll-separated EPCs surface markers, including CD31, CD133
and VEGFR. BMSCs, bone marrow stromal cells; EPCs, endothelial
progenitor cells; CD44, CD44 antigen; CD71, transferrin receptor
protein 1; CD45, receptor-type tyrosine-protein phosphatase C;
CD31, platelet endothelial cell adhesion molecule; CD133,
prominin-1; VEGFR, vascular endothelial growth factor receptor;
FITC, fluorescein isothiocyanate; PE, phycoerythrin. |
ad-BMP-2-BMSCs/EPCs system accelerates
cell proliferation, promotes persistent BMP-2 secretion and
activates ALP activity in the BMSCs and EPCs co-culture system
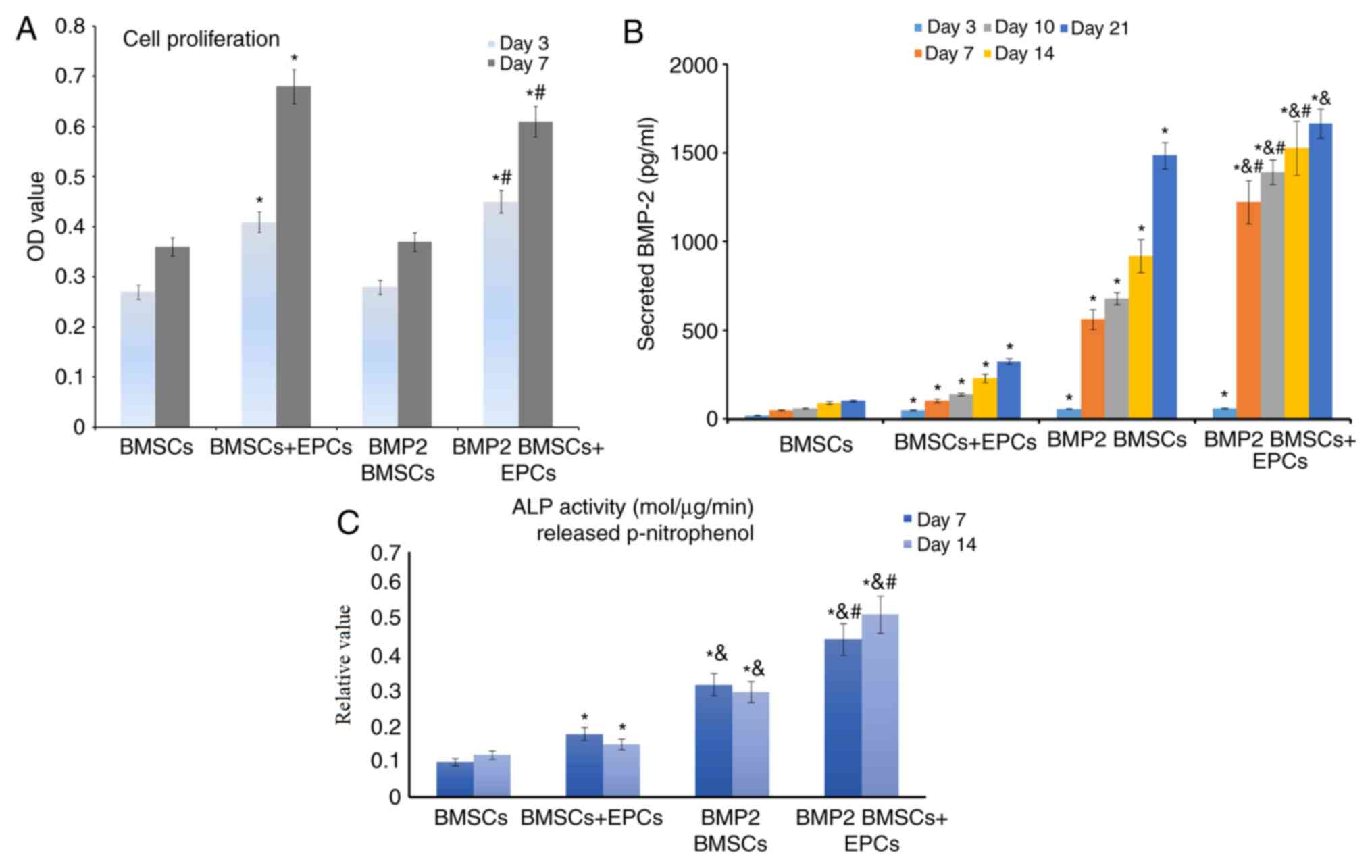
A CCK-8 assay was used to quantify levels of cell
proliferation. It was demonstrated that the OD values after 7 days
were increased compared with those after 3 days in all groups
(Fig. 2A). In addition, the cells in
the BMP-2-modified BMSCs + EPCs group grew at an increased rate
compared with those in the other groups. BMP2 secretion and ALP
activity in the BMSCs and EPCs co-culture system were markedly
increased in a time-dependent manner (Fig. 2B and C). Collectively, these data
concluded that the BMSCs and EPCs co-culture system may promote
bone cell proliferation and differentiation.
ad-BMP-2-BMSCs/EPCs system promotes
the mRNA expression of Runx2, Dlx5, ALP and Ibsp
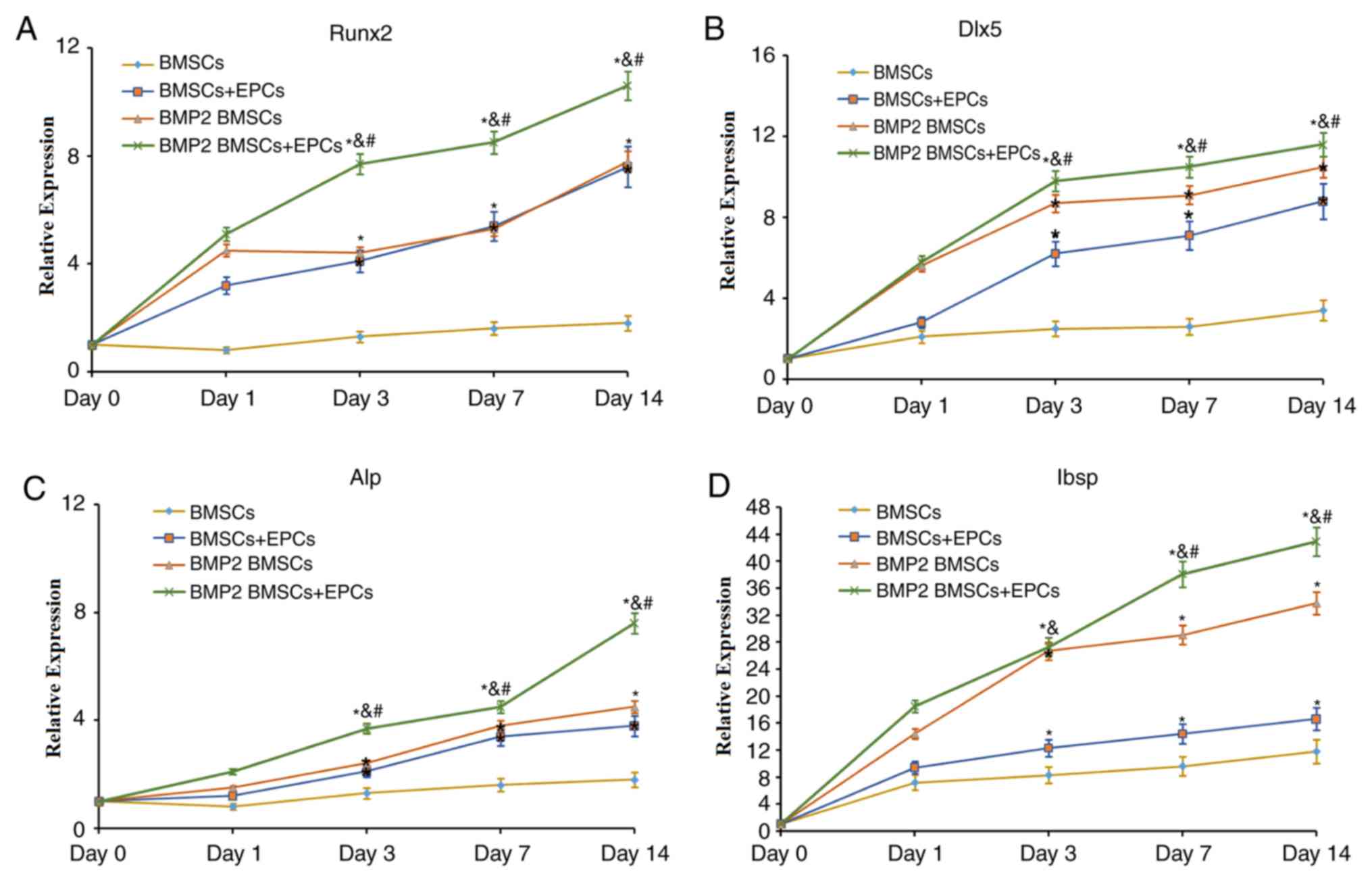
Next, the expression levels of osteoblast-associated
genes and proteins, including Runx2, Dlx5, ALP and Ibsp, were
examined to evaluate osteoblast differentiation and bone formation
in each group. As indicated in Fig.
3, the expression levels of Runx2 (Fig. 3A), Dlx5 (Fig. 3B), ALP (Fig. 3C) and Ibsp (Fig. 3D), which are essential in bone
formation, increased with time in all groups. In addition, the most
notable changes in expression of these genes were in the
BMP-2-modified BMSCs/EPCs group. Concomitantly, the expression
levels of these genes in BMP-2-modified BMSCs were significantly
increased compared with those in the BMSCs and the BMSCs + EPCs
group at all times. Therefore, these data indicated that BMP-2 may
facilitate bone formation in a co-culture of BMSCs and EPCs.
ad-BMP-2-BMSCs/EPCs system promotes
the protein expression of Runx2, Dlx, ALP, Ibsp, VEGF, osteonectin
and collagen I
As demonstrated in Fig.
4, Runx2, Dlx, ALP, Ibsp, VEGF, osteonectin and collagen I
protein levels were markedly increased in the BMP-2-modified BMSCs
+ EPCs group compared with the other groups. These results
suggested that BMP-2-modified BMSCs may affect the expression of
Runx2, Dlx, ALP, Ibsp, VEGF, osteonectin and collagen I, which may
contribute to bone formation in a co-culture of BMSCs and EPCs.
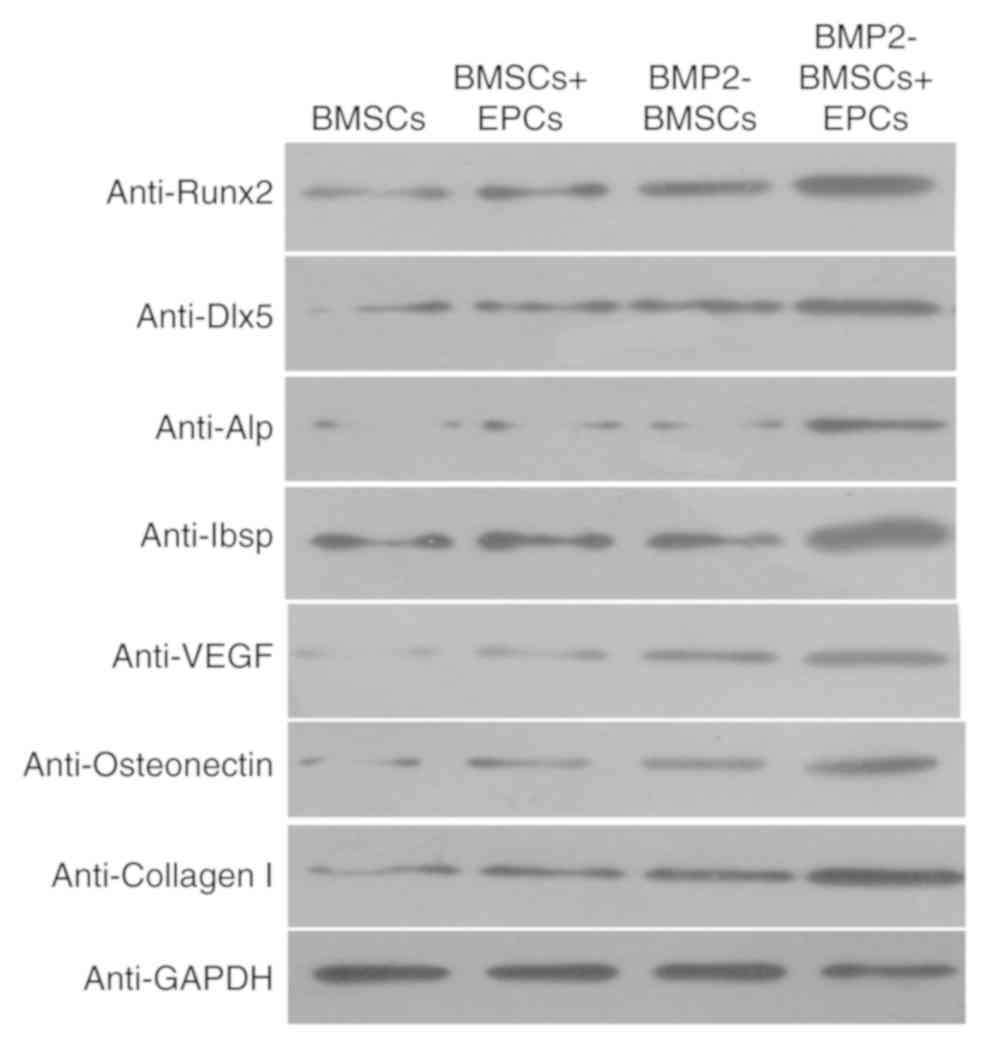 | Figure 4.Protein expression of
osteogenesis-associated genes. Runx2, Dlx5, ALP, Ibsp, VEGF,
osteonectin and collagen I protein levels in BMSCs, BMSCs + EPCs,
BMP2 BMSCs and BMP2 BMSCs + EPCs cultures by western blot analysis.
Runx2, runt related transcription factor 2; Dlx5, distal-less
homeobox 5; ALP, alkaline phosphatase; Ibsp, integrin binding
sialoprotein; VEGF, vascular endothelial growth factor; collagen I;
type I collagen BMSCs, bone marrow stromal cells; EPCs, endothelial
progenitor cells; BMP2, bone morphogenic protein 2. |
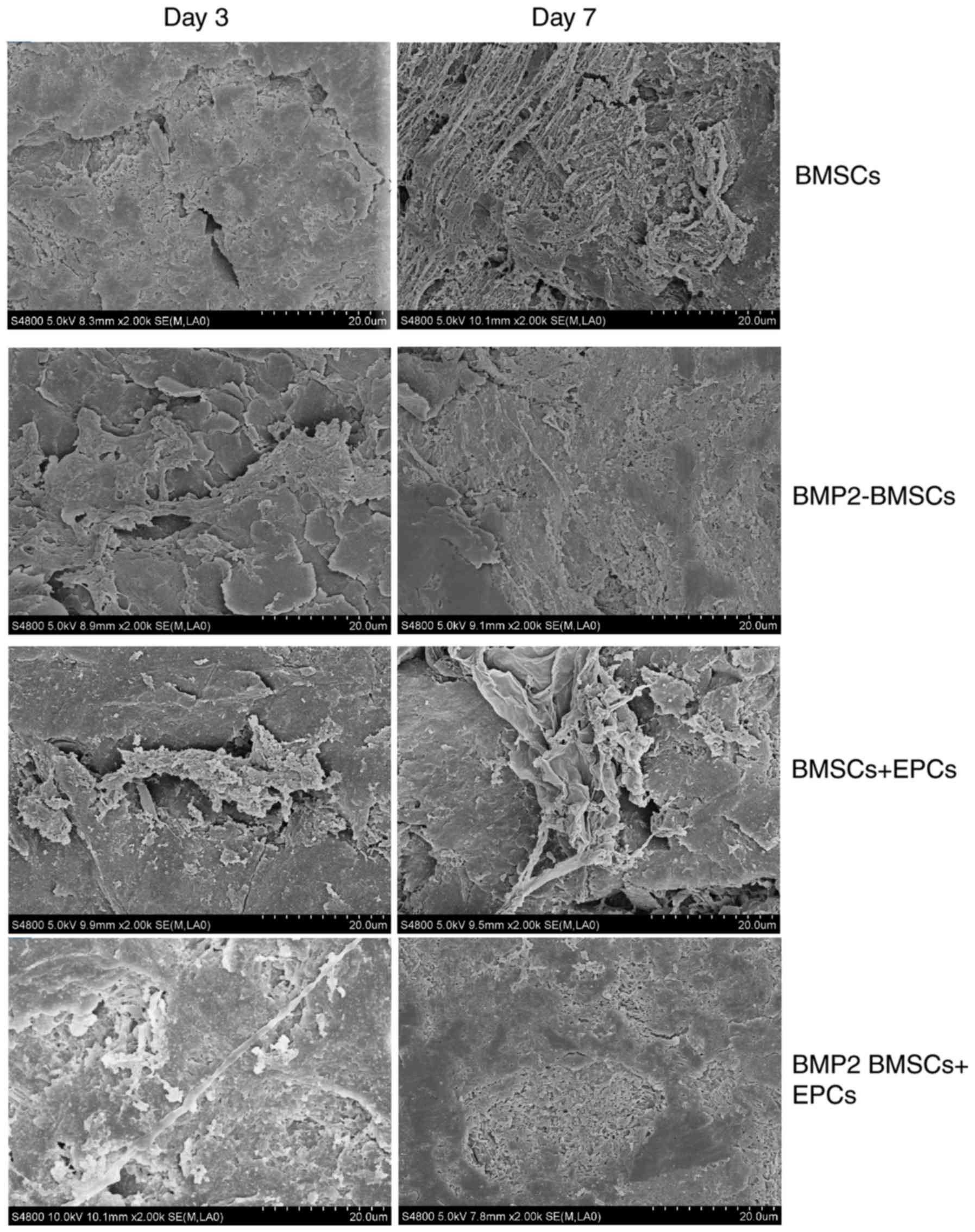
SEM observation of cell sheets
To examine the osteoblast-oriented differentiation
of the BMP2-modified BMSCs in culture conditions with EPCs, SEM was
employed to observe the morphological appearance and ultrastructure
of the BMP2-modified BMSCs/EPCs construct. It was clearly observed
that the BMP2-modified BMSCs presented as ball-shaped, and they
adhered well to the surface of EPCs through their pseudopods
(Fig. 5). Concomitantly, the
BMP2-modified BMSCs secreted extracellular matrix, expanded well
and extended multiple cell processes into the pores of the EPCs
(Fig. 5). Therefore, from these
results it was concluded that tissue engineering approaches using
BMP2-modified BMSCs in combination with EPCs may be a suitable cell
delivery strategy for treating bone defects.
Discussion
Bone defects or nonunion, which are serious
consequences for conditions including surgical resections,
infection, trauma, spinal degenerative diseases, fall fractures in
patients with osteoporosis, tumors or other systemic problems that
negatively affect the bone healing process, remain a major clinical
challenge in orthopedic surgery (22,23).
Presently, ideal bone tissue engineering should not only mimic
local tissue architecture but also support robust osteogenic
differentiation of cells (24,25).
BMSCs have been confirmed to exhibit osteogenetic potential
(26,27). In addition, EPCs have also been
demonstrated to contribute to enhanced bone formation to bridge
bone defects during bone repair (28,29).
Therefore, BMSCs and EPCs are considered attractive cell sources
for tissue engineering applications and key candidates for cell
therapy in bone defects. In the present study, surface markers were
used to identify, isolate and culture the BMSCs and EPCs cells from
the bone marrow of SD rats. The results revealed that the isolated
and cultured cells exhibited typical characteristics of BMSCs and
EPCs. Following verification of the phenotypes of the BMSCs and
EPCs, the BMP2 gene was transferred into BMSCs. BMP2, a
multifunctional growth factor belonging to the transforming growth
factor β superfamily, is a potent osteoinductive molecule that
induces osteogenic differentiation of immature osteoblasts and
non-committed cells, stimulates bone formation and accelerates
callus remodeling and fracture healing (15,30,31).
Therefore, to additionally investigate the effects of BMP2 in a
mixed culture of BMP2-modified BMSCs and EPCs, a co-culture system
of BMP2-modified BMSCs and EPCs was constructed at a ratio of 1:5.
Concomitantly, a series of biochemical experiments were performed.
It was identified that the levels of cell proliferation in the
BMP2-modified BMSCs + EPCs group were remarkably increased compared
with those in the other groups. Additionally, the secreted BMP2
levels and ALP activity were markedly increased in the
BMP2-modified BMSCs + EPCs group as compared with the other groups.
Cell proliferation is an important factor for the evaluation of
cell growth ability; therefore, the data from the present study
implied that BMP2 may accelerate cell proliferation in the cell
sheet system. It is known that BMP2 is a crucial factor for the
regulation of angiogenesis and bone formation, and serves as a
marker for angiogenesis (31,32).
Therefore, the increase in its secretion observed in the present
study indicated that the capacity of bone formation was notably
increased in the BMP2-modified BMSCs + EPCs group. Furthermore, ALP
is an indicator of bone tissue maturation (33,34),
suggesting that BMP2 may quickly promote bone maturation. In
addition, the expression levels of bone formation-associated genes
and proteins were examined using RT-qPCR and western blot analysis,
respectively. Tissue engineering offers a novel approach to
regenerate diseased or damaged tissues, including bone, which is a
natural organic-inorganic composite consisting of collagen fibrils
containing embedded, well-arrayed, nanocrystalline and plate-like
inorganic materials (24). During
the bone formation process, a number of osteogenesis-associated
genes and proteins serve important regulatory roles. In the present
study, the levels of the osteogenesis-associated genes and proteins
examined were all augmented in the BMP2-modified BMSCs + EPCs
group. Furthermore, the SEM results indicated the successful
formation of a composite scaffold of BMP2-modified BMSCs and EPCs,
which implied that the BMP2-modified BMSCs and EPCs constructs may
have the ability to facilitate bone regeneration.
Taken together, the data from the present study
indicated that BMSCs and EPCs were successfully isolated, and
demonstrated that BMP2-modified BMSCs combined with EPCs may
promote cell proliferation, promote BMP2 secretion and enhance ALP
activity. In addition, the expression levels of
osteogenesis-associated genes and proteins were markedly increased
in cell sheets of the BMP2-modified BMSCs + EPCs group. Finally,
SEM observation also revealed that the correct combination of
BMP2-modified BMSCs and EPCs may integrate to generate cell sheets.
Therefore, the use of cell sheets of BMP2-modified BMSCs and EPCs
may be a promising and effective clinical treatment modality for
patients with bone defects.
Acknowledgements
Not applicable.
Funding
This study is supported by The National Natural
Science Foundation of China (grant nos. 81460298 and 81460296).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JE and XH performed the molecular and animal
experiments, data acquisition and revised the article; SW, YZ and
XD analyzed and interpreted the data. BL performed the molecular
experiment during the revision and edited the revision; LL and XZ
designed the concept and wrote the manuscript. All of the authors
agree to publish the manuscript.
Ethics approval and consent to
participate
All experiments in the present study were performed
in compliance with the recommendations in the Guide for the Care
and Use of Laboratory Animals of the National Institutes of Health
and approved by the Ethics Committee of The First Hospital of
Kunming Medical College (Kunming, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Younger EM and Chapman MW: Morbidity at
bone graft donor sites. J Orthop Trauma. 3:192–195. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banwart JC, Asher MA and Hassanein RS:
Iliac crest bone graft harvest donor site morbidity. A statistical
evaluation. Spine (Phila Pa 1976). 20:1055–1060. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Meng G, Zhang L, Xiong Z and Liu
J: Physical properties and biocompatibility of a core-sheath
structure composite scaffold for bone tissue engineering in vitro.
J Biomed Biotechnol. 2012:5791412012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie H, Wang Z, Zhang L, Lei Q, Zhao A,
Wang H, Li Q, Chen Z and Zhang W: Development of an
angiogenesis-promoting microvesicle-alginate-polycaprolactone
composite graft for bone tissue engineering applications. PeerJ.
4:e20402016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu W, Le AV, Mendez JJ, Chang J, Niklason
LE and Steinbacher DM: Osteogenic performance of donor-matched
human adipose and bone marrow mesenchymal cells under dynamic
culture. Tissue engineering. Part A. 21:1621–1632. 2015.
|
|
6
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Girolamo L, Lucarelli E, Alessandri G,
Avanzini MA, Bernardo ME, Biagi E, Brini AT, D'Amico G, Fagioli F,
Ferrero I, et al: Mesenchymal stem/stromal cells: A new ‘cells as
drugs’ paradigm. Efficacy and critical aspects in cell therapy.
Curr Pharm Des. 19:2459–2473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Egashira K, Sumita Y, Zhong W, Ohba S,
Nagai K and Asahina I: Bone marrow concentrate promotes bone
regeneration with a suboptimal-dose of rhBMP-2. PLoS One.
13:e01910992018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hokugo A, Saito T, Li A, Sato K, Tabata Y
and Jarrahy R: Stimulation of bone regeneration following the
controlled release of water-insoluble oxysterol from biodegradable
hydrogel. Biomaterials. 35:5565–5571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Atesok K, Li R, Stewart DJ and Schemitsch
EH: Endothelial progenitor cells promote fracture healing in a
segmental bone defect model. J Orthop Res. 28:1007–1014.
2010.PubMed/NCBI
|
|
12
|
Shirota T, Yasui H, Shimokawa H and
Matsuda T: Fabrication of endothelial progenitor cell (EPC)-seeded
intravascular stent devices and in vitro endothelialization on
hybrid vascular tissue. Biomaterials. 24:2295–2302. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li R, Atesok K, Nauth A, Wright D,
Qamirani E, Whyne CM and Schemitsch EH: Endothelial progenitor
cells for fracture healing: A microcomputed tomography and
biomechanical analysis. J Orthop Trauma. 25:467–471. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seebach C, Henrich D, Kähling C, Wilhelm
K, Tami AE, Alini M and Marzi I: Endothelial progenitor cells and
mesenchymal stem cells seeded onto beta-TCP granules enhance early
vascularization and bone healing in a critical-sized bone defect in
rats. Tissue Eng Part A. 16:1961–1970. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carreira AC, Lojudice FH, Halcsik E,
Navarro RD, Sogayar MC and Granjeiro JM: Bone morphogenetic
proteins: Facts, challenges, and future perspectives. J Dent Res.
93:335–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ho SS, Vollmer NL, Refaat MI, Jeon O,
Alsberg E, Lee MA and Leach JK: Bone morphogenetic protein-2
promotes human mesenchymal stem cell survival and resultant bone
formation when entrapped in photocrosslinked alginate hydrogels.
Adv Healthc Mater. 5:2501–2509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carreira AC, Zambuzzi WF, Rossi MC,
Astorino Filho R, Sogayar MC and Granjeiro JM: Bone morphogenetic
proteins: Promising molecules for bone healing, bioengineering, and
regenerative medicine. Vitam Horm. 99:293–322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garrison KR, Donell S, Ryder J, Shemilt I,
Mugford M, Harvey I and Song F: Clinical effectiveness and
cost-effectiveness of bone morphogenetic proteins in the
non-healing of fractures and spinal fusion: A systematic review.
Health Technol Assess. 11:1–150, iii-iv. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bloomsmith MA, Perlman JE, Hutchinson E
and Sharpless M: Behavioral management programs to promote
laboratory animal welfareManagement of animal care and use programs
in research, education, and testing. Weichbrod RH, Thompson GAH and
Norton JN: 2nd. CRC Press/Taylor & Francis; Boca Raton, FL:
2018
|
|
20
|
He X, Dziak R, Yuan X, Mao K, Genco R,
Swihart M, Sarkar D, Li C, Wang C, Lu L, et al: BMP2 genetically
engineered MSCs and EPCs promote vascularized bone regeneration in
rat critical-sized calvarial bone defects. PLoS One. 8:e604732013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amstein CF and Hartman PA: Adaptation of
plastic surfaces for tissue culture by glow discharge. J Clin
Microbiol. 2:46–54. 1975.PubMed/NCBI
|
|
23
|
Shi S, Wang XH, Guo G, Fan M, Huang MJ and
Qian ZY: Preparation and characterization of microporous
poly(D,L-lactic acid) film for tissue engineering scaffold. Int J
Nanomedicine. 5:1049–1055. 2010.PubMed/NCBI
|
|
24
|
Alghazali KM, Nima ZA, Hamzah RN, Dhar MS,
Anderson DE and Biris AS: Bone-tissue engineering: Complex tunable
structural and biological responses to injury, drug delivery, and
cell-based therapies. Drug Metab Rev. 47:431–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
An ideal cell source for bone tissue
engineering? Bonekey Rep. 1:2222012.PubMed/NCBI
|
|
26
|
Sun J, Li J, Li C and Yu Y: Role of bone
morphogenetic protein-2 in osteogenic differentiation of
mesenchymal stem cells. Mol Med Rep. 12:4230–4237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smajilagić A, Aljičević M, Redžić A,
Filipović S and Lagumdžija A: Rat bone marrow stem cells isolation
and culture as a bone formative experimental system. Bosn J Basic
Med Sci. 13:27–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Denecke B, Horsch LD, Radtke S, Fischer
JC, Horn PA and Giebel B: Human endothelial colony-forming cells
expanded with an improved protocol are a useful endothelial cell
source for scaffold-based tissue engineering. J Tissue Eng Regen
Med. 9:E84–E97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
James AW, LaChaud G, Shen J, Asatrian G,
Nguyen V, Zhang X, Ting K and Soo C: A review of the clinical side
effects of bone morphogenetic protein-2. Tissue Eng Part B Rev.
22:284–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo X, Chen J, Song WX, Tang N, Luo J,
Deng ZL, Sharff KA, He G, Bi Y, He BC, et al: Osteogenic BMPs
promote tumor growth of human osteosarcomas that harbor
differentiation defects. Lab Invest. 88:1264–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen D, Zhao M and Mundy GR: Bone
morphogenetic proteins. Growth Factors. 22:233–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan T, Chen J, Pan P, Zhang Y, Hu Y, Liu
X, Shi X and Zhang Q: Bioinspired double polysaccharides-based
nanohybrid scaffold for bone tissue engineering. Colloids Surf B
Biointerfaces. 147:217–223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z,
Zhang Y, Li Y, Mao Q, Wang J, et al: An inactivated enterovirus 71
vaccine in healthy children. N Engl J Med. 370:829–837. 2014.
View Article : Google Scholar : PubMed/NCBI
|