Introduction
Multiple sclerosis (MS) is a chronic inflammatory
disorder of the central nervous system characterized by lymphocyte,
dendritic cell and macrophage infiltration, microglial activation,
oligodendrocyte death, demyelination and axonal destruction
(1,2). MS is one of the primary causes of
neurologic disability in young adults between 20 and 45 years of
age (3). The specific pathogenic
mechanisms of MS are well understood, and a combination of
environmental, genetic and infectious factors are implicated in the
occurrence and progression of the disease (4). Although the treatment of MS has greatly
improved over the past 20 years, further investigation into
therapeutic options for this complex disease is required.
Necrostatin-1 (Nec-1) is a potent and specific
inhibitor of necroptosis that allosterically suppresses the
activity of receptor-interacting serine/threonine protein kinase
(RIPK)1, blocking formation of the RIPK1-RIPK3 complex (5). The protective effects of Nec-1 have
been reported in a number of experimental models, including models
of ischemic brain injury, inflammatory kidney disease, myocardial
infarction, Parkinson's disease and various types of cancer
(6–11). However, the effects of Nec-1 in MS
remain unknown. Therefore, the present study aimed to investigate
the functions of Nec-1 in an experimental autoimmune
encephalomyelitis (EAE) mouse model.
Necroptosis is a type of programmed cell death with
the morphological features of necrosis. Necroptosis is involved in
various physiological and pathological conditions, including tissue
homeostasis, organ development, ischemia-reperfusion injury,
rheumatoid arthritis and neurodegenerative diseases (12,13). It
is induced by toll-like receptor and death receptor activation,
tumor necrosis factor α (TNFα), interferons and DNA damage
(14), which further activate RIPK3
and its substrate, pseudo-kinase mixed lineage kinase domain-like
protein (MLKL) (15,16). Phosphorylated (p)-MLKL is
subsequently transformed from the monomeric to the oligomeric form
in order to activate downstream signaling cascades and induce
programmed cell death (12).
Numerous studies have demonstrated the involvement of necroptosis
in MS pathogenesis, suggesting that the inhibition of RIPK1 may be
an effective means of treating MS (17,18).
TNFα is an immunomodulatory cytokine that regulates
various physiological and pathological functions, including
apoptosis, proliferation, inflammation and cancer (19–22).
TNFα expression levels are elevated in the serum of patients with
MS, which correlates with disease severity (23–26).
TNFα-induced oligodendrocyte death morphologically resembles
necrosis, and results in the activation of RIPK1 through TNF
receptor 1 signaling (27,28).
In the present study, in vivo and in
vitro experiments were performed to investigate the functions
of Nec-1 in EAE and primary oligodendrocytes. Nec-1 significantly
attenuated the pathogenesis of EAE by reducing inflammatory factors
and suppressing apoptosis and necroptosis. Furthermore, Nec-1
treatment restricted TNFα + zVAD-fmk-induced apoptosis and
necroptosis in oligodendrocyte precursor cells (OPCs).
Materials and methods
Animal maintenance
A total of 24 eight-week-old female C57BL/6 mice
(18–20 g) were purchased from Vital River Laboratory Animal
Technology Co., Ltd. The mice were housed under specific
pathogen-free conditions (temperature, 24±2°C; humidity, 50–60%) at
the animal facility of the Second Hospital of Hebei Medical
University (Shijiazhuang, China), with a 12-h light/dark cycle and
free access to a standard rodent diet and water. All animal
experiments were approved by the animal ethics committee of Hebei
Medical University.
EAE induction
Prior to animal experiments, an anesthesia chamber
was charged with 1.5% isoflurane and 100% oxygen (2 l/min oxygen
flow) for 15 min, and then mice were put into the chamber for 30
min to anaesthetize. Each mouse was subcutaneously immunized in the
hindquarters with myelin oligodendrocyte glycoprotein
(MOG)35-55 (Lysine Biosystem; 250 µg for 4 sites)
emulsified in an equivalent volume of Complete Freund's Adjuvant
(CFA; 90% paraffin oil and 10% mannide monooleate, with 4 mg/ml
Mycobacterium tuberculosis, strain H37Ra). Immunized mice
also received two intraperitoneal injections of 500 ng pertussis
toxin (Alexis Biochemicals; Enzo Life Sciences, Inc.) at days 0 and
2. On day 2, the EAE model was establised. Normal, untreated mice
served as the control group.
Nec-1 treatment in vivo
EAE mice were intrathecally injected with 1.65 mg/kg
Nec-1 (Med Chem Express LLC) from day 2 every 3 days for 15 days.
Mice in the control group were treated with saline solution
only.
Disease scoring
Mice were weighed and evaluated on a daily basis,
using the Weaver score method to assess neurological function as
previously described (29). The
total score ranged from 0 to 14, with a score of between 0 and 2
for the tail, and between 0 and 3 for each of the 4 limbs. For the
tail, scoring was defined as follows: i) 0-no disease; ii)
1-partial paralysis; and iii) 2-paralysis. The limbs were evaluated
separately as follows: i) 0-no disease; ii) 1-altered gait or
weakness; iii) 2-paresis; and iv) 3-completely paralyzed. Following
experiments, at day 30, mice were sacrificed by CO2
asphyxiation.
Hematoxylin and eosin (H&E)
staining and histopathological scoring
To evaluate inflammatory infiltration, on day 30,
5-µm-thick spinal cord tissues sections were fixed with 4% (w/v)
paraformaldehyde solution overnight at the room temperature in PBS,
embedded in paraffin and stained using a conventional H&E
staining method (30). Briefly,
tissues were fixed with 10% paraformaldehyde overnight at room
temperature and embedded in paraffin for 2 h to prepare the
paraffin blocks, which were later sliced into 5-µm-thick sections.
Slides were then stained with H&E staining kit (cat. no.
E607318; Sangon Biotech Co., Ltd.) for 5–10 min at 20°C. Following
H&E staining, the samples were evaluated using light microscopy
at a magnfication of ×100. Histopathological scores were calculated
as previously described (18): i)
0-normal; ii) 1-mild inflammation, lymphocyte infiltrates partially
surrounding the meninges and blood vessels; iii) 2-moderate
inflammation, 1–10 lymphocyte infiltrates in the spinal cord; iv) 3
-severe inflammation, 11–100 lymphocyte infiltrates in the spinal
cord; and v) 4-massive inflammation, >100 lymphocyte infiltrates
in the spinal cord.
Primary cell isolation and
culture
At day 16, OPCs were isolated from the cerebrum of
C57BL/6 mouse embryos (E16) as described by Chen et al
(31). Cells were cultured at 37°C
in a 5% CO2 incubator in DMEM/F12 medium (Thermo Fisher
Scientific, Inc.) supplemented with 5 ng/ml neurotrophin 3, 10
ng/ml ciliary neurotrophic factor, 20 ng/ml fibroblast growth
factor-basic and 20 ng/ml platelet derived growth factor-AA (all
from R&D Systems, Inc.). Isolated OPCs were identified by
staining with anti-platelet derived growth factor receptor α (cat.
no. ab134123) and neural/glial antigen 2 (cat. no. ab129051; both
1:200; Abcam) antibodies, and analyzed by flow cytometry using the
BD FACSVia™ system (BD Biosciences). In addition, the primary OPCs
were treated with 40 ng/ml TNFα and 10 µM pan-caspase inhibitor
zVAD-fmk (MedChemExpress LLC) for 6 h to induce apoptosis and
necroptosis. For the Nec-1 treatment groups, 20 or 50 µM of Nec-1
were applied into the culture medium at the same time TNFα and
zVAD-fmk. All the cells were cultured at 37°C in a 5%
CO2 incubator in DMEM/F12 medium.
ELISA
Concentrations of the cytokines TNFα (Mouse TNFα
Quantikine ELISA Kit; cat. no. PMTA00B), interferon γ (IFNγ; Mouse
IFN-γ Quantikine ELISA Kit; cat. no. MIF00) and interleukin-1β
(IL1β; Mouse IL-1β/IL-1F2 Quantikine ELISA Kit; cat. no. MLB00C) in
tissue lysates were determined using the associated ELISA kits
(R&D Systems, Inc.), according to the manufacturer's
protocol.
Flow cytometric analysis of apoptosis
and necroptosis
Using the fluorescein isothiocyanate-Annexin V
Apoptosis Detection kit I (BD Biosciences) according to the
manufacturer's protocol, Annexin V/propidium iodide (PI) staining
was performed followed by flow cytometry to determine apoptosis and
necroptosis. As described previously, PI−/Annexin
V+ staining was defined as early apoptosis,
PI+/Annexin V+ staining as late apoptosis,
and PI+/Annexin V− staining as pure
necroptosis (32).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed as previously described
(33). Total RNA was extracted from
the spinal cord tissue and treated cells using Qiagen's RNeasy kit
(Qiagen, Inc.) according to the manufacturer's instructions., and
cDNA was synthesized using the RevertAid™ First Strand cDNA
synthesis kit (Thermo Fisher Scientific, Inc.). RT-qPCR was
detected by the SYBR method [TB Green® Premix Ex Taq™ II
(Tli RNaseH Plus); Takara Bio, Inc.]. A total of 1 µg of total RNA
was reversely transcribed using oligo(dT) primer at 42°C for 1 h,
and 2 µl of the reverse transcription reaction mix was amplified by
PCR with denaturation at 95°C for 2 min, and 50 cycles at 95°C for
30 sec, 55°C for 30 sec, and 72°C for 1 min. GAPDH was used as an
internal control. The 2−ΔΔCq method was applied to
calculate the relative expression (34). Primers for the apoptosis regulators
were as follows: Bax forward, 5′-GGAAGGCCTCCTCTCCTACTTC-3′ and
reverse, 5′-GAGGACTCCAGCCACAAAGATG-3′; Bcl2 forward,
5′-TTCGCAGCGATGTCCAGTCAGCT-3′ and reverse,
5′-TGAAGAGTTCTTCCACCACCGT-3′; Bcl2 like 11 (Bim) forward,
5′-GAGGCGGAGGATGATCCCG-3′ and reverse, 5′-CGAGGAGGCAAGGGAAACA-3′
and GAPDH forward, 5′-CTGGGCTACACTGAGCACC-3′ and reverse,
5′-AAGTGGTCGTTGAGGGCAATG-3′.
Western blot analysis
Western blot analysis was performed as previously
described (35). Briefly, the
samples were lysed with RIPA Lysis Buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology) and the protein concentrations
were determined by the BCA Protein Assay Kit (cat. no. P0012S;
Beyotime Institute of Biotechnology). For each lane, 25 µg sample
was loaded into 10% gels; the proteins were separated by SDS-PAGE
and transferred to a PVDF membrane. After the transfer, the
membrane was blocked with 5% milk in PBS at room temperature for 1
h. Then, the membrane was incubated with primary antibodies at 4°C
overnight. On the second day, the membrane was washed with 1X PBST
three times (5 min each) before being incubated with horseradish
peroxidase (HRP)-conjuated secondary antibodies for 1 h at room
temperature. The primary antibodies used were antibodies against
Bax (cat. no. ab182734), Bim (cat. no. ab7888), Bcl2 (cat. no.
ab692), phosporylated-dynamin related protein 1 (p-DRP1; S637; cat.
no. ab193216), p-MLKL (S345; cat. no. ab196436), DRP1 (cat. no.
ab184248), MLKL (cat. no. ab184718; all 1:1,000; Abcam) and β-actin
(cat. no. AA128; 1:3,000; Beyotime Institute of Biotechnology).
HRP-conjugated goat anti-rabbit (cat. no. ZDR5306) or anti-mouse
(cat. no. ZDR5307) secondary antibodies (both 1:5,000; OriGene
Technologies, Inc.). BeyoECL Plus kit (cat. no. P0018M; Beyotime
Institute of Biotechnology) was used to detect the signal. The
results were analyzed by ECL detection system (ChemiScope 6000 Ex;
Clinx Science Instruments Co., Ltd.) and analyzed by ImageJ
software (version 1.52p; National Institutes of Health).
Immunofluorescence staining and
confocal imaging
Immunofluorescence staining and confocal imaging
were performed as previously described (36). Briefly, treated cells were seeded
into a 24-well plate with a polylysine-pretreated coverslip at a
density of 1×104 cells per well. After treatment, cells
were fixed with 4% paraformaldehyde in PBS for 20 min at room
temperature. The coverslip was then washed twice with PBS (5 min
each), incubated with 5% BSA in 1X TBST in a humidified chamber at
room temperature for 1 h and then washed three times. The slides
were incubated with primary p-MLKL antibodies (cat. no. ab196436;
1:200; Abcam) and Cy3®-conjugated goat anti-rabbit
antibodies (cat. no. ab97075; 1:1,000; Abcam). The slides were then
washed three times, mounted directly using fluromount with DAPI
(Olink Bioscience) and images were collected at a magnfication of
×400 using a charge-coupled-device camera (AxioCam MRm) with
AxioVision software (version 3; both Carl Zeiss AG). The
immunofluorescence staining was analyzed using ImageJ software
(version 1.52p).
Statistical analysis
Flow cytometric data were analyzed using FlowJo
software 7.6.1 and other data were analyzed using GraphPad Prism 5
software (GraphPad Software, Inc.). For multiple comparisons,
one-way analysis of variance was performed followed by Tukey's
test. Data are presented as the mean ± standard error of the mean.
P<0.05 was considered to indicate statistical significance.
Results
Nec-1 reduces the severity of EAE and
associated tissue inflammation
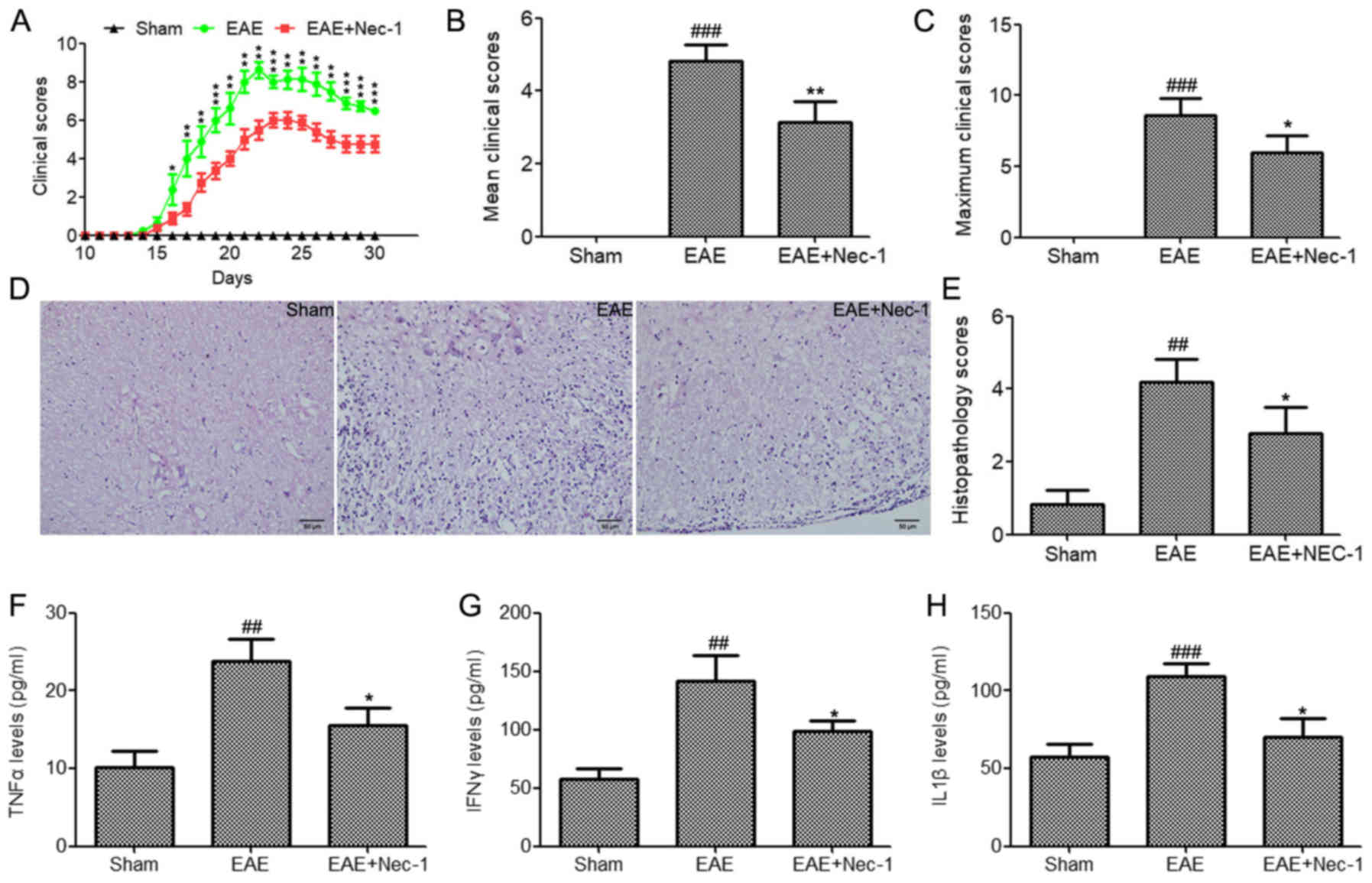
To determine whether Nec-1 treatment is able to
reduce the disease severity of MS, EAE was induced in C57BL/6 mice
using MOG35-55/CFA. Nec-1 (1.65 mg/kg) or saline
solution were intrathecally administered every 3 days until the end
of the experiment (day 30). Nec-1 treatment significantly reduced
the severity of EAE in mice (Fig.
1A-C). Furthermore, histological analyses demonstrated that EAE
mice possessed a greater number of CNS lesions and inflammatory
cell infiltrates in their tissues compared with the sham group
(Fig. 1D). This presented as a
higher histopathological score for the spinal cord tissue in the
EAE group compared with the sham group (Fig. 1E). However, Nec-1 treatment reduced
inflammatory cell infiltration and decreased the histopathological
score of the spinal cord tissues compared with that in the EAE
group (Fig. 1D and E). Inflammatory
factors in the tissue lysates of the mice, namely TNFα, IFNγ and
IL1β, were evaluated using ELISA kits. The data demonstrated that
TNFα, IFNγ and IL-1β were significantly upregulated in the spinal
cord tissues of mice with EAE compared with the sham group
(Fig. 1F-H). By contrast, the levels
of these inflammatory factors were significantly reduced in
Nec-1-treated EAE mice (Fig. 1F-H).
These findings suggest that Nec-1 contributed to the reduction of
EAE disease severity.
Nec-1 protects spinal cord tissues by
reducing MS-associated apoptosis and necroptosis
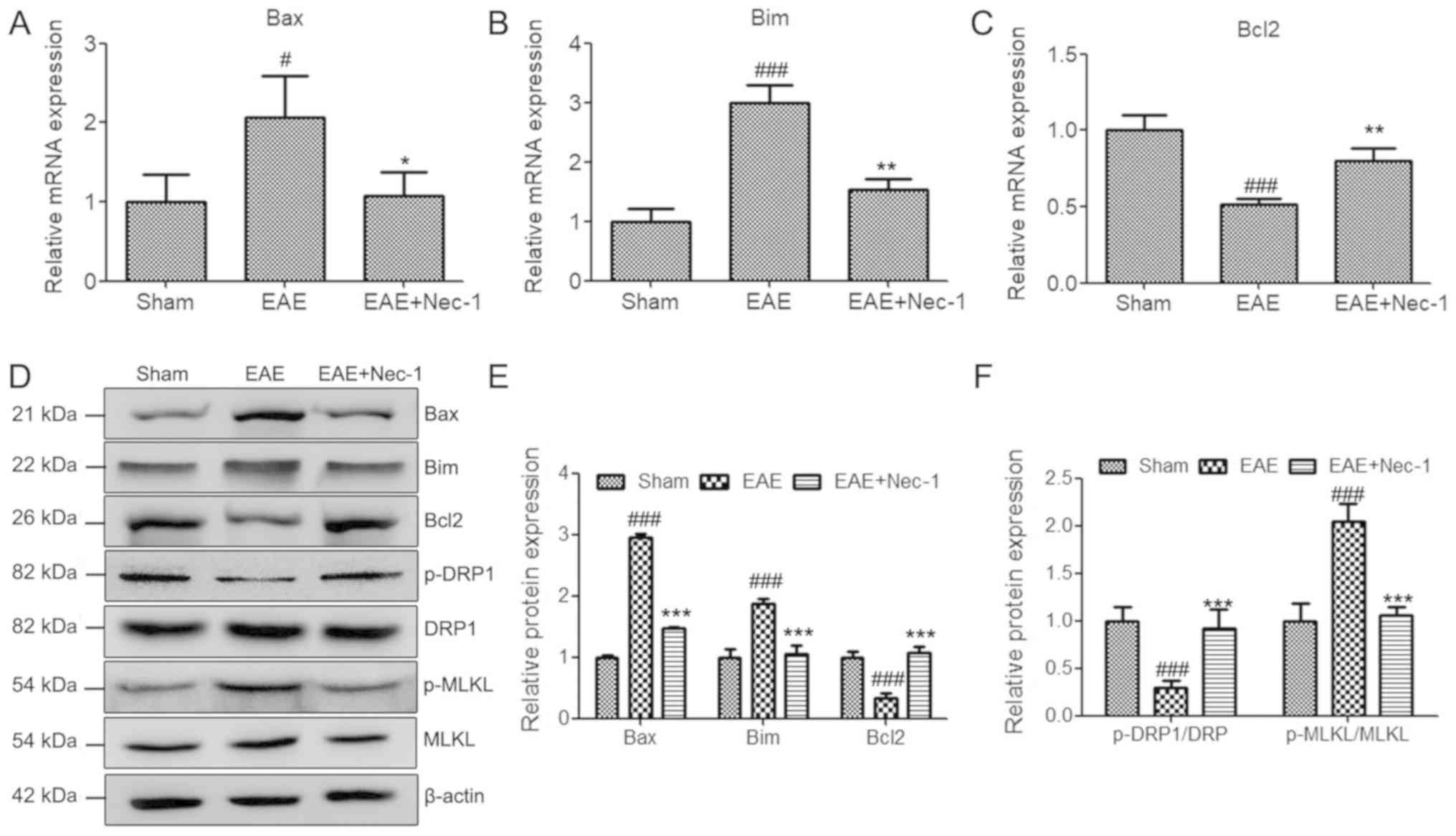
To further investigate the protective role of Nec-1,
the expression levels of apoptosis and necroptosis-associated genes
were detected in the spinal cord tissues of mice with EAE, using
RT-qPCR and western blotting. The results revealed that EAE induced
the expression of pro-apoptotic genes Bax and Bim, and suppressed
the expression of anti-apoptotic gene Bcl2. However, Nec-1
treatment significantly decreased the mRNA and protein expression
levels of apoptosis-promoting genes Bax and Bim, and increased
those of the anti-apoptosis gene Bcl2 following establishment of
the EAE model (Fig. 2A-E). This
suggests that Nec-1 reduced EAE-associated apoptosis in spinal cord
tissues. In addition, EAE suppressed the ratio of pDRP1/DRP and
increased the ratio of p-MLKL/MLKL, which suggests that EAE induced
cell necroptosis in this model. Administration of Nec-1 markedly
reversed the changes in the phosphorylation levels of DRP1 and MLKL
that were induced in the EAE group, which indicates that Nec-1 also
reduced EAE-induced necroptosis in spinal cord tissues (Fig. 2D-F).
Nec-1 inhibits apoptosis and
necroptosis in primary OPCs induced using TNFα + zVAD-fmk
It has previously been reported that the apoptosis
and necrosis of oligodendrocytes is fundamental to the initiation
and progression of EAE (37).
Therefore, the protective effect of Nec-1 on oliogodendrocytes in
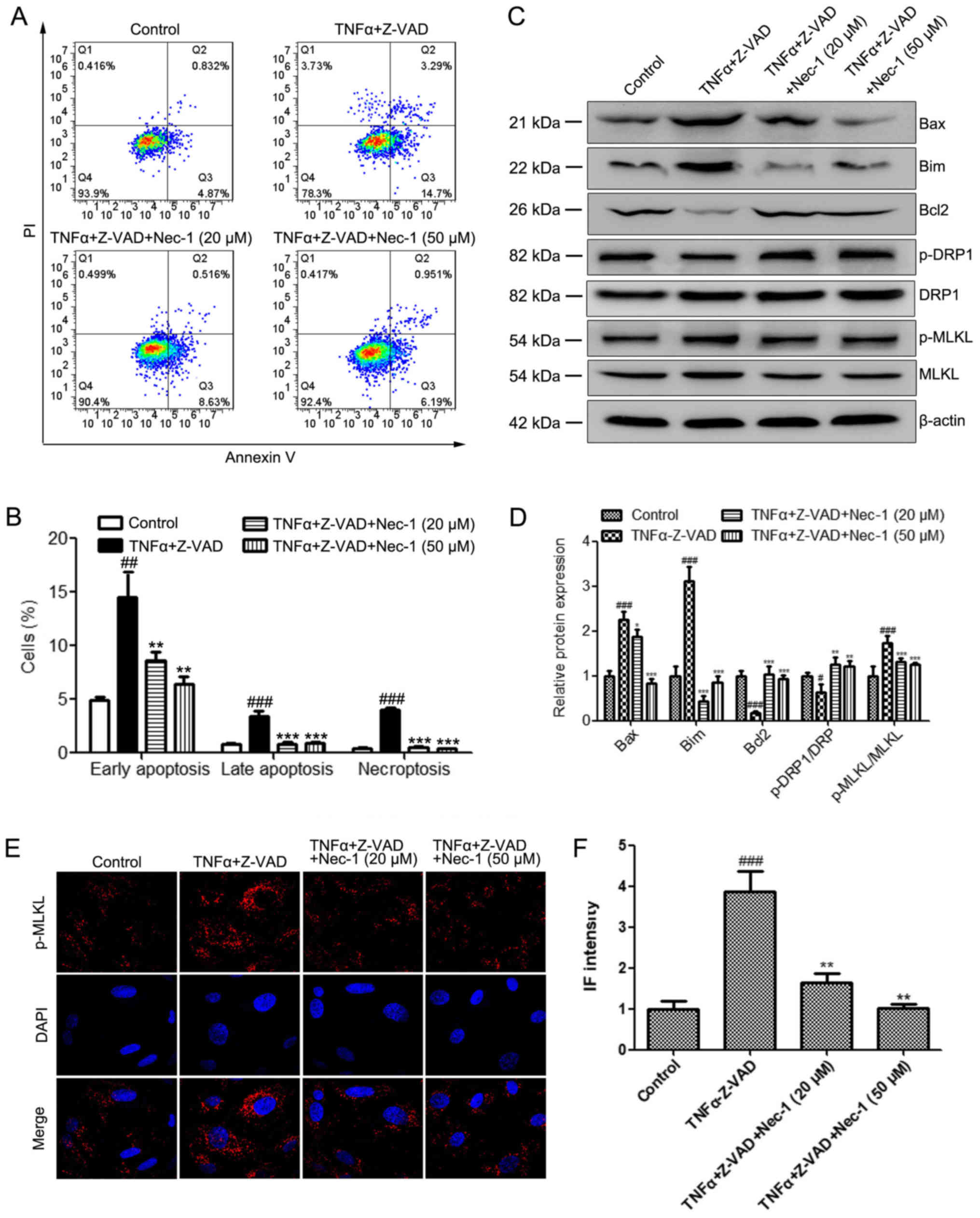
EAE was investigated. Primary OPCs were isolated (Fig. S1) and TNFα and the pan-caspase
inhibitor zVAD-fmk were administered to primary OPCs to induce
apoptosis and necroptosis. The results demonstrated that Nec-1
treatment (20 and 50 µM) significantly reduced the number of
necroptotic and apoptotic cells induced by TNFα + zVAD-fmk
(Fig. 3A and B). In addition,
western blot analyses were performed to evaluate the protein levels
of Bax, Bim, Bcl2, p-DRP1 and p-MLKL in these groups. In agreement
with the in vivo results, the administration of TNFα +
zVAD-fmk significantly upregulated the expression levels of Bax and
Bim, and the phosphorylation of MLKL, and reduced DRP1
phosphorylation and Bcl2 expression in OPCs (Fig. 3C and D). Nec-1 treatment (20 and 50
µM) reversed the EAE-induced changes in the levels of these
proteins (Fig. 3D). Similar trends
were observed for the immunofluorescence staining of p-MLKL in the
indicated groups, where TNFα + zVAD-fmk significantly increased
p-MLKL staining intensity which was then attenuated by Nec-1
treatment at both concentrations (Fig.
3E and F). These results demonstrated that Nec-1 suppressed the
TNFα + zVAD-fmk-induced apoptosis and necroptosis of OPCs.
Discussion
EAE is a widely adopted animal model that mimics the
clinical characteristics and pathogenic mechanisms of MS, a disease
affecting 2.5 million individuals worldwide (38). The EAE animal model provides a
suitable tool for understanding the pathogenesis and underlying
mechanisms of MS and also how best to manage the condition
(39). Using the EAE model, the
present study established that necroptosis inhibitor Nec-1 reduced
the pathogenesis of MS by the suppression of apoptosis and
necroptosis in OPCs. To the best of our knowledge, this is the
first study to directly demonstrate the protective role of Nec-1 in
EAE and MS, with the results providing background for the potential
discovery of novel therapies.
A large number of studies have demonstrated that
necroptosis may contribute to the pathogenesis of various
neurodegenerative disorders, including MS (13,40,41). The
activation of RIPK1, RIPK3 and MLKL has been observed in cortical
lesions from MS brain specimens (17). Ofengeim et al (18) demonstrated that necroptosis is
involved in MS, and suggested that targeting RIPK1 may represent a
therapeutic strategy for treating the disease. The inhibition of
necroptosis may also provide effective relief from the symptoms of
MS. Previous studies have illustrated that Nec-1 has a significant
neuroprotective effect in ischemic stroke (6,42,43).
However, the role of Nec-1 in MS remains unknown. In the present
study, the use of in vivo and in vitro models
revealed for the first time that Nec-1 effectively alleviates the
symptoms of EAE. Mechanistically, Nec-1 reduced cellular apoptosis
and necroptosis induced by EAE in vitro and in vivo,
providing an improved understanding of the role of Nec-1 in MS.
Microglial cells are resident innate immune cells,
primarily accountable for the inflammatory response in
neurodegenerative diseases (43,44).
Since RIPK1 is more highly expressed than RIPK3 in activated
microglia (17), targeting RIPK1 may
selectively inhibit microglial-mediated inflammatory signaling. The
present study demonstrated that Nec-1 suppressed the expression of
inflammatory cytokines TNFα, IFNγ and IL1β in spinal cord tissues.
Microglia-associated inflammation triggers necroptosis in
oligodendrocytes, which is critical for the pathogenesis of MS
(17,44). Therefore, the present study
illustrated that Nec-1 may effectively regulate inflammation in
microglial cells, and necroptosis in oligodendrocytes.
Several limitations exist for the present study. For
instance, although it was determined that Nec-1 suppressed the
apoptosis and necroptosis of primary oligodendrocyte precursor
cells in vitro, no histological staining for the apoptosis
or necroptosis of oligodendrocytes in the spinal cord tissues in
vivo was performed. Future studies will involve more in-depth
in vivo experiments.
In conclusion, the present study demonstrated that
Nec-1 suppressed the apoptosis and necroptosis of oligodendrocytes
by inhibiting the release of inflammatory mediators. The promising
therapeutic value of Nec-1 for the treatment of MS was
demonstrated, suggesting that it may have potential positive
neuroprotective effects on patients with MS.
Supplementary Material
Supporting Data
Acknowledgements
The authors greatly thank the Neurology Laboratory
of The Second Hospital of Hebei Medical University (Shijiazhuang,
China).
Funding
The current study was supported by the National
Natural Science Foundation of China (grant no. 81873759).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BL proposed the current study and drafted the
manuscript. LG designed the experiments. YW performed the
experiments and wrote the manuscript. JW collected and analyzed
experimental data. ZX and WS performed histological analysis. All
authors read and approved the final manuscript for publication.
Ethics approval and consent to
participate
All animal experiments were approved by the animal
ethics committee of Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sedal L, Winkel A, Laing J, Law LY and
McDonald E: Current concepts in multiple sclerosis therapy. Degener
Neurol Neuromuscul Dis. 7:109–125. 2017.PubMed/NCBI
|
|
2
|
Lemus HN, Warrington AE and Rodriguez M:
Multiple Sclerosis: Mechanisms of disease and strategies for myelin
and axonal repair. Neurol Clin. 36:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kacperska MJ, Walenczak J and Tomasik B:
Plasmatic microRNA as potential biomarkers of multiple sclerosis:
Literature review. Adv Clin Exp Med. 25:775–779. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simon M, Ipek R, Homola GA, Rovituso DM,
Schampel A, Kleinschnitz C and Kuerten S: Anti-CD52 antibody
treatment depletes B cell aggregates in the central nervous system
in a mouse model of multiple sclerosis. J Neuroinflammation.
15:2252018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Linkermann A, Hackl MJ, Kunzendorf U,
Walczak H, Krautwald S and Jevnikar AM: Necroptosis in immunity and
ischemia-reperfusion injury. Am J Transplant. 13:2797–2804. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang R, Hu K, Chen J, Zhu S, Li L, Lu H,
Li P and Dong R: Necrostatin-1 protects hippocampal neurons against
ischemia/reperfusion injury via the RIP3/DAXX signaling pathway in
rats. Neurosci Lett. 651:207–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tristao VR, Goncalves PF, Dalboni MA,
Batista MC, Durao Mde S Jr and Monte JC: Nec-1 protects against
nonapoptotic cell death in cisplatin-induced kidney injury. Ren
Fail. 34:373–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koudstaal S, Oerlemans MI, Van der Spoel
TI, Janssen AW, Hoefer IE, Doevendans PA, Sluijter JP and Chamuleau
SA: Necrostatin-1 alleviates reperfusion injury following acute
myocardial infarction in pigs. Eur J Clin Invest. 45:150–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dionisio PE, Oliveira SR, Amaral JS and
Rodrigues CM: Loss of microglial parkin inhibits necroptosis and
contributes to neuroinflammation. Mol Neurobiol. 56:2990–3004.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, Xu
BQ, Li JH, Jing L, Jiang JL, Tang J and Chen ZN: Necrostatin-1
reduces intestinal inflammation and colitis-associated
tumorigenesis in mice. Am J Cancer Res. 5:3174–3185.
2015.PubMed/NCBI
|
|
11
|
Han W, Xie J, Fang Y, Wang Z and Pan H:
Nec-1 enhances shikonin-induced apoptosis in leukemia cells by
inhibition of RIP-1 and ERK1/2. Int J Mol Sci. 13:7212–7225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhe-Wei S, Li-Sha G and Yue-Chun L: The
role of necroptosis in cardiovascular disease. Front Pharmacol.
9:7212018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shan B, Pan H, Najafov A and Yuan J:
Necroptosis in development and diseases. Genes Dev. 32:327–340.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhuriya YK and Sharma D: Necroptosis: A
regulated inflammatory mode of cell death. J Neuroinflammation.
15:1992018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Linkermann A, Hackl MJ, Kunzendorf U,
Walczak H, Krautwald S and Jevnikar AM: Necroptosis in immunity and
ischemia-reperfusion injury. Am J Transplant. 13:2797–2804. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu W, Liu P and Li J: Necroptosis: An
emerging form of programmed cell death. Crit Rev Oncol Hematol.
82:249–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dhib-Jalbut S and Kalvakolanu DV:
Microglia and necroptosis: The culprits of neuronal cell death in
multiple sclerosis. Cytokine. 76:583–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ofengeim D, Ito Y, Najafov A, Zhang Y,
Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg
H, et al: Activation of necroptosis in multiple sclerosis. Cell
Rep. 10:1836–1849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu C, Chen X, Wang Q, Xu X and Xu B: TNFα
promotes glioblastoma A172 cell mitochondrial apoptosis via
augmenting mitochondrial fission and repression of MAPK-ERK-YAP
signaling pathways. Onco Targets Ther. 11:7213–7227. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shioda M, Muneta T, Tsuji K, Mizuno M,
Komori K, Koga H and Sekiya I: TNFα promotes proliferation of human
synovial MSCs while maintaining chondrogenic potential. PLoS One.
12:e01777712017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bryan C, Sammour I, Guerra K, Sharma M,
Dapaah-Siakwan F, Huang J, Zambrano R, Benny M, Wu S and Young K:
TNFα-stimulated protein 6 (TSG-6) reduces lung inflammation in an
experimental model of bronchopulmonary dysplasia. Pediatr Res.
85:390–397. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee E, Ouzounova M, Piranlioglu R, Ma MT,
Guzel M, Marasco D, Chadli A, Gestwicki JE, Cowell JK, Wicha MS, et
al: The pleiotropic effects of TNFα in breast cancer subtypes is
regulated by TNFAIP3/A20. Oncogene. 38:469–482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pegoretti V, Baron W, Laman JD and Eisel
ULM: Selective modulation of TNF-TNFRs signaling: Insights for
multiple sclerosis treatment. Front Immunol. 9:9252018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Taylor B, van der Mei I, Stewart
N, Charlesworth J, Blizzard L, Ponsonby AL, Dwyer T, Pittas F and
Simpson S Jr: Genetic variation in PBMC-produced IFN-γ and TNF-α
associations with relapse in multiple sclerosis. J Neurol Sci.
349:40–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dendrou CA, Bell JI and Fugger L: A
clinical conundrum: The detrimental effect of TNF antagonists in
multiple sclerosis. Pharmacogenomics. 14:1397–1404. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haji N, Mandolesi G, Gentile A, Sacchetti
L, Fresegna D, Rossi S, Musella A, Sepman H, Motta C, Studer V, et
al: TNF-α-mediated anxiety in a mouse model of multiple sclerosis.
Exp Neurol. 237:296–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jurewicz AM, Walczak AK and Selmaj KW:
Shedding of TNF receptors in multiple sclerosis patients.
Neurology. 53:1409–1414. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kamali-Sarvestani E, Nikseresht A, Aflaki
E, Sarvari J and Gharesi-Fard B: TNF-alpha, TNF-beta and IL-4 gene
polymorphisms in Iranian patients with multiple sclerosis. Acta
Neurol Scand. 115:161–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Bi Y, Xia Z, Shi W, Li B, Li B,
Chen L and Guo L: Butylphthalide ameliorates experimental
autoimmune encephalomyelitis by suppressing PGAM5-induced
necroptosis and inflammation in microglia. Biochem Biophys Res
Commun. 497:80–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alwahaibi NY, Alkhatri AS and Kumar JS:
Hematoxylin and eosin stain shows a high sensitivity but
sub-optimal specificity in demonstrating iron pigment in liver
biopsies. Int J Appl Basic Med Res. 5:169–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, Balasubramaniyan V, Peng J,
Hurlock EC, Tallquist M, Li J and Lu QR: Isolation and culture of
rat and mouse oligodendrocyte precursor cells. Nat Protoc.
2:1044–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tao F, Tian X, Lu M and Zhang Z: A novel
lncRNA, Lnc-OC1, promotes ovarian cancer cell proliferation and
migration by sponging miR-34a and miR-34c. J Genet Genomics.
45:137–145. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Zhang B, Li W, Fu L, Fu L, Zhu Z
and Dong JT: Epigenetic silencing of miR-203 upregulates SNAI2 and
contributes to the invasiveness of malignant breast cancer cells.
Genes Cancer. 2:782–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lenhausen AM, Wilkinson AS, Lewis EM,
Dailey KM, Scott AJ, Khan S and Wilkinson JC: Apoptosis inducing
factor binding protein PGAM5 triggers mitophagic cell death that is
inhibited by the ubiquitin ligase activity of X-linked inhibitor of
apoptosis. Biochemistry. 55:3285–3302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao L, Shang L, Li N, Wang S, Wang M,
Huang Y, Chen D, Huang J and Xiong K: Mixed lineage kinase
domain-like protein induces RGC-5 necroptosis following elevated
hydrostatic pressure. Acta Biochim Biophys Sin (Shanghai).
49:879–889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cudrici C, Niculescu T, Niculescu F, Shin
ML and Rus H: Oligodendrocyte cell death in pathogenesis of
multiple sclerosis: Protection of oligodendrocytes from apoptosis
by complement. J Rehabil Res Dev. 43:123–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Kaer L, Postoak JL, Wang C, Yang G and
Wu L: Innate, innate-like and adaptive lymphocytes in the
pathogenesis of MS and EAE. Cell Mol Immunol. 16:531–539. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Robinson AP, Harp CT, Noronha A and Miller
SD: The experimental autoimmune encephalomyelitis (EAE) model of
MS: Utility for understanding disease pathophysiology and
treatment. Handb Clin Neurol. 122:173–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yuan J, Amin P and Ofengeim D: Necroptosis
and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev
Neurosci. 20:19–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shao L, Yu S, Ji W, Li H and Gao Y: The
Contribution of Necroptosis in Neurodegenerative Diseases.
Neurochem Res. 42:2117–2126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang S, Wang Y, Li D, Wu J, Si W and Wu
Y: Necrostatin-1 attenuates inflammatory response and improves
cognitive function in chronic ischemic stroke mice. Medicines
(Basel). 3:E162016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Y, Zhang L, Yu H, Song K, Shi J, Chen
L and Cheng J: Necrostatin-1 improves long-term functional recovery
through protecting oligodendrocyte precursor cells after transient
focal cerebral ischemia in mice. Neuroscience. 371:229–241. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fan H, Tang HB, Kang J, Shan L, Song H,
Zhu K, Wang J, Ju G and Wang YZ: Involvement of endoplasmic
reticulum stress in the necroptosis of microglia/macrophages after
spinal cord injury. Neuroscience. 311:362–373. 2015. View Article : Google Scholar : PubMed/NCBI
|