Introduction
Spinal tuberculosis (TB) was first reported by
Sternbach (1) in 1779, and it was
named as Pott disease. Spinal TB is one of the most common
extrapulmonary forms of TB, accounting for ~1% of total TB cases
and 50–60% of cases of osteoarticular TB (2). The early clinical manifestations of
spinal TB are insidious, usually manifesting first as back pain and
local tenderness, and with the subsequent progression of the
disease, posterior kyphosis and nerve compression symptoms may
develop.
Chemotherapy is an important part of the treatment
of spinal TB, and is an effective means of eliminating spinal
lesions and prevent recurrence. When kyphotic deformity,
neurological deficits or a huge abscess occurs, surgery becomes an
efficient treatment option. Surgery is effective in eradicating TB
foci, relieving spinal cord compression, re-establishing spinal
stability and correcting the deformity (3).
According to a clinical epidemiological survey, the
incidence of spinal TB is the highest in developing countries, and
it has also been reported to be increasingly prevalent among
immigrants in western countries (4).
However, as compared to other diseases, early or atypical spinal TB
is prone to being misdiagnosed due to the lack of specific clinical
manifestations, and misleading negative results of various
laboratory analyses and imaging examinations. Thus, atypical spinal
TB is thought to be a diagnostic dilemma for surgeons. Atypical
spinal TB has frequently been reported as case reports or case
series (5); the available literature
provides insufficient evidence and is not comprehensive enough to
guide its treatment, particularly regarding the selection of
surgical options, which may lead to inappropriate treatment.
From 2015 to 2018, 10 patients with atypical spinal
TB were treated by surgery at our department. The present study
summarizes the radiographic and clinical characteristics, and the
therapeutic outcomes of these atypical spinal TB patients.
Materials and methods
The present study was approved by the Ethics
Committee of the Wuhan No. 1 Hospital, Wuhan Integrated TCM &
Western Medicine Hospital (Wuhan, China). Written informed consent
was obtained from all patients. From March 2015 to March 2018, 118
patients with spinal TB were diagnosed and treated by surgery at
the Department of Orthopedics of Wuhan No. 1 Hospital, Wuhan
Integrated TCM & Western Medicine Hospital (Wuhan, China). Of
these patients, 10 exhibited lesion involvement with an atypical
radiographic presentation. Histopathological examinations and/or
bacteriological cultures were used for the final diagnoses
(1).
Patients were diagnosed using and X-ray machine. The
test sites were imaged manually or by Automatic Exposure Control.
Conventional plain CT was performed using dual-source CT, the
instrument voltage was adjusted to 120 kV, the current was 200 mA,
the layer thickness was 2–3 mm and the layer spacing was 5 mm. The
images were processed by multiple planner reconstruction and
surface shaded display in the post-processing station of spiral CT,
and the vertebral body and spinal canal lesions were displayed.
General MRI scanning was performed using 3T superconducting
whole-body MRI scanners, and the scanning sequences included
transverse T2WI, sagittal T2WI, sagittal T1WI and fat-suppressed
T2WI. Transverse, coronal and sagittal axial helical scans were
performed, and sagittal and axial enhanced scans were performed
when necessary.
In all of the 10 patients of the present study, the
diagnoses were confirmed by histopathological examinations and/or
bacteriological cultures. Bone tissue or abscess samples were
stained for acid-fast bacilli, mycobacterial organisms, in isolated
cultures. Pus or bone tissues (1×1×1 cm) and necrotic
intervertebral discs (2 ml), and 2 ml SDS-NaOH were added to the
sterile centrifuge tube, which was agitated by a vortex shaker for
a few sec. Then, the sample was agitated by a shaker at room
temperature for 20 min and 50 ml PBS was added. After
centrifugation for 20 min at 3,000 × g at room temperature, a
suspension was prepared with PBS and 0.5 ml of the centrifugal
precipitate in a sterile syringe. The pellets of the centrifugal
precipitate were applied to the slide evenly, and the Ziehl-Neelsen
acid fast staining was performed according to a previously
described standard protocol (6).
Dedicated liquid medium containing the specimens was incubated in a
BACT/ALERT 3D system (cat. no. BTA3D; BioMérieux SA) at 37°C for 40
days. During the growth of mycobacterium tuberculosis, the
metabolism of CO2 resulted in a change in pH, which
caused the color of the sensor to change from green to yellow, and
the color sensor in the BACT/ALERT 3D system reported the results
automatically and continuously.
CT- or ultrasonography-guided needle biopsies, or
surgical biopsies were used to confirm the diagnosis. As a standard
treatment, anti-TB drugs were administered for 9–12 months
post-operatively and at all follow-ups until 36 months after the
operation.
Results
Characteristics of patients
In the present study, 6 males and 4 females aged
8–56 years were included, and the median age was 34 years. The
cohort included 3 cases with thoracic vertebra lesions, 1 with a
thoracolumbar vertebra lesion, 5 with lumbar spine lesions and 1
with a sacral vertebra lesion. Medical records from 1–14 months
previously were available and the average time-point was 3 months
previously. Two patients were not diagnosed, whereas 8 patients
were misdiagnosed. Among these misdiagnosed patients, 2 were
diagnosed with lumbar muscle fasciitis, 3 with spinal tumors, 1
with intercostal neuralgia, 2 with osteoporotic fractures and 2
with lumbar disc herniation. Of the 10 patients, two had a history
of chest TB and the remaining eight reported not to have been
previously diagnosed with TB. In the present study, all of the
patients had local pain and 2 cases (20%) had a TB toxin reaction,
including hypothermia, night sweats and weakness. Lower limb pain
or numbness was present in 5 patients (50%) and 6 cases (60%) had a
spinal activity limitation.
Laboratory test results
The erythrocyte sedimentation rate (ESR) was normal
in 6 patients, while it ranged between 25–40 mm/h in 4 patients,
and it was clearly higher than the normal reference value (adult
males, 0–10 mm/h; adult females, 0–12 mm/h) (2). Polymerase chain reaction (PCR) analyses
of Mycobacterium tuberculi DNA content in peripheral blood
may also be used to monitor TB treatment. These examinations are
more specific than the tuberculin skin test (TST) for the diagnosis
of TB infection, and their sensitivity varies. Among those
individuals with low immunity, the T-SPOT is more sensitive than
the TST. Although these tests detect TB, it is not possible to
distinguish between an occult and active TB infection, and a
further clinical assessment of the extent or activity of the
disease is required if the T-SPOT/TST test is positive. Of the 10
patients, 3 patients had a normal blood sedimentation (25–122 mm/h)
and 3 patients had a positive tuberculin purified protein
derivative test result. The ESR, PCR, TST and T-SPOT tests were
performed by the clinical laboratory department of Wuhan No. 1
Hospital, Wuhan Integrated TCM & Western Medicine Hospital.
Imaging examination results
In terms of diagnosis, typical cases are easy to
diagnose, but the 10 cases with atypical spinal TB in the present
study were difficult to diagnose as they has few symptoms and those
they did exhibit were slight. Therefore, the X-ray could not
sufficiently reveal their pathological features. In the present
study, X-ray images exhibiting various degrees of bone destruction,
narrowing of the intervertebral space, bone necrosis or shadows of
cold abscesses were present in seven cases (70%). A further eight
cases (80%) had positive signs on CT or MRI examinations (Table I). No recurrence in TB infections or
lesions was identified, and all of the 10 patients presented with
satisfactory clinical and radiological results.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Item | N |
|---|
| Sex |
|
| Male | 6 |
|
Female | 4 |
| Lesion location |
|
| Thoracic
vertebra | 3 |
|
Thoracolumbar vertebra | 1 |
| Lumbar
vertebra | 5 |
| Sacral
vertebra | 1 |
| Known history of
chest TB |
|
| Yes | 2 |
| No | 8 |
| Clinical
manifestations |
|
| Local
pain | 10 |
|
Tuberculous toxin
reaction | 2 |
| Lower
limb pain/numbness | 5 |
| Spinal
activity limitation | 6 |
| Auxiliary
examination |
|
| ESR
(+) | 4 |
| PPD
(+) | 3 |
| X-ray
(+) | 7 |
| CT/MRI
(+) | 8 |
| Initial
diagnosis |
|
| Spinal
TB | 2 |
| Lumbar
muscle fasciitis | 2 |
| Spinal
tumors | 3 |
| Spinal
intercostal neuralgia | 1 |
|
Osteoporotic fractures | 2 |
| Lumbar
disc herniation | 2 |
Case report
A specific case of misdiagnosis is presented below.
A 55 year-old female patient, whose chief complaint was lumbago for
>1 year, presented with a 2 week history of limited motion. She
was previously diagnosed with lupus erythematosus and chronic renal
failure. The visual analogue scale (VAS) scores of the waist and
lower limbs were 4 and 1, respectively. The sensory and motor
functions were normal and no pathological sign was observed. Her
ESR was 16 mm/h and the C-reactive protein (CRP) level was 34.3
mg/l. The T-SPOT test was negative. The MRI of the lumbar vertebral
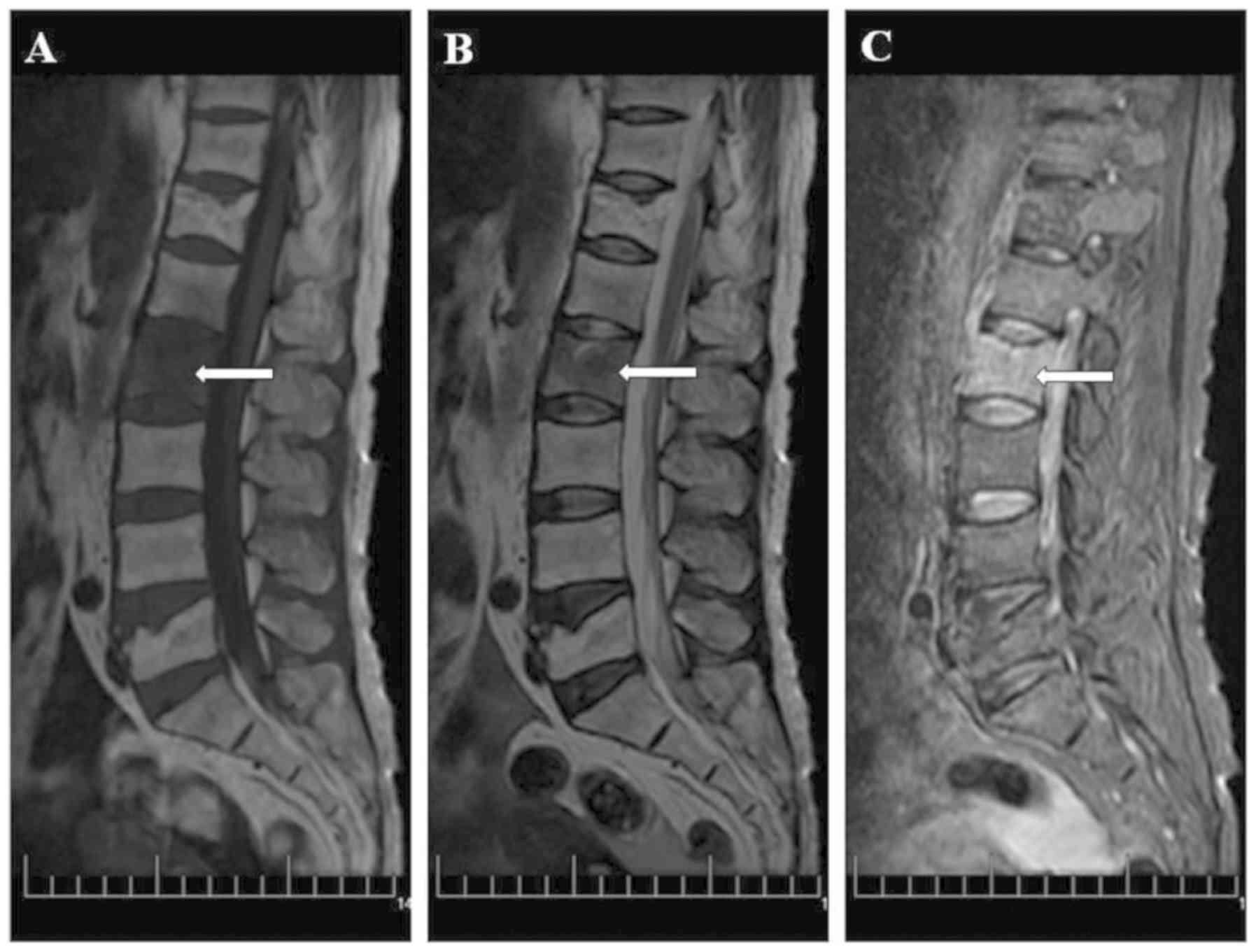
body exhibited patchy long T1 and T2 signals, and high short T
inversion recovery (STIR) signals (Fig.
1). The patient was diagnosed with an osteoporotic compression
fracture. Her back pain did not cease significantly even after one
month; hence, she underwent a lumbar CT and MRI. The CT images
revealed a small spot of lamellar low-density shadow and hardening
of the edge around the L2 vertebral body. The MRI exhibited patchy
long T1 and T2 signals and high STIR signals at the L1 and L2
vertebral bodies, but the disc was not invaded (Fig. 2). The patient denied any history of
trauma and refused to undergo a biopsy. After 1 year the patient's
waist pain became significantly worse and the VAS score were
elevated to 6. The patient's ESR was 71 mm/h and the CRP level was
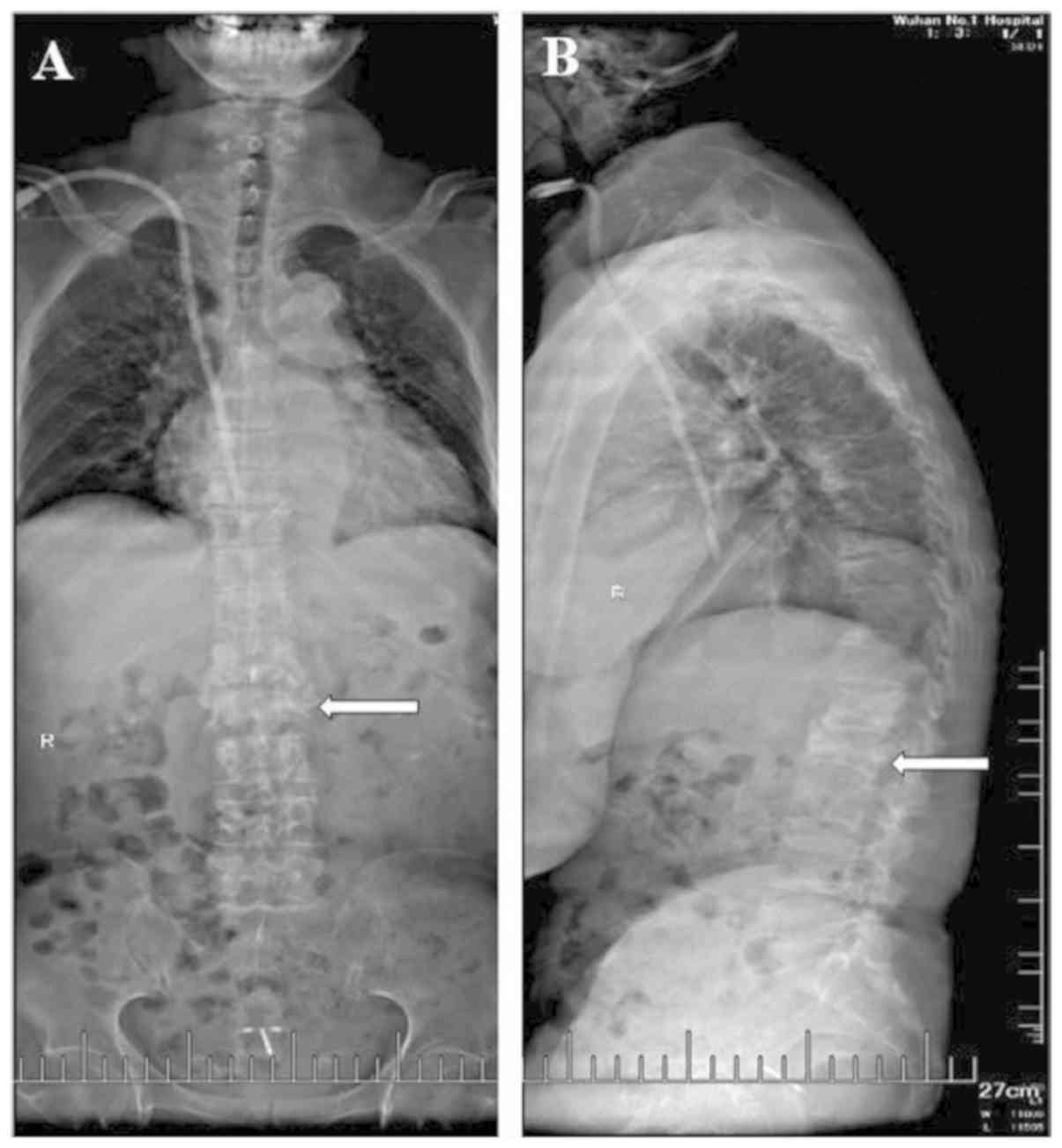
67 mg/l. The T-SPOT test was weakly positive. An X-ray and CT
revealed vertebral bone destruction, narrowing of the
intervertebral space and partial necrosis (Fig. 3). The MRI exhibited patchy long T1
and T2 signals and high STIR signals at the L1 and L2 vertebral
bodies; these indicated heterogeneous signal changes in multiple
vertebrae and a paravertebral abscess with intervertebral disc
involvement (Figs. 3 and 4). Anti-TB drugs were prescribed for 2
weeks although the physical examination revealed no obvious
positive indicators. The patient was subjected to excision of
anterior L2-3 foci, abscess debridement and autograft fusion with
instrumentation. The diagnosis was confirmed by bacteriological
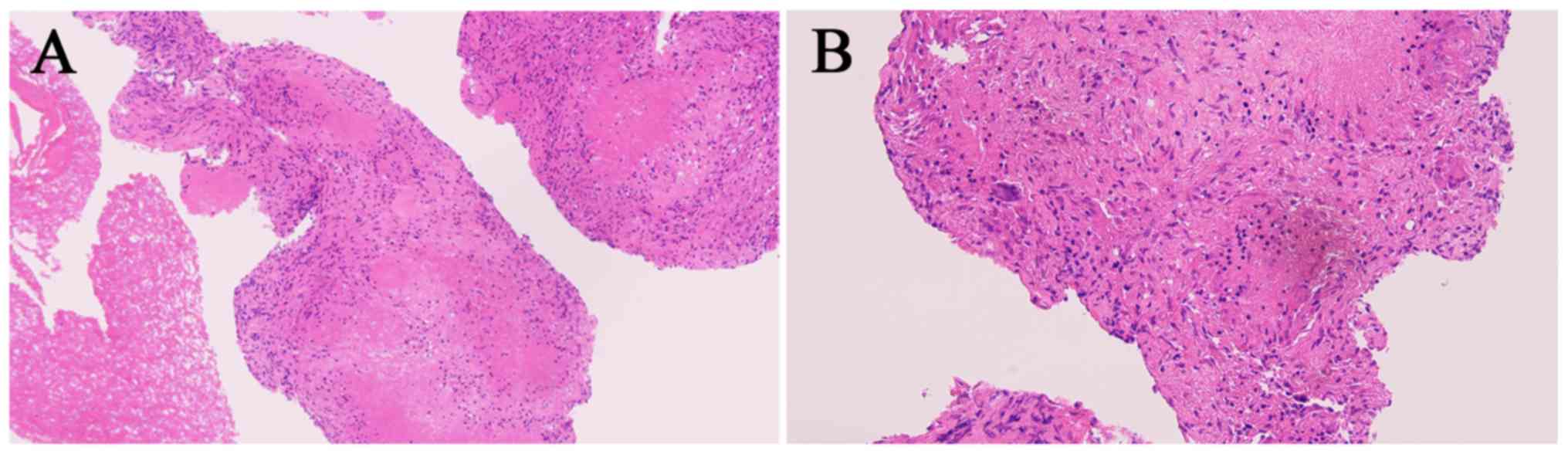
cultures of the abscess. A post-operative pathological section
exhibited large areas of inflammatory granulation tissue, dense
fibrous connective tissue and cartilage, and a small number of dead
bone fragments (Fig. 5). During the
post-operative follow-ups, no abscess was detected. Thus far, the
patient has continued with chemotherapy and no recurrence was
detected during the 15 month follow-up.
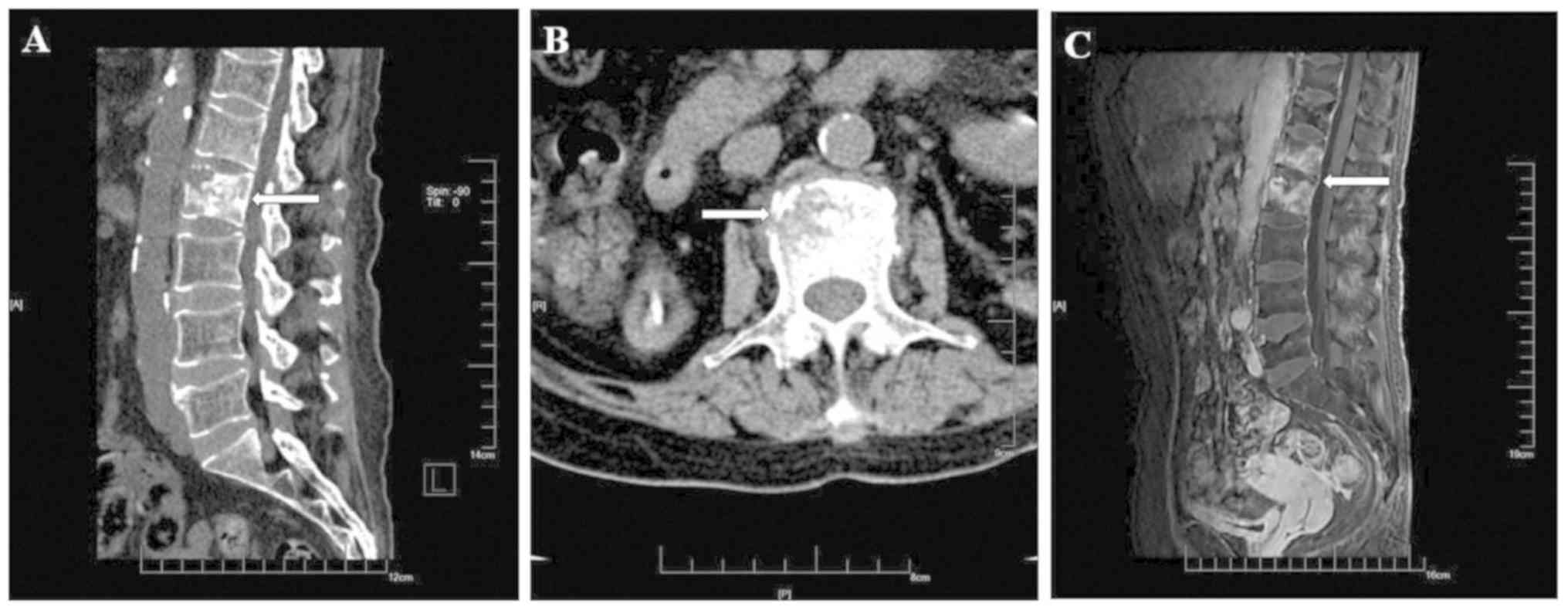 | Figure 2.Computed tomography and magnetic
resonance imaging images of the lumbar vertebral body in a
54-year-old female patient on December 30, 2016. The patient did
not receive any treatment after the first examination. (A) On
computed tomography, a small-spot lamellar low-density shadow and a
hardening edge around the L2 vertebral body in radiographs in the
sagittal plane were visible, with no significant compression in
vertebral height. (B) In the transverse plane of the vertebral
body, the destruction of the vertebrae did not encroach on the
spinal canal, and there was no compression of the dura or spinal
cord. (C) On magnetic resonance imaging (visual field, 40×40×40 cm;
layer thickness, 5 mm; layer spacing, 3 mm; b, 800
sec/mm2), patchy long T1 and T2 signals, and a high
short T inversion recovery signal of the L1 and L2 vertebral body
in the sagittal plane were observed, without invasion of the disk.
Small grid, 1 cm. The lesion is indicated by white arrows. |
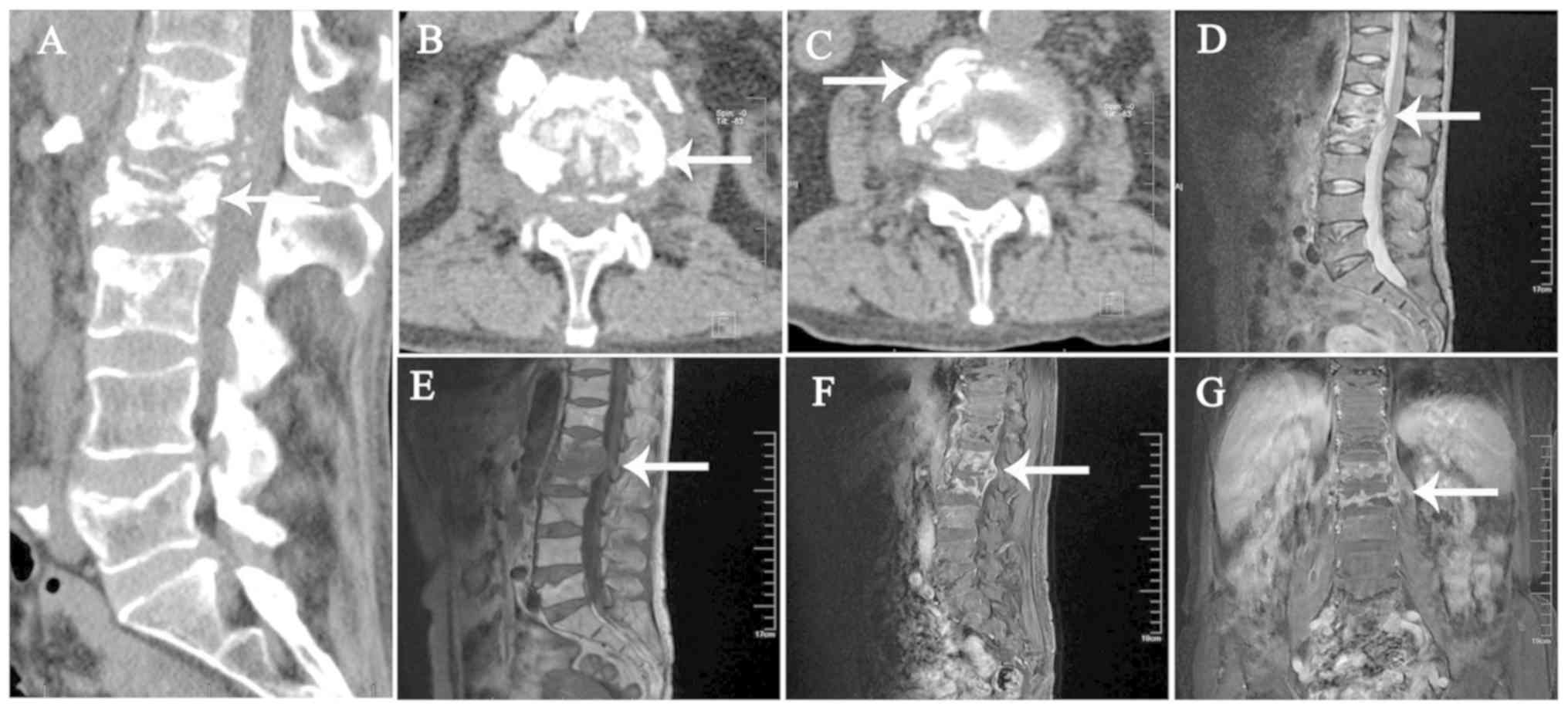 | Figure 4.CT and MRI images of the lumbar
vertebral body in a 54 year-old female patient on June 26, 2017.
The patient did not receive any treatment after examination. (A) In
the sagittal plane on CT, the destruction of the vertebral body and
local bone sclerosis were apparent, and the vertebral body was
highly compressed. (B) Cross-sectional view of the vertebral body
revealing bone destruction and compression of local dura in the
transverse plane. (C) CT cross-sectional view indicates partial
necrosis of the vertebral body in the transverse plane. (D-G) MRI
(visual field, 40×40×40 cm; layer thickness, 5 mm; layer spacing, 3
mm; b, 800 sec/mm2). (D) Patchy long T1 signal of the L1
and L2 vertebral body in the sagittal plane, (E) patchy long T2
signal of the L1 and L2 vertebral body in the sagittal plane and
(F) high short T inversion recovery signal of the L1 and L2
vertebral body in the sagittal plane. Heterogeneous signal changes
in multiple vertebrae and a paravertebral abscess, with
intervertebral disc involvement were observed. (G)
Contrast-enhanced MRI revealed intervertebral disc involvement in
the coronal plane. Small grid, 1 cm. The lesion is indicated by
white arrows. CT, computed tomography; MRI, magnetic resonance
imaging. |
Discussion
Early clinical manifestations of spinal TB are
atypical and insidious. The earliest signs are stiffness in the
upper back with muscle contraction; this may be due to the lesion
area developing tension to produce the corresponding
spine-chest-neck stiffness. Spinal rigidity is not a specific
manifestation for the diagnosis of the disease; however, it
provides a powerful clue. It is important to note that these
clinical manifestations are also common in other types of spinal
disease; therefore, it is necessary to inquire about the patients'
history and confirm whether a history of a pulmonary disease or TB
is present.
The causes of pain in the spine from the cervical
segment to the sacral segment are complex; back pain is a common
and the earliest clinical symptom of a spinal tumor. In addition, a
spinal tumor and spinal TB may occur as a result of mild to severe
progression of spinal cord dysfunction, including nerve root pain,
sensorimotor dysfunction, urinary incontinence and even paraplegia.
Bone destruction may result in pathologies including vertebral body
collapse, cuneate and spinal deformity; in fact, certain spinal
tumors may produce similar bone destruction and spinal deformity
(7).
Radiographic examination is of great diagnostic
value, and the relevant pathological features include vertebral
space stenosis and paravertebral shadow, as well as vertebral body
destruction and compression. In terms of diagnosis, typical cases
are easy to diagnose, spinal TB has typical imaging
characteristics, including adjacent vertebral body bone
destruction, intervertebral disc destruction and vertebral abscess
formation. In general, spinal TB results in extensive destruction
of intervertebral disc and then the vertebral body; however, the
narrowing or disappearance of the intervertebral space is
associated with this type of lesion. Central lesions of spinal TB
affect the perivertebral body at a late stage. In elderly patients,
spinal TB generally does not result in paravertebral abscess or
smaller lesions, which is associated with the weak response in
those patients.
The gold standard for the traditional diagnosis is
the examination of clinical samples of the patients, namely a blood
smear test for Mycobacterium tuberculi, along with typical
histological examinations and laboratory tests including complete
blood count, ESR and CRP, as well as further auxiliary examinations
to diagnose the corner curriculum (8,9).
However, due to the growth requirements and slow growth rate of TB
bacilli, their culture is challenging. The TST is frequently
performed, but its sensitivity and specificity are limited, even in
high-incidence areas of TB; even after in individuals with repeated
exposure to TB bacillus, a ~20% negative rate of the TST has been
estimated (8).
There are 3 types of non-cultivation laboratory
examination methods to detect TB bacillus: i) Immunological
detection including the tuberculin experiment (in vitro
detection of the antibody against mycobacterium TB in the white
blood cells) using ICT Tuberculosis (AMRAD-ICT; Sydney, Australia)
to detect mycobacterium TB in whole blood. It allows for a rapid
and accurate diagnosis of TB (10).
ii) Detection of metabolites: a) In vitro interferon (IFN)
test-when T-lymphocytes come into contact with mycobacterium, the
lymph cells secrete a significant amount of IFN-γ; b) T-SPOT
technology of ELISA (11); iii) PCR
technology to amplify the DNA of the TB mycobacterium (including
repeated sequencing of IS6110), and the results may be obtained
within a few hours (12).
X-ray imaging may roughly determine the location of
the TB lesion and affected area of the spine, and it is conducive
to the detection of spinal TB (13).
The X-ray image may appear normal if the lesions are in the early
stage, while progressive aggravation of the lesions may lead to
intervertebral space stenosis following intervertebral disc
involvement. Attention should be paid regarding changes in bone
density, osteoporotic lesions, vertebral collapse and abnormalities
in the physiological curvature of the spine. X-ray imaging may also
fail to detect osteoporotic fractures and congenital diseases. It
also has the following disadvantages: Slight bone changes and small
calcification areas may not be easily identified and it is also
difficult to diagnose cold abscesses by X-ray imaging.
A CT scan is able to detect even small bone damages
at an early stage, even at specific sites, including the
atlantoaxial and cervicothoracic junctions, which are difficult to
be observed on X-ray imaging. A three-dimensional CT image may be
used to assess the damage of the spine as a whole, indicating the
location of TB, invasion into the spinal canal and the degree of
spinal stenosis. The damage is mainly categorized into the debris
type, osteolysis type and sub-periosteum type. However, the CT
examination also has certain disadvantages: The original image does
not reveal any abnormalities in the intervertebral space and it is
difficult to visualize soft tissue lesions (14,15).
Among all the imaging modalities, MRI is the best
method to diagnose spinal TB, particularly for the early diagnosis.
When no abnormality is detected on X-ray imaging and even the CT
image is not clear, the MRI screening does not only indicate the
number and range of the involved vertebral segments clearly, but
also reveals the condition of the paravertebral soft tissues
(16,17). The MRI characteristics of spinal TB
are as follows: Lesions in 2 or more adjacent vertebral bodies are
most frequent; vertebral body destruction originating from anterior
and central segments may be present; T1-weighted imaging (T1WI)
signals are low and T2WI signals are mixed; vertebral body damage
may display as multiple necrosis; and the abscess area may vary in
size. Obvious enhancement of cricoid attachment is involved in rare
cases.
The MRI analysis of the representative cases in the
present study revealed that the intervertebral disc was deformed
and flattened with an unclear edge; abnormal signal intensities
were recorded and the severely damaged intervertebral disc
disappeared, leading to vertebral body fusion. The vertebral body
around the vertebral disc corresponded to the bone damage and the
shape of the vertebral body was incomplete, mostly wedge-shaped.
However, if the vertebral body of the spine is free from
intervertebral discitis and soft tissue signal abnormalities during
the inflammation, it is difficult to distinguish spinal TB from a
spinal tumor, and a biopsy may be performed to confirm the
diagnosis.
B-ultrasonography has obvious advantages in spinal
TB with paravertebral or lumbar abscess. The area of the abscess
may be observed as a dark area of fluid and the dead bone area is
strongly echogenic. The use of B-ultrasonography is simple, safe
and fast. It may also be used to guide the local positioning of a
puncture drainage, an indwelling drainage tube or the injection of
therapeutic drugs (14).
The clinical features of early spinal TB are not
typical and may be misdiagnosed as those of other diseases
(2,8), including the following: i) If one
vertebral body or several discontinuous segments are involved
during the imaging for spinal TB, this may easily be misdiagnosed
as a spinal tumor; ii) intervertebral disc inflammation: The
patient has a high fever, which is different from the afternoon low
fever of TB patients. The imaging may display no damage to the
cartilage endplate and no paravertebral cold abscess; iii)
ankylosing spondylitis: X-ray images of the spine showed vertebral
osteoporosis, quadrate degeneration, facet joint fuzziness,
calcification of the paravertebral ligaments, and the formation of
bone bridges. ‘Bamboo-like’ changes in the vertebral column may be
observed on X-ray imaging. However, there are certain further
spinal diseases that require to be differentiated from spinal TB,
including suppurative infection of the spine and eosinophilic
granuloma. Hence, for the definitive diagnosis of spinal TB,
comprehensive judgment of the latest detection methods such as
AMRAD ICT, T-SPOT, PCR, spinal biopsy and sepsis bacteriology
studies are required.
In conclusion, if spinal TB is diagnosed at an early
stage, early treatment may be initiated. This does not only
interfere with the progression of the disease and shorten the
course of treatment, but also reduces the economic burden and the
risk of the occurrence of spinal deformity. It is apparent that
surgery is an important adjunct, but does not cure active TB. An
early and more accurate diagnosis of spinal TB will become feasible
with the continuous improvement of the examination methods.
Acknowledgements
The authors would like to thank Dr Wei Liu and Dr
Xiaolong Zhao from Department of Orthopedics, Wuhan No. 1 Hospital,
Wuhan Integrated TCM & Western Medicine Hospital, Tongji
Medical College, Huazhong University of Science and Technology
(Wuhan, China) for providing technical assistance.
Funding
This work was supported by grants from the Research
Project of Hubei Province Health and Family Planning Commission
(grant no. WJ2017Z022), the Natural Science Fund Project of Science
and Technology Department of Hubei Province (grant no. 2016CFB666),
a Special Clinical Research Fund of Wu Jieping Medical Foundation
(grant no. 320.6750.17566), the Scientific Research Program of
Wuhan Health and Family Planning Commission (grant nos. WX17Q38 and
WZ18Q05) and the Research Program of Wuhan No. 1 Hospital, Wuhan
Integrated TCM & Western Medicine Hospital (grant no.
2017Y01).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to patient privacy and
copyright of case data, but are available from the corresponding
author on reasonable request.
Authors' contributions
FP and JF conceived and designed the experiments of
the current study. LY and LZ contributed to the admission and
treatment of the patients. LY, LZ, FP and PX performed the
collection and collation of data. PX analyzed and interpreted the
data. All authors have written the manuscript, and read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Wuhan No. 1 Hospital, Wuhan Integrated TCM &
Western Medicine Hospital (Wuhan, China). Written informed consent
was obtained from all patients.
Patient consent for publication
Consent for publication of images was obtained from
all patients.
Competing interests
The authors declare that they have no competing
interests regarding this study.
References
|
1
|
Sternbach G: Percivall Pott: Tuberculous
spondylitis. J Emerg Med. 14:79–83. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dunn RN and Ben Husien M: Spinal
tuberculosis: Review of current managment. Bone Joint J.
100:425–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Varatharajah S, Charles YP, Buy X, Walter
A and Steib JP: Update on the surgical management of pott's
disease. Orthop Traumatol Surg Res. 100:229–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Y, Wang Q, Zhu R, Yang C, Chen Z, Bai
Y, Li M and Zhai X: Trends of spinal tuberculosis research
(1994–2015): A bibliometric study. Medicine (Baltimore).
95:e49232016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'souza MM, Mondal A, Sharma R, Jaimini A
and Khanna U: Tuberculosis the great mimicker: 18F-fludeoxyglucose
positron emission tomography/computed tomography in a case of
atypical spinal tuberculosis. Indian J Nucl Med. 29:99–101. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marks J: Notes on the ziehl-neelsen
staining of sputum. Tubercle. 55:241–244. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garg RK and Somvanshi DS: Spinal
tuberculosis: A review. J Spinal Cord Med. 34:440–454. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CH, Chen YM, Lee CW, Chang YJ, Cheng
CY and Hung JK: Early diagnosis of spinal tuberculosis. J Formos
Med Assoc. 115:825–836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Merino P, Candel FJ, Gestoso I, Baos E and
Picazo J: Microbiological diagnosis of spinal tuberculosis. Int
Orthop. 36:233–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delogu G, Zumbo A and Fadda G:
Microbiological and immunological diagnosis of tuberculous
spondylodiscitis. Eur Rev Med Pharmacol Sci. 16:73–78.
2012.PubMed/NCBI
|
|
11
|
Yuan K, Zhong ZM, Zhang Q, Xu SC and Chen
JT: Evaluation of an enzyme-linked immunospot assay for the
immunodiagnosis of atypical spinal tuberculosis (atypical clinical
presentation/atypical radiographic presentation) in China. Braz J
Infect Dis. 17:529–537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharma K, Meena RK, Aggarwal A and Chhabra
R: Multiplex PCR as a novel method in the diagnosis of spinal
tuberculosis-a pilot study. Acta Neurochir (Wien). 159:503–507.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H and Lu Z: Atypical imaging of
spinal tuberculosis: A case report and review of literature. Pan
Afr Med J. 24:1012016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ansari S, Amanullah MF, Ahmad K and
Rauniyar RK: Pott's spine: Diagnostic imaging modalities and
technology advancements. N Am J Med Sci. 5:404–411. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rivas-Garcia A, Sarria-Estrada S,
Torrents-Odin C, Casas-Gomila L and Franquet E: Imaging findings of
pott's disease. Eur Spine J. 22:567–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kilborn T, Janse van Rensburg P and Candy
S: Pediatric and adult spinal tuberculosis: Imaging and
pathophysiology. Neuroimaging Clin N Am. 25:209–231. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar Y, Gupta N, Chhabra A, Fukuda T,
Soni N and Hayashi D: Magnetic resonance imaging of bacterial and
tuberculous spondylodiscitis with associated complications and
non-infectious spinal pathology mimicking infections: A pictorial
review. BMC Musculoskelet Disord. 18:2442017. View Article : Google Scholar : PubMed/NCBI
|