Introduction
Until the introduction of direct-acting antiviral
(DAA) therapy, interferon (IFN)-based therapy for patients with
chronic hepatitis C was the standard treatment. Although the
incidence of hepatocellular carcinoma (HCC) has decreased after
achieving a sustained virological response (SVR), some reports
indicate that HCC develops in 0.5–8.8% of patients during an
observation period of 3 to 5 years (1–9). In
liver tissue, inflammation and fibrosis improve after achieving
SVR. Shiratori et al reported that the activity grade
improved in 89% of patients and fibrosis regressed at a rate of
0.282 U/year in SVR patients during an average observation period
of 3.7 years (10).
On the other hand, Nirei et al (11) reported persistent hepatic
inflammation in patients who developed HCC after IFN-based SVR.
Motoyama et al (12) reported
that lack of fibrosis improvement is a risk factor for HCC after
SVR. However, there are no reports of immunohistochemistry for
inflammatory cells in the portal area of patients who developed HCC
after achieving SVR. Therefore, we examined pathological changes
before IFN therapy and after HCC development with a focus on
hepatic inflammation, fibrosis, and immunology.
Immunologically, eradication of hepatitis C virus
can be achieved by vigorous antiviral T cell response. On the other
hand, a weak cellular immune response results in HCV persistence
(13). In the immune response,
CD4+ T cells support CD8+ T cells and B cells
by secreting cytokines (14,15). To clarify changes after SVR in
immunity, we investigated the immunological markers CD3, CD4, CD8
and CD20 (16,17).
We also investigated granzyme because it is a marker
for CTL. We also investigated forkhead box P3 (FOXP3) because it is
a specific marker for regulatory T cells (Tregs), which are
immunosuppressive cells. In cancerous tissue, Tregs have a positive
effect on tumor proliferation and thus are associated with a poor
prognosis (18–20). Sakaki et al (21) reported that the frequency of FOXP3 in
portal tracts in patients with chronic hepatitis C was
significantly higher than that in normal controls. FOXP3 is also
strongly correlated with the portal inflammation score (22). Transforming growth factor β1 (TGF-β1)
was also examined because TGF-β1 suppresses liver regeneration and
promotes tissue fibrosis in the liver (23).
In this study, we retrospectively examined the
pathological changes before IFN therapy and after HCC development
and used immunohistochemistry of infiltrating lymphocytes in the
portal area to assess histological characteristics.
Materials and methods
Patients and controls
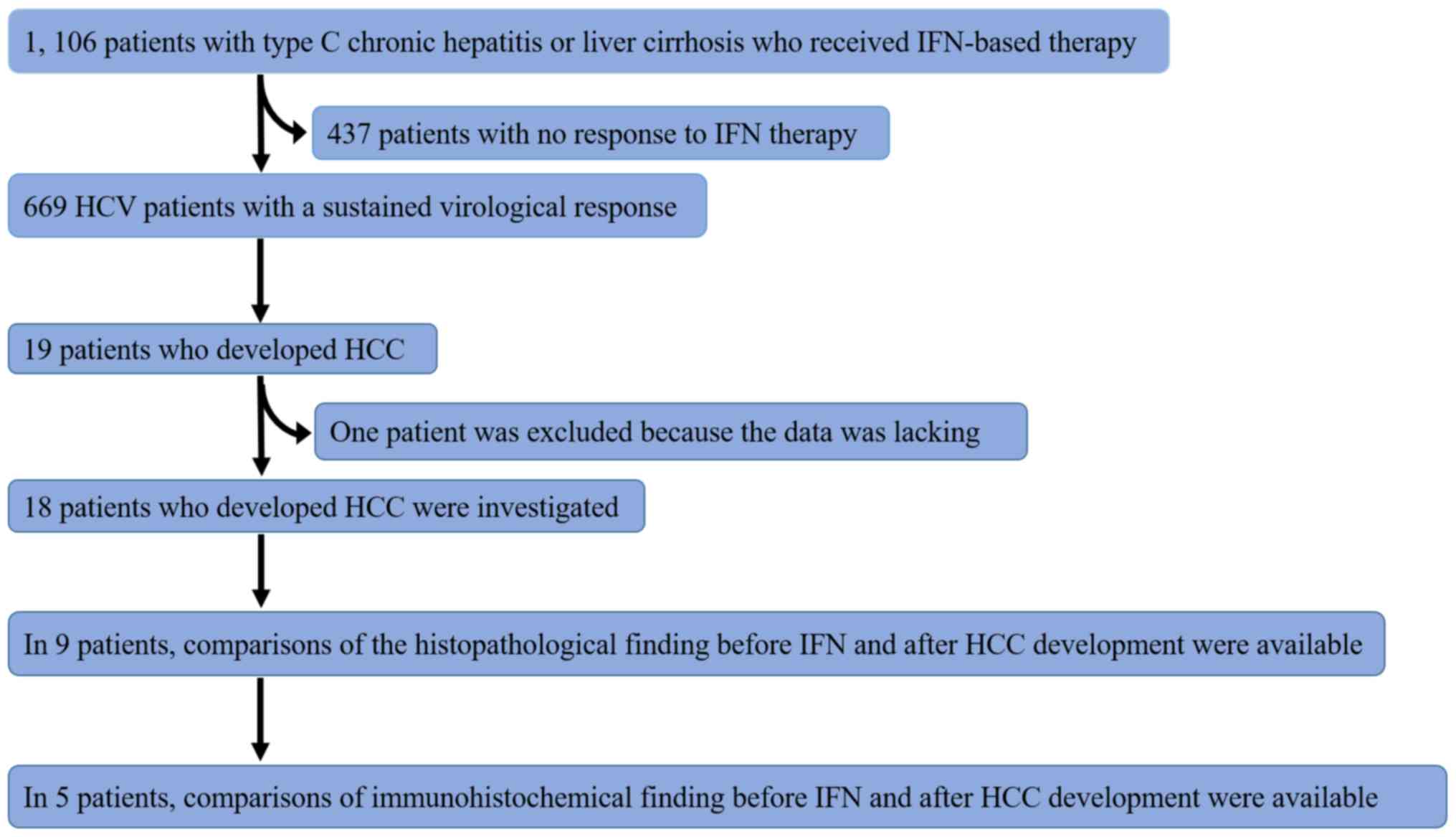
A total of 1,106 Japanese patients with type C
chronic hepatitis or liver cirrhosis who visited Kurume University
Hospital and were treated with IFN-based therapy between January
2003 and December 2016 were enrolled. Before IFN administration,
baseline data were evaluated. All patients were positive for HCV
antibody (by 2nd generation ELISA; Abbot, Tokyo, Japan). HCV RNA
levels were measured using a Roche COBAS Taq Man test. Patients
were considered to have achieved SVR if they remained negative for
serum HCV RNA 24 weeks after the end of IFN therapy. We excluded
patients who had hepatitis B surface antigen, a history of HCC
before IFN-based therapy, and developed HCC within one year after
the end of therapy. SVR was achieved in 669 patients by IFN-based
therapy, and 19 patients who developed HCC were selected. We
excluded one patient whose data were lacking. From among patients
with HCV-related chronic liver disease who developed HCC and were
treated at Kurume University in 2009 and 2010, we randomly selected
6 patients whose non-cancerous liver tissue was obtained as
controls. Exclusion criteria for the control samples included
previous IFN therapy. Four patients had chronic hepatitis, and 2
patients had cirrhosis. In control patients, laboratory data were
obtained at the time when they were admitted to our hospital for
initial HCC therapy. They were negative for hepatitis B surface
antigen. All patients gave written informed consent according to a
protocol approved by the Ethical Committee of Kurume University
(approval No. 16244).
Histological examination
We examined 18 patients who developed HCC after
IFN-based SVR. Specimens were obtained before IFN therapy by
ultrasound-guided biopsy using 14-gauge needles. After developing
HCC, non-cancerous liver tissues around the tumor were obtained by
hepatic resection or by tumor biopsy before radiofrequency
ablation. We compared 9 patients histologically before IFN therapy
and after HCC development to evaluate histological changes. We also
obtained non-cancerous liver specimens from six control patients
before treatment for HCC.
We investigated specimens stained with haematoxylin
and eosin (H&E). The degrees of hepatic inflammation and
fibrosis were scored according to the classification of Desmet
(24), Knodell (25), and Ishak (26) from A0 to A3 and from F0 to F4,
respectively. When fat in the liver exceeded 5% histologically, the
liver was defined as a liver steatosis. For the immunohistochemical
examination, liver tissue was fixed with 10% formalin, embedded in
paraffin, cut into 4-µm sections, examined on a coated slide glass,
and then used for immunohistochemical analyses. The following
primary antibodies were used: Mouse monoclonal anti-CD3 antibody
(×300; Leica Biosystems, Nussloch, Germany), mouse monoclonal
anti-CD4 antibody (×200; Leica Biosystems, Nussloch, Germany),
mouse monoclonal anti-CD8 antibody (×200; Leica Biosystems,
Nussloch, Germany), mouse monoclonal anti-CD20 antibody (×1,200;
Dako, Glostrup, Denmark), mouse monoclonal anti-FOXP3 antibody
(×600; Abcam, Cambridge, MA, USA), mouse polyclonal anti-TGF-β1
antibody (×300; Santa Cruz Biotechnology, Heidelberg, Germany), and
mouse monoclonal anti-granzyme B antibody (×50; Leica Biosystems,
Nussloch, Germany). Immunohistochemical examinations with CD3, CD4,
CD8, CD20, TGF-β1, and granzyme B were performed on the same fully
automated Bond-Max system (Leica Biosystems, Newcastle, UK) using
onboard heat-induced antigen retrieval with ER2 for 10 min and the
Refine polymer detection system. The chromogen
3,3′-diaminobenzidine-tetrachloride (DAB) was used for all
immunostaining. FOXP3 immunostaining was performed using the Dako
autostainer (Dako, Glostrup, Denmark). Briefly, specimens were
boiled in a microwave oven for 30 min in 1 mmol/l
ethylenediaminetetraacetic acid pH 9.0 and target retrieval
solution for antigen recovery, and the specimens were then
incubated with the antibody at 4°C overnight. After washing with
Tris-buffered saline (TBS), slides were incubated with labeled
polymer-horseradish peroxidase secondary antibody for 30 min at
room temperature. After washing with TBS, the slides were
visualized using DAB.
Evaluation of histology
(immunohistochemical staining)
Immunohistochemical examination of CD3+,
CD4+, CD8+, and CD20+ lymphocytes,
and FOXP3, TGF-β1, and granzyme B-positive cells were performed in
the portal area. Two relatively small-to-medium size portal tracts
were investigated with a microscope to count positive cells. To
investigate specimens, positive cells were counted in visual fields
with clear inflammatory cell infiltration. The number of positive
cells was counted twice. Because of the difference in the sizes of
the portal tract, the positive rate of immunohistochemical staining
was calculated as follows: Positive cell rate = (number of positive
cells/number of total mononuclear cells) × 100.
Statistical analyses
Values were expressed as the median (IQR,
interquartile range). Statistical analysis was performed using the
JMP software package (release 13, SAS Institute, Cary, NC, USA).
For comparison of variables, Mann-Whitney and Wilcoxon tests were
performed as appropriate. Bonferroni's correction was used for
multiple comparisons. P<0.05 was used as a critical P-value, and
following Bonferroni's correction, P<0.017 was considered to
indicate a statistically significant difference.
Results
Clinical findings
A flow chart of the subjects is shown in Fig. 1. Tables
I and II show the
characteristics of 18 patients who developed HCC after IFN-based
SVR. These tables show characteristics obtained before IFN
treatment (Table I) and at the time
HCC was diagnosed (Table II). To
estimate the degree of liver fibrosis, the Fibrosis-4 index
(27) was calculated. Two patients
(cases 4 and 17) had a splenectomy before IFN-based therapy. Risk
factors, such as age ≥65, male sex, or advanced fibrosis F≥3, were
present in all cases except one (case 10). There were 11 of 18
patients (61%) that had a risk factor such as alcohol intake (≥60
mg/day), diabetes mellitus, or liver steatosis. In 9 patients
(cases 1–9) whose specimens were available for comparison, 4
patients (cases 6–9) developed HCC 3 years after end of IFN
therapy. In case 8, the duration between the end of IFN and HCC
development was 112 months. That patient had risk factors for HCC
including diabetes mellitus and alcohol intake 60 g/day.
 | Table I.Characteristics of 18 patients before
IFN therapy who developed hepatocellular carcinoma after an
IFN-based SVR. |
Table I.
Characteristics of 18 patients before
IFN therapy who developed hepatocellular carcinoma after an
IFN-based SVR.
| Case | Age (year) | Sex | Geno/Sero type | IFN therapy | BMI | AST (U/l) | ALT (U/l) | PLT
(104/µl) | AFP (ng/ml) | FIB4 index | HBc Ab | Liver
steatosis | DM | Alcohol)
(g/day) |
Activity/fibrosis |
|---|
| 1 | 67 | M | 1b | IFNα-2b+Rib | 22.5 | 45 | 64 | 12.7 | 12.2 | 2.97 | (+) | (+) | (−) | 20 | A1F2 |
| 2 | 49 | M | 1b | PEG-IFN+Rib | 27.7 | 161 | 133 | 10.8 | 10.8 | 6.33 | (−) | (−) | (−) | 100 | A3F3 |
| 3 | 67 | F | 1b |
PEG-IFN+Rib+Simeprevir | 24.2 | 24 | 37 | 14.2 | 3.2 | 1.86 | (−) | (−) | (−) | (−) | A2F3 |
| 4 | 50 | F | 1b | PEG-IFN+Rib | 19.7 | 78 | 79 | 6.7 | 19.1 | 6.55 | (−) | (−) | (−) | (−) | A2F3 |
| 5 | 65 | M | 1b | PEG-IFN+Rib | 17.7 | 94 | 133 | 13.1 | 10.0 | 4.04 | (+-) | (−) | (+) | (−) | A2F2 |
| 6 | 49 | F | 1b | IFNβ | 26.3 | 47 | 39 | 10.5 | 1.7 | 3.51 | (−) | (−) | (+) | (−) | A1F4 |
| 7 | 69 | F | 1b | PEG-IFN+Rib | 21.9 | 92 | 125 | 10.6 | 5.1 | 5.36 | (−) | (−) | (−) | (−) | A2F2 |
| 8 | 46 | M | 2 | IFNβ | 29.4 | 44 | 62 | 18.3 | 8.3 | 1.40 | (+) | (−) | (+) | 60 | A2F2 |
| 9 | 50 | M | 2a | PEG-IFN | 23.1 | 61 | 59 | 19.3 | 6.6 | 2.06 | (+) | (−) | (+) | 60 | A2F4 |
| 10 | 51 | F | 2 | IFNα+Rib | 20.4 | 18 | 18 | 14.4 | 4.0 | 1.50 | (+) | (−) | (−) | 200 | A1F2 |
| 11 | 67 | F | 2b | PEG-IFN+Rib | 22.9 | 24 | 24 | 12.3 | 2.4 | 2.67 | n.d. | (−) | (−) | (−) | A1F1 |
| 12 | 56 | M | 1b | PEG-IFN+Rib | 19.4 | 47 | 53 | 10.5 | 4.9 | 3.44 | (+) | (−) | (+) | (−) | A2F3 |
| 13 | 55 | M | 1b | PEG-IFN+Rib | 28.9 | 133 | 210 | 14.5 | 22.6 | 3.48 | (−) | (+) | (−) | (−) | A2F2 |
| 14 | 72 | F | 1b | PEG-IFN+Rib | 24.1 | 49 | 37 | 13.6 | 18.6 | 4.03 | (−) | n.d. | (−) | (−) | n.d. |
| 15 | 66 | M | 1b |
PEG-IFN+Rib+Telaprevir | 21.3 | 55 | 60 | 9.2 | 17.5 | 5.09 | (−) | n.d. | (−) | (−) | n.d. |
| 16 | 48 | M | 1b |
PEG-IFN+Rib+Telaprevir | 30.2 | 37 | 35 | 11.0 | 4.7 | 4.06 | (−) | n.d. | (−) | (−) | n.d. |
| 17 | 52 | M | 2a | IFNα-2b+Rib | 33.0 | 104 | 90 | 13.5 | 6.7 | 4.22 | (+) | n.d. | (+) | (−) | n.d. |
| 18 | 58 | M | 2a | PEG-IFN | 29.3 | 33 | 38 | 14.6 | 6.4 | 2.13 | (−) | n.d. | (+) | (−) | n.d. |
 | Table II.Characteristics of 18 patients after
HCC development. |
Table II.
Characteristics of 18 patients after
HCC development.
| Case | Age (years) | Sex | BMI | AST (U/l) | ALT (U/l) | PLT
(104/µl) | AFP 6 months) end
of IFN therapy (ng/ml | FIB4 index | Last HCVAb
(COI) | Therapy for
HCC | Duration between
end of IFN and HCC development (months) | Liver
steatosis |
Activity/fibrosis |
|---|
| 1 | 69 | M | 21.8 | 17 | 12 | 14.3 | n.d. | 2.37 | 8.2 | Resection | 19 | (−) | A2F1 |
| 2 | 53 | M | 28.2 | 69 | 52 | 12.1 | 5.3 | 4.19 | 10.0 | Resection | 29 | (−) | A2F4 |
| 3 | 68 | F | 25.3 | 22 | 13 | 16.7 | n.d. | 2.48 | 11.6 | Resection | 13 | (−) | A1F4 |
| 4 | 53 | F | 20.0 | 20 | 13 | 8.6 | 9.5 | 3.42 | 13.0 | Resection | 19 | (−) | A2F3 |
| 5 | 67 | M | 21.8 | 20 | 19 | 18.5 | 2.6 | 1.71 | 7.9 | RFA | 18 | (−) | A1F1 |
| 6 | 54 | F | 29.1 | 28 | 39 | 9.0 | 4.3 | 2.69 | 32.1 | Resection | 45 | (+) | A1F4 |
| 7 | 75 | F | 22.3 | 21 | 12 | 15.7 | 2.0 | 2.90 | n.d. | Resection | 64 | (−) | A1F1 |
| 8 | 56 | M | 27.7 | 26 | 27 | 21.5 | n.d. | 1.30 | 6.9 | Resection | 112 | (−) | A2F2 |
| 9 | 56 | M | 25.2 | 28 | 22 | 16.8 | 3.6 | 1.99 | 17.1 | RFA | 65 | (−) | A1F2 |
| 10 | 58 | F | 23.4 | 20 | 16 | 15.3 | n.d. | 1.90 | 32.8 | RFA | 88 | n.d. | n.d. |
| 11 | 73 | F | 21.9 | 337 | 407 | 11.4 | 2.0 | 10.7 | n.d. | Chemotherapy | 61 | n.d. | n.d. |
| 12 | 61 | M | 18.9 | 31 | 20 | 8.8 | n.d. | 4.81 | 14.6 | Radiation,
HAIC | 51 | n.d. | n.d. |
| 13 | 61 | M | 29.4 | 60 | 56 | 25.0 | 4.4 | 1.96 | 12.5 | TACE | 59 | n.d. | n.d. |
| 14 | 77 | F | 23.2 | 25 | 13 | 12.6 | 13.8 | 4.24 | n.d. | Resection | 44 | (−) | A1F1 |
| 15 | 69 | M | 20.7 | 23 | 16 | 10.6 | 4.6 | 3.74 | 15.6 | Resection | 26 | (−) | A1F3 |
| 16 | 53 | M | 30.8 | 21 | 28 | 12.7 | 5.3 | 1.66 | 13.9 | Resection | 24 | (+) | A1F4 |
| 17 | 56 | M | 32.6 | 39 | 36 | 15.1 | 4.8 | 2.41 | 33.6 | RFA | 24 | n.d. | n.d. |
| 18 | 64 | M | 26.9 | 12 | 13 | 10.4 | 6.3 | 2.05 | 8.3 | RFA | 66 | n.d. | n.d. |
Histological findings
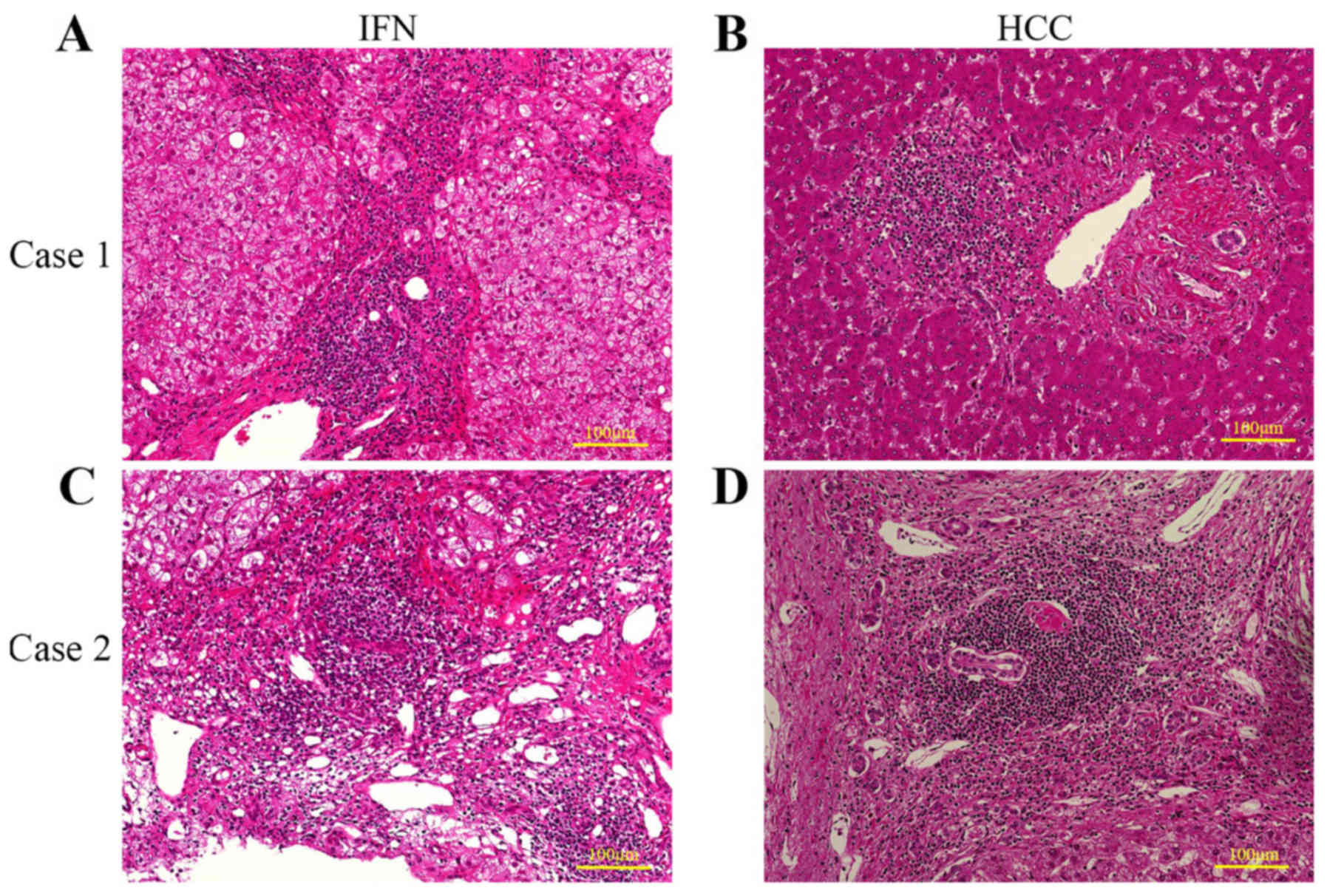
Inflammatory cell infiltration consisting mostly of
lymphocytes was observed histologically in the portal area of all
12 specimens after HCC development. In 9 patients, the degree of
hepatic inflammation and fibrosis according to the classification
of Desmet et al (24),
Knodell et al (25), and
Ishak et al (26) were
comparable. Inflammation improved in 5 patients, worsened in 1
patient, and was unchanged in 3 patients. Fibrosis improved in 4
patients, worsened in 2 patients, and was unchanged in 3 patients.
Fig. 2 shows representative
specimens from 5 patients.
Immunohistochemical findings
From a total of 9 patients, we compared the
specimens in 5 patients by immunohistochemical staining. We did not
collect specimens before IFN and after HCC development in the
remaining 4 patients because the specimens for immunohistochemical
staining were lost or degraded. Fig.
3 shows the average frequency of CD3+,
CD4+, CD8+, and CD20+ lymphocytes,
and FOXP3, TGF-β1, and granzyme B-positive cells in the portal area
from cases 1–5. In many specimens, the majority of infiltrating
inflammatory cells were predominantly CD3+ lymphocytes.
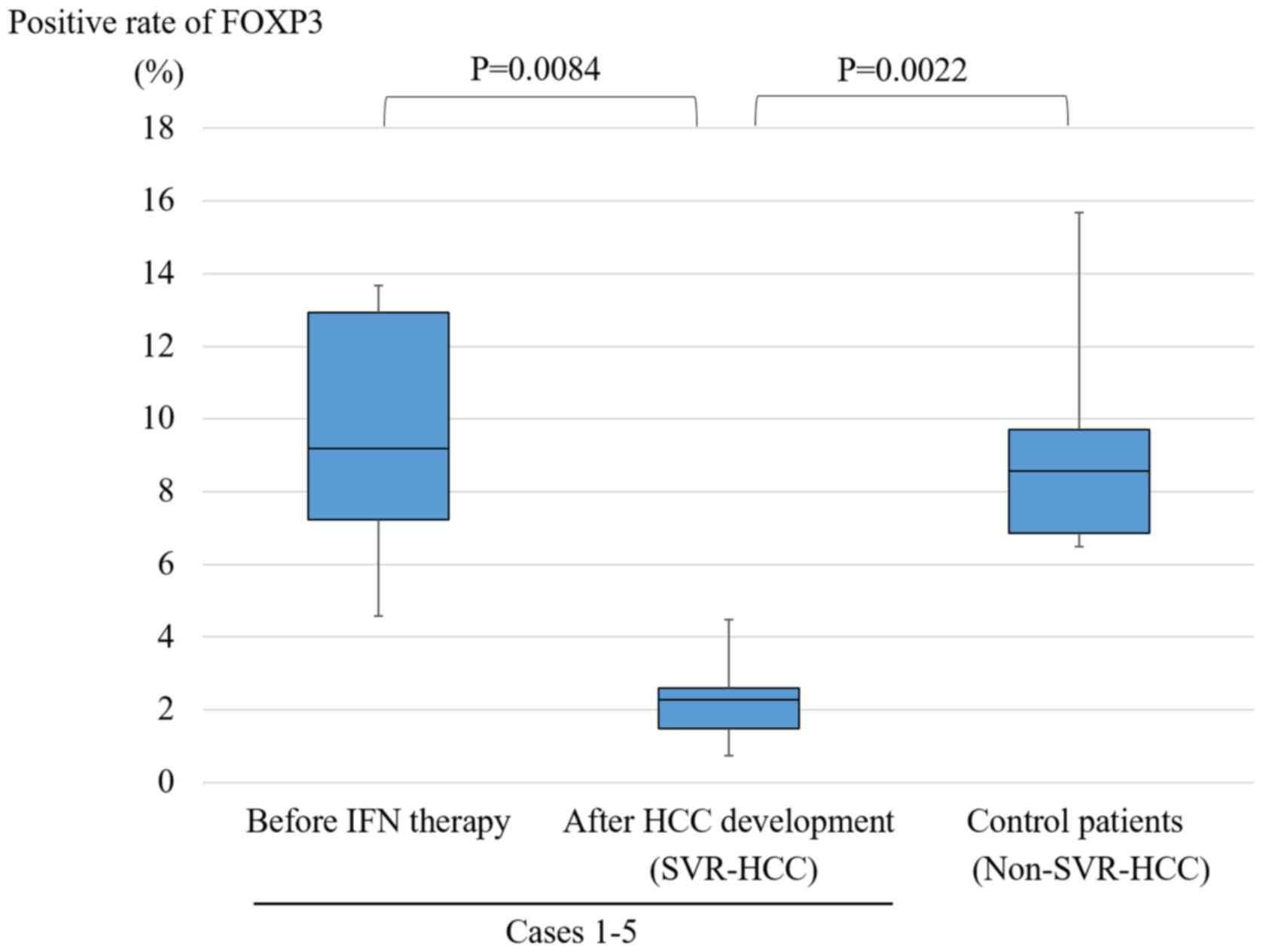
The median positive rate of FOXP3 was 9.20% (7.23–12.93%) before
IFN therapy and 2.28% (1.48–2.59%) after HCC development. Table III shows the ratio of positive
cells after HCC development to that before IFN therapy for CD3,
CD4, CD8, CD20, FOXP3, TGF-β1, and granzyme B. In all 5 cases, the
ratio of FOXP3 was less than 1.0 (0.11–0.36). However, there was no
change in CD3+, CD4+, CD8+, and
CD20+ lymphocytes, and TGF-β1 and granzyme B-positive
cells. Table IV shows the clinical
characteristics and positive rate of FOXP3 in control patients. The
median positive rate of FOXP3 was 8.58% (6.86–9.70%). Fig. 4 shows the positive rate of FOXP3 in
cases 1–5 and control patients. For cases 1–5, there was a
significant difference in the positive rate (P=0.0084) before IFN
and after HCC development. In addition, there was a significant
difference between after HCC development of cases 1–5 and control
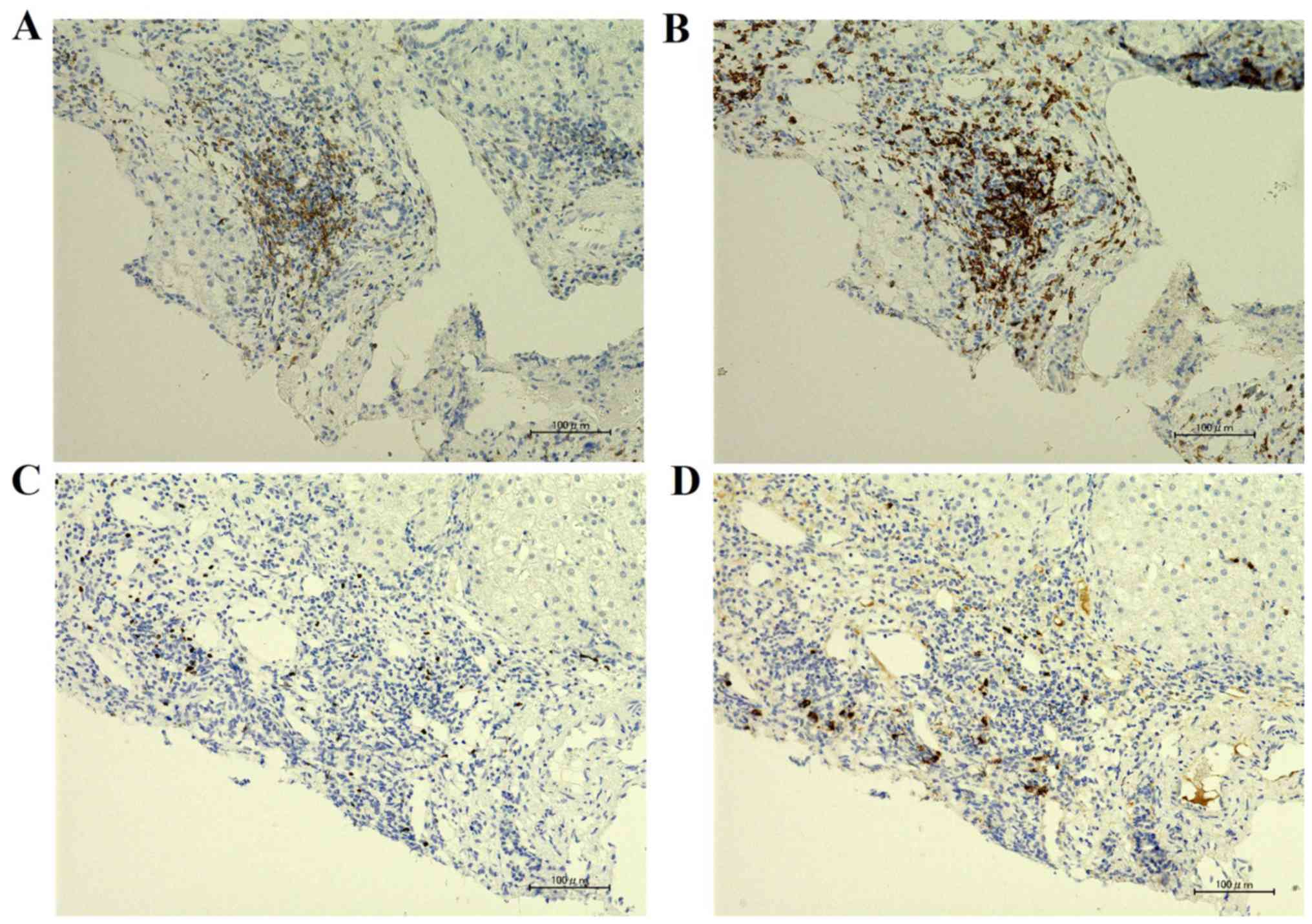
patients (P=0.0022). We examined liver specimens by
immunohistochemical staining of CD4, CD8, FOXP3, and TGF-β1 in case
2 (Fig. 5).
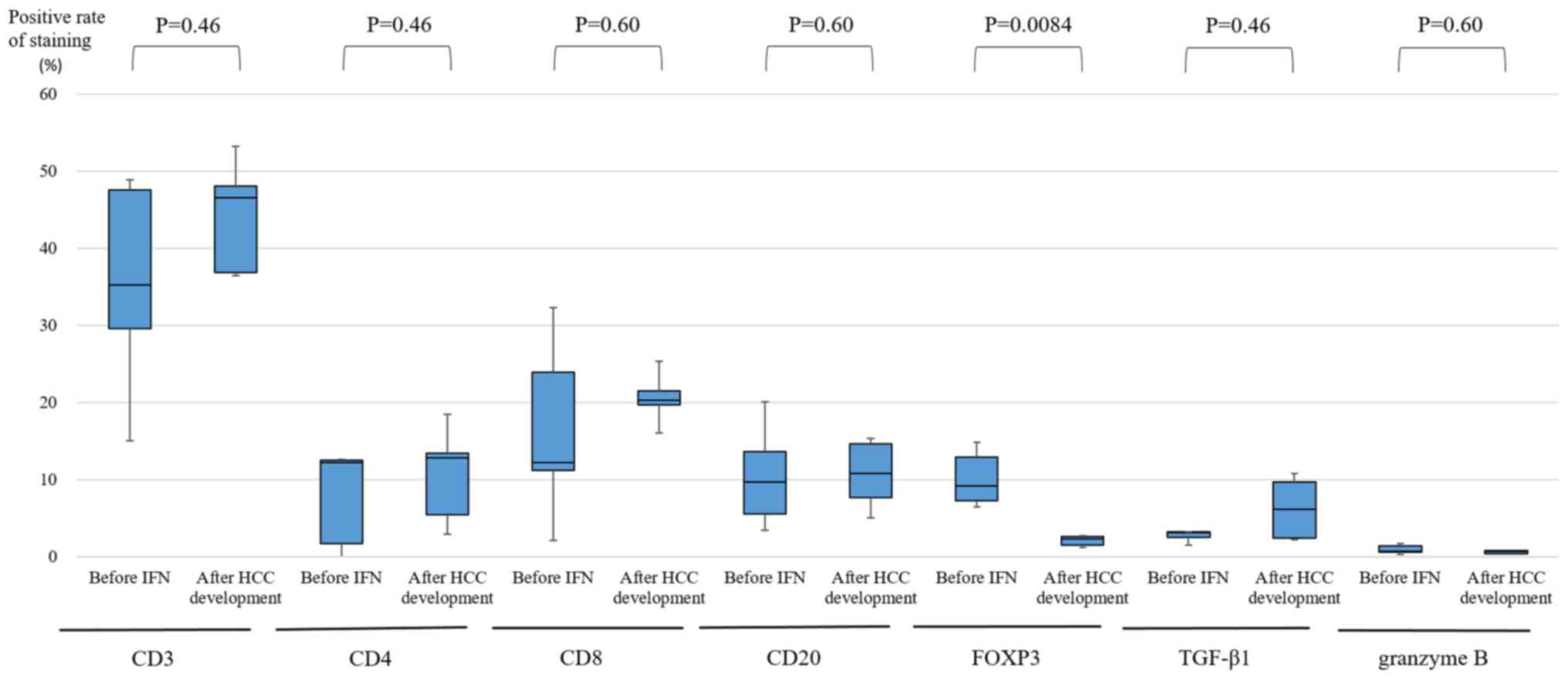 | Figure 3.Average frequency of CD3+,
CD4+, CD8+ and CD20+ lymphocytes,
and FOXP3, TGF-β1, and granzyme B-positive cells in cases 1–5
within the portal area. For the enumeration of positive mononuclear
cells, all mononuclear cells were counted in two microscopic fields
of the portal tract, twice. For each sample, the mean percentage of
positive cells was used. In FOXP3, there was a significant
difference before IFN therapy and after HCC development (P=0.0084),
as determined by the Wilcoxon test. No significant differences were
identified in levels of CD3 (P=0.46), CD4 (P=0.46), CD8 (P=0.60),
CD20 (P=0.60), TGF-β1 (P=0.46) and granzyme B (P=0.60) before IFN
therapy compared with after HCC development. FOXP3, forkhead box
P3; TGF-β1, transforming growth factor-β1; IFN, interferon; HCC,
hepatocellular carcinoma; SVR, sustained virological response. |
 | Table III.Ratio of positive staining after HCC
development to that of staining before IFN therapy (Positive rate
of staining after HCC development/that of staining before IFN
therapy). |
Table III.
Ratio of positive staining after HCC
development to that of staining before IFN therapy (Positive rate
of staining after HCC development/that of staining before IFN
therapy).
| Case number | CD3 | CD4 | CD8 | CD20 | FOXP3 | TGF-β1 | Granzyme B |
|---|
| Case 1 | 2.73 | 3.25 | 1.21 | 2.90 | 0.11 | 3.09 | 0.78 |
| Case 2 | 0.99 | 0.73 | 0.74 | 1.21 | 0.36 | 1.85 | 1.09 |
| Case 3 | 0.47 | 0.22 | 0.85 | 0.79 | 0.16 | 0.96 | 0.23 |
| Case 4 | 1.04 | 10.3 | 1.74 | 2.63 | 0.25 | 0.60 | 1.11 |
| Case 5 | 1.57 | 1.09 | 2.43 | 0.80 | 0.35 | 3.01 | 0.28 |
 | Table IV.Characteristics and the positive
rates of FOXP3 in control patients. |
Table IV.
Characteristics and the positive
rates of FOXP3 in control patients.
| Case | Age (years) | Sex | BMI | AST (U/l) | ALT (U/l) | PLT
(104/µl) | FIB4 index | Therapy for
HCC | Liver
steatosis | DM | Alcohol
(g/day) |
Activity/Fibrosis | Positive rate of
FOXP3 (%) |
|---|
| 1 | 65 | F | 24.1 | 78 | 52 | 4.7 | 14.96 | RFA | (−) | (−) | (−) | A2F2 | 7.68 |
| 2 | 51 | M | 21.6 | 110 | 128 | 10.3 | 4.81 | Resection, RFA | (−) | (−) | 14 | A2F2 | 9.47 |
| 3 | 73 | M | 22.8 | 70 | 45 | 4.9 | 15.55 | Resection, RFA | (−) | (−) | (−) | A2F3 | 6.59 |
| 4 | 66 | F | 26.7 | 47 | 27 | 4.4 | 13.57 | RFA | (−) | (−) | (−) | A2F4 | 6.50 |
| 5 | 75 | F | 24.1 | 83 | 39 | 7.0 | 14.24 | HAIC | (−) | (−) | (−) | A2F3 | 9.78 |
| 6 | 53 | F | 21.7 | 52 | 48 | 3.7 | 10.75 | Laparoscopic
RFA | (−) | (−) | (−) | A2F4 | 15.69 |
Discussion
Previous studies have revealed improvement in liver
tissue when SVR is achieved after IFN-based therapy. In 1991,
Schvarcz et al (28)
evaluated liver tissue before and 9 months after IFN monotherapy
and observed improvement in fibrosis, necrosis, and inflammation in
liver tissue after therapy. Terada et al (29) found improvement of staging and
grading to A0 (or A1) and F0 (or F1) three years after achievement
of SVR. Shiratori et al (10)
followed patients for a mean of 3.7 years (1–10 years) and showed
improvements of grading in 89% and fibrosis regression at a rate of
0.282 U/year. On the other hand, George et al (30). followed patients for 5 years on
average and observed improvement of liver fibrosis in 82%,
improvement of inflammation score in 92%, and improvement to a
normal liver state in 20% of all patients. Thus, fibrosis and
inflammation improved even with longer follow-up periods. In our
study, although improvement of inflammation and fibrosis was
observed in some of the 9 patients, inflammation and fibrosis still
remained in these patients. Staging and grading improved after SVR
in general, but some patients who developed HCC after SVR did not
have improved staging and grading. These results differed from the
general course of staging and grading after SVR. Nirei et al
(11) reported that specimens in all
10 patients who developed HCC after SVR had persistent
inflammation, which suggests an association with carcinogenesis.
Motoyama et al (12) reported
that hepatic stellate cell activation may inhibit improvement in
fibrosis after SVR and potentially contribute to
hepatocarcinogenesis. Ikeda et al (31) suggested that long-standing chronic
liver inflammation and liver regeneration after SVR may trigger
tumor development.
Clinical risk factors for carcinogenesis after SVR
include advanced age, male sex, advanced fibrosis (3 major
factors), alcohol abuse, diabetes mellitus, and liver steatosis
(9). All 18 patients who developed
HCC after SVR had at least one of these risk factors.
In immunohistochemical staining, Sakaki et al
(21) evaluated FOXP3 expression in
the portal tracts of patients with hepatitis C and normal controls
and found significantly higher FOXP3 expression in the former
group. Our immunohistological evaluation revealed a significant
decrease in the positive rate of FOXP3 in all 5 patients at the
time of HCC development, and the positive rate of FOXP3 in these
patients was also significantly lower than that in the control
group. HCV itself, especially the NS3 region, induces the
infiltration of Tregs in liver tissues (32), which may be why the rate of Tregs
decreased after achieving SVR. FOXP3 expression in the portal area
before IFN therapy and after SVR (without HCC) has not been
reported, but a previous report found that FOXP3 is strongly
correlated with the portal inflammation score (22). Therefore, if portal inflammation
improves after SVR, it is likely that FOXP3 expression has
decreased. We found that FOXP3 was not related to HCC development
because the expression levels of FOXP3 were higher in HCC patients
with HCV-related chronic liver disease. Although FOXP3 expression
decreased, there was continuous inflammation in patients who
developed HCC after achieving SVR. Therefore, a different
inflammatory mechanism may participate in hepatocarcinogenesis
after SVR compared with that in the presence of HCV.
Immunohistochemical examination of immune markers, such as CD3, 4,
8, 20, TGF-β, granzyme B, and FOXP3, of non-cancerous liver tissue
of patients that achieved SVR with and without HCC is needed, but
we could not collect non-cancerous liver tissue from patients who
achieved SVR without HCC.
There was no change in CD3+,
CD4+, CD8+, and CD20+ lymphocytes,
and TGF-β1 and granzyme B-positive cells. Sakaki et al
(21) also evaluated the portal
tracts in patients with hepatitis C and normal controls
(HCV-negative) and found there was no significant difference
between the two groups in the frequency of CD4+ and
CD8+. We also found no significant difference in the
frequency of CD4+ and CD8+ before IFN therapy
and after SVR (with HCC). This study was limited because the number
of enrolled patients by immunohistochemical staining was small.
This is mainly because the incidence of HCC development after
achieving SVR is low in general (1–9). In
addition, we did not collect many specimens before IFN therapy.
After HCC development, non-cancerous liver tissues around the tumor
were obtained in cases of hepatic resection or tumor biopsy before
radiofrequency ablation was performed. Further cases are needed to
draw more statistically robust conclusions.
Alcohol intake and liver steatosis may be involved
in the inflammatory mechanism. Portal inflammation occurs in the
livers of patients with alcoholic liver disease (ALD) or
non-alcoholic fatty liver disease (NAFLD) (33–35), and
portal inflammation is associated with advanced histological
changes in fatty liver disease (both alcoholic and non-alcoholic)
(36). In 9 patients (case 1–9)
whose specimens were compared, 4 patients drank alcohol (≥20
mg/day). In 3 out of 4 patients, moderate inflammation (A2) was
observed after HCC development in the portal area. Mild activity
(A1) was observed in the portal area in the remaining 1 patient. In
addition, we observed liver steatosis before IFN therapy in case 1.
However, at the time of HCC development, liver steatosis was
absent. Another possible reason why persistent hepatic inflammation
remains after SVR may be cellular senescence. Cellular senescence
is a state of irreversible cell cycle arrest caused by intrinsic
and extrinsic stress (37).
Senescent cells secrete senescence-associated secretory phenotype
(SASP), which affects neighboring cells and causes chronic
inflammation and tumorigenesis (38). SASP has an essential role in the
pathological process of liver cirrhosis (39), and these factors may be involved in
the inflammation mechanism.
In conclusion, we histopathologically evaluated
patients who developed HCC after SVR and found continuous
inflammation and fibrosis in the portal area after SVR.
Immunohistological analysis indicated a different inflammatory
mechanism may participate in hepatocarcinogenesis after SVR
compared with that in the presence of HCV. These results suggest
that factors other than hepatitis C, such as advanced age, male
sex, advanced fibrosis, fatty liver (36), diabetes mellitus, or alcohol intake
(33), are involved in the
inflammation and fibrosis that remains. Further studies with
additional cases are needed.
Acknowledgements
The authors would like to thank Professor Tatsuyuki
Kakuma and Mr. Obara Hitoshi (Biostatistics Center, Kurume
University School of Medicine) for their technical assistance.
Funding
No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
TK, TI, RKo and YN conceived and designed the
current study; TK, TI, RKo, YN, TAH, RKu, KA, TS, JA, KO, HY and TT
acquired and analyzed the data. TT revised the manuscript. All
authors read and approved the final manuscript.
Ethics and approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of Kurume University School of Medicine (approval
no. 16244). Informed consent was obtained from all the patients
enrolled in the study.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
SVR
|
sustained virological response
|
|
DAA
|
direct-acting antiviral
|
|
FOXP3
|
forkhead box P3
|
|
TGF-β1
|
transforming growth factor β1
|
|
NAFLD
|
non-nalcoholic fatty liver disease
|
|
SASP
|
senescence-associated secretory
phenotype
|
References
|
1
|
Iwasaki Y, Takaguchi K, Ikeda H, Makino Y,
Araki Y, Ando M, Kobashi H, Kobatake T, Tanaka R, Tomita M, et al:
Risk factors for hepatocellular carcinoma in hepatitis C patients
with sustained virologic response to interferon therapy. Liver Int.
24:603–610. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enokimura N, Shiraki K, Kawakita T, Saitou
Y, Inoue H, Okano H, Yamamoto N, Deguchi M, Sakai T, Ohmori S, et
al: Hepatocellular carcinoma development in sustained viral
responders to interferon therapy in patients with chronic hepatitis
C. Anticancer Res. 23:593–596. 2003.PubMed/NCBI
|
|
3
|
Okanoue T, Itoh Y, Minami M, Sakamoto S,
Yasui K, Sakamoto M, Nishioji K, Murakami Y and Kashima K:
Interferon therapy lowers the rate of progression to hepatocellular
carcinoma in chronic hepatitis C but not significantly in an
advanced stage: A retrospective study in 1148 patients. Viral
hepatitis therapy study group. J Hepatol. 30:653–659. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshida H, Shiratori Y, Moriyama M,
Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, et
al: Interferon therapy reduces the risk for hepatocellular
carcinoma: National surveillance program of cirrhotic and
noncirrhotic patients with chronic hepatitis C in Japan. IHIT study
group. Inhibition of hepatocarcinogenesis by interferon therapy.
Ann Intern Med. 131:174–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahina Y, Tsuchiya K, Tamaki N, Hirayama
I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T, et al:
Effect of aging on risk for hepatocellular carcinoma in chronic
hepatitis C virus infection. Hepatology. 52:518–527. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shindo M, Hamada K, Oda Y and Okuno T:
Long-term follow-up study of sustained biochemical responders with
interferon therapy. Hepatology. 33:1299–1302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takimoto M, Ohkoshi S, Ichida T, Takeda Y,
Nomoto M, Asakura H, Naito A, Mori S, Hata K, Igarashi K, et al:
Interferon inhibits progression of liver fibrosis and reduces the
risk of hepatocarcinogenesis in patients with chronic hepatitis C:
A retrospective multicenter analysis of 652 patients. Dig Dis Sci.
47:170–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka H, Tsukuma H, Kasahara A, Hayashi
N, Yoshihara H, Masuzawa M, Kanda T, Kashiwagi T, Inoue A, Kato M,
et al: Effect of interferon therapy on the incidence of
hepatocellular carcinoma and mortality of patients with chronic
hepatitis C: A retrospective cohort study of 738 patients. Int J
Cancer. 87:741–749. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hiramatsu N, Oze T and Takehara T:
Suppression of hepatocellular carcinoma development in hepatitis C
patients given interferon-based antiviral therapy. Hepatol Res.
45:152–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiratori Y, Imazeki F, Moriyama M, Yano
M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G,
et al: Histologic improvement of fibrosis in patients with
hepatitis C who have sustained response to interferon therapy. Ann
Intern Med. 132:517–524. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nirei K, Kanda T, Nakamura H, Matsuoka S,
Takayama T, Sugitani M and Moriyama M: Persistent hepatic
inflammation plays a role in hepatocellular carcinoma after
sustained virological response in patients with HCV infection. Int
J Med Sci. 15:466–474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Motoyama H, Tamori A, Kubo S,
Uchida-Kobayashi S, Takemura S, Tanaka S, Ohfuji S, Teranishi Y,
Kozuka R, Kawamura E, et al: Stagnation of histopathological
improvement is a predictor of hepatocellular carcinoma development
after hepatitis C virus eradication. PLoS One. 13:e01941632018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rehermann B, Chang KM, McHutchison JG,
Kokka R, Houghton M and Chisari FV: Quantitative analysis of the
peripheral blood cytotoxic T lymphocyte response in patients with
chronic hepatitis C virus infection. J Clin Invest. 98:1432–1440.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang KM, Thimme R, Melpolder JJ, Oldach
D, Pemberton J, Moorhead-Loudis J, McHutchison JG, Alter HJ and
Chisari FV: Differential CD4(+) and CD8(+) T-cell responsiveness in
hepatitis C virus infection. Hepatology. 33:267–276. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aslan N, Yurdaydin C, Wiegand J, Greten T,
Ciner A, Meyer MF, Heiken H, Kuhlmann B, Kaiser T, Bozkaya H, et
al: Cytotoxic CD4 T cells in viral hepatitis. J Viral Hepat.
13:505–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
R-Viso AT, Duarte MI, Pagliari C,
Fernandes ER, Brasil RA, Benard G, Romano CC, Ogusuku S, Cavalheiro
NP, Melo CE and Barone AA: Tissue and serum immune response in
chronic hepatitis C with mild histological lesions. Mem Inst
Oswaldo Cruz. 105:25–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dimitropoulou D, Karakantza M, Tsamandas
AC, Mouzaki A, Theodorou G and Gogos CA: T-lymphocyte subsets in
peripheral blood and liver tissue of patients with chronic
hepatitis B and C. In Vivo. 25:833–840. 2011.PubMed/NCBI
|
|
18
|
Kretschmer K, Apostolou I, Hawiger D,
Khazaie K, Nussenzweig MC and von Boehmer H: Inducing and expanding
regulatory T cell populations by foreign antigen. Nat Immunol.
6:1219–1227. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi N, Hiraoka N, Yamagami W, Ojima
H, Kanai Y, Kosuge T, Nakajima A and Hirohashi S: FOXP3+
regulatory T cells affect the development and progression of
hepatocarcinogenesis. Clin Cancer Res. 13:902–911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakaki M, Hiroishi K, Baba T, Ito T,
Hirayama Y, Saito K, Tonoike T, Kushima M and Imawari M:
Intrahepatic status of regulatory T cells in autoimmune liver
diseases and chronic viral hepatitis. Hepatol Res. 38:354–361.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ward SM, Fox BC, Brown PJ, Worthington J,
Fox SB, Chapman RW, Fleming KA, Banham AH and Klenerman P:
Quantification and localisation of FOXP3+ T lymphocytes
and relation to hepatic inflammation during chronic HCV infection.
J Hepatol. 47:316–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fausto N, Mead JE, Gruppuso PA and Braun
L: TGF-beta in liver development, regeneration, and carcinogenesis.
Ann N Y Acad Sci. 593:231–242. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Desmet VJ, Gerber M, Hoofnagle JH, Manns M
and Scheuer PJ: Classification of chronic hepatitis: Diagnosis,
grading and staging. Hepatology. 19:1513–1520. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Knodell RG, Ishak KG, Black WC, Chen TS,
Craig R, Kaplowitz N, Kiernan TW and Wollman J: Formulation and
application of a numerical scoring system for assessing
histological activity in asymptomatic chronic active hepatitis.
Hepatology. 1:431–435. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishak K, Baptista A, Bianchi L, Callea F,
De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al:
Histological grading and staging of chronic hepatitis. J Hepatol.
22:696–699. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schvarcz R, Glaumann H, Weiland O,
Norkrans G, Wejstål R and Fryden A: Histological outcome in
interferon alpha-2b treated patients with chronic posttransfusion
non-A, non-B hepatitis. Liver. 11:30–38. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Terada M, Ikegami F, Oota M, Ooyama T,
Sezai S, Ito M, Sakurai Y, Kamisaka K, Abe T, Takasu S and Tanaka
Y: A long-term histological prognosis after IFN therapy for chronic
hepatitis C. Nihon Shokakibyo Gakkai Zasshi. 94:163–171. 1997.(In
Japanese). PubMed/NCBI
|
|
30
|
George SL, Bacon BR, Brunt EM,
Mihindukulasuriya KL, Hoffmann J and Di Bisceglie AM: Clinical,
virologic, histologic, and biochemical outcomes after successful
HCV therapy: A 5-year follow-up of 150 patients. Hepatology.
49:729–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide
T, Yatsuhashi H and Watanabe H: Clinical features of hepatocellular
carcinoma that occur after sustained virological response to
interferon for chronic hepatitis C. J Gastroenterol Hepatol.
21:122–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tajimi M, Ugajin T, Ota M, Hiroishi K,
Nakamura I and Imawari M: Immune responses of liver-infiltrating
lymphocytes and peripheral blood mononuclear cells to hepatitis C
virus core and NS3 antigens. Hepatol Res. 35:250–255. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Colombat M, Charlotte F, Ratziu V and
Poynard T: Portal lymphocytic infiltrate in alcoholic liver
disease. Hum Pathol. 33:1170–1174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brunt EM, Kleiner DE, Wilson LA, Unalp A,
Behling CE, Lavine JE and Neuschwander-Tetri BA; NASH Clinical
Research NetworkA list of members of the Nonalcoholic
Steatohepatitis Clinical Research Network can be found in the
Appendix, : Portal chronic inflammation in nonalcoholic fatty liver
disease (NAFLD): A histologic marker of advanced
NAFLD-Clinicopathologic correlations from the nonalcoholic
steatohepatitis clinical research network. Hepatology. 49:809–820.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yeh MM and Brunt EM: Pathological features
of fatty liver disease. Gastroenterology. 147:754–764. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rakha EA, Adamson L, Bell E, Neal K, Ryder
SD, Kaye PV and Aithal GP: Portal inflammation is associated with
advanced histological changes in alcoholic and non-alcoholic fatty
liver disease. J Clin Pathol. 63:790–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lujambio A, Akkari L, Simon J, Grace D,
Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA,
Krizhanovsky V and Lowe SW: Non-cell-autonomous tumor suppression
by p53. Cell. 153:449–460. 2013. View Article : Google Scholar : PubMed/NCBI
|