Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and malignant tumors worldwide, and mortality associated
with HCC is the fourth highest in the world (1). The pathogenesis of HCC is unclear,
early diagnosis is difficult and the tumor develops rapidly
(2,3). The majority of patients are at later or
advanced stages of the disease at the time of diagnosis, and thus
few patients are eligible to undergo radical surgical resection
(4,5). Resection rate is low and the degree of
recurrence is high for HCC patients. Other treatments, including
radiotherapy, chemotherapy and interventional therapy, have side
effects and a limited influence on inhibiting the recurrence and
metastasis of HCC (6,7). Therefore, it is urgent to seek novel
and effective therapeutic drugs.
Norcantharidin increases the number of white blood
cells, as well as inhibits the proliferation, invasion and
metastasis, and induces apoptosis in tumor cells. This agent is
often used in the treatment of various tumor types, including HCC
(8,9). Yeh et al (10) demonstrated that norcantharidin
exhibits antimetastatic effects on HCC by transcriptional
inhibition of matrix metalloproteinase-9 through the modulation of
nuclear factor-κB activity. In addition, Sun et al (11) reported that norcantharidin treatment
induces autophagic cell death in HCC cells. A study by Gao et
al (12) also indicated that
norcantharidin inhibits interleukin-6-induced
epithelial-mesenchymal transition via the JAK2/STAT3/TWIST
signaling pathway in HCC cells.
Interferon-stimulated gene 15 (ISG15), a
ubiquitin-type molecule, is strongly upregulated by type I
interferons as a primary response to diverse microbial and cellular
stress stimuli (13). ISG15
expression is increased in various cancer types and is considered
as a potential prognostic marker (14–16).
Additionally, ISG15 is overexpressed in HCC and acts as a trigger
for tumorigenesis and metastasis (17,18).
Desai et al (19)
demonstrated that ISG15 is a potential tumor biomarker for
camptothecin (CPT) sensitivity. Tessema et al (15) reported that ISG15 sensitizes
non-small cell lung cancer cells to topoisomerase-1 inhibitor and
CPT treatment. However, the effect of ISG15 on the sensitivity of
HCC cells to norcantharidin remains to be established.
In the present study, the objective was to examine
the association between norcantharidin and ISG15 expression on the
viability of HCC. ISG5 expression was examined in clinical HCC
samples, and it was demonstrated that aberrant ISG15 expression in
HCC tissues was correlated with multiple malignant
clinicopathological characteristics. Furthermore, ISG15 knockdown
sensitized HCC cells to apoptosis induced by norcantharidin.
Materials and methods
Patients and tissue samples
Paired HCC tissues (n=37) and matched non-tumor
samples were obtained from a tissue bank of samples collected from
patients that underwent surgical treatment between May 2010 and
March 2016 at The First Affiliated Hospital of Soochow University
(Suzhou, China). Patient characteristics are listed in Table I. The clinicopathological
significance of ISG15 levels in patients with HCC was studied
according to methods described in the previous studies (20–22).
Following surgical resection, the tissue samples were immediately
snap frozen in liquid nitrogen and stored at −80°C. Patients that
had received radiotherapy and/or immunotherapy prior to surgical
treatment were excluded from the current study. Written informed
consent was obtained from all patients. The study protocol
conformed to the ethical guidelines of the 1975 Declaration of
Helsinki and was approved by The First Affiliated Hospital of
Soochow University.
 | Table I.Association between ISG15 expression
and the clinicopathological features of hepatocellular carcinoma
patients. |
Table I.
Association between ISG15 expression
and the clinicopathological features of hepatocellular carcinoma
patients.
|
| ISG15 expression
(n) |
|
|---|
|
|
|
|
|---|
| Features | Low | High | P-value |
|---|
| Cases (n) | 15 | 22 |
|
| Age (years) |
|
| 0.516 |
|
<60 | 12 | 7 |
|
|
≥60 | 9 | 9 |
|
| Gender |
|
| 0.674 |
|
Male | 11 | 9 |
|
|
Female | 10 | 7 |
|
| α-fetoprotein
(µg/l) |
|
| 0.33 |
|
<400 | 9 | 9 |
|
|
≥400 | 6 | 13 |
|
| Size (cm) |
|
| 0.026 |
|
<3 | 11 | 8 |
|
| ≥3 | 4 | 14 |
|
| TNM stage |
|
| 0.014 |
|
I/II | 12 | 8 |
|
|
III/IV | 3 | 14 |
|
| Differentiation
degree |
|
| 0.033 |
|
Moderate to well | 10 | 17 |
|
|
Poor | 5 | 5 |
|
Immunohistochemical (IHC)
analysis
ISG15 protein expression in 37 paired
paraffin-embedded tumor and adjacent normal tissues was detected
using an immunoperoxidase method. In brief, following
deparaffinization of samples, antigen retrieval was performed in a
sodium citrate solution (pH 6.0). Tumor and adjacent normal tissues
were cut into 3-µm-thick sections, and the sections were blocked
with normal goat serum at 37°C for 60 min and incubated with rabbit
anti-ISG15 antibody (1:100; cat. no. ab131119; Abcam) overnight at
4°C. Subsequently, sections were washed and incubated with
horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody
(1:1,000; cat. no. ab6721; Abcam) at room temperature for 1 h.
Finally, 3,3′-diaminobenzidine tetrahydrochloride (DAB; ZSGB-BIO;
OriGene Technologies, Inc.) was used as a substrate to visualize
the bound antibody for 30 min at 20°C, following which the sections
were counterstained with hematoxylin for 10 min at 20°C, dehydrated
with an ascending series of ethanol concentrations and mounted with
neutral resin. The samples observed with a light microscope
(magnification, ×400).
Cell lines
Four HCC cell lines Hep3B, Huh7, MHCC97-H and
MHCC97-L (23,24) were purchased from the Type Culture
Collection of the Shanghai Institute of Biochemistry and Cell
Biology, Chinese Academy of Sciences (Shanghai, China). Cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA)
at 37°C with 5% CO2.
Cell transfection
For ISG15 knockdown, small interfering RNA (siRNA)
targeting ISG15 (referred to as siISG15) was purchased from
Shanghai GenePharma Co., Ltd., and its sequence was as follows:
5′-TGAGCACCGTGTTCATGAATCTGCG-3′. A negative control siRNA (siNC)
was also obtained, as follows: 5′-UCUCCGAACGUGUCACGUTT-3′. HCC
cells were transfected with siISG15 or siNC using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocols. For overexpression of ISG15, human ISG15
was cloned into a pcDNA3.1 expression vector. The ISG15 expression
vector or control vector (NC) was then transfected into the cells
using Lipofectamine 2000, according to the manufacturer's protocol.
In order to confirm the knockdown and overexpression, ISG15 mRNA
and protein expression levels were detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis, respectively.
RT-qPCR
The expression of ISG15 was evaluated by RT-qPCR.
Briefly, cells were seeded in 6-well plates at a density of
5×105 cells/well, cultured overnight and then subjected
to transfection for 48 h. Total RNA was extracted from the clinical
specimens and cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The total RNA was then reverse transcribed into cDNA
using the TransCript One-step gDNA Removal and cDNA Synthesis
SuperMix (TransGen Biotech Co., Ltd.). ISG15 expression in cells
was detected by qPCR using SYBR Green I chemistry (TransStart Top
Green qPCR SuperMix; TransGen Biotech Co., Ltd.). The cycling
parameters defined as follows: Initial cycling for 5 min at 95°C,
followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C
for 30 sec. The primers used for the amplification of the indicated
genes were designed using the Primer Express Software (Applied
Biosystems; Thermo Fisher Scientific, Inc.), and were as follows:
RPN2 forward, 5′-GGACCTGACGGTGAAGATG-3′, and reverse,
5′-TGGTGTTCCGAAGTTGGTCA-3′ (product, 142 bp); GAPDH forward,
5′-ACCCAGAAGACTGTGGATGG-3′, and reverse, 5′-TCAGCTCAGGGATGACCTTG-3′
(product, 124 bp). The relative expression level was calculated
according to the 2−ΔΔCq method, where ΔCq=Cq (gene of
interest)-Cq (housekeeping gene) (25). GAPDH served as an internal control.
All RT-qPCR reactions were performed in triplicate.
Cell proliferation assay
Cells were seeded at a density of 5,000 cells/well
in 96-well plates at 24 h after transfection and then treated with
norcantharidin (CAS: 5442-12-6; 40 µg/ml; Shanghai Yuanye
Biotechnology Co., Ltd.) or DMSO. Cell proliferation was assessed
using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc.) assay at 24, 48 and 72 h following seeding. Absorbance was
determined at 450 nm using a microplate spectrophotometer.
Colony formation assay
Colony formation assays were used to assess the
clonogenic ability of HCC cells. After 24 h transfection and then
48 h treatment with norcantharidin, cells (2×103/well)
were trypsinized into single-cell suspensions and seeded in 6-well
dishes. Subsequently, cells were maintained in DMEM supplemented
with 10% FBS for ~2 weeks. Subsequently, visible colonies were
fixed in 4% paraformaldehyde for 4 h at 37°C and stained with 0.5%
crystal violet for 2 h at 37°C (Beyotime Institute of
Biotechnology). Colony numbers were counted under a light
microscope.
Cell apoptosis assay
Cells were harvested, washed with ice-cold PBS and
stained with Annexin V-fluorescein isothiocyanate (FITC)/propidium
iodide (PI) apoptosis detection kits (Nanjing KeyGen Biotech Co.,
Ltd.) according to the manufacturer's protocol. Cell apoptosis was
measured using a flow cytometer, and the results were analyzed with
FlowJo software, version 7.6.1 (FlowJo LLC).
Western blot analysis
HCC cells were dissociated using the Total Protein
Extraction kit (Wuhan Goodbio Technology Co., Ltd.) according to
the manufacturer's instructions, and the extracted protein was
examined by western blotting. Protein concentrations were assessed
using a bicinchoninic acid assay prior to loading. Total protein
lysates (40 µg protein/lane) were resolved by SDS-PAGE on 10% gels
and then transferred to polyvinyl difluoride membranes (EMD
Millipore). Following blocking in TBS containing 0.1% Tween-20
(TBS-T) with 5% nonfat dry milk for 30 min at 37°C, the membranes
were washed with TBS-T (4X) and incubated overnight at 4°C with
primary antibodies. All the primary antibodies used in this
experiment were obtained from Abcam and were as follows:
Anti-caspase-9 (1:800; cat. no. ab202068), anti-caspase-3 (1:500;
cat. no. ab13847), anti-GAPDH (1:1,000; cat. no. ab5174),
anti-B-cell lymphoma 2 (Bcl-2; 1:1,000; cat. no. 196495),
anti-Bcl-2-like protein 4 (Bax; 1:1,000; cat. no. ab32503) and
anti-ISG15 (1:2,000; cat. no. ab131119). Following extensive
washing, the membranes were incubated with polyclonal
HRP-conjugated goat anti-rabbit IgG secondary antibody (cat. no.
7074; CST Biological Reagents Co., Ltd.) at a dilution of 1:2,000
for 1 h at 25°C. Immunoreactivity was detected with an enhanced
chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.),
and subsequently visualized using a ChemiDoc XRS imaging system and
analysis software (Bio-Rad Laboratories, Inc.). GAPDH served as the
internal loading control.
Statistical analysis
Data from three independent experiments are
expressed as the mean ± standard deviation. Statistical analysis
was performed using SPSS software, version 19.0 (IBM Corp.).
Student's t-test was used to compare the mean values between two
groups, while one-way analysis of ANOVA with Dunnett's multiple
comparisons test was used to compare the mean values among multiple
groups. Pearson's χ2 test was conducted for correlation
analysis between the clinicopathological features and ISG15
expression of patients with HCC. P<0.05 was considered to
indicate a statistically significant difference.
Results
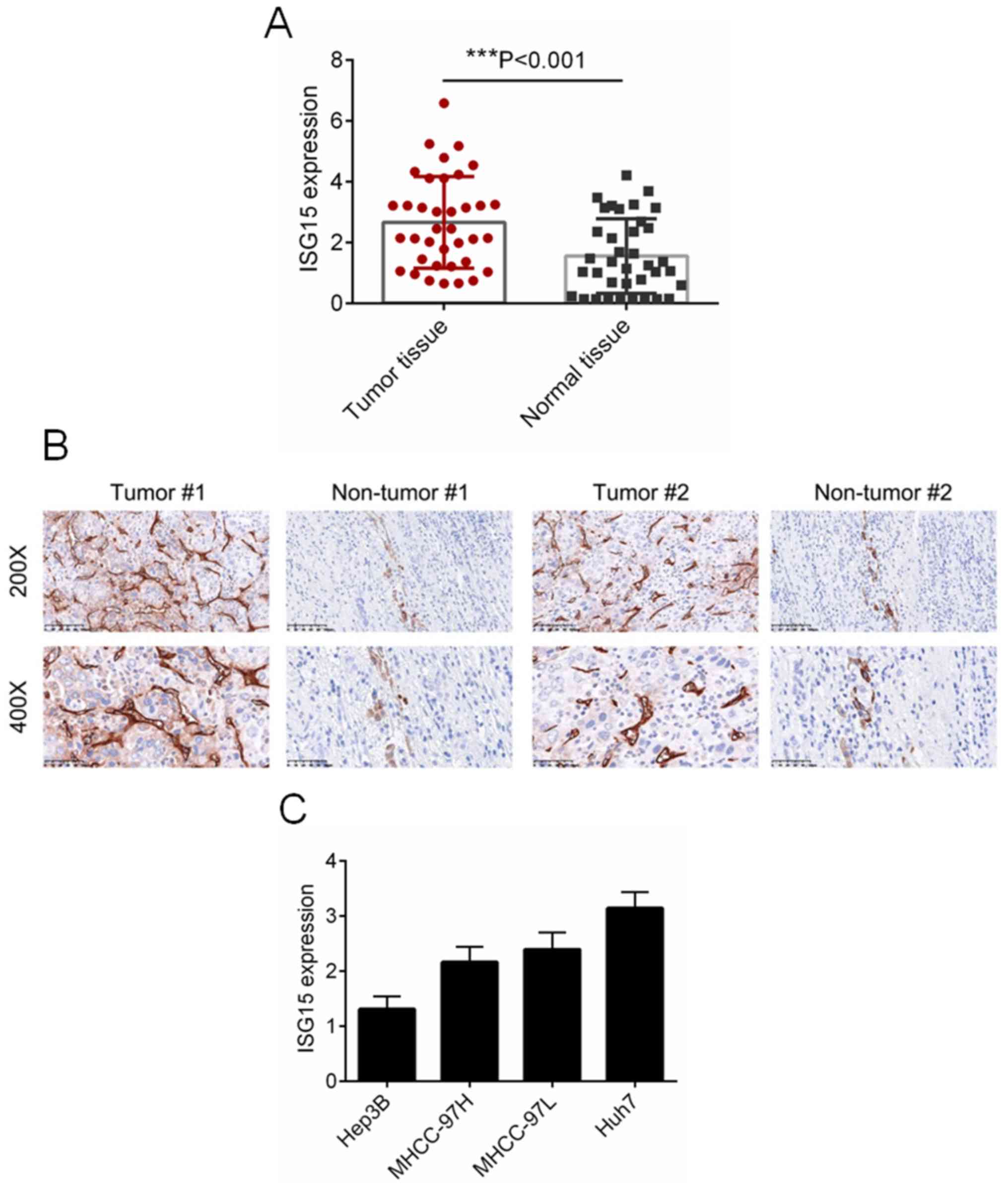
ISG15 is overexpressed in HCC tissues
and cell lines
To identify the biological role of ISG15 in HCC, the
expression of ISG15 was detected in 37 paired HCC and normal
tissues. As presented in Fig. 1A,
the expression of ISG5 was significantly increased in HCC tissues,
as compared with that in normal tissues (P<0.001). The protein
expression of ISG15 was examined by IHC analysis, and
representative images are presented in Fig. 1B. It was observed that ISG15 was
evidently overexpressed in HCC tissues as compared with the
adjacent non-tumor tissues (Fig.
1B). Additionally, the mRNA levels of ISG15 in a number of HCC
cell lines, including Hep3B, Huh7, MHCC97-H and MHCC97-L were
evaluated by RT-qPCR. Among the HCC cell lines assessed, Huh7 cells
exhibited the highest expression of ISG15, while Hep3B cells
exhibited the lowest expression of ISG15. For this reason,
downregulation of ISG15 was performed in Huh7 cells, whereas
overexpression of ISG15 was performed in Hep3B cells.
As described in previous studies (21–23), the
clinicopathological significance of ISG15 levels in patients with
HCC was further investigated. The 37 patients were divided into two
subgroups based on the mean expression value, which included the
low (n=15) and high (n=22) ISG15 expression groups. As presented in
Table I, ISG15 levels in HCC tissues
were positively correlated with the tumor size, TNM stage and tumor
differentiation (P<0.05). Taken together, these results
indicated that ISG15 may function as an oncogene in HCC.
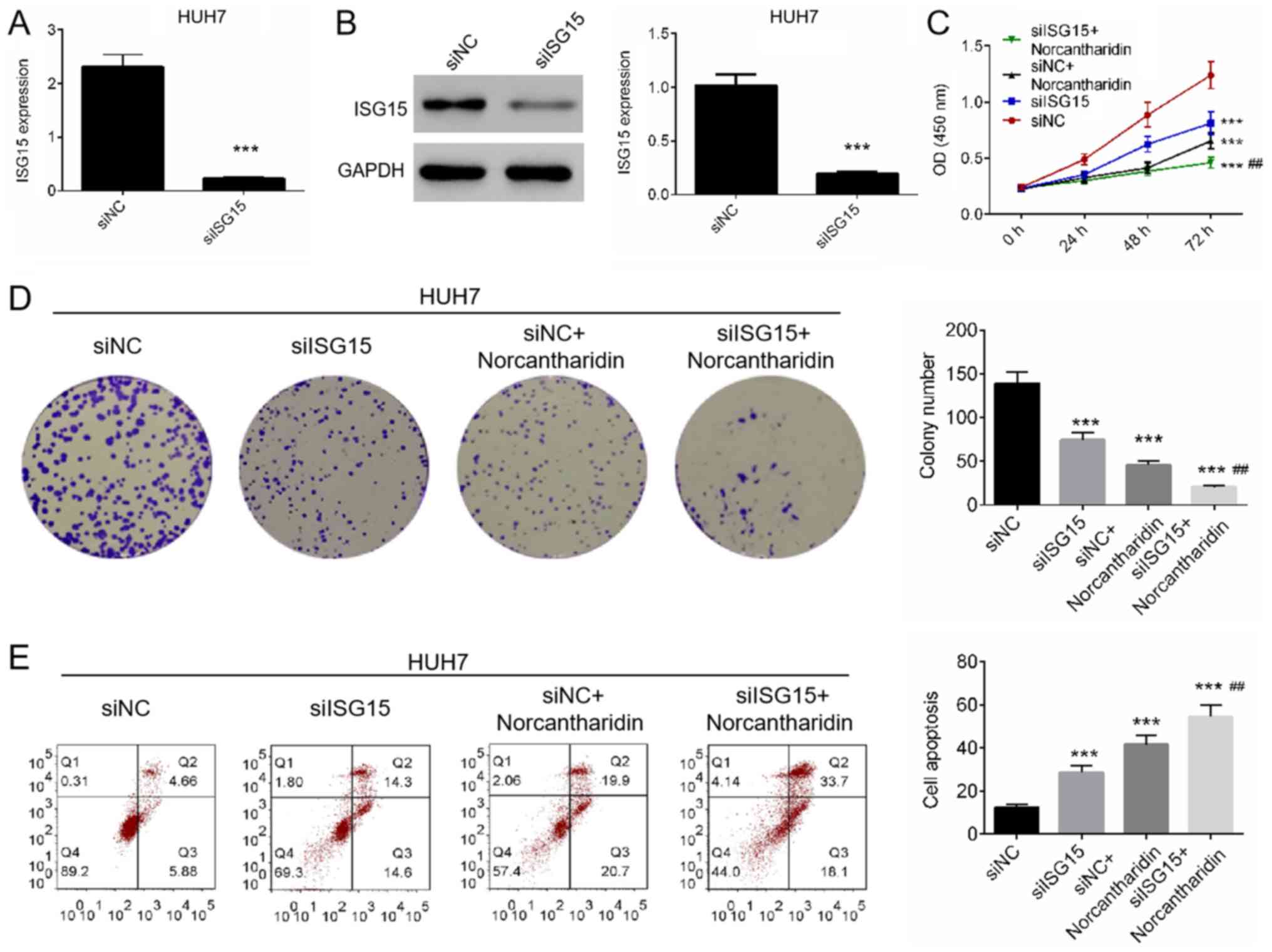
Downregulation of ISG15 increases the
sensitivity of HCC cells to norcantharidin
To identify the effect of ISG15 on the sensitivity
of HCC to norcantharidin, Huh7 cells were transfected with siISG15
and then treated with norcantharidin. The expression of ISG15 in
Huh7 cells was assessed by RT-qPCR and western blot analysis,
confirming that downregulation was successfully established in the
siISG15 group (Fig. 2A and B). Next,
cell proliferation and apoptosis were measured by CCK-8, colony
formation and Annexin V/PI staining assays. The results revealed
that cell proliferation and colony formation were significantly
decreased in cells treated with siISG15 and/or norcantharidin, with
the combination of siISG15 and norcantharidin leading to the lowest
cell viability and number of colonies (P<0.01; Fig. 3C and D). Annexin V/PI staining
demonstrated that treatment with siISG15 and/or norcantharidin
significantly induced apoptosis in Huh7 cells (P<0.01; Fig. 3E). Combined treatment with siISG15
and norcantharidin significantly promoted apoptosis when compared
with the siISG15 or norcantharidin single treatments (P<0.01;
Fig. 3E). Taken together, these
results suggested that downregulation of ISG15 may promote the
antitumor effect of norcantharidin on Huh7 cells.
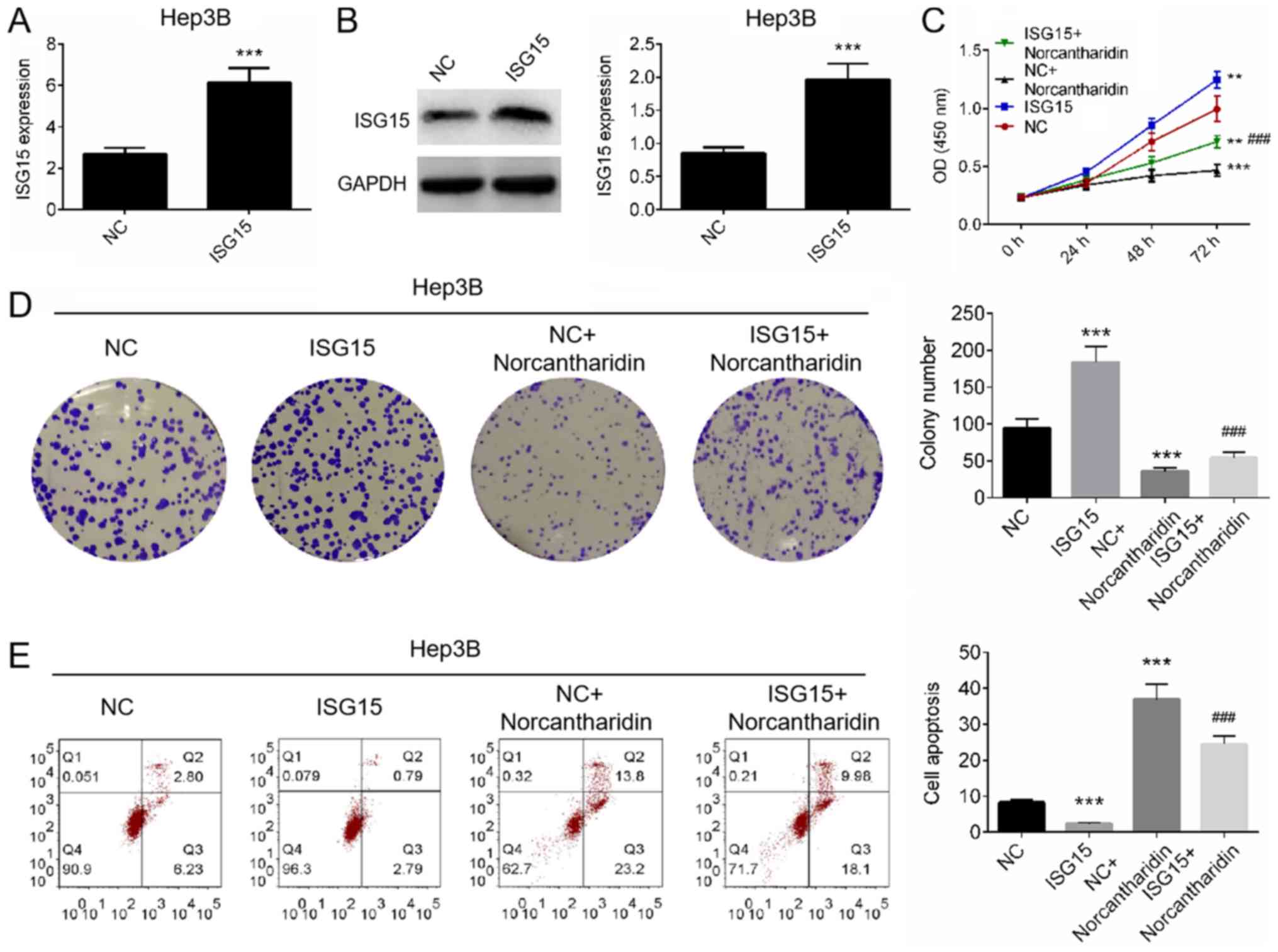
Norcantharidin reverses the
tumor-promoting effects of ISG15 in HCC cells
To investigated whether norcantharidin reverses the
tumor-promoting effects of ISG15 in HCC cells, Hep3B cells were
transfected with an ISG15 overexpression plasmid and then treated
with norcantharidin. The transfection efficiency was measured using
RT-qPCR and western blot analysis. It was revealed that ISG15 was
significantly overexpressed in Hep3B cells transfected with the
plasmid, as compared with the control group (P<0.001; Fig. 3A and B). Cell proliferation and
apoptosis were then analyzed. As presented in Fig. 3C and D, ISG15 overexpression alone
significantly increased the cell proliferation and colony formation
ability compared with the control group (P<0.001). By contrast,
treatment with norcantharidin resulted in marked reduction in cell
proliferation and colony formation compared with the control
(P<0.001). However, the combination treatment significantly
reduced the norcantharidin-induced inhibition of cell proliferation
and colony formation, suggesting that ISG15 overexpression may
reverse the antiproliferative effect of norcantharidin (P<0.001;
Fig. 3C and D). Furthermore, the
results of Annexin V/PI staining analysis revealed that
norcantharidin induced marked cell apoptosis (P<0.001; Fig. 3E), while ISG15 overexpression
reversed the effect of norcantharidin on the apoptosis of Hep3B
cells (P<0.001; Fig. 3E). The
results suggest that ISG15 overexpression can reverse the
inhibitory effects of norcantharidin on HCC cells.
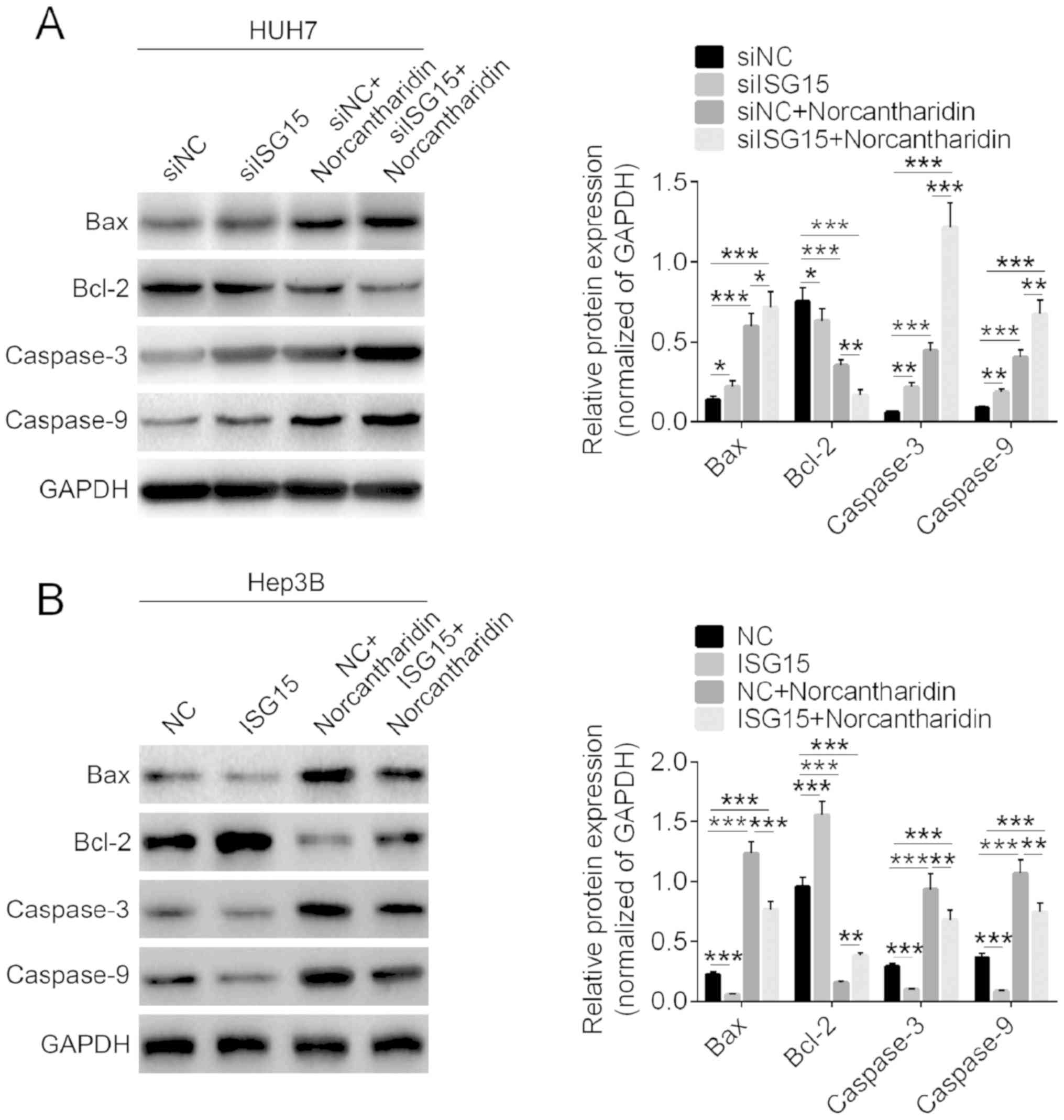
Apoptosis-associated proteins are
regulated by ISG15 and norcantharidin
The expression levels of apoptosis-associated
proteins in HCC cells were detected by western blot analysis. As
presented in Fig. 4A, treatment with
norcantharidin alone significantly increased the protein expression
levels of Bax, caspase-3 and caspase-9, and decreased Bcl-2
expression (P<0.01). Downregulation of ISG15 markedly aggravated
the effects of norcantharidin on expression of apoptosis-associated
proteins in HCC cells. Furthermore, overexpression of ISG15 was
observed to significantly inhibit the expression levels of Bax,
caspase-3 and caspase-9, and increase Bcl-2 expression, as compared
with the control group (P<0.05; Fig.
4B). Upon combined treatment with the plasmid and
norcantharidin, ISG15 overexpression was found to markedly reverse
the effect of norcantharidin on the protein expression. These
findings suggest that proteins associated with apoptosis were
regulated by ISG15 and norcantharidin treatment.
Discussion
Various studies have reported that regulating
prognostic markers increases the sensitivity of malignant tumor
cells to chemotherapy drugs, which may result in an important
benefit in the treatment of malignant tumors (26,27). The
study by Fang et al (28)
reported that downregulation of UBC9 increased the sensitivity of
HCC cells to doxorubicin. In addition, Meng et al (29) indicated that MEK inhibitors enhance
the sensitivity of multidrug resistant HCC cells to
chemotherapeutic drugs. Previous studies further reported that
norcantharidin suppresses proliferation, inhibits invasion and
metastasis, and induces apoptosis in HCC cells, which is a
potential treatment of HCC (30,31).
ISG15 expression has been reported to be increased
in breast (14), lung (15) and bladder cancer (16), as well as in HCC (17). Knockdown of ISG15 using siRNAs
inhibited xenograft tumor growth and prolonged the survival of
tumor-bearing mice with HCC (18).
ISG15 is further considered as a potential tumor biomarker for drug
sensitivity (19,20). In the present study, the mRNA and
protein expression levels of ISG15 in HCC tissues were found to be
significantly increased in HCC tissues. Furthermore, according to
the previous studies (20–22), the clinicopathological significance
of ISG15 levels in patients with HCC was further investigated in
the current study. It was observed that ISG15 levels in HCC tissues
were significantly correlated with tumor size, TNM stage and
differentiation. These results indicated that ISG15 may function as
an oncogene in HCC.
Although norcantharidin has evident curative
effects, it also exhibits organ toxicity, which limits its clinical
application to a certain extent (10). Therefore, it is important to increase
the sensitivity of HCC cells to norcantharidin. Zhang et al
(30) reported that FAM46C serves a
critical role in the antiproliferative and proapoptotic effects of
norcantharidin in HCC cells. Xiong et al (32) demonstrated that Atg5 siRNA treatment
affected cell autophagy and enhanced the anticancer action of
norcantharidin through reactive oxygen species generation and the
mitochondrial apoptosis pathway. The current study examined how
ISG15 downregulation in Huh7 cells and ISG15 upregulation in Hep3B
cells affected the proliferation, colony formation ability and
apoptosis following norcantharidin treatment. The results indicated
that ISG15 knockdown significantly accentuated the
antiproliferation and apoptosis-promoting effects of norcantharidin
on HCC cells. By contrast, overexpression of ISG15 increased the
proliferation and decreased the apoptosis of HCC cells, while
norcantharidin notably reversed the effects of ISG15.
The expression levels of apoptosis-associated
proteins Bax, Bcl-2, caspase-3 and caspase-9 were also investigated
in the current study. Bcl-2 is an oncogenic protein that inhibits
apoptosis, while overexpression of Bax antagonizes the protective
effects of Bcl-2 and causes cell death (33). Caspase-9 is considered to be a
caspase initiator, the activation of which subsequently activates
caspase-3 to induce apoptosis (34).
In the present study, the expression levels of Bax, Bcl-2,
caspase-3 and caspase-9 were found to be regulated by siISG15
and/or norcantharidin treatment. The results revealed that ISG15
knockdown sensitized HCC cells to norcantharidin-induced apoptosis
by regulating apoptosis-associated proteins.
In conclusion, the present study reported that ISG15
expression was increased in HCC tissues, suggesting that it may
serve as a potential prognostic marker in HCC. Furthermore,
downregulation of ISG15 effectively sensitized HCC cells to
norcantharidin-induced apoptosis; however, the underlying mechanism
involved needs to be further studied. The current study may provide
novel insights for the clinical application of norcantharidin and
molecular targeted therapy for HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BXC and CFN collected the clinical samples and
performed most of the experiments. SQJ and YSL collected and
analyzed data from public datasets, and performed the RT-qPCR assay
and statistical analysis. BB and ZL conceived and designed the
study, and assisted in the drafting of the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of The First Affiliated
Hospital of Soochow University reviewed and approved the study, and
written informed consent was obtained from each participant at the
examination phase. The study complied with the principles of the
Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zakharia K, Luther CA, Alsabbak H and
Roberts LR: Hepatocellular carcinoma: Epidemiology, pathogenesis
and surveillance-implications for sub-Saharan Africa. S Afr Med J.
108:35–40. 2018.PubMed/NCBI
|
|
2
|
Kim WJ, Kim KH, Kim SH, Kang WH and Lee
SG: Laparoscopic versus open liver resection for centrally located
hepatocellular carcinoma in patients with cirrhosis: A propensity
score-matching analysis. Surg Laparosc Endosc Percutan Tech.
28:394–400. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie W, Qiao X, Shang L, Dou J, Yang X,
Qiao S and Wu Y: Knockdown of ZNF233 suppresses hepatocellular
carcinoma cell proliferation and tumorigenesis. Gene. 679:179–185.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdel-Rahman O and Cheung WY: The
expanding role of systemic therapy in the management of
hepatocellular carcinoma. Can J Gastroenterol Hepatol.
2018:47638322018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka H, Hijioka S, Iwaya H, Mizuno N,
Kuwahara T, Okuno N, Ito A, Kuraoka N, Matsumoto S, Obata M, et al:
Fibrolamellar hepatocellular carcinoma with multiple lung
metastases treated with multidisciplinary therapy. Intern Med.
57:3537–3543. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH,
An JH, Lee HC and Lim YS: Efficacy and safety of transarterial
chemoembolization plus external beam radiotherapy vs. sorafenib in
hepatocellular carcinoma with macroscopic vascular invasion: A
randomized clinical trial. JAMA Oncol. 4:661–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi SH and Seong J: Strategic application
of radiotherapy for hepatocellular carcinoma. Clin Mol Hepatol.
24:114–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang PY, Chen MF, Tsai CH, Hu DN, Chang FR
and Wu YC: Involvement of caspase and MAPK activities in
norcantharidin-induced colorectal cancer cell apoptosis. Toxicol In
Vitro. 24:766–775. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng C, Liu X, Liu E, Xu K, Niu W, Chen R,
Wang J, Zhang Z, Lin P, Wang J, et al: Norcantharidin induces HT-29
colon cancer cell apoptosis through the alphavbeta6-extracellular
signal-related kinase signaling pathway. Cancer Sci. 100:2302–2308.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeh CB, Hsieh MJ, Hsieh YH, Chien MH,
Chiou HL and Yang SF: Antimetastatic effects of norcantharidin on
hepatocellular carcinoma by transcriptional inhibition of MMP-9
through modulation of NF-kB activity. PLoS One. 7:e310552012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun CY, Zhu Y, Li XF, Tang LP, Su ZQ, Wang
XQ, Li CY, Yang HM, Zheng GJ and Feng B: Norcantharidin alone or in
combination with crizotinib induces autophagic cell death in
hepatocellular carcinoma by repressing c-Met-mTOR signaling.
Oncotarget. 8:114945–114955. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Li W, Liu R, Guo Q, Li J, Bao Y,
Zheng H, Jiang S and Hua B: Norcantharidin inhibits IL-6-induced
epithelialmesenchymal transition via the JAK2/STAT3/TWIST signaling
pathway in hepatocellular carcinoma cells. Oncol Rep. 38:1224–1232.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bektas N, Noetzel E, Veeck J, Press MF,
Kristiansen G, Naami A, Hartmann A, Dimmler A, Beckmann MW, Knüchel
R, et al: The ubiquitin-like molecule interferon-stimulated gene 15
(ISG15) is a potential prognostic marker in human breast cancer.
Breast Cancer Res. 10:R582008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hadjivasiliou A: ISG15 implicated in
cytoskeleton disruption and promotion of breast cancer. Expert Rev
Proteomics. 9:72012.
|
|
15
|
Tessema M, Yingling CM, Thomas CL, Klinge
DM, Bernauer AM, Liu Y, Dacic S, Siegfried JM, Dahlberg SE,
Schiller JH and Belinsky SA: SULF2 methylation is prognostic for
lung cancer survival and increases sensitivity to topoisomerase-I
inhibitors via induction of ISG15. Oncogene. 31:4107–4116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andersen JB, Aaboe M, Borden EC, Goloubeva
OG, Hassel BA and Orntoft TF: Stage-associated overexpression of
the ubiquitin-like protein, ISG15, in bladder cancer. Br J Cancer.
94:1465–1471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu X, Hong Y, Yang D, Xia M, Zhu H, Li Q,
Xie H, Wu Q, Liu C and Zuo C: ISG15 as a novel prognostic biomarker
for hepatitis B virus-related hepatocellular carcinoma. Int J Clin
Exp Med. 8:17140–17150. 2015.PubMed/NCBI
|
|
18
|
Li C, Wang J, Zhang H, Zhu M, Chen F, Hu
Y, Liu H and Zhu H: Interferon-stimulated gene 15 (ISG15) is a
trigger for tumorigenesis and metastasis of hepatocellular
carcinoma. Oncotarget. 5:8429–8441. 2014.PubMed/NCBI
|
|
19
|
Desai SD, Wood LM, Tsai YC, Hsieh TS,
Marks JR, Scott GL, Giovanella BC and Liu LF: ISG15 as a novel
tumor biomarker for drug sensitivity. Mol Cancer Ther. 7:1430–1439.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Zhao Q, Qi J, Wang W, Zhang D, Li Z
and Qin C: lncRNA Ftx promotes aerobic glycolysis and tumor
progression through the PPARγ pathway in hepatocellular carcinoma.
Int J Oncol. 53:551–566. 2018.PubMed/NCBI
|
|
21
|
Lin Q, Peng S and Yang Y: Inhibition of
CD9 expression reduces the metastatic capacity of human
hepatocellular carcinoma cell line MHCC97-H. Int J Oncol.
53:266–274. 2018.PubMed/NCBI
|
|
22
|
Kondo R, Ishino K, Wada R, Takata H, Peng
WX, Kudo M, Kure S, Kaneya Y, Taniai N, Yoshida H and Naito Z:
Downregulation of protein disulfideisomerase A3 expression inhibits
cell proliferation and induces apoptosis through STAT3 signaling in
hepatocellular carcinoma. INT J Oncol. 54:1409–1421.
2019.PubMed/NCBI
|
|
23
|
Tan X, Liao Z, Liang H, Chen X, Zhang B
and Chu L: Upregulation of liver kinase B1 predicts poor prognosis
in hepatocellular carcinoma. Int J Oncol. 53:1913–1926.
2018.PubMed/NCBI
|
|
24
|
Iwai N, Yasui K, Tomie A, Gen Y, Terasaki
K, Kitaichi T, Soda T, Yamada N, Dohi O, Seko Y, et al: Oncogenic
miR-96-5p inhibits apoptosis by targeting the caspase-9 gene in
hepatocellular carcinoma. Int J Oncol. 53:237–245. 2018.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu WJ and Liu HL: Induction of pancreatic
cancer cell apoptosis, invasion, migration, and enhancement of
chemotherapy sensitivity of gemcitabine, 5-FU, and oxaliplatin by
hnRNP A2/B1 siRNA. Anticancer Drugs. 24:566–576. 2013.PubMed/NCBI
|
|
27
|
Liu T, Xiong J, Yi S, Zhang H, Zhou S, Gu
L and Zhou M: FKBP12 enhances sensitivity to chemotherapy-induced
cancer cell apoptosis by inhibiting MDM2. Oncogene. 36:1678–1686.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang S, Qiu J, Wu Z, Bai T and Guo W:
Down-regulation of UBC9 increases the sensitivity of hepatocellular
carcinoma to doxorubicin. Oncotarget. 8:49783–49795. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meng Q, He X, Xie G, Tian Q, Shu X, Li J
and Xiao Y: MEK inhibitor enhances sensitivity to chemotherapeutic
drugs in multidrug resistant hepatocellular carcinoma cells. Oncol
Lett. 14:3089–3095. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang QY, Yue XQ, Jiang YP, Han T and Xin
HL: FAM46C is critical for the anti-proliferation and pro-apoptotic
effects of norcantharidin in hepatocellular carcinoma cells. Sci
Rep. 7:3962017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang S, Li G, Ma X, Wang Y, Liu G, Feng
L, Zhao Y, Zhang G, Wu Y, Ye X, et al: Norcantharidin enhances
ABT-737-induced apoptosis in hepatocellular carcinoma cells by
transcriptional repression of Mcl-1. Cell Signal. 24:1803–1809.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiong X, Wu M, Zhang H, Li J, Lu B, Guo Y,
Zhou T, Guo H, Peng R, Li X, et al: Atg5 siRNA inhibits autophagy
and enhances norcantharidin-induced apoptosis in hepatocellular
carcinoma. Int J Oncol. 47:1321–1328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao G, Zhu Y, Eno CO, Liu Y, Deleeuw L,
Burlison JA, Chaires JB, Trent JO and Li C: Activation of the
proapoptotic Bcl-2 protein Bax by a small molecule induces tumor
cell apoptosis. Mol Cell Biol. 34:1198–1207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim B, Srivastava SK and Kim SH: Caspase-9
as a therapeutic target for treating cancer. Expert Opin Ther
Targets. 19:113–127. 2015. View Article : Google Scholar : PubMed/NCBI
|