Introduction
Cardiac arrest (CA) is a leading cause of mortality
that affects >325,000 people in the United States of America
each year. Sudden cardiac arrest may lead to a loss of blood
supply, ischemia and hypoxia in various organs of the body, causing
serious damage to organ function and potentially mortality
(1,2). Although knowledge and proficiency of
cardiopulmonary resuscitation (CPR) have expanded amongst the
general population, the rate of survival to hospital discharge
following pulseless cardiac arrest remains low (2). However, although resumption of
spontaneous circulation (ROSC) following prolonged and complete,
whole-body ischemia is an unnatural pathophysiological state
created by CPR, in a recent study of 24,132 patients in the United
Kingdom who were admitted to critical care units following a
cardiac arrest, the in-hospital mortality rate was 71% (3).
High rates of morbidity and mortality are observed
in patients in which ROSC occurs following out-of-hospital CA,
which is largely due to the cerebral and cardiac dysfunction that
accompanies prolonged whole-body ischemia (4,5),
referred to as post cardiac arrest syndrome (PACS). PACS is
comprised of anoxic brain injury, post-cardiac arrest myocardial
dysfunction, systemic ischemia/reperfusion (I/R) response and
persistent precipitating pathology (6). The intestine is equally or potentially
even more sensitive to ischemia compared with the brain or muscles
following CPR (7). CA causes a
prolonged decrease of blood flow to the intestine (7). The I/R response in the intestine
increases intestinal barrier permeability and results in the
translocation of pathogenic bacteria and endotoxins, which is
considered as an important mechanism leading to the initiation of
sepsis and multiple organ failure (8,9).
Mild therapeutic hypothermia (MTH), also referred to
as targeted temperature management, was introduced into the
clinical management of cardiac arrest survivors >10 years ago
(10,11). Therapeutic hypothermia (TH) is now
recommended in international resuscitation guidelines, and its use
has been extended to cardiac arrest of other origins, with other
dysrhythmias and in the hospital setting (12,13).
Previous studies have confirmed that TH and postconditioning with
sevoflurane affected the expression and activity of several small
intestinal proteins that may be involved in intestinal I/R-mediated
events following successful CPR (14,15).
Ulinastatin (UTI), a urinary trypsin inhibitor, is a
serine protease inhibitor which has anti-inflammatory properties at
sites of inflammation and has been widely used as a treatment for
patients with inflammatory disorders. UTI has been demonstrated to
represent an attractive ‘rescue’ therapeutic option for
endotoxin-associated inflammatory disorders including disseminated
intravascular coagulation, acute lung injury and acute liver injury
(16–18). UTI treatment at the onset of CPR also
alleviates the inflammatory responses following resuscitation and
improves neurological function (19,20). In
a meta-analysis, UTI decreased the plasma levels of
pro-inflammatory cytokines and increased the levels of
anti-inflammatory cytokines in patients from China and Japan
undergoing cardiac surgery with cardiopulmonary bypass. UTI
treatment may have protective effects on myocardial injury and may
increase the frequency of auto resuscitation, and shorten the
duration of intubation and mechanical ventilation (21).
However, the effects of UTI combined with MTH on
intestinal barrier dysfunction in vivo have yet to be
determined. The aim of the present study was to investigate the
effect of UTI alone or combined with MTH on intestinal barrier
dysfunction following cardiopulmonary resuscitation in rats.
Materials and methods
Animals and chemicals
The experiments performed in the present study were
approved by The Animal Research Committee of Xiangya Hospital of
Central South University (Changsha, China), and the animals were
housed and subsequently used in the Experimental Animal Center of
the Xiangya Hospital of Central South University (Changsha, China).
A total of 25 adult male Sprague-Dawley rats (400±40 g) were
purchased from Shanghai Laboratory Animal Center, Chinese Academy
of Science. During the entire experiment the animals were housed in
stainless steel cages (1 rat/cage) at conventional controlled
conditions (temperature 25±2°C; relative humidity 50±10%; 12-h
light: dark cycle) and had ad libitum access to standard
laboratory food and tap water. The rats were randomly assigned to
five groups of 5 rats each. The first group was the sham group, in
which anesthesia, endotracheal intubation and insertion of arterial
and venous catheters were performed, and asphyxiated CA was not
induced. Rats in the other four groups, including the control
group, the UTI group, the MTH group and the combined group (UTI
combined with MTH) were established based on the rat model of CPR
following asphyxiated CA. Rats in these four groups underwent the
following interventions: Anesthesia; endotracheal intubation; and
insertion of arterial and venous catheters; and asphyxiated CA. The
UTI group received 1.5 mg/100 g UTI (Guangdong Techpool Bio-pharma,
Co., Ltd.) at the onset of resuscitation within the first 2 min,
whereas the MTH group were cooled over 5 min through the
application of 70% isopropyl alcohol to their ventral surface
followed by hand fanning after 2 min of ROSC to achieve a target
rectal temperature (32.5±0.5°C) in 30 min. On reaching the target
temperature of 32.5±0.5°C, it was maintained with a cooling blanket
[model no, P&C-A II; Heng Bang (Beijing) Technology Development
Co., Ltd.] for an additional 6 h. For rats not in the MTH or UTI
and MTH groups, the rectal temperature was maintained at 37°C for
an additional 6 h.
Establishment of a rat model of CPR
following asphyxiated CA
The rats acclimated to conditions for 1 week prior
to the experiment and fasted overnight prior to surgery, with ad
libitum access to water. The asphyxiated CA rat model was
induced as previously described (22). Briefly, rats were anesthetized with
10% chloral hydrate 0.3 ml/100 g (300 mg/kg), orally intubated and
mechanically ventilated using a small-animal ventilator (PE 200
catheter) to maintain an end-tidal CO2 level between 35
and 45 mmHg. The rats were intravenously injected with 0.2 mg/100 g
vecuronium bromide to prevent spontaneous respiration. The left
femoral artery and vein were separately cannulated with PE-50 tubes
to measure arterial pressure, heart rate (HR) and arterial blood
gas and in order to provide fluid and medications. A standard II
lead electrocardiogram was also recorded. Following surgical
preparation, the animals were observed for 30 min to ensure
hemodynamic stability. Asphyxiated CA was induced by switching off
the ventilator for 30 min. When the electrocardiogram indicated
ventricular fibrillation (VF), no pulse electrical activity or
electrical static and mean arterial pressure (MAP) below 20 mmHg,
this was defined as CA (22).
Cardiopulmonary resuscitation was performed on the
asphyxiated CA rats after 1 min of CA. CPR was performed with
continuous ventilation at a tidal volume of 0.50 ml/100 g with 21%
O2, at a frequency of 100 breaths/min and external
cardiac compressions at a rate of 200/min. The compression depth
was 1/3 of the anteroposterior diameter of the thorax, along with
an intravenous injection of 0.02 mg/100 g epinephrine. If VF
persisted or an organized rhythm with a mean aortic pressure ≤25
mmHg ensued, defibrillation was attempted after 1 min of chest
compression by delivering up to 2 J electric shock biphasic
waveform electrical shocks across the chest (Heartstream XL;
Philips Medical Systems, Inc.). After 30 sec of CPR, 1 3-J
monophasic electric shock was administered. Successful CPR was
defined as achievement of ROSC, and ROSC was defined as the return
of an organized rhythm with a systolic pressure >80 mmHg for
>5 min (22). CPR was considered
to have failed if no sign of ROSC was observed during the 5 min
post-resuscitation period (22).
Assessment of the circulatory
function
MAP, HR and rectal temperature were recorded for all
the animals during the experimental process.
Histological examination
All rats were re-anesthetized as described above
[10% chloral hydrate 0.3 ml/100 g (300 mg/kg)] and then sacrificed
by cervical spinal cord dissection after 6 h treatment. No signs of
peritonitis were observed. Tissue section samples of the ileum (1.0
cm) were snap-frozen in liquid nitrogen and stored at −70°C prior
to analysis. For analysis, the samples were cut with a cryostat
into 2×2 mm thick sections, fixed in 2.5% glutaric acid at room
temperature for 24 h, 2.0% osmic acid for 2 h and subsequently
dehydrated in a series of 50, 70, 90 and 100% acetone for 3 cycles,
10 min/cycle. Following dehydration, all the samples were infused
for 24 h in 1:1 epoxy resin mixture and pure acetone. The samples
were then embedded in ethoxyline resin, dodecenylsuccinic
anhydride, methyl nadic anhydride, dimethylaminomethyl phenol at
60°C for 24 h. Following embedding, the samples were dyed with 1%
toluidine blue at 50°C for 20 min and cut with an LKB-III ultrathin
slicing machine (LKB Ltd.) into 500 Å slices. The slices were then
double-stained with 7.7% uranium acetate for 10 min and 4% lead
nitrate for 5 min at room temperature and examined using a HT7700
type transmission electron microscope (Hitachi, Ltd.).
Malondialdehyde (MDA) and superoxide
dismutase (SOD) assays in intestinal tissues
Intestinal tissues (1.0 cm segments from 5 cm of the
terminal ileum) were harvested, frozen immediately and stored at
−80°C until assessment. The levels of MDA in the ileum samples were
measured using MDA detection kit (cat. no. A003-1; Nanjing
Jiancheng Bioengineering Institute), according to the
manufacturer's protocol. Briefly, tissues from the small intestine
were homogenized in 1.5% cold KCl solution at a ratio of 1:10
(weight: Volume). The lipid peroxide level in the supernatant
(1,000 × g; 10 min; room temperature) was measured as described
previously (23). Absorbance of the
reaction was measured at 532 nm (Shimadzu UV-1700; Shimadzu
Corporation). The lipid peroxide levels are expressed as nmol of
MDA/mg of tissue protein. The content of MDA in the intestine was
calculated according to the following formula: MDA (nmol/mg
protein)={[(sample tube absorbance-blank tube absorbance)/(standard
tube absorbance-blank tube absorbance)] × standard
concentration}/protein content.
Intestinal SOD activity was determined using
Superoxide Dismutase assay kit (cat. no. A001-3) according to the
manufacturer's protocol (Nanjing Jiancheng Bioengineering
Institute). The tissue homogenate and reagent were placed in a
water bath at 37°C for 40 min following thorough mixing. After 10
min at room temperature, the absorbance value of each supernatant
was determined at 550 nm. The activity of SOD in the intestine was
calculated according to the following formula: SOD (U/ml)=[(control
tube absorbance-determination tube absorbance)/control tube
absorbance]/(50% × reaction system dilution multiple × sample
dilution multiple).
Statistical analysis
All results statistics were analyzed by GraphPad
Prism v.5 (GraphPad Software, Inc.) for statistical evaluation.
Data are expressed as the mean ± standard error of the mean. The
statistical analysis of differences between experimental groups was
performed using a Student's t-test. A one-way analysis of variance
followed by Student-Newman-Keuls test was used to analyze the
differences among three experimental groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Changes in physiological parameters in
rats from each group
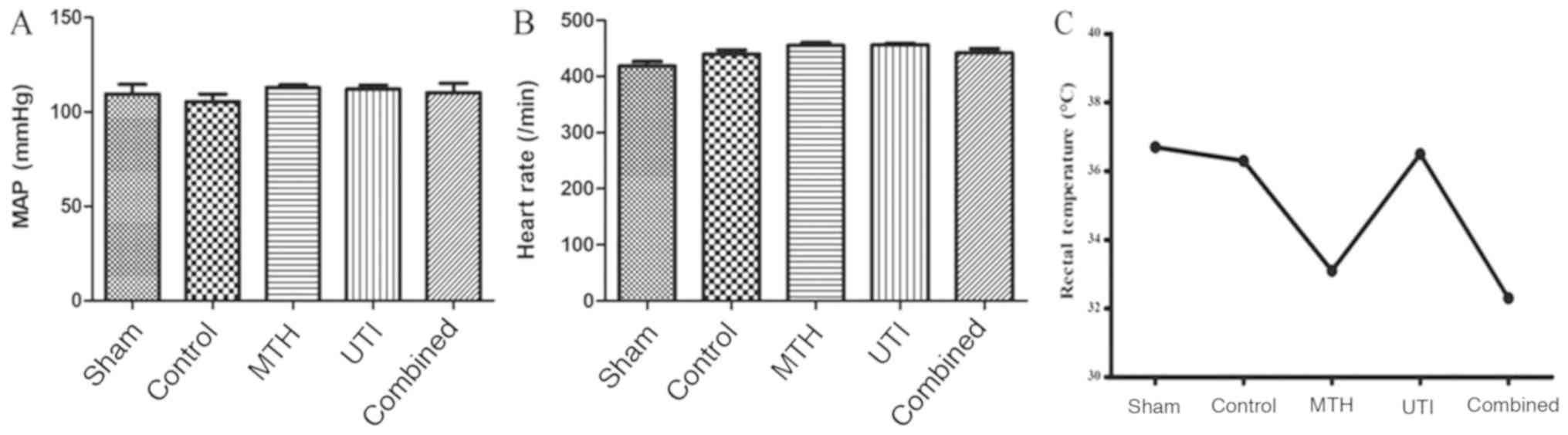
MAP, HR and rectal temperature of each group were
measured prior to euthanizing the rats following the various
treatments for 6 h after ROSC (Fig.
1). MAP and HR values of each group were not significantly
different (Fig. 2A and B). The MTH
group and combined group rats successfully attained the target
temperature, and the other three groups all survived at a normal
temperature (Fig. 2C).
Histological changes
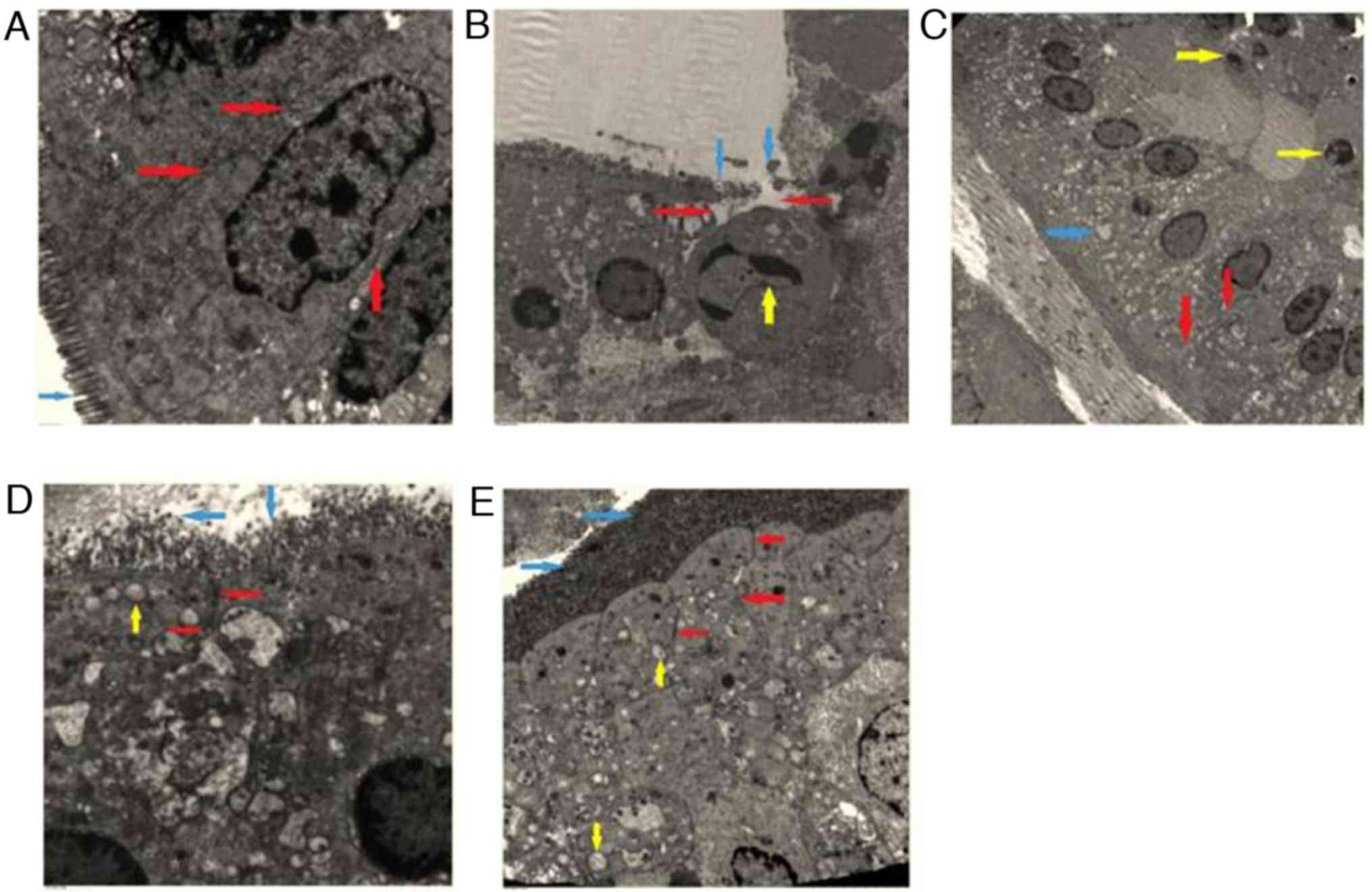
The epithelial cells of the small intestinal mucosa
were altered following different treatments for 6 h (Fig. 3). Fig.
3A demonstrates that the sham group had the normal epithelial
cells, whereas the epithelial cells in the rats in the combined
group exhibited indications of slight changes including
mitochondrial edema (Fig. 3C). The
epithelial cells in the UTI and the MTH groups demonstrated more
marked pathological changes, including shortened and partially
opened intercellular tight junctions and disorderly epithelial cell
surface microvilli (Fig. 3D and E).
The control group exhibited the most notable changes to the
epithelial cells, including disappearance of the intercellular
space and broken microvilli, and the nucleus had indications of
chromatin concentration and edge accumulation (Fig. 3B).
MDA and SOD expression assays in
intestinal tissue samples
After the 6 h treatment steps, the levels of MDA in
the intestine of the sham, combined, UTI and MTH groups were
decreased when compared with the control group (all P<0.05;
Table I; Fig. 4A). The MDA levels in the combined,
UTI and MTH groups were significantly increased compared with the
sham group (P<0.05) and the MDA levels in UTI and MTH groups
were significantly increased compared with the combined group
(P<0.05), but there were no significant difference between the
UTI and MTH groups (P>0.05). The results indicated that the
levels of MDA in each group were significantly different, with the
exception of the comparison between the UTI and MTH groups.
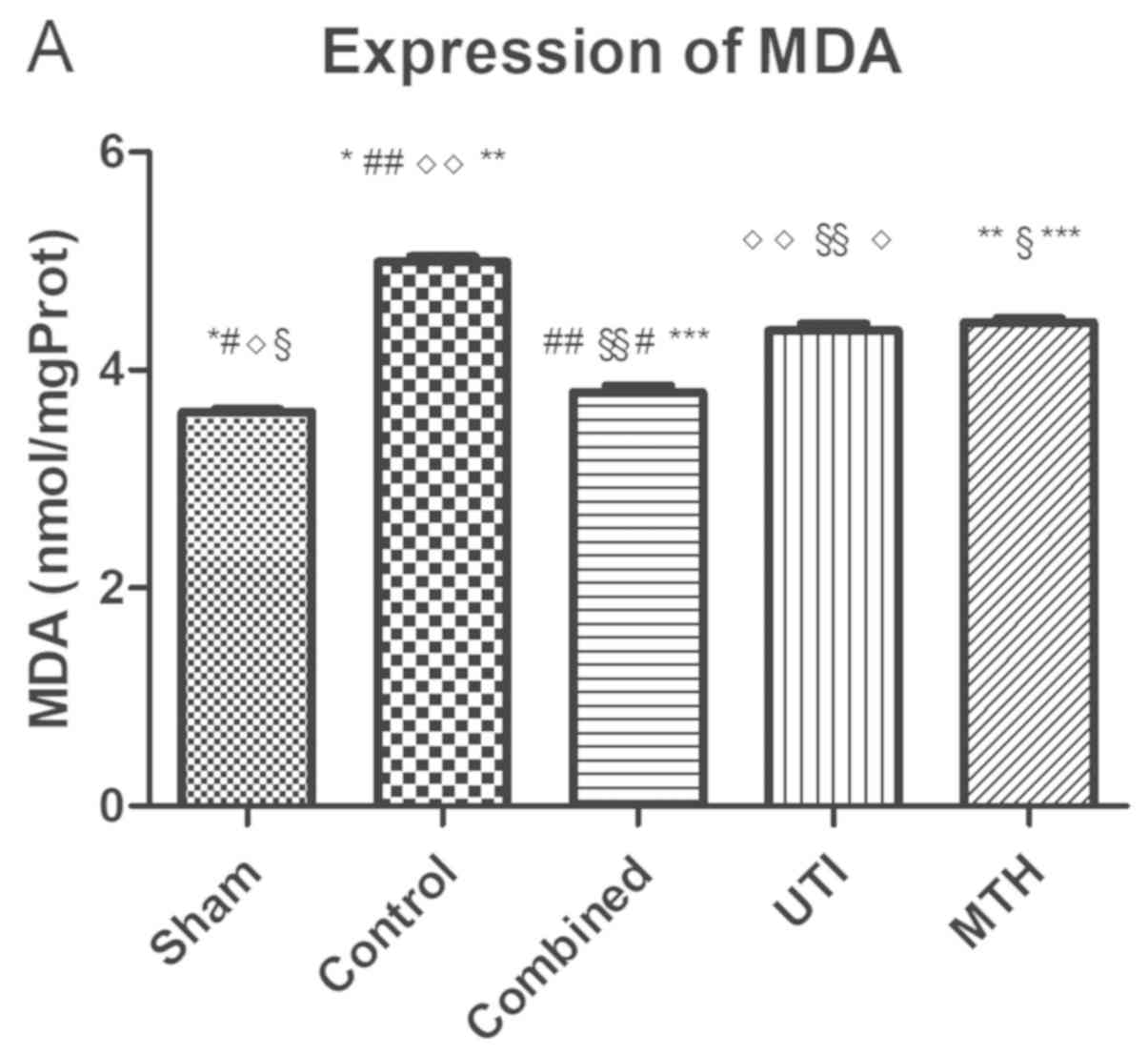 | Figure 4.MDA and SOD expression measurements in
intestinal tissue samples from rats from each experimental group.
(A) Expression of MDA in each group. There was no significant
difference between the UTI and MTH groups (P>0.05). (B)
Expression of SOD in each group. There was no significant
difference between the UTI and MTH groups (P>0.05). *P<0.05,
Sham vs. Control; #P<0.05, Sham vs. Combined;
◊P<0.05, Sham vs. UTI; and §P<0.05, and
Sham vs. MTH. ##P<0.05, Control vs. Combined;
◊◊P<0.05, Control vs. UTI; **P<0.05, Control vs.
MTH; §§P<0.05, Combined vs. UTI; and ***P<0.05,
Combined vs. MTH. MDA, malondialdehyde; SOD, superoxide dismutase;
UTI, ulinastatin; MTH, mild therapeutic hypothermia. |
 | Table I.Levels of MDA in each group. |
Table I.
Levels of MDA in each group.
| Group | Sample | MDA (nmol/mg
protein) |
|---|
| Sham | 5 |
3.61±0.06a–d |
| Control | 5 |
4.99±0.10a,e–g |
| UTI | 5 |
4.39±0.12b,e,h,i |
| MTH | 5 |
4.43±0.09c,f,h |
| Combined | 5 |
3.79±0.14d,g,i |
The levels of SOD in the intestinal tissues of the
sham, combined, UTI and MTH groups were significantly increased
compared with the control group (P<0.05; Fig. 4B; Table
II). The SOD levels in the UTI and MTH groups were decreased
compared with the combined group (P<0.05) and there was no
significant difference between the UTI and MTH groups (P>0.05).
These results suggested that the levels of SOD in each group was
statistically significant, with the exception of the comparison
between the UTI and MTH groups.
 | Table II.Levels of SOD in the intestinal
tissues of each group. |
Table II.
Levels of SOD in the intestinal
tissues of each group.
| Group | Samples | SOD (U/ml) |
|---|
| Sham | 5 |
46.46±1.88a–d |
| Control | 5 |
190.20±7.68a,e–g |
| UTI | 5 |
130.61±10.426b,e,h,i |
| MTH | 5 |
128.53±5.836c,f,h |
| Combined | 5 |
179.15±11.17d,g,i |
Taken together, the results indicated that when
compared with the control group, the sham group exhibited
relatively decreased levels of MDA and increased levels of SOD.
However, the UTI and MTH groups demonstrated decreased levels of
MDA and increased levels of SOD compared with the sham group, but
there was no difference between them. The combined group exhibited
the lowest levels of MDA and the highest levels of SOD.
Discussion
Intestinal ischemia is one of the major causes
leading to multiple organ failure in patients in intensive care
facilities and has been described as a consequence of low
splanchnic blood flow following cardiac arrest (7). The intestine is not only a vulnerable
target to ischemia but a potential secondary source of inflammatory
cytokines. Clinically, intestinal injuries following cardiac arrest
are often underestimated, even though they represent a potentially
devastating complication following successful resuscitation
(24). Loss of intestinal barrier
integrity serves a pivotal role in the development of systemic
inflammatory response syndrome, sepsis and multiple organ failure.
The formation of the intestinal mucosal barrier may be associated
with the peculiar anatomical-physiological arrangements of the
intestinal villi and the epithelial cells (23). Intestinal mucosal surfaces are lined
by epithelial cells. These cells establish a barrier between
occasionally hostile external environments and the internal milieu.
The intestinal epithelial cells serve as a central mediator of
interactions between the mucosal immune system and luminal
contents, including dietary antigens and microbial products
(25). Tight junctions seal the
paracellular space between epithelial cells, are the rate-limiting
step in transepithelial transport and are the principal determinant
of mucosal permeability to regulate the absorption of nutrients
(25). Therefore, the epithelium and
tight junctions are modulators of mucosal homeostasis.
Previous studies have suggested that UTI may
attenuate multiple organ injury in a cardiac arrest model by
suppressing inflammation, central autophagy and apoptosis, and
antioxidative mechanisms (20,26,27). The
present study demonstrated that in the in the control group, the
epithelial cells demonstrated the most notable pathological
changes; although the rats in this group achieved ROSC following
CA, they did not receive any effective intervention and the small
intestinal mucosa endured an unnatural pathophysiological ischemic
state as a result of CA, which caused a prolonged decrease of blood
flow to the intestinal wall (7).
Even when treated with UTI or MTH, the epithelial cells in rats
demonstrated pathophysiological changes, including alterations to
the tight junctions and epithelial cell surface microvilli when
compared with the sham group. These changes suggested that UTI or
MTH only had a partly protective effect on the small intestinal
mucosa barrier. The rats that underwent the combined treatment had
epithelial cells that presented with only slight pathophysiological
changes, including mitochondrial edema, but in all other respects
were highly similar to the sham group. The results of the present
study suggest that UTI combined with MTH may significantly decrease
I/R injury in the small intestinal mucosa.
The levels of the MDA and SOD in the intestinal
tissues were additionally examined in the present study. The
results of the MDA and SOD assays corroborated the results of the
electron microscopy examination of the ileum tissues. UTI and MTH
separately partly protected against injury induced as a result of
I/R of the small intestinal mucosa; however, UTI combined with MTH
significantly improved intestinal mucosa barrier function in rats
that achieved ROSC following CA. MDA is often used as an indicator
of oxidative stress, as it is degradation product of
polyunsaturated lipids by reactive oxygen species and may cause
toxic stress in cells, whereas SOD is important antioxidant in
cells exposed to oxygen (8).
Therefore, UTI combined with MTH may improve antioxidant defense
and suppress the oxidative stress in the small intestinal
mucosa.
The intestinal mucosa is an organ with a rich blood
supply and extensive metabolic activities, and therefore it is also
vulnerable to metabolic disorders caused by hypoxia. In addition,
I/R injury often occurs when reperfusion is achieved due to
abnormal oxygen metabolism, the generation of oxygen free radicals,
calcium overload and endothelial swelling (28). When intestinal injury is caused by
reperfusion, the primary characteristics are expression of adhesion
molecules, production of inflammatory cytokines, activation of the
complement system, infiltration of neutrophil and release of
proteases, and UTI may directly inhibit the production of these
molecular and cellular factors, or directly affect neutrophils,
inhibiting its infiltration into the tissues which would otherwise
cause inflammatory damage (29).
Previous studies have also confirmed that UTI had a significant
effect in protecting the mucosal barrier function particularly
against early pathological changes (30,31) and
that UTI significantly ameliorated injury to the small intestinal
and subsequent bacterial translocation by inhibiting degranulation
of mast cells in septic rats (32).
In addition, a protective effect of UTI on intestinal injury during
the perioperative period of acute superior mesenteric artery
ischemia has also been observed (29). Previous studies additionally
suggested that UTI significantly decreased the levels of MDA in the
plasma of patients following early CPR (26), and the systemic administration of UTI
also largely restored SOD in CA models (20). The present study confirmed that UTI
treatment alone or combined with MTH may serve a protective role by
suppressing the expression of SOD and MDA in rats. Patients are
prone to multiple organ dysfunction following CPR, but effective
treatments are limited at present. UTI has been widely applied in
clinical practice in China, and previous studies have confirmed its
protective effects (18–21). The present study may provide novel
insights into the development of potential therapeutics for
clinical treatment of patients following CPR.
Therapeutic hypothermia is now recommended in the
international resuscitation guidelines to treat patients post-CA
(12). Hypothermia applied as a
rescue therapy for intestinal I/R resulted in decreased mortality
even following returning to normothermia (33,34).
Hypothermic protection during early reperfusion appears to be
mediated by several events, including prevention of intestinal and
pulmonary neutrophil infiltration, a decrease in oxidative stress
in the ileum, and preservation of cardiac and hepatic energy
metabolism. Moderate hypothermia may improve outcomes in clinical
conditions associated with intestinal I/R (33,34).
However, relatively few studies have determined the mechanisms
underlying the protective effects of MTH on small intestinal
injury. Perioperative hypothermia decreased the activity of the
antioxidant enzymes catalase and SOD, decreased glutathione levels
and increased lipid peroxidation in the scar tissue of colonic
anastomoses in rats (35–37). Hypothermia altered the expression and
activity of several small intestinal proteins that may be involved
in intestinal I/R-mediated events following successful
cardiopulmonary resuscitation (14).
The present study has several limitations: Firstly,
only the small intestine changes within 6 h after CPR in rats were
observed, but without real-time dynamic monitoring; secondly, the
pharmacological mechanisms of MTH and UTI activity were not
examined in further detail, to provide additional understanding of
the pathways underlying the protective effects of MTH and UTI;
thirdly, although UTI and MTH have been widely used in the clinical
treatment of patients following CPR, there are certain differences
between animal models and actual clinical situations, and therefore
the results of the present study may not accurately reflect the
results of any potential clinical application; fourthly, as the
levels of MDA content and SOD activity are the primary indicators
for evaluating the degree of oxidative stress damage in various
tissues, and are considered to reflect the initial stage of
oxidative stress and I/R injury changes, no other molecules or
biomarkers were analyzed in the present study. However, additional
molecules or biomarkers involved in intestinal barrier injury will
be examined in subsequent studies.
In conclusion, both UTI and MTH alone demonstrated
partial protective effects on intestinal mucosal barrier function;
however, UTI combined with MTH exhibited an improved protective
effect by suppressing oxidative stress in the small intestinal
mucosa following CPR in rats.
Acknowledgments
Not applicable.
Funding
The present study was supported by the grants from
the National Natural Science Foundation of China (grant no.,
81501923) and the Provincial Natural Science Foundation of Hunan
(grant no., 2018JJ2639) and the Rui E (Ruiyi) Emergency Medical
Research Special Funding Project (grant no. R201907).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJZ wrote the manuscript and analyzed the data. XML
designed the experiments. FJZ and HQS established the rat models.
HQS analyzed all experimental data. FJZ and XML provided the
experiment fees. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The experiments performed in the present study were
approved by The Animal Research Committee of Xiangya Hospital of
Central South University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nichol G, Thomas E, Callaway CW, Hedges J,
Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, et al:
Regional variation in out-of-hospital cardiac arrest incidence and
outcome. JAMA. 300:1423–1431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu A and Zhang J: Meta-analysis of
outcomes of the 2005 and 2010 cardiopulmonary resuscitation
guidelines for adults with in-hospital cardiac arrest. Am J Emerg
Med. 34:1133–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nolan JP, Laver SR, Welch CA, Harrison DA,
Gupta V and Rowan K: Outcome following admission to UK intensive
care units after cardiac arrest: A secondary analysis of the ICNARC
Case Mix programme database. Anaesthesia. 62:1207–1216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stub D, Bernard S, Duffy SJ and Kaye DM:
Post cardiac arrest syndrome: A review of therapeutic strategies.
Circulation. 123:1428–1435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Z, Ye Z, Huang G, Wang N, Wang E and
Guo Q: Sevoflurane Post-conditioning enhanced hippocampal neuron
resistance to global cerebral ischemia induced by cardiac arrest in
rats through PI3K/Akt survival pathway. Front Cell Neurosci.
10:2712016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nolan JP, Neumar RW, Adrie C, Aibiki M,
Berg RA, Bbttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC,
et al: Post-cardiac arrest syndrome: Epidemiology, pathophysiology,
treatment, and prognostication. A scientific statement from the
international liaison committee on resuscitation; the american
heart association emergency cardiovascular care committee; the
council on cardiovascular surgery and anesthesia; the council on
cardiopulmonary, perioperative, and critical care; the council on
clinical cardiology; the council on stroke. Resuscitation.
79:350–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Korth U, Krieter H, Denz C, Janke C,
Ellinger K, Bertsch T, Henn C and Klein J: Intestinal ischaemia
during cardiac arrest and resuscitation: Comparative analysis of
extracellular metabolites by microdialysis. Resuscitation.
58:209–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan H, Chen D, Liu B, Xie X, Zhang J and
Yang G: Effects of sodium hydrosulfide on intestinal mucosal injury
in a rat model of cardiac arrest and cardiopulmonary resuscitation.
Life Sci. 93:24–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stallion A, Kou TD, Latifi SQ, Miller KA,
Dahms BB, Dudgeon DL and Levine AD: Ischemia/reperfusion: A
clinically relevant model of intestinal injury yielding systemic
inflammation. J Pediatr Surg. 40:470–477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hypothermia after Cardiac Arrest Study
Group, : Mild therapeutic hypothermia to improve the neurologic
outcome after cardiac arrest. N Engl J Med. 346:549–556. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bernard SA, Gray TW, Buist MD, Jones BM,
Silvester W, Gutteridge G and Smith K: Treatment of comatose
survivors of out-of-hospital cardiac arrest with induced
hypothermia. N Engl J Med. 346:557–563. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nolan JP, Hazinski MF, Aickin R, Bhanji F,
Billi JE, Callaway CW, Castren M, de Caen AR, Ferrer JM, Finn JC,
et al: Part 1: Executive summary: 2015 International consensus on
cardiopulmonary resuscitation and emergency cardiovascular care
science with treatment recommendations. Resuscitation. 95:e1–e31.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng W, Jin L, Xie Q, Huang L, Jiang Z, Ji
Y, Li C, Yang L and Wang D: Eugenol protects the transplanted heart
against ischemia/reperfusion injury in rats by inhibiting the
inflammatory response and apoptosis. Exp Ther Med. 16:3464–3470.
2018.PubMed/NCBI
|
|
14
|
Albrecht M, Gruenewald M, Zitta K,
Zacharowski K, Scholz J, Bein B and Meybohm P: Hypothermia and
anesthetic postconditioning influence the expression and activity
of small intestinal proteins possibly involved in
ischemia/reperfusion-mediated events following cardiopulmonary
resuscitation. Resuscitation. 83:113–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang GS, Zhou XY, An XF, Liu XJ, Zhang YJ
and Yu D: mTOR is involved in stroke-induced seizures and the
anti-seizure effect of mild hypothermia. Mol Med Rep. 17:5821–5829.
2018.PubMed/NCBI
|
|
16
|
Inoue K and Takano H: Urinary trypsin
inhibitor as a therapeutic option for endotoxin-related
inflammatory disorders. Expert Opin Investig Drugs. 19:513–520.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karnad DR, Bhadade R, Verma PK, Moulick
ND, Daga MK, Chafekar ND and Iyer S: Intravenous administration of
ulinastatin (human urinary trypsin inhibitor) in severe sepsis: A
multicenter randomized controlled study. Intensive Care Med.
40:830–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang B, Gao M, Wang K, Jiang Y, Peng Y,
Zhang H, Yang M and Xiao X: Intraintestinal administration of
ulinastatin protects against sepsis by relieving intestinal damage.
J Surg Res. 211:70–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sui B, Li Y and Ma L: Postconditioning
improvement effects of ulinastatin on brain injury following
cardiopulmonary resuscitation. Exp Ther Med. 8:1301–1307. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang XM, Hu JH, Wang LL, Ma C, Wang X and
Liu XL: Ulinastatin alleviates neurological deficiencies evoked by
transient cerebral ischemia via improving autophagy, Nrf-2-ARE and
apoptosis signals in hippocampus. Physiol Res. 67:637–646. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Zeng Z, Cao Y, Du X and Wan Z:
Effect of urinary protease inhibitor (ulinastatin) on
cardiopulmonary bypass: A meta-analysis for China and Japan. PLoS
One. 9:e1139732014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han F, Boller M, Guo W, Merchant RM, Lampe
JW, Smith TM and Becker LB: A rodent model of emergency
cardiopulmonary bypass resuscitation with different temperatures
after sphyxial cardiac arrest. Resuscitation. 81:93–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghali MG and Marchenko V: Dynamic changes
in phrenic motor output following high cervical hemisection in the
decerebrate rat. Exp Neurol. 271:379–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He W, Liu Y, Geng H and Li Y: The
regulation effect of ulinastatin on the expression of SSAT2 and
AQP4 in myocardial tissue of rats after cardiopulmonary
resuscitation. Int J Clin Exp Pathol. 8:10792–10799.
2015.PubMed/NCBI
|
|
25
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I. A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katsoulis IE, Balanika A, Sakalidou M,
Gogoulou I, Stathoulopoulos A and Digalakis MK: Extensive colonic
necrosis following cardiac arrest and successful cardiopulmonary
resuscitation: Report of a case and literature review. World J
Emerg Surg. 7:352012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turner JR: Intestinal mucosal barrier
function in health and disease. Nat Rev Immunol. 9:799–809. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Q, Peng J, Zhou Y, Zeng W, Xiao S,
Cheng H, Zhong Z, Liao X, Xiao X, Luo L and Liu X: Clinical
observation of ulinastatin combined with CRRT in the treatment of
early cardiopulmonary resuscitation. Exp Ther Med. 14:6064–6068.
2017.PubMed/NCBI
|
|
29
|
Liu S, Xu J, Gao Y, Shen P, Xia S, Li Z
and Zhang M: Multi-organ protection of ulinastatin in traumatic
cardiac arrest model. World J Emerg Surg. 13:512018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sasaki M and Joh T: Oxidative stress and
ischemia-reperfusion injury in gastrointestinal tract and
antioxidant, protective agents. J Clin Biochem Nutr. 40:1–12. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qian ZY, Yang MF, Zuo KQ, Xiao HB, Ding WX
and Cheng J: Protective effects of ulinastatin on intestinal injury
during the perioperative period of acute superior mesenteric artery
ischemia. Eur Rev Med Pharmacol Sci. 18:3726–3732. 2014.PubMed/NCBI
|
|
32
|
Tian YF, Li Y, Zhao Q, Fan LQ, Zhao WJ, Xu
BL, Song ZC, Kuang G, Dong ZM and Zhang QF: Effect of ulinastatin
on intestinal mucosal barrier function of rats with obstructive
jaundice. Nan Fang Yi Ke Da Xue Xue Bao. 27:987–990. 2007.(In
Chinese). PubMed/NCBI
|
|
33
|
Ivary SHA, Jajarmy N, Shahri MK, Shokoohi
M, Shoorei H, Ebadi A, Moghimian M and Sigaroodi F: Effect of fish
and flaxseed oil supplementation on isoprenaline-induced myocardial
infarction in rats: Inhibition of mitochondrial permeability
transition pore opening. Crescent J Med Biol Sci. 6:158–163.
2019.
|
|
34
|
Zhang YJ, Li M, Meng M, Feng M and Qin CY:
The effect of ulinastatin on the small intestine injury and mast
cell degranulation in a rat model of sepsis induced by CLP. Exp
Toxicol Pathol. 61:481–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stefanutti G, Pierro A, Parkinson EJ,
Smith VV and Eaton S: Moderate hypothermia as a rescue therapy
against intestinal ischemia and reperfusion injury in the rat. Crit
Care Med. 36:1564–1572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang GS, Zhou XY, An XF, Liu XJ, Zhang YJ
and Yu D: Mild hypothermia inhibits the Notch 3 and Notch 4
activation and seizure after stroke in the rat model. Pathol Res
Pract. 214:1008–1016. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oliveira JC, Oliveira CH, Oliveira HE,
Pereira A, Maraschin M and D'Acampora AJ: Effects of perioperative
hypothermia and reactive oxygen species in the healing of colonic
anastomosis in rats. Acta Cir Bras. 29:742–747. 2014. View Article : Google Scholar : PubMed/NCBI
|