Introduction
The majority of hepatocellular carcinoma (HCC) cases
occur in developing countries, and it is the most commonly
diagnosed cancer in patients <60 years of age, particularly in
males (1,2). One of the primary reasons for the high
HCC mortality rate is late diagnosis. Although advances have been
made in therapeutic strategies and surgical procedures, prognosis
for patients with HCC remains unsatisfactory (3,4).
Therefore, potential biomarkers of HCC are required to improve
early detection and prognostic assessment.
MicroRNAs (miRNAs/miRs) are well conserved, small
RNA molecules consisting of 18–22 nucleotides that do not encode
proteins. The earliest report of an association between miRNAs and
cancer was found in 2002 (5). In
particular, certain miRNAs have been found to be upregulated in the
serum or plasma samples in patients with HCC, including miR-21,
miR-199 and miR-221, whilst others such as miR-122 have been found
to be downregulated (6,7). These previous findings suggest that
they can serve as potential biomarkers for the early diagnosis of
HCC. Previous studies have reported that miR-142-3p inhibits cell
viability and aerobic glycolysis (8)
whereas miR-199 suppressed cell viability, migration and invasion
in HCC (9), suggesting that the
miRNAs serve roles in HCC physiology. One particular miRNA,
miR-552, has been previous demonstrated to serve crucial roles in
cell viability and migration of colorectal cancer, by targeting a
number of mRNAs, including dachshund family transcription factor 1
and a disintergin and metalloprotease family member 28 (10,11).
However, to the best of our knowledge, no research has investigated
the role of miR-552 in HCC.
Runt-related transcription factor 3 (RUNX3) is a
tumor suppressor gene that regulates gene expression associated
with cell viability and metastasis (12). Emerging evidence has indicated that
RUNX3 is expressed in different types of cancer. It has been
reported that miR-20a directly targeted RUNX3 expression, whereas
miR-186 reversed RUNX3-induced inhibition of HCC cell metastasis,
demonstrating functional interactions between miRNAs and RUNX3
(13); however, to the best of our
knowledge, the association between RUNX3 and miR-552 has not been
investigated previously.
In the present study, the role of miR-552 in HCC
cell lines was investigated in vitro to determine whether an
interaction between miR-552 and RUNX3 exists in this system. The
results of the present study may be useful in providing a potential
biomarker for detection of HCC.
Materials and methods
Clinical samples
Fresh HCC and adjacent normal liver tissues were
collected from patients that underwent hepatectomy at The First
Affiliated Hospital of Xinjiang Medical University (Urumqi, China)
from January 2017 to June 2018. Inclusion and exclusion criteria
were as follows: i) The patients, including men and women, were
18–75 years old and all pathologically demonstrated to exhibit
hepatocellular carcinoma; ii) no distant metastasis; iii) no
chemotherapy prior to surgery. A total of 15 patients aged 27–74
years old, with 12 male and 3 female patients, were enrolled in the
current study. The present study was approved by the Ethics Review
Committees of The First Affiliated Hospital of Xinjiang Medical
University and performed in accordance with Declaration of
Helsinki. All patients provided informed consent.
Cell lines and culture
PLC/PRF/5 and Huh-7 cells were purchased from the
Type Culture Collection of the Chinese Academy of Sciences. Huh-7
cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS. PLC/PRF/5 cells cultured in minimum
essential medium (MEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS. All cells were maintained in a
humidified atmosphere maintained at 37°C with 5%
CO2.
Cell transfection
To knock down endogenous miR-552 expression,
PLC/PRF/5 and Huh-7 cells, at ~30–50% confluence, were transfected
with 20 µM miR-552 inhibitor (cat. no. miRB0026615-2-1; Guangzhou
RiboBio Co., Ltd.) or miR-552 negative control (miR-NC; cat. no.
miR2N0000001-1-5; Guangzhou RiboBio Co., Ltd.) for 48 h using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The transfected cells were performed to extract RNA,
proteins or to detect cell viability, migration, apoptosis and
luciferase activity immediately. Untransfected cells served as an
additional negative control (NC).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells and tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. RNA quality and
quantity were determined using Qubit™ 4 fluorometer (Invitrogen;
Thermo Fisher Scientific, Inc.). Reverse transcription and
isolation of cDNA was performed using HiScript® II or
III RT supermix plus gDNA wiper (Vazyme) according the
manufacturer's protocols. The levels of miR-552 and RUNX3
expression were measured using specific primers and
SYBR® Green fluorophore probes (Vazyme). The sequences
of primers used in this study are presented in Table I. Relative expression was quantified
by using the 2−ΔΔCq method (14). GAPDH and U6 were used as internal
controls for mRNA and miRNA expression, respectively. The
thermocycling conditions were as follows: Initial denaturation
(50°C for 2 min), and enzyme denaturation (95°C for 10 min)
followed by 40 cycles of denaturation (95°C for 15 sec), annealing
(60°C for 30 sec), elongation (70°C for 1 min) and a final
extension (72°C for 10 min).
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| miR-552 |
CCGCACAGGTGACTGGTTAGA | GTGCAGGGTCCGAGGT |
| RUNX3 | TGGCAGGCAATGACG | CAGGGAACGGCTTGGT |
| GAPDH |
CCCACTCCTCCACCTTTGAC |
TGTTGCTGTAGCCAAATTCGT |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
Western blot analysis
Total protein was extracted from the PLC/PRF/5 and
Huh-7 cells using cell lysis buffer (RIPA; Thermo Fisher
Scientific, Inc.) containing protease inhibitors. Protein
concentration was quantified using bicinchoninic acid assay
(Pierce; Thermo Fisher Scientific, Inc.). Total protein (40 µg per
lane) were separated by SDS-PAGE (10% for 30–205 kDa proteins; 12%
for 14–66 kDa proteins) and subsequently transferred to
polyvinylidene difluoride membranes (EMD Millipore). The PVDF
membranes were blocked using 5% (m/v) skim milk powder (Thermo
Fisher Scientific, Inc.) dissolved in TBST (Tris Buffered Saline
Tween-20; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. The PVDF membranes were incubated with specific
primary antibodies (caspase-3; 1:1,000; cat. no. 9662; bcl-2;
1:1,000; cat. no. 4223; bax; 1:1,000; cat. no. 5023; RUNX3;
1:1,000; cat. no. 9647; All antibodies were purchased from Cell
Signaling Technology) for 12 h at 4°C, before subsequent incubation
with corresponding HRP-linked anti-rabbit secondary antibody
(1:2,000; cat. no. 7074; Cell Signaling Technology) for 1 h at room
temperature. Protein bands were visualized using Immobilon Western
Chemiluminescent HRP substrate (Merck KGaA). Densitometric analysis
was performed using ImageJ v1.8.0 (National Institutes of
Health).
Cell viability analysis using MTT
assay
PLC/PRF/5 and Huh-7 cells (1.5×104
cells/ml) were seeded into 96-well plates with 100 µl medium/well
and were allocated into three separate groups (miR-552 inhibitor,
miR-552 NC and NC) and cell viability was measured after 24, 48 and
72 h. MTT solution was added at a concentration of 20 µl/well prior
to incubation at 37°C for 4 h. Following incubation, 150 µl
dimethylsulfoxide was added to each well to dissolve the formazan
crystals and the absorbance values at 490 nm were detected using
the Thermo Multiskan™ FC Microplate Reader for each well (Thermo
Fisher Scientific, Inc.).
Cell migration analysis by
wound-healing assay
PLC/PRF/5 and Huh-7 cells were first transfected
with either miR-552 inhibitor or miR-NC and subsequently seeded at
~70–80% confluence into six-well plates and cultured to confluence.
Following the attainment of 100% confluence, wounds were created by
scratching using 200 µl pipette tips. The wound monolayers of
PLC/PRF/5 and Huh-7 were cultured in serum-free MEM (Gibco; Thermo
Fisher Scientific, Inc.) and 1640 culture medium (Gibco, Thermo
Fisher Scientific), respectively. Cells were then treated with
mitomycin c (10 µg/ml; Selleck Chemicals) for 30 min. The wound
area was recorded using light microscopy (scale bar, 200 µm for the
wound healing assay; 100 µm for the Transwell assay) at 0 and 24
h.
Transwell assay
PLC/PRF/5 and Huh-7 cell migration was measured
using Transwell® chambers (8-µm pore size; Corning
Inc.). PLC/PRF/5 and Huh7 cells (4×104 cells/ml) were
diluted, respectively, in 100 µl of MEM and 100 µl of 1640 culture
medium supplemented with 10% FBS (transfected with miR-552
inhibitor, transfected with miR-NC and NC) and were seeded into the
upper chamber; MEM or 1640 medium supplemented with 20% FBS was
added into lower chamber. Following 12-h incubation, cells that
migrated through the membrane and adhered to the bottom of the
chamber were stained using crystal violet and counted under a light
microscope at ×100 magnification (5 field of views).
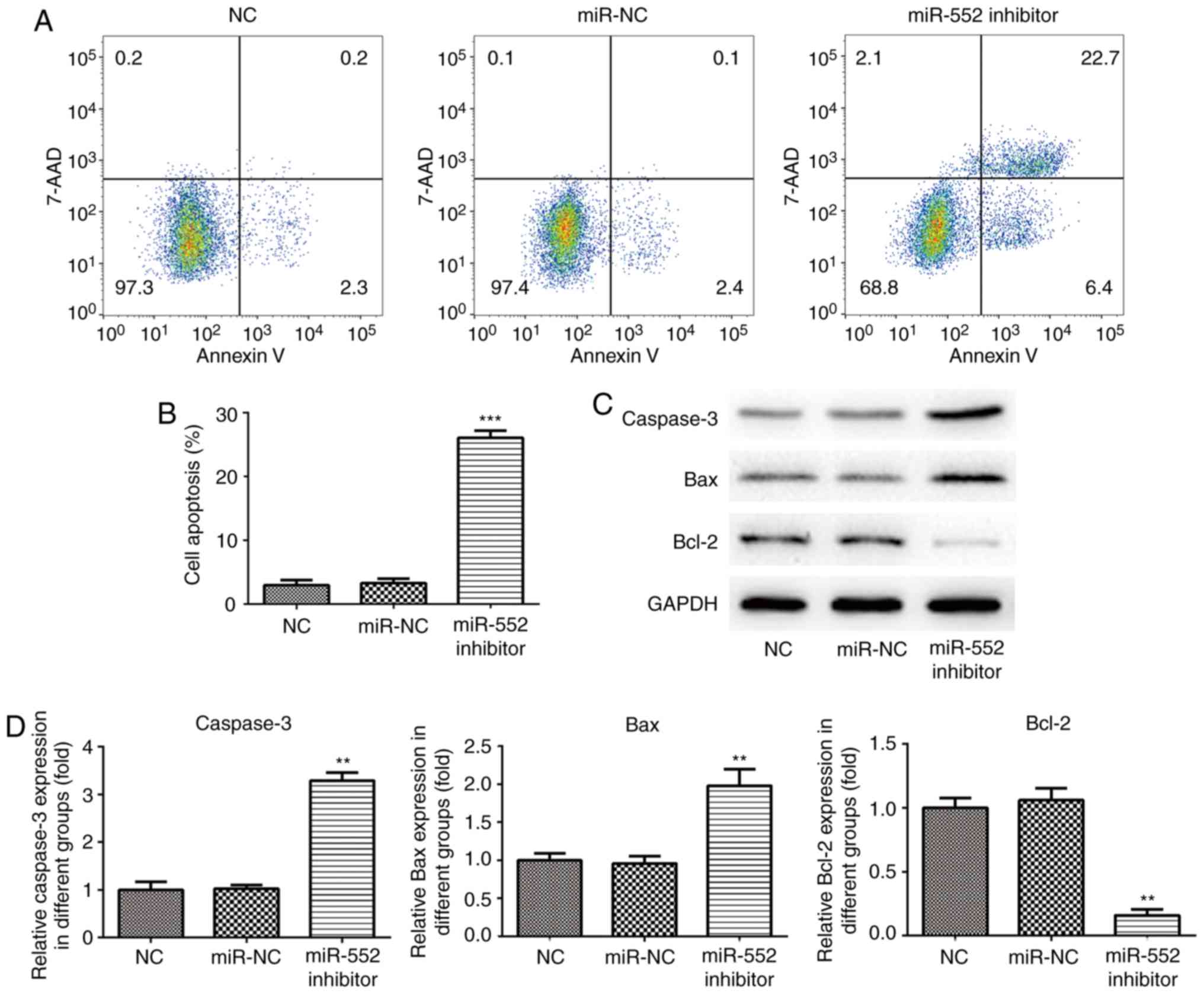
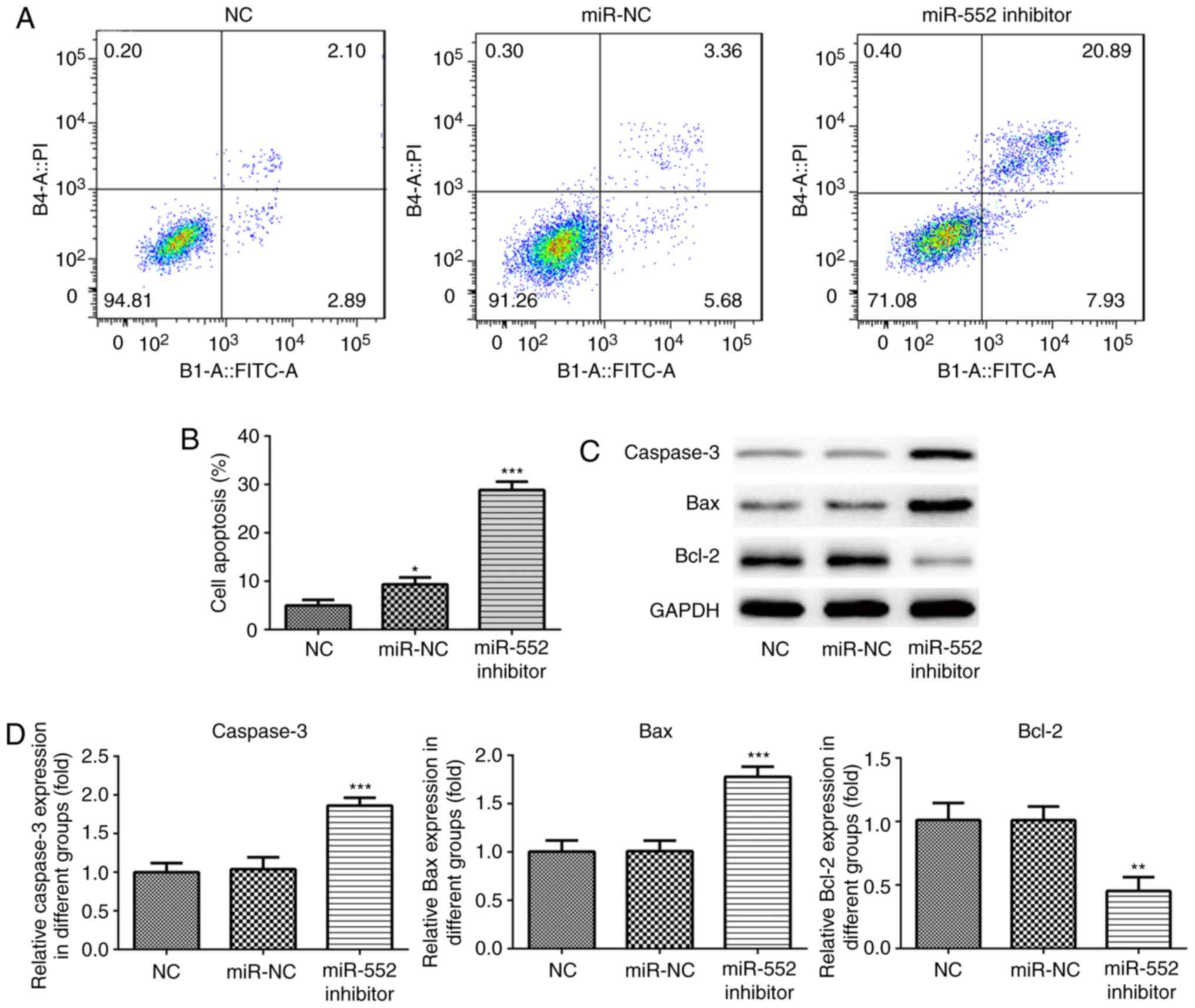
Cell apoptosis assay
PLC/PRF/5 and Huh-7 cells (4×105
cells/ml) were seeded into six-well plates. Following adherence,
cells were transfected with either miR-552 inhibitor or miR-552 NC
in addition to NC. PLC/PRF/5 cell apoptosis was measured using
Annexin V-FITC/7-AAD Apoptosis Detection kit (E-CK-A212;
Elabscience Biotechnology Inc.) according to manufacturer's
protocol. Huh-7 cell apoptosis was measured using Annexin V-FITC/PI
apoptosis detection kit (Beijing Solarbio Science & Technology
Co., Ltd.). Cell apoptosis detected using BD FACSCanto II (BD
Biosciences). Viable cells were indicated by FITC-Annexin V and
7-AAD negative (left lower). Cells that were in early apoptosis
were indicated by FITC-Annexin V-positive and 7-AAD negative (right
lower). Cells that are were in end stage apoptosis were indicated
by FITC positive and 7-AAD positive (right up and left up). Viable
Huh7 cells FITC-Annexin V and PI negative cells are indicated (left
lower); Cells that are in early apoptosis are FITC-Annexin
V-positive and PI negative (right lower); Cells that are in end
stage apoptosis and death are FITC positive and PI positive (right
up and left up). Cell apoptosis was detected using BD FACSCanto II
(BD Biosciences) (15,16); (Figs.
4 and 5).
Dual luciferase reporter assay
To validate that RUNX3 mRNA is a target of miR-552
(miR-552 was validated using mirbase.org:
Accession no. MIMAT0003215), a wild-type (WT) or a mutated (MUT)
fragment of the human RUNX3 3′-untranslated region (3′-UTR)
sequence encoding a potential miR-552 binding site was amplified
using RT-qPCR. Subsequently, the WT and MUT 3′-UTR of RUNX3 was
cloned into the pMIR-Report vector (BioVector NTCC, Inc.).
PLC/PRF/5 cells were seeded in 96-well plate at a density of
1×105 cells/ml. A total of 1 µg pSicoR/miR-552
(EK-Bioscience Biotechnology Co., Ltd.) or 1 µg pSicoR/miR-NC
(EK-Bioscience Biotechnology Co., Ltd.) was co-transfected with 0.5
µg pMIR-Report/RUNX3 WT or 0.5 µg pMIR-Report/RUNX3 MUT group,
respectively. All plasmids were diluted into 25 µl diluted buffer
(MEM). A total of 25 µl of MEM was added into pMIR-report/RUNX3 WT
or MUT groups and was used as a control for the buffer. A total of
0.2 µg pRL-TK (EK-Bioscience Biotechnology Co., Ltd.) plasmids were
added in each group to normalize the luciferase activity. Plasmids
were transfected into cells using Lipofectamine 2000®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocol. Relative luciferase activity was detected
by measuring relative activity of firefly luciferase unit at 48 h
using a Dual-Luciferase Reporter assay kit (Promega Corporation).
Luciferase activity=luc (firefly luciferase)/R-luc (Renin
luciferase).
Statistical analysis
Experiments were performed in triplicate, with
results presented as the mean ± standard deviation. The level of
significance between two groups was determined using Student's
t-test (used to analyze differences between tumor and adjacent
tissues) and one-way ANOVA followed by Turkey's multiple
comparisons for multiple group comparisons. Analyzes were performed
using GraphPad Prism 6 software (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
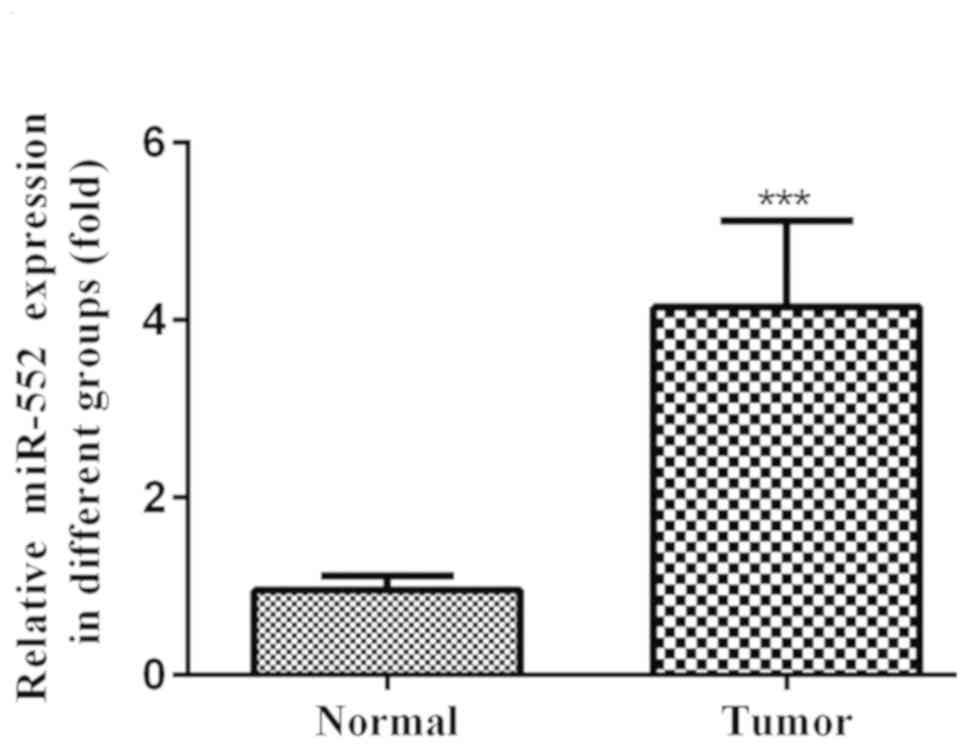
miR-552 is upregulated in HCC tissues
and cell lines
However, an association between the endogenous
miR-552 and HCC cell lines has not been reported. Therefore, in the
present study the relative expression of miR-552 in HCC tumor
tissues was validated using RT-qPCR. Compared with adjacent normal
liver tissues, tumor tissues exhibited significantly higher miR-552
expression (Fig. 1).
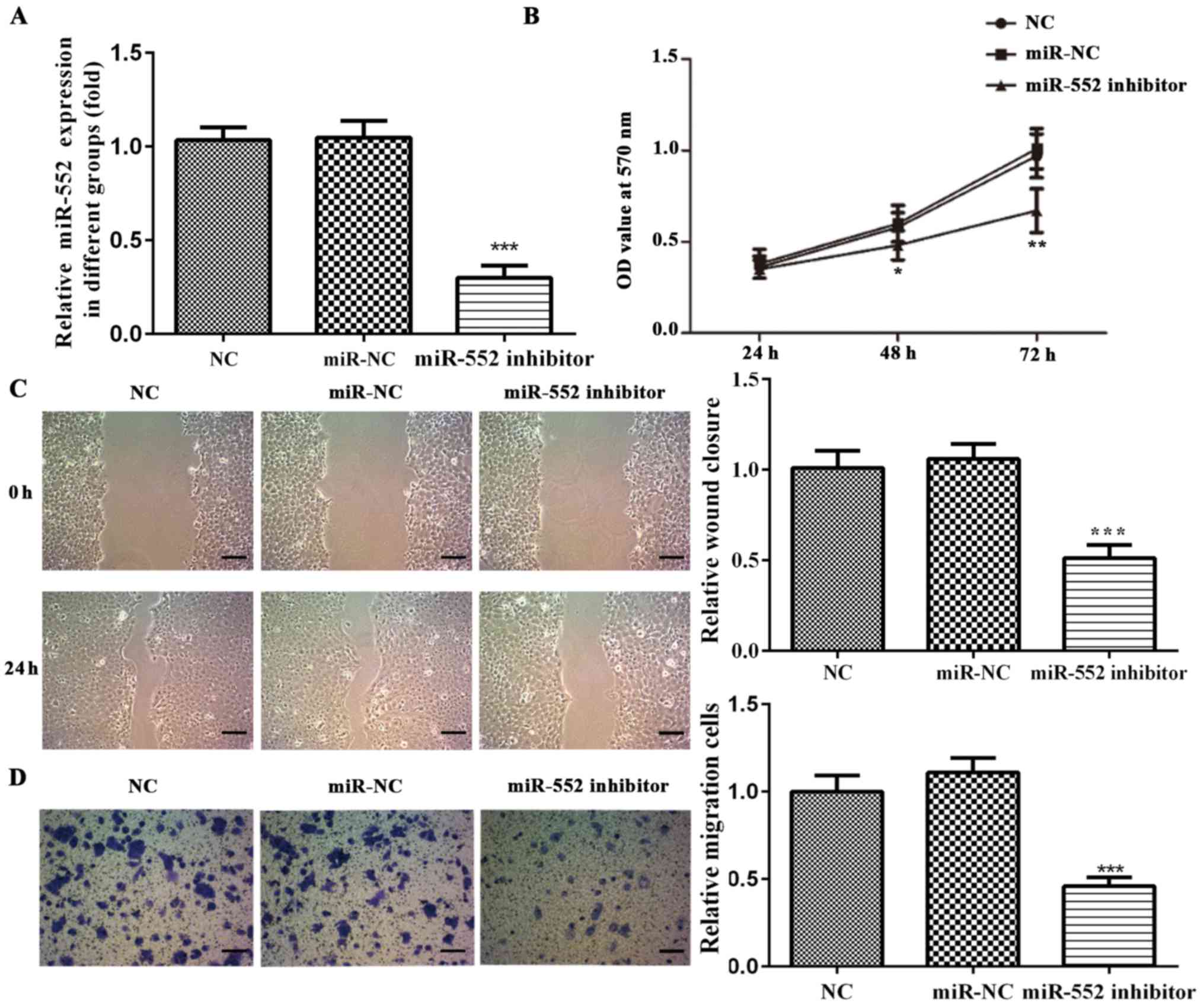
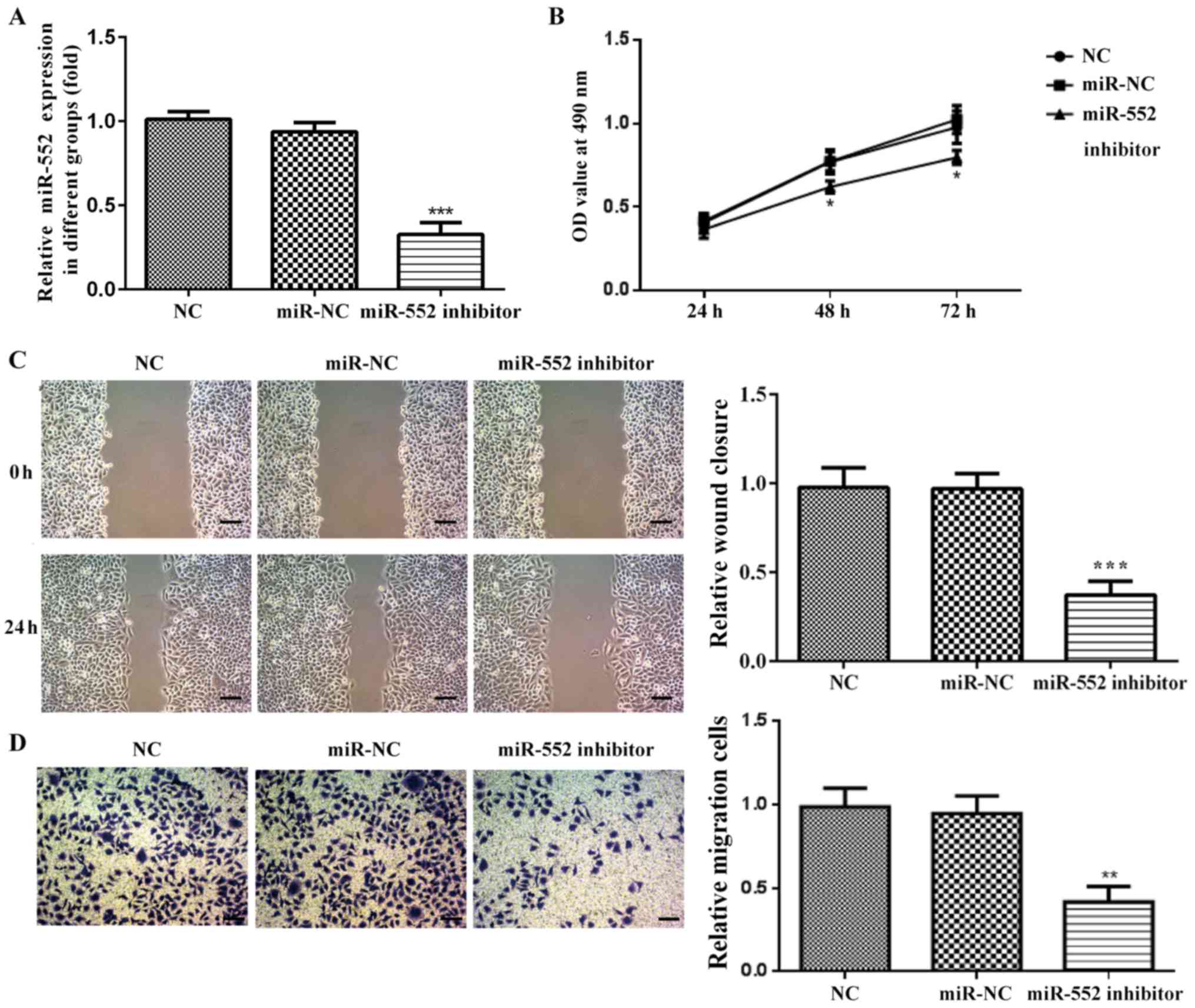
Transfection efficiency of miR-552
inhibitor in HCC cell lines
In order to determine the role of miR-552 in HCC,
PLC/PRF/5 and Huh-7 cells were transfected with a miR-552 inhibitor
or corresponding miR-NC. miR-552 expression was significantly
downregulated in cells transfected with miR-552 inhibitor compared
with the miR-NC or NC group, whilst no significant differences were
observed between the miR-NC and NC groups (Figs. 2A and 3A). These results suggest that miR-552
levels were successfully reduced by the miR inhibitor.
miR-552 knockdown reduces HCC cell
viability
Viability of PLC/PRF/5 and Huh-7 cells was analyzed
using MTT assay. miR-552 knockdown significantly decreased
PLC/PRF/5 and Huh-7 cell viability compared with miR-NC and NC
cells after 48 and 72 h (Figs. 2B
and 3B) whilst no significant
differences were observed between the miR-NC and NC groups. These
findings suggest that miR-552 regulated HCC cell viability.
miR-552 knockdown reduces HCC cell
migration
Wound healing and Transwell assays were performed to
determine the effect of miR-552 on HCC cell migration. miR-552
knockdown significantly inhibited PLC/PRF/5 (Fig. 2C and D) and Huh-7 migration (Fig. 3C and D) compared with the miR-NC
group. These observations suggest that miR-552 promoted HCC cell
migration.
Effect of miR-552 knockdown on HCC
apoptosis
Bax, Bcl-2 and caspase-3, proteins associated with
cell apoptosis, were measured to investigate the effects of miR-552
knockdown on HCC cell apoptosis. Flow cytometry analysis
demonstrated that PLC/PRF/5 cells transfected with miR-NC or NC
exhibited a low quantity of Annexin V-positive cells (Fig. 4A and B). However, apoptosis was
significantly increased in cells transfected with the miR-552
inhibitor. Western blot analysis demonstrated that transfection
with the miR-552 inhibitor increased the expression of caspase-3
and Bax compared with the miR-NC group and NC cells whilst
significantly reducing Bcl-2 expression in PLC/PRF/5 cells
(Fig. 4C and D). Similar results
were obtained using Huh-7 cells (Fig.
5). These results suggest that miR-552 inhibits HCC cell
apoptosis.
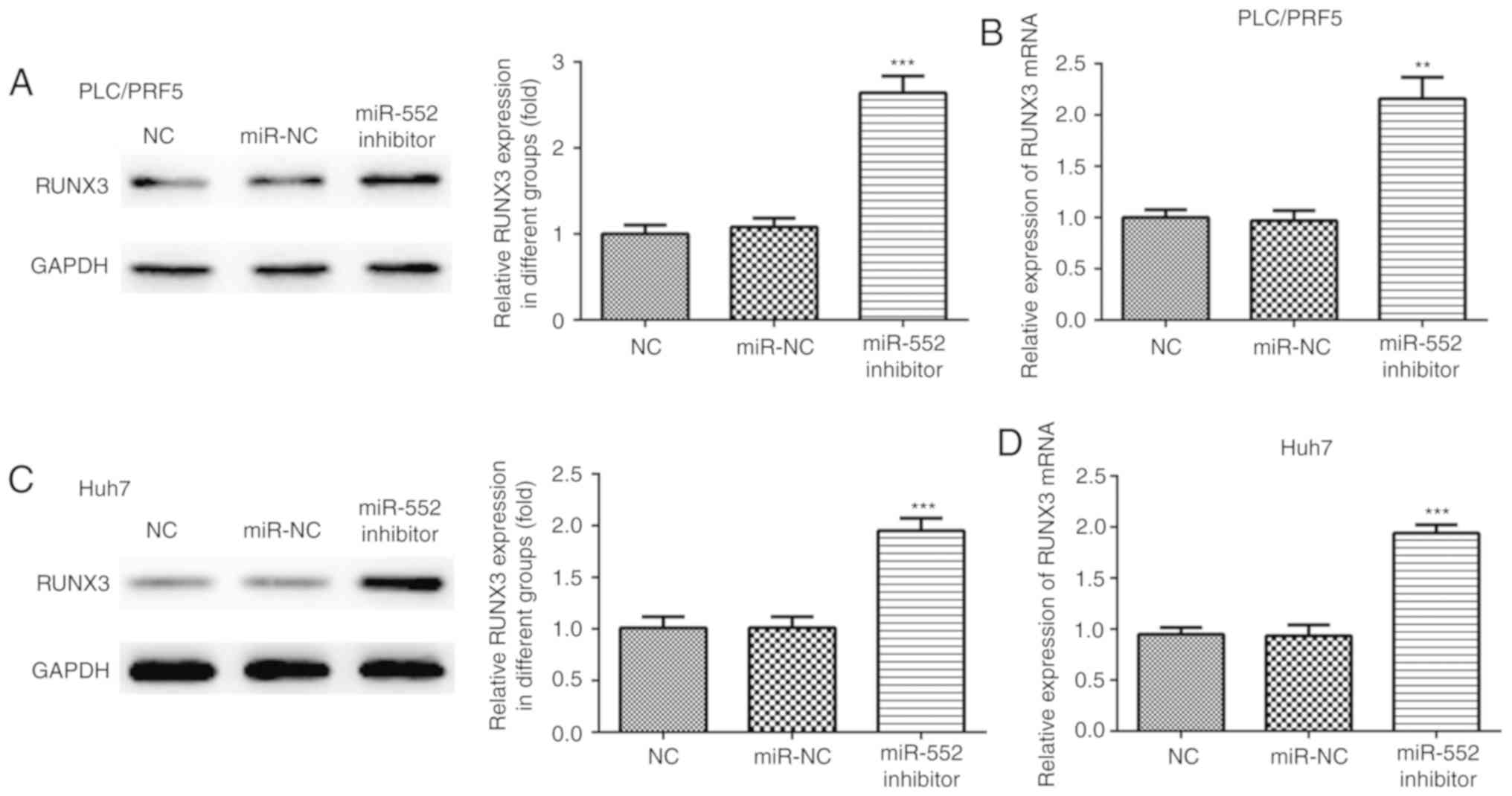
RUNX3 is directly targeted by miR-552
in HCC
Further analysis was performed to determine whether
miR-552 regulates the expression of RUNX3, a protein previously
found to be involved in the regulation of HCC physiology (17). Western blot and RT-qPCR analyzes
revealed that miR-552 knockdown increased the expression of RUNX3
in PLC/PRF/5 (Fig. 6A and B) and
Huh-7 cells (Fig. 6C and D). To
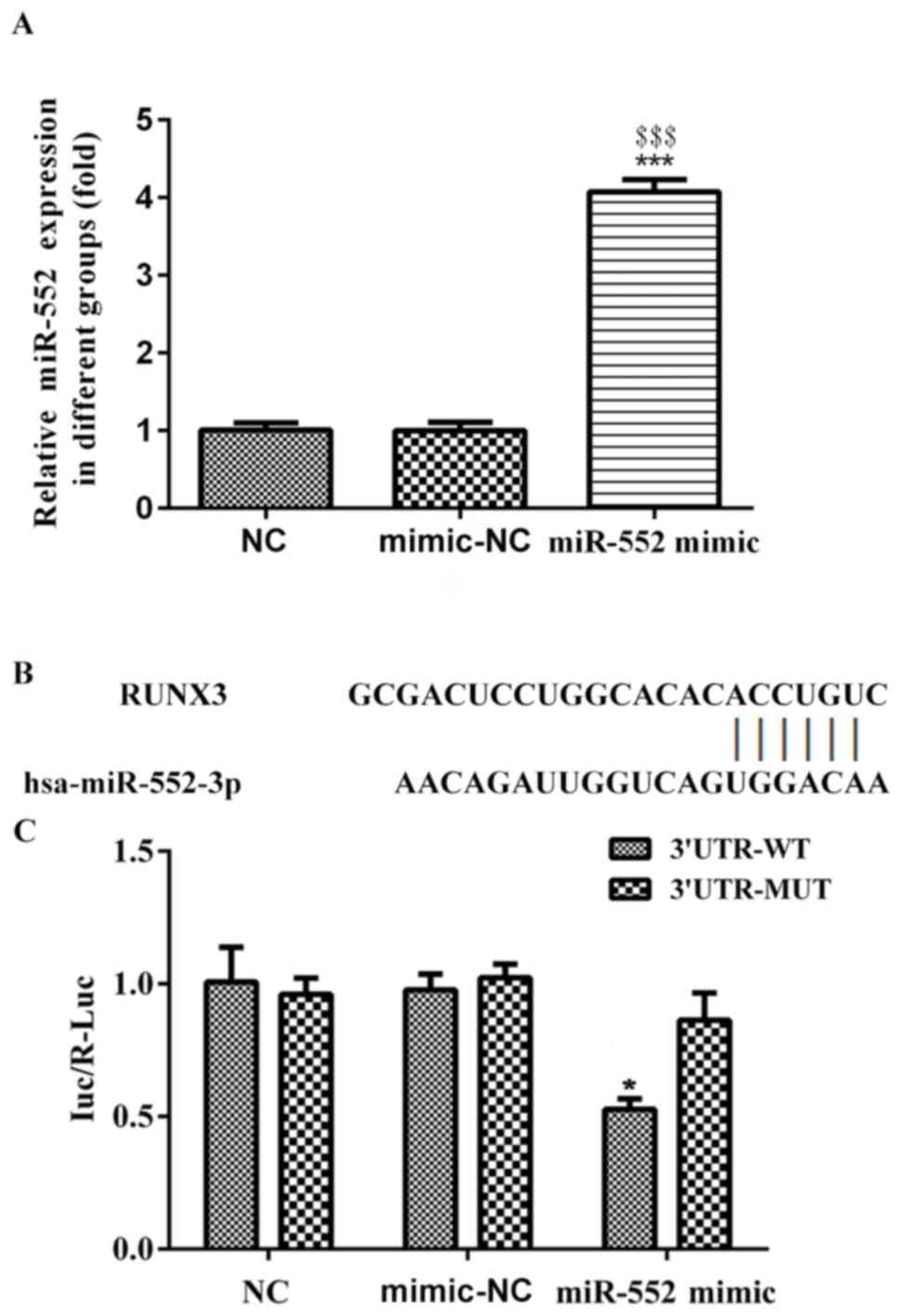
confirm whether RUNX3 was directly targeted by miR-552, dual
luciferase assay was performed. The transfection of miR-552 mimic
was efficient in PLC/PRF/5 (Fig.
7A). miR-552 mimic or miR-NC with plasmids containing
3′untranslated regions (UTR) of wt-RUNX3 or mut-RUNX3 were
transfected into PLC/PRF/5 cells. miR-552 mimics and mimic-NC were
transfected into RUNX3 wild-type and mutant reporter plasmids and
subjected to luciferase activity, which exhibited that RUNX3
luciferase activity in WT instead of mutant group significantly
reduced, compared with the control (Fig.
7B and C). Therefore, RUNX3 was directly targeted and
downregulated by miR-552 in PLC/PRF/5 cells.
Discussion
HCC is the primary cancer of the liver (18). Although advances in HCC therapy have
been made, overall survival has not improved over the previous
decades (3). HCC is commonly
accompanied by cirrhosis, which is associated with poor prognosis
(19). miRNAs are involved in the
post-transcriptional regulation of gene expression by binding to
3′-UTRs of target mRNAs, resulting in degradation of the mRNA
targets (20). Aberrant miRNA
expression is associated with a number of molecular pathways
including cyclin-dependent kinases, Bcl-2 family proteins, matrix
metalloproteinase and the phosphatidyl inositol 3-kinase (PI3K)-Akt
kinase signaling pathway, and biological processes involved in the
pathogenesis of HCC, including cell viability, apoptosis,
angiogenesis and metastasis (21,22).
miR-552 expression is significantly increased in
colon cancer and enhances proliferation, migration and
clonogenicity (11). As the abnormal
expression of miR-552 and subsequent downstream effects have been
previously reported in colon, breast and lung cancer (23,24), it
was hypothesized that miR-552 may also have an effect in HCC. In
the present study, since aberrant expression of miR-552 was
detected in HCC tissues and liver cancer cell lines, the role of
miR-552 in HCC was investigated in more depth. To the best of our
knowledge, the present study is the first report to demonstrate
that miR-552 regulates cell viability, migration, invasion and
apoptosis in HCC cell lines, suggesting that miR-552 can be used as
a potential biomarker for HCC diagnosis and determining prognosis.
A limitation of the present study is insufficient sample size for
the validation of miR-552 as a biomarker for diagnosis and
prognosis, and the lack of a correlation analysis between HCC and
miR-552 expression, including age, gender, tumor size, lymph node
and distance metastases, clinical stage and histological grade. The
expression of miR-552 in the blood can be as a potential biomarker
for the early diagnosis and prognosis of HCC. Larger sample sizes
will need to be collected for further studies.
Caspase-3 is an important apoptotic mediator
involved in endogenous and exogenous apoptotic pathways (25). Caspase-3 activation by the
mitochondrial release of cytochrome c is a key mechanism that
mediates cell apoptosis (26).
Overexpression of Bcl-2 inhibits the dimerization of Bax which in
turn inhibits the expression of cytochrome c, reducing caspase-3
activation and subsequent cell apoptosis (27). In the present study, inhibition of
miR-552 significantly decreased the expression of Bcl-2 and
increased the expression of caspase 3 and Bax. These results
suggest that the inhibition of miR-552 may promote apoptosis by
activating the mitochondrial apoptosis pathway.
In a previous meta-analysis, a screen for potential
biomarkers in HCC was conducted, which resulted in identification
of 17 aberrantly methylated genes (28). Within this list, RUNX3 DNA
methylation was found to be significant higher in HCC tissues and
serum of patients with HCC compared with normal controls (28). The findings of the present study
demonstrated that miR-552 directly targeted RUNX3 mRNA, resulting
in downstream effects on cell viability, metastasis and apoptosis.
However, further research is required to validate the mechanism of
miR-552 in HCC cell lines.
Taken together, the present study indicated that
miR-552 may have oncogenic properties in HCC by enhancing cancer
cell viability, migration and invasion whilst reducing cell
apoptosis. miR-552 directly targeted RUNX3 in HCC and this
mechanism may be involved in the mode of action of miR-522 and
subsequent biological effects.
Acknowledgements
Not applicable.
Funding
Natural Science Foundation of China-Joint Fund of
Xinjiang Uygur Autonomous Region (grant no. 2018D01C186).
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM designed the design, drafted manuscript,
performed the experiments and participated in data analysis. MM and
LM collected the experimental samples and performed RT-qPCR,
western blotting and cell apoptosis. XM and YL analyzed the data.
XM also contributed to the study design and revised the manuscript
critically. FZ produced the figures, performed data analysis and
picture production, performed literature research for this
investigation which is essential for the methods establishment and
revised the draft manuscript. All authors have approved to publish
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
Committees of The First Affiliated Hospital of Xinjiang Medical
University and performed in accordance with Declaration of
Helsinki.
Patient consent for publication
All patients provided informed consent and approved
the publication of data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Hessheimer AJ, Isabel Real M and
Bruix J: Treatment of hepatocellular carcinoma. Crit Rev Oncol
Hematol. 60:89–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guglielmi A, Ruzzenente A, Conci S,
Valdegamberi A, Vitali M, Bertuzzo F, De Angelis M, Mantovani G and
Iacono C: Hepatocellular carcinoma: Surgical perspectives beyond
the barcelona clinic liver cancer recommendations. World J
Gastroenterol. 20:7525–7533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro- RNA genes miR15
and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad
Sci USA. 99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fiorino S, Bacchi-Reggiani ML, Visani M,
Acquaviva G, Fornelli A, Masetti M, Tura A, Grizzi F, Zanello M,
Mastrangelo L, et al: MicroRNAs as possible biomarkers for
diagnosis and prognosis of hepatitis B- and
C-related-hepatocellular-carcinoma. World J Gastroenterol.
22:3907–3936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hayes CN and Chayama K: MicroRNAs as
biomarkers for liver disease and hepatocellular carcinoma. Int J
Mol Sci. 17:2802016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hua S, Liu C, Liu L and Wu D: miR-142-3p
inhibits aerobic glycolysis and cell proliferation in
hepatocellular carcinoma via targeting LDHA. Biochem Biophys Res
Commun. 496:947–954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang W, Qian S, Yang G, Zhu L, Zhou B,
Wang J, Liu R, Yan Z and Qu X: MicroRNA-199 suppresses cell
proliferation, migration and invasion by downregulating RGS17 in
hepatocellular carcinoma. Gene. 659:22–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao J, Yan XR, Liu T, Han XB, Yu JJ, Liu
SH and Wang LB: MicroRNA-552 promotes tumor cell proliferation and
migration by directly targeting DACH1 via the Wnt/β-catenin
signaling pathway in colorectal cancer. Oncol Lett. 14:3795–3802.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Li H, Wang Y, Wang L, Yan X, Zhang
D, Ma X, Du Y, Liu X and Yang Y: MicroRNA-552 enhances metastatic
capacity of colorectal cancer cells by targeting a disintegrin and
metalloprotease 28. Oncotarget. 7:70194–70210. 2016.PubMed/NCBI
|
|
12
|
Gou Y, Zhai F, Zhang L and Cui L: RUNX3
regulates hepatocellular carcinoma cell metastasis via targeting
miR-186/E-cadherin/EMT pathway. Oncotarget. 8:61475–61486. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Wang X, Cheng J, Wang Z, Jiang T,
Hou N, Liu N, Song T and Huang C: MicroRNA-20a-5p targets RUNX3 to
regulate proliferation and migration of human hepatocellular cancer
cells. Oncol Rep. 36:3379–3386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt VM, Isachenko E, Rappl G, Rahimi
G, Hanstein B, Morgenstern B, Mallmann P and Isachenko V:
Construction of human artificial ovary from cryopreserved ovarian
tissue: Appearance of apoptosis and necrosis after enzymatic
isolation of follicles. Cryobiology. 84:10–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong Z, Cao X, Li N, Zhang Y, Lan L, Zhou
Y, Pan X, Shen L, Yin Z and Luo L: Luteolin is effective in the
non-small cell lung cancer model with L858R/T790M EGF receptor
mutation and erlotinib resistance. Br J Pharmacol. 171:2842–2853.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Zuo X, Pu L, Zhang Y, Han G, Zhang
L, Wu J and Wang X: circLARP4 induces cellular senescence through
regulating miR-761/RUNX3/p53/p21 signaling in hepatocellular
carcinoma. Cancer Sci. 110:568–581. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kondili LA, Lala A, Gunson B, Hubscher S,
Olliff S, Elias E, Bramhall S and Mutimer D: Primary hepatocellular
cancer in the explanted liver: Outcome of transplantation and risk
factors for HCC recurrence. Eur J Surg Oncol. 33:868–873. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Duan J, Hu C, Qiu Q, Zhang J, Meng H, Wang
K, Dong H, Wei H and Yin Y: Characterization of microvessels and
parenchyma in in-line phase contrast imaging CT: Healthy liver,
cirrhosis and hepatocellular carcinoma. Quant Imaging Med Surg.
9:1037–1046. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin K, Li T, Sanchez-Duffhues G, Zhou F
and Zhang L: Involvement of inflammation and its related microRNAs
in hepatocellular carcinoma. Oncotarget. 8:22145–22165.
2017.PubMed/NCBI
|
|
21
|
Chu R, Mo G, Duan Z, Huang M, Chang J, Li
X and Liu P: miRNAs affect the development of hepatocellular
carcinoma via dysregulation of their biogenesis and expression.
Cell Commun Signal. 12:452014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Callegari E, Gramantieri L, Domenicali M,
D'Abundo L, Sabbioni S and Negrini M: MicroRNAs in liver cancer: A
model for investigating pathogenesis and novel therapeutic
approaches. Cell Death Differ. 22:46–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J, Lim NJ, Jang SG, Kim HK and Lee GK:
miR-592 and miR-552 can distinguish between primary lung
adenocarcinoma and colorectal cancer metastases in the lung.
Anticancer Res. 34:2297–2302. 2014.PubMed/NCBI
|
|
24
|
Leivonen SK, Sahlberg KK, Mäkelä R, Due
EU, Kallioniemi O, Børresen-Dale AL and Perälä M: High-throughput
screens identify microRNAs essential for HER2 positive breast
cancer cell growth. Mol Oncol. 8:93–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Juraver-Geslin HA and Durand BC: Early
development of the neural plate: New roles for apoptosis and for
one of its main effectors caspase-3. Genesis. 53:203–224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garrido C, Galluzzi L, Brunet M, Puig PE,
Didelot C and Kroemer G: Mechanisms of cytochrome c release from
mitochondria. Cell Death Differ. 13:1423–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo J, Zhang K, Ji Y, Jiang X and Zuo S:
Effects of ethyl pyruvate on myocardial apoptosis and expression of
Bcl-2 and Bax proteins after ischemia-reperfusion in rats. J
Huazhong Univ Sci Technolog Med Sci. 28:281–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Li J, Huang T, Duan S, Dai D,
Jiang D, Sui X, Li D, Chen Y, Ding F, et al: Meta-analysis of DNA
methylation biomarkers in hepatocellular carcinoma. Oncotarget.
7:81255–81267. 2016. View Article : Google Scholar : PubMed/NCBI
|