Introduction
Lung cancer is the leading cause of cancer-related
mortality in developed countries (1,2). Based
on the treatment strategy, lung cancer is clinically classified
into non-small-cell lung cancer (NSCLC) and small-cell lung cancer.
NSCLC accounts for 85% of all lung cancer cases, and it includes
several types of histological subtypes, including adenocarcinoma
and squamous cell carcinoma. Due to the advances in molecular
biology, several molecular-targeted therapies have been developed
for lung adenocarcinomas. Epidermal growth factor receptor
(EGFR)-tyrosine kinase inhibitors (TKIs), such as gefitinib, have
been proven to be effective against lung adenocarcinomas with
activating EGFR mutations (3).
Anaplastic lymphoma kinase (ALK) inhibitors, such as alectinib,
have also been reported to be effective against lung
adenocarcinomas with the ALK fusion gene (4). However, molecular-targeted therapies
are not applicable to cancers without mutations of these target
genes. Recently, immune checkpoint inhibitors (ICIs), such as
nivolumab, have been demonstrated to exhibit prominent clinical
efficacy against various types of cancer, including NSCLC (5). However, these ICIs have been reported
to be less effective against patients with programmed death-ligand
1 (PD-L1)-negative tumors (6).
Therefore, it is crucial to fully elucidate tumor biology in order
to develop novel treatment strategies against NSCLC without these
mutations of target genes or PD-L1-positive expression.
The evaluation of infiltrating macrophages in
tumors, referred to as tumor-associated macrophages (TAMs), has
been reported to be important. TAMs are key components of the tumor
microenvironment (TME) that influence tumor growth and progression
(7,8). Tumor cells release various chemokines
to attract macrophages, as well as other inflammatory cells, into
the tumor stroma, and a number of substances secreted by TAMs may
stimulate the proliferation and metastasis of tumor cells (9,10).
Several clinical studies on TAMs have been reported in various
human cancers, including NSCLC, colon and breast cancer (11–14).
However, previous studies using only immunostaining for CD68, the
most common pan-macrophage marker, yielded confusing results
regarding its prognostic potential in NSCLC. For example, these
studies reported that increased levels of TAMs in tumor islets were
associated with good prognosis (15–19),
whereas the increased levels of TAMs in the tumor stroma were found
to be associated with poor prognosis (15,18).
There could be several factors responsible for such confusing
results.
First, macrophages are particularly heterogeneous in
their phenotype and function. Under physiological or pathological
conditions, macrophages can be polarized into different phenotypes,
namely tumor-inhibiting M1 and tumor-promoting M2 macrophages
(11,20,21). M1
TAM-derived cytokines have the ability to kill pathogens. On the
other hand, M2 macrophages are pro-angiogenic and participate in
wound healing by downregulating inflammatory response to promote
connective tissue remodeling (14).
Th2-derived cytokines, such as interleukin (IL)4, IL10 and IL13,
transforming growth factor-β, prostaglandin E2 or
colony-stimulating factor 1, may promote M2 differentiation of
macrophages (8,21). During tumor progression, these
signals originating from tumor and stromal cells may induce the
production of M2 TAMs in the TME. M2 TAMs may induce angiogenesis
by secretion of cytokines, such as vascular endothelial growth
factor (VEGF) (22), and promote
tumor growth and metastasis. Experimental studies also demonstrated
that M2 macrophages may promote tumor cell proliferation (23).
Second, macrophages are distributed in various
tissue compartments in lung cancer, such as tumor stroma, tumor
islets and alveolar space, and the TAMs present in different tissue
components may display different biological properties (11,12). In
fact, previous clinical studies in NSCLC demonstrated that high
infiltration of tumor islets by M1 TAMs was associated with
increased survival (20,24), whereas infiltration of tumor islets
and tumor stroma by high numbers of M2 TAMs was associated with
reduced survival (20,25). In addition, the levels of M2
macrophages were reported to be higher compared with those of M1
macrophages in NSCLC (24).
Taking these factors into consideration, in order to
elucidate the biological and clinical significance of M2 TAMs in
NSCLC, a comprehensive clinical study on M2 TAMs in terms of tissue
distribution was performed, using immunostaining for CD163, an M2
macrophage marker (20,24,25).
Materials and methods
Patients
A total of 160 consecutive NSCLC patients who
underwent surgery at the Department of Thoracic Surgery of Kitano
Hospital between November 2011 and October 2014 were
retrospectively investigated. The study protocol was approved by
the Institutional Ethics Committee of Kitano Hospital (P181200300),
and written informed consent was obtained from all the patients.
Pathological staging was determined using the 8th
tumor-node-metastasis (TNM) classification system (26). Invasive size was defined as the
maximum dimension of the invasive component, excluding the lepidic
growth component (26). The
histological type and grade of differentiation of the tumors were
determined according to the World Health Organization
classification system (27). The
patients' medical records and histopathological diagnoses were
fully documented. The patient records included follow-up data as of
August 2018. The overall median follow-up period was 42.8
months.
Immunohistochemistry
Immunohistochemical studies were performed to
evaluate the M2 TAM distribution by CD163 staining and the tumor
proliferation rate by Ki-67 proliferation index, using the Ventana
BenchMark GX system (Roche/Ventana Medical Systems), according to
the recommended protocol. The following antibodies were used: Mouse
monoclonal anti-human CD163 antibody (clone 760-4437,
Roche/VentanaMedical Systems), and rabbit monoclonal anti-human
Ki-67 antibody (clone 30-9, Roche/VentanaMedical Systems).
Formalin-fixed paraffin-embedded tissues were cut into 4-µm
sections and mounted on poly-L-lysine-coated slides. The sections
were deparaffinized and rehydrated, and antigen retrieval was
performed with Cell Conditioner 1 (32 min for CD163 and 64 min for
Ki-67). The sections were then incubated with the specific primary
antibody against CD163 (16 min) and Ki-67 (8 min). Subsequently,
the sections were treated with the OptiView HQ Linker for 8 min and
the OptiView HRP Multimer for 8 min. Finally, counterstaining was
performed with Mayer's hematoxylin and Scott's tap water bluing
reagent.
Evaluation of
immunohistochemistry
Evaluation of stained tissue sections was performed
by two investigators (RS and TH) who were blinded to the patients'
clinical status. Cases with discrepancies were jointly re-evaluated
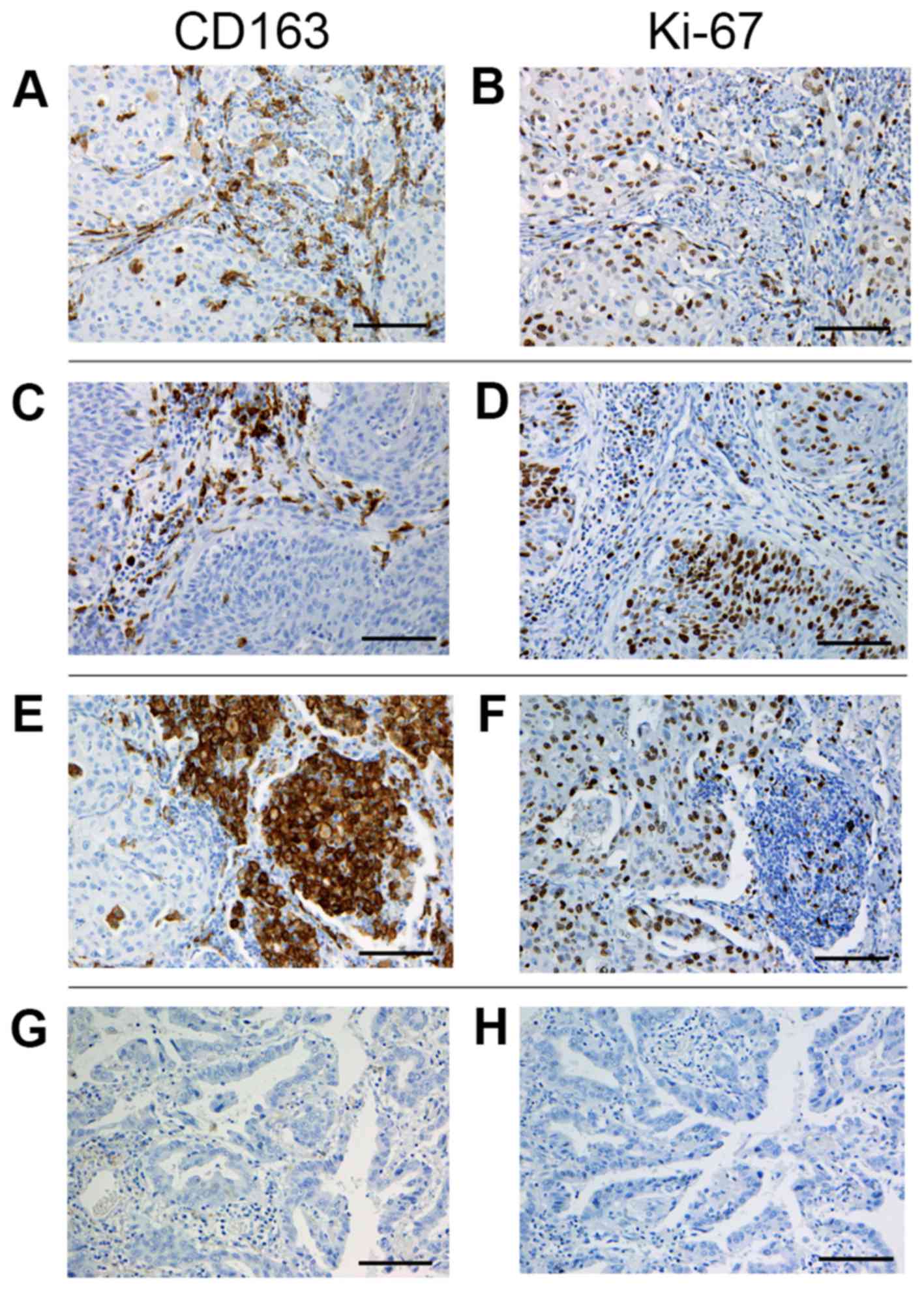
and a consensus was reached. For CD163 staining, the five most
representative high-power fields (magnification, ×400; 0.0625
mm2) of the tumor stroma, tumor islets and alveolar
space per tissue section were selected (Fig. 1). Tumor stroma was defined as the
area where tumor stromal cells accounted for >70% of the total
cells (28). In adenocarcinoma in
situ cases with a scant stromal component, the perivascular or
peribronchiolar stromal tissue inside the tumor was analyzed as
tumor stroma. Tumor islets were defined as areas where tumor cells
accounted for >70% of the total cells. The alveolar space was
defined as the air space inside the main tumor or outside within
three alveoli. The number of CD163-positive cells in each area was
counted manually, and the mean number of fields in each area was
calculated. Finally, the CD163-positive macrophage (M2 TAM) density
was defined as cell number per mm2 in the tumor stroma
(stromal M2 TAM), tumor islets (islet M2 TAM) and alveolar space
(alveolar M2 TAM). The percentage of carcinoma cells with a
positive staining for Ki-67 in a given specimen was defined as the
Ki-67 proliferation index (29).
Statistical analysis
As stromal M2 TAM density (P=0.1648), islet M2 TAM
density (P=0.2845), alveolar M2 TAM density (P=0.1936), C-reactive
protein (CRP) level (P=0.3056), total tumor size (P=0.1387),
invasive size (P=0.1211) and Ki-67 proliferation index (P=0.1734)
exhibited normal distribution (Kolmogorov-Smirnov analysis),
statistical significance was assessed by the t-test, ANOVA with
Bonferroni/Dunn test or Pearson's correlation coefficient.
Categorical variables were compared using χ2 test. As
previous clinical studies reported that the level of CRP, a marker
of inflammatory response, was related to cancer risk and prognosis
(30,31), receiver operating characteristic
(ROC) curve analysis was performed to determine the optimal cut-off
value of each M2 TAM density with maximal sensitivity and
specificity for distinguishing between <1 mg/l and ≥1 mg/l of
CRP (30,31). The sample was classified as stromal
M2 TAM-high when the stromal M2 TAM density was >380 [area under
the curve (AUC)=0.521]. The sample was classified as alveolar M2
TAM-high when the alveolar M2 TAM density was >400 (AUC=0.628).
On the other hand, the sample was classified as islet M2 TAM-high
when the islet M2 TAM density was >35, its median value, because
the islet M2 TAM density was not associated with the CRP level.
Disease-free survival (DFS) was defined as the time from treatment
initiation (surgical resection, chemotherapy or radiation) to the
date of disease recurrence or death from any cause. Overall
survival (OS) was defined as the time from treatment initiation to
the date of death from any cause. The Kaplan-Meier method was used
to estimate the probability of DFS and OS as a function of time,
and differences in the survival of subgroups of patients were
compared using Mantel's log-rank test. The univariate analysis
using the Cox regression model was used to evaluate the effects on
survival. P-values obtained used a t-test, Mantel's log-rank test
or Bonferroni/Dunn post-hoc test were based on the two-sided
statistical analysis, and a P<0.05 was considered to indicate a
statistically significant difference.
Results
Distribution of M2 TAMs in the tumor
stroma, tumor islets and alveolar space
The stromal M2 TAM density varied greatly among the
160 tumor tissues investigated (mean, 407.0±389.2). A total of 93
tumors (58.1%) were classified as stromal M2 TAM-low tumors, and 67
(41.9%) as stromal M2 TAM-high tumors. In addition, the islet M2
TAM density also varied greatly among the 160 tumor tissues (mean,
82.3±143.4). A total of 80 tumors (50.0%) were islet M2 TAM-low
tumors, and 80 (50.0%) were islet M2 TAM-high tumors. The stromal
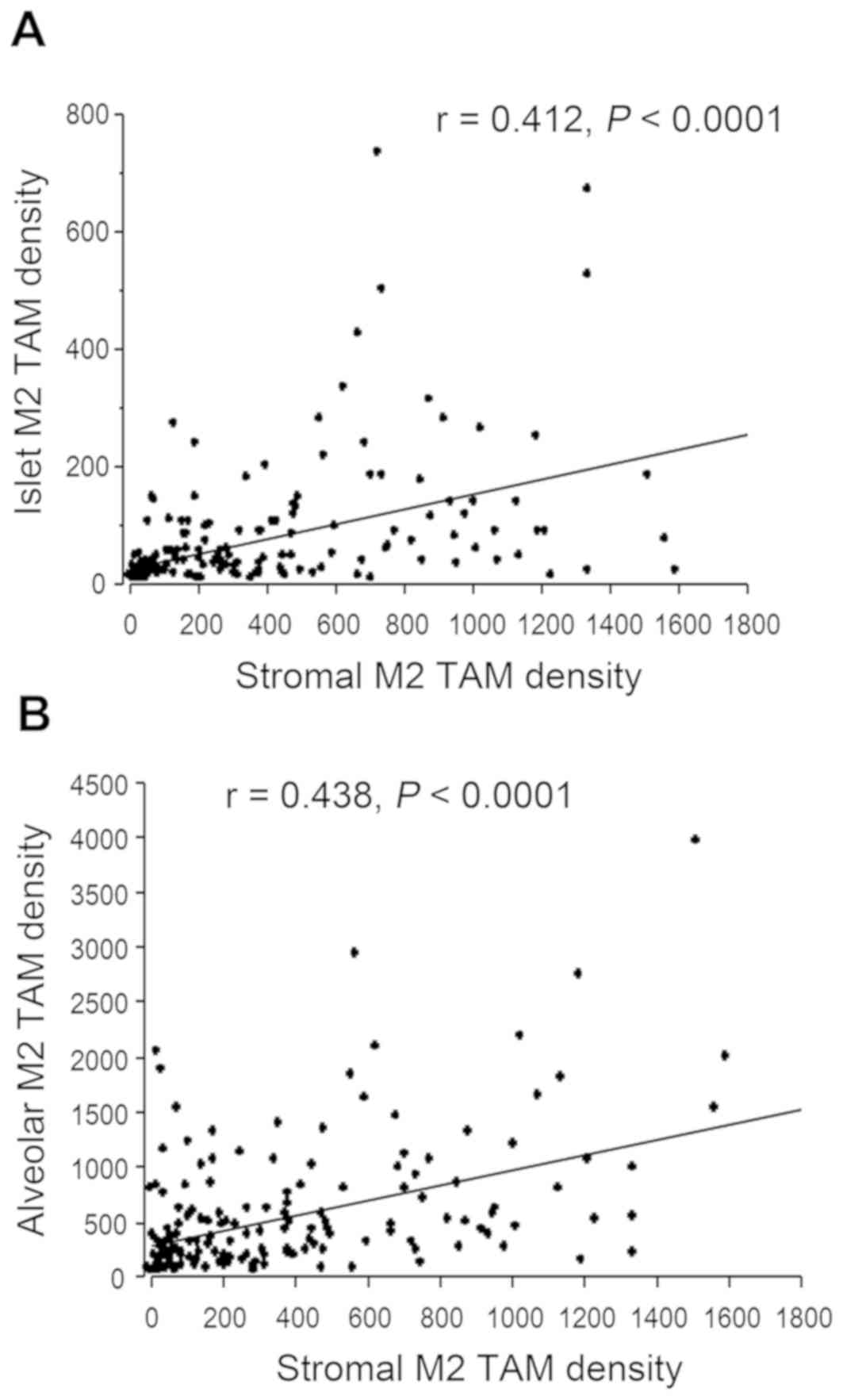
M2 TAM density was moderately correlated with the islet M2 TAM
density (r=0.412; Fig. 2A). However,
the islet M2 TAM density was significantly lower compared with the
stromal M2 TAM density in each tumor tissue (P<0.001).
The alveolar M2 TAM density also varied greatly
among the 160 tumor tissues (mean, 560.6±612.9). A total of 88
tumors (55.0%) were alveolar M2 TAM-low tumors, and 72 (45.0%) were
alveolar M2 TAM-high tumors. The alveolar M2 TAM density was also
moderately correlated with the stromal M2 TAM density (r=0.438;
Fig. 2B). However, the correlation
between the islet M2 TAM density and the alveolar M2 TAM density
was low (r=0.212).
Biological and clinical significance
of M2 TAMs in the tumor stroma among resected NSCLC
The biological significance of the M2 TAMs in the
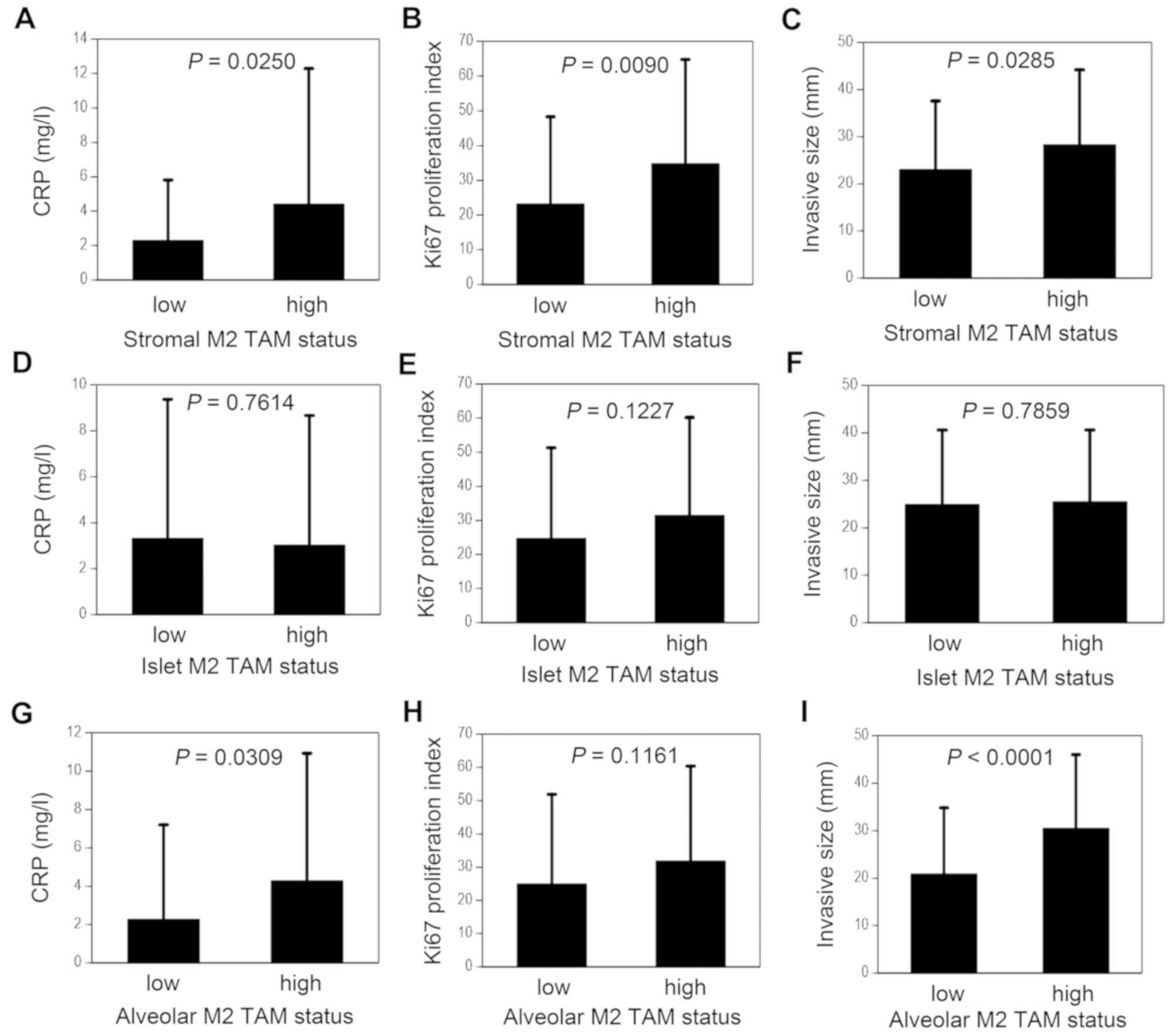
tumor stroma is shown in Fig. 3. The
CRP level was significantly higher in the stromal M2 TAM-high group
compared with that in the stromal M2 TAM-low group (4.41±7.88 vs.
2.29±3.52 mg/l, P=0.0250; Fig. 3A).
In addition, the Ki-67 proliferation index was significantly higher
in the stromal M2 TAM-high group compared with that in the stromal
M2 TAM-low group (34.8±30.0 vs. 23.2±25.1%, P=0.0090; Fig. 3B). The invasive size was also
significantly higher in the stromal M2 TAM-high group compared with
that in the stromal M2 TAM-low group (28.3±15.9 vs. 23.0±14.6 mm,
P=0.0285; Fig. 3C).
The distribution of the stromal M2 TAM density
according to clinicopathological characteristics is shown in
Table I. With respect to tumor
histology, the stromal M2 TAM density was significantly higher in
squamous cell carcinomas compared with that in adenocarcinomas
(P=0.0034). In addition, the stromal M2 TAM density was
significantly associated with tumor differentiation (P=0.0018), and
was significantly higher in poorly differentiated tumors compared
with that in well-differentiated and moderately differentiated
tumors (P=0.0004 and P=0.0149, respectively). With respect to nodal
status, the stromal M2 TAM density was significantly higher in
node-positive tumors compared with that in node-negative tumors
(P=0.0347). Furthermore, the stromal M2 TAM density was
significantly associated with pathological stage (P=0.0412).
 | Table I.Distribution of M2 tumor-associated
macrophage density in patients with NSCLC according to
clinicopathological characteristics. |
Table I.
Distribution of M2 tumor-associated
macrophage density in patients with NSCLC according to
clinicopathological characteristics.
|
Characteristics | n | Tumor stroma
(cells/mm2) | P-value | Tumor islet
(cells/mm2) | P-value | Alveolar space
(cells/mm2) | P-value |
|---|
| Smoking |
|
|
|
|
|
|
|
|
Non-smoker | 85 | 370.6±367.4 | 0.2092a | 76.6±102.2 | 0.5960a | 525.1±684.3 | 0.4374a |
|
Smoker | 75 | 448.2±411.0 |
| 88.7±179.7 |
| 600.8±522.1 |
|
| Tumor status |
|
|
|
|
|
|
|
| T0 | 8 | 207.0±330.1 | 0.1726b | 52.2±52.1 | 0.0714b | 239.0±168.3 | 0.0108b |
| T1 | 72 | 379.7±386.6 |
| 56.8±67.7 |
| 442.9±625.8 |
|
|
T2-4 | 80 | 451.6±392.6 |
| 108.3±188.9 |
| 698.7±599.4 |
|
| Nodal status |
|
|
|
|
|
|
|
| N0 | 123 | 371.4±344.9 | 0.0347a | 74.1±106.7 | 0.1864a | 517.1±575.0 | 0.1017a |
|
N1-3 | 37 | 525.2±497.1 |
| 109.7±226.5 |
| 705.2±714.8 |
|
| Pathological
Stage |
|
|
|
|
|
|
|
| 0 | 7 | 95.4±104.6 | 0.0412b | 43.8±50.1 | 0.8020b | 241.1±181.7 | 0.0110b |
| I | 100 | 385.3±359.4 |
| 89.1±166.9 |
| 472.2±594.7 |
|
| II | 24 | 548.0±428.9 |
| 66.9±83.5 |
| 851.3±657.4 |
|
|
III | 29 | 440.3±453.5 |
| 80.9±106.4 |
| 701.9±611.6 |
|
|
Differentiation |
|
|
|
|
|
|
|
|
Well | 33 | 251.8±278.7 | 0.0018b | 80.4±123.2 | 0.0522b | 405.9±393.6 | 0.0192b |
|
Moderately | 93 | 397.7±369.4 |
| 59.2±68.5 |
| 526.1±561.4 |
|
|
Poorly | 34 | 499.9±154.0 |
| 147.3±255.9 |
| 805.1±832.0 |
|
| Histology |
|
|
|
|
|
|
|
|
Adenocarcinoma | 128 | 357.3±365.5 | 0.0034b | 71.8±95.1 | 0.0692b | 535.6±637.2 | 0.3053b |
|
Squamous cell carcinoma | 25 | 635.3±469.4 |
| 106.0±261.9 |
| 726.4±534.0 |
|
| Large
cell carcinoma | 7 | 499.9±154.0 |
| 190.0±247.6 |
| 424.9±266.8 |
|
| Total number of
patients | 160 | 407.0±389.2 |
| 82.3±143.4 |
| 560.6±612.9 |
|
Biological and clinical significance
of M2 TAMs in the tumor islets among resected NSCLC
The islet M2 TAM was not associated with the CRP
level, the Ki-67 proliferation index or the invasive size (Fig. 3D-F). In addition, the islet M2 TAM
density was not associated with tumor histology, tumor
differentiation, tumor status, nodal status or pathological stage
(Table I).
Biological and clinical significance
of M2 TAMs in the alveolar space among resected NSCLC
With respect to biological significance, the CRP
level was significantly higher in the alveolar M2 TAM-high group
compared with that in the alveolar M2 TAM-low group (4.29±6.64 vs.
2.27±4.93 mg/l, P=0.0309; Fig. 3G).
On the other hand, the alveolar M2 TAM was not significantly
associated with the Ki-67 proliferation index (Fig. 3H). However, the total tumor size was
significantly higher in the alveolar M2 TAM-high group compared
with that in the alveolar M2 TAM-low group (31.7±14.8 vs. 23.4±12.8
mm, P=0.0005). In addition, the invasive size was also
significantly higher in the alveolar M2 TAM-high group compared
with that in the alveolar M2 TAM-low group (30.5±15.5 vs. 20.9±13.9
mm, P<0.0001; Fig. 3I).
With respect to clinical significance, the alveolar
M2 TAM density was significantly associated with tumor
differentiation (P=0.0192) (Table
I), and the alveolar M2 TAM density was significantly higher in
poorly differentiated tumors compared with that in
well-differentiated and moderately differentiated tumors (P=0.0073
and P=0.0219, respectively). In addition, the alveolar M2 TAM
density was significantly associated with tumor status and
pathological stage (P=0.0108 and P=0.0110, respectively).
DFS of patients with resected NSCLC in
relation to M2 TAM status
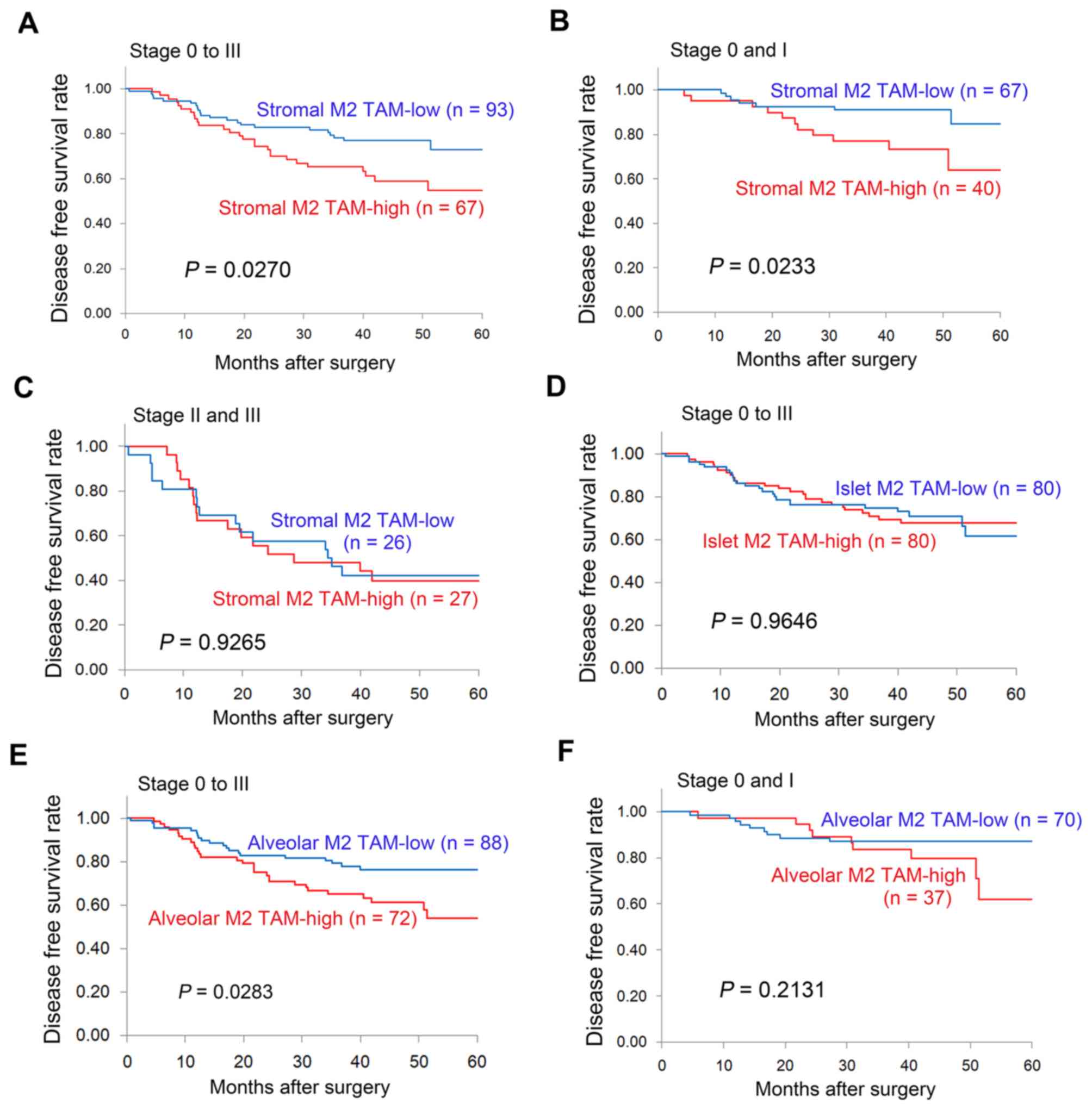
With respect to the stromal M2 TAM status, the
5-year DFS rate was significantly lower in patients with stromal M2
TAM-high tumors compared with stromal M2 TAM-low tumors (54.7 vs.
72.9%, P=0.0270; Fig. 4A). In
particular, among patients with early-stage disease (stage 0 and
I), the 5-year DFS rate was significantly lower in patients with
stromal M2 TAM-high tumors compared with those with stromal M2
TAM-low tumors (64.0 vs. 84.5%, P=0.0233; Fig. 4B). However, among patients with
advanced disease (stage II and III), no significant difference was
observed in the DFS of patients with resected NSCLC patients in
relation to the stromal M2 TAM status (Fig. 4C). In addition, no difference was
observed in the DFS of patients with resected NSCLC according to
islet M2 TAM status (Fig. 4D). With
respect to the alveolar M2 TAM status, the 5-year DFS rate was
significantly lower in patients with alveolar M2 TAM-high tumors
compared with those with alveolar M2 TAM-low tumors (54.0 vs.
76.2%, P=0.0283; Fig. 4E). However,
among patients with early-stage disease (stage 0 and I), no
significant difference was observed in the DFS of patients with
resected NSCLC patients in relation to the alveolar M2 TAM status
(Fig. 4F). Univariate analyses using
the Cox regression model also demonstrated that the stromal M2 TAM
status [HR=1.869 (95% CI: 1.064–3.283); P=0.0296] and the alveolar
M2 TAM status [HR=1.873 (95% CI: 1.059–3.311); P=0.0310] were
significant factors for predicting the DFS of patients with
resected NSCLC.
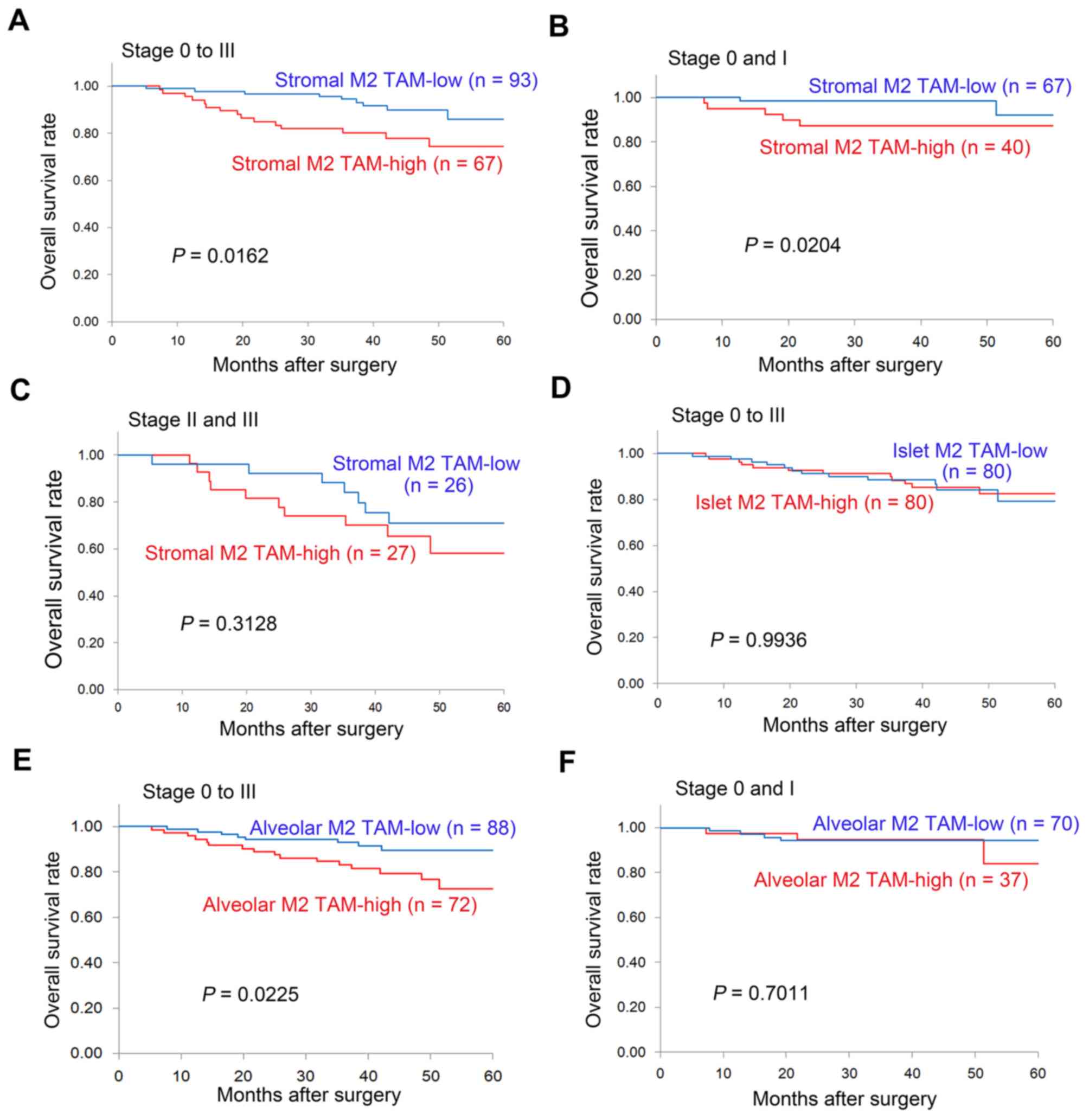
OS of patients with resected NSCLC in
relation to M2 TAM status
With respect to the stromal M2 TAM status, the
5-year OS rate was significantly lower in patients with stromal M2
TAM-high tumors compared with those with stromal M2 TAM-low tumors
(74.5 vs. 85.8%, P=0.0162; Fig. 5A).
In particular, for early-stage disease (stage 0 and I), the 3-year
OS was significantly lower in patients with stromal M2 TAM-high
tumors compared with those with stromal M2 TAM-low tumors (87.3 vs.
98.5%, P=0.0204; Fig. 5B). However,
for advanced disease (stage II and III), no difference was observed
in the OS of patients with resected NSCLC in relation to the
stromal M2 TAM status (Fig. 5C). In
addition, no difference was observed in the OS of patients with
resected NSCLC according to islet M2 TAM status (Fig. 5D). With respect to the alveolar M2
TAM status, the 5-year OS was significantly lower in patients with
alveolar M2 TAM-high tumors compared with those with alveolar M2
TAM-low tumors (72.7 vs. 89.5%, P=0.0225; Fig. 5E). However, among patients with
early-stage disease (stage 0 and I), no significant difference was
observed in the OS of patients with resected NSCLC patients in
relation to the alveolar M2 TAM status (Fig. 5F). Univariate analyses using the Cox
regression model also demonstrated that the stromal M2 TAM status
[HR=2.630 (95% CI: 1.160–5.964); P=0.0207] and the alveolar M2 TAM
status [HR=2.573 (95% CI: 1.109–5.972); P=0.0278] were significant
factors for predicting OS in patients with resected NSCLC.
Discussion
In order to elucidate the biological and clinical
significance of M2 TAMs in NSCLC, a comprehensive clinical study on
M2 TAMs with respect to tissue distribution was performed.
Regarding M2 macrophage markers, such as CD163 and CD204 (28), CD163 immunostaining was used, as
previous clinical studies using this immunostaining demonstrated
the clinical significance of M2 TAMs in NSCLC patients (20,24,25). The
findings of the present study demonstrated that the stromal M2 TAM
density in NSCLC was associated with tumor differentiation, CRP
level, tumor growth, invasive size, lymph node metastasis,
pathological stage and poor prognosis. In addition, the alveolar M2
TAM density was also associated with tumor differentiation, CRP
level, tumor status, invasive size, pathological stage and poor
prognosis. By contrast, the islet TAM density was significantly
lower compared with the stromal M2 TAM density, and no association
was observed between the islet M2 TAM and the abovementioned
biological and clinical factors.
First, TAMs originate from circulating blood cells,
such as monocytes. Chemotactic signals originating from tumor or
stromal cells in the TME could recruit monocytic precursors to the
tumor site. The present study demonstrated that the CRP level was
associated with both the stromal and alveolar M2 TAM density. A
previous clinical study also reported that a higher density of
CD163-positive macrophages was associated with elevated CRP levels
(32). These results suggest the
existence of crosstalk between cancer-related inflammation and M2
TAMs in TME (8). During tumor
progression, this crosstalk may generate more aggressive tumors. In
fact, previous clinical studies reported that an elevated CRP level
was a poor prognostic factor in NSCLC patients (33,34).
Next, the present study demonstrated that the
stromal M2 TAM density was associated with tumor proliferation and
invasive size, and that the alveolar M2 TAM density was also
associated with invasive size. M2 TAMs produce various
tumor-promoting cytokines, including VEGF, which affect tumor
growth and metastasis (8,21). In addition, invasive size was found
to be correlated with various prognostic pathological factors
(35), and it is an important factor
for TNM classification (26). To the
best of our knowledge, the present study was the first to
demonstrate that stromal M2 TAM density is correlated with tumor
proliferation and invasive size, which indicate a more aggressive
malignant potential.
Therefore, the present study revealed that the
stromal M2 TAM density is associated with lymph node metastasis,
pathological stage, reduced DFS and reduced OS. The alveolar M2 TAM
density was also associated with reduced DFS and reduced OS.
Previous clinical studies also reported that stromal M2 macrophages
are associated with poor prognosis in lung cancer patients
(20,25,36).
Former clinical studies using immunostaining for only CD68 also
demonstrated that the stromal macrophages, which were primarily M2
TAMs, were associated with a poor prognosis in NSCLC (15,18).
Although the prognostic significance of M2 TAMs was found only on
univariate analysis using the Cox regression model in the present
study, the statistical analyses regarding prognosis did not reach
statistical significance on multivariate analysis using the Cox
regression model. These results may be partly due to the relatively
small number of patients, which was a limitation of the present
study. Further clinical studies using a higher number of patients
are required.
Considering the results of the present study, during
NSCLC progression, TME may produce M2 TAMs, thereby promoting tumor
aggressiveness. M2 TAMs are predominantly located in the tumor
stroma, and may move into the alveolar space. The stromal M2 TAM
density is a potential marker for predicting malignant potential
and clinical outcome. Therefore, postoperative adjuvant
chemotherapy may be required for patients with stromal M2 TAM-high
tumors, even in the early stages. In addition, further
investigation on TAMs may enable the development of novel
treatments, such as TAM repolarization strategies using ‘M2-to-M1’
macrophage reprogramming molecules (8,21).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS, MF and CH designed the present study. RS, TH and
CH designed and performed the experiments. RS, HM and YO collected
the data. RS, HM, CH and YO analyzed and interpreted the data and
wrote the manuscript. All authors have read and approved the final
version of the manuscript for publication.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Ethics Committee of the Kitano Hospital (approval no. P181200300),
and written informed consent was obtained from each patient. The
research was conducted in compliance with the principles outlined
in the Declaration of Helsinki.
Patient consent for publication
Written informed consent for publication of patient
data/accompanying images was obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu WH, Yang JC, Mok TS and Loong HH:
Overview of current systemic management of EGFR-mutant NSCLC. Ann
Oncol. 29 (Suppl_1):i3–i9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan J, Fong T, Xia Z, Zhang J and Luo P:
The efficacy and safety of ALK inhibitors in the treatment of
ALK-positive non-small cell lung cancer: A network meta-analysis.
Cancer Med. 7:4993–5005. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantovani A, Marchesl F, Malesci A, Laghi
L and Allavena P: Tumor-associated macrophages as treatment targets
in oncololy. Nat Rev Clin Oncol. 14:399–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis CE and Pollard JW: Distinct role of
macrophages in different tumor microenvironments. Cancer Res.
66:605–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Astekar M, Metqud R, Sharma A and Soni A:
Hidden keys in stroma: Unlocking the tumor progression. J Oral
Macxilofac Pathol. 17:82–88. 2013. View Article : Google Scholar
|
|
11
|
Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C,
Lin F, Liao H, You Z and Liu L: Prognostic impact of
tumor-associated macrophage infiltration in non-small cell lung
cancer: A systemic review and meta-analysis. Oncotarget.
7:34217–34228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu P, Wu D, Zhao L, Huang L, Chen G, Shen
G, Huang J and Chai Y: Inverse role of distinct subsets and
distribution of macrophage in lung cancer prognosis: A
meta-analysis. Oncotarget. 7:40451–40460. 2016.PubMed/NCBI
|
|
13
|
Ong SM, Tan YC, Beretta O, Jiang D, Yeap
WH, Tai JJ, Wong WC, Yang H, Schwarz H, Lim KH, et al: Macrophages
in human colorectal cancer are pro-inflammatory and prime T cells
towards an anti-tumour type-1 inflammatory response. Eur J Immunol.
42:89–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Medrek C, Ponten F, Jirstrom K and
Leandersson K: The presence of tumor associated macrophages in
tumor stroma as a prognostic marker for breast cancer patients. BMC
Cancer. 12:3062012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Welsh TJ, Green RH, Richardson D, Waller
DA, O'Byrne KJ and Bradding P: Macrophage and mast-cell invasion of
tumor cell islets confers a marked survival advantage in
non-small-cell lung cancer. J Clin Oncol. 23:8959–8967. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim DW, Min HS, Lee KH, Kim YJ, Oh DY,
Jeon YK, Lee SH, Im SA, Chung DH, Kim YT, et al: High tumour islet
macrophage infiltration correlates with improved patient survival
but not with EGFR mutations, gene copy number or protein expression
in resected non-small cell lung cancer. Br J Cancer. 98:1118–1124.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawai O, Ishii G, Kubota K, Murata Y,
Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al:
Predominant infiltration of macrophages and CD8(+) T cells in
cancer nests is a significant predictor of survival in stage IV
nonsmall cell lung cancer. Cancer. 113:1387–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S,
Ma J, Ma L and You Z: The number and microlocalization of
tumor-associated immune cells are associated with patient's
survival time in non-small cell lung cancer. BMC Cancer.
10:2202010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng PH, Yu CT, Wu CY, Lee MJ, Lee WH,
Wang LS, Lin SM, Fu JF, Lee KY and Yen TH: Tumor-associated
macrophages in stage IIIA pN2 non-small cell lung cancer after
neoadjuvant chemotherapy and surgery. Am J Trans Res. 6:593–603.
2014.
|
|
20
|
Jackute J, Zemaitis M, Pranys D,
Sitkauskiene B, Miliauskas S, Vaitkiene S and Sakalauskas R:
Distribution of M1 and M2 macrophages in tumor islets and stroma in
relation to prognosis of non-small cell lung cancer. BMC Immunol.
19:32018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Dalen FJ, van Stevendaal MHME,
Fennemann FL, Verdoes M and Ilina O: Molecular repolarisation of
tumour-associated macrophages. Molecules. 24:E92018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT,
Kuo ML, Lee YC and Yang PC: Up-regulation of tumor interleukin-8
expression by infiltrating macrophages: Its correlation with tumor
angiogenesis and patient survival in non-small cell lung cancer.
Clin Cancer Res. 9:729–737. 2003.PubMed/NCBI
|
|
23
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma J, Liu L, Che G, Yu N, Dai F and You Z:
The M1 form of tumor-associated macrophages in non-small cell lung
cancer is positively associated with survival time. BMC Cancer.
10:1122010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung FT, Lee KY, Wang CW, Heh CC, Chan
YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH, et al:
Tumor-associated macrophages correlate with response to epidermal
growth factor receptor-tyrosine kinase inhibitors in advanced
non-small cell lung cancer. Int J Cancer. 131:E227–E235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amin MB, Edge S and Greene F: AJCC Cancer
Staging Manual8th. Springer; New York, NY, USA: 2017
|
|
27
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: WHO Classification of Tumours of the Lung,
Pleura, Thymus and Heart4th. International Agency for Research on
Cancer; Lyon, France: 2015
|
|
28
|
Li Z, Maeda D, Yoshida M, Umakoshi M,
Nanjo H, Shiraishi K, Saito M, Kohno T, Konno H, Saito H, et al:
The intratumoral distribution influences the prognostic impact of
CD68- and CD204-positive macrophages in non-small cell lung cancer.
Lung Cancer. 123:127–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scagliotti GV, Micela M, Gubetta L,
Leonardo E, Cappia S, Borasio P and Pozzi E: Prognostic
significance of Ki67 labelling in resected non small cell lung
cancer. Eur J Cancer 29A. 363–365. 1993. View Article : Google Scholar
|
|
30
|
Siemes C, Visser LE, Coebergh JW, Splinter
TA, Witteman JC, Uitterlinden AG, Hofman A, Pols HA and Stricker
BH: C-reactive protein levels, variation in the C-reactive protein
gene, and cancer risk: The Rotterdam Study. J Clin Oncol.
24:5216–5222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Allin KH, Bojesen SE and Nordestgaard BG:
Baseline C-reactive protein is associated with incident cancer and
survival in patients with cancer. J Clin Oncol. 27:2217–2224. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carus A, Ladekarl M, Hager H, Pilegaard H,
Nielsen PS and Donskov F: Tumor-associated neutrophils and
macrophages in non-small cell lung cancer: No immediate impact on
patient outcome. Lung Cancer. 81:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lopez-Pastorini A, Riedel R, Koryllos A,
Beckers F, Ludwig C and Stoelben E: The impact of preoperative
elevated serum C-reactive protein on postoperative morbidity and
mortality after anatomic resection of lung cancer. Lung Cancer.
109:68–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leuzzi G, Galeone C, Gisabella M, Duranti
L, Taverna F, Suatoni P, Morelli D and Pastorino U: Baseline
C-reactive protein level predicts survival of early-stage lung
cancer: Evidence from a systematic review and meta-analysis.
Tumori. 102:441–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kameda K, Eguchi T, Lu S, Qu Y, Tan KS,
Kadota K, Adusumilli PS and Travis WD: Implications of the eighth
edition of the TNM proposal: Invasive versus total tumor size for
the T descriptor in pathologic stage I–IIA lung adenocarcinoma. J
Thorac Oncol. 13:1919–1929. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohtaki Y, Ishii G, Nagai K, Ashimine S,
Kuwata T, Hishida T, Nishimura M, Yoshida J, Takeyoshi I and Ochiai
A: Stromal macrophage expressing CD204 is associated with tumor
aggressiveness in lung adenocarcinoma. J Thorac Oncol. 5:1507–1515.
2010. View Article : Google Scholar : PubMed/NCBI
|