Introduction
Neonatal asphyxia refers to an anomaly in the
respiration of neonates, caused by various factors, such as
inadequate oxygen levels in the mother's blood or lowered
respiration rates, before, during or after birth, that can damage
multiple systems, and involves severe sequelae or can even cause
mortality (1). Neonatal asphyxia
usually arises as hypoxia, ischemia and abnormal metabolism;
respiratory failure blocks alveolar gas exchange, which causes
hypoxia (2). In neonates, the
systemic and organ damage severity is proportionately associated
with the severity of hypoxia (3).
Out of all neonates, ~4,000,000 succumb to neonatal asphyxia,
accounting for nearly 23% of cases of mortality in developing
countries in 2013 (4,5). A previous study demonstrated that
neonatal asphyxia is a major cause of mortality within 1 week after
birth, and a factor contributing to perinatal mortality, neurologic
handicap and developmental dysfunction (6). The Apgar scale is a convenient and
widely accepted method that is frequently used to evaluate neonatal
asphyxia; a 1-min Apgar score <7 suggests mild asphyxia, while a
score <3 suggests severe asphyxia (7,8).
Perinatal hypoxia and infection may result in potent
postnatal stress responses, impacting the neuroendocrine and
gastrointestinal systems (9,10). The decrease in gastrointestinal
permeability and increased intestinal inflammation further
contribute to systemic organ dysfunction, since inflammatory
factors pass through the intestinal mucosa (11). Probiotics are characterized by their
ability to sustain the microecological balance within the
intestines, and regulate the neuroendocrine functions in stress
responses. They can protect the intestinal mucosa and ameliorate
intestinal barrier function by facilitating cell proliferation and
migration, which prevents cell apoptosis and enhance sprotein
synthesis (12). A study
demonstrated that probiotics can serve as an auxiliary treatment
for stress-induced intestinal responses in neonatal mice, with
significant improvements noted in gastrointestinal function
(13).
Since, to the best of our knowledge, no studies have
reported the stress response and intestinal permeability of term
neonates with low Apgar scores, the present study aimed to
elucidate the effect of probiotics on the stress responses and
intestinal permeability of term neonates with low Apgar scores to
provide a theoretical basis for clinical treatment.
Materials and methods
General materials
The present study retrospectively analyzed the
clinical data of 78 term neonates (42 males and 36 females) who
were admitted to the Department of Neonatology at The Second
Hospital of Lanzhou University (Lanzhou, China) for treatment
between March 2017 and March 2018. Neonates born to mothers with a
history of smoking or drug abuse, metabolic depression,
hypertension during pregnancy, and acute infectious diseases were
excluded from the present study. Additionally, all neonates with
congenital heart disease, early-stage infection, aspiration
pneumonia, gastrointestinal deformity or bleeding were excluded
from the present study. Neonates were born at 37–41 weeks'
gestation, weighed 2–4 kg and had Apgar scores of <7 points
(14). In the control group (n=38),
total parenteral nutrition and comprehensive treatment
(anti-infection therapy) were provided; in the observation group
(n=40), Lactobacillus Complex Capsules were administered to
the neonates in addition to the control group treatment. Due to
ethical reasons, a placebo only treatment was not approved. The
present study was approved by the Ethics Committee of The Second
Hospital of Lanzhou University, and the family of each neonate
provided written informed consent.
Treatment methods
Following resuscitation, neonates in the control
group received total parenteral nutrition and anti-infection
therapy, in addition to regular nursing, which is standard
treatment. Breast feeding was prioritized, but formula milk (Mead
Johnson & Company, LLC) was considered, and the milk intake was
increased according to the condition of the neonate. During the
treatment period, a number of neonates were infected and then
received anti-infection therapy. The anti-infection therapy regimen
was: Neonates were given ampicillin (100 mg/kg/day) for 3 days.
Neonates that had a positive penicillin skin test were given
cefazolin (50 mg/kg/day) but not ampicillin for 3 days. On the
third day, neonates with normal peripheral blood C-reactive protein
(CRP) levels stopped antibiotics treatment. When the intake was
≥120 ml/kg/day, the feeds were gradually transitioned to total
parenteral nutrition. In addition to the treatment provided in the
control group, neonates in the observation group were given
probiotics treatment and were administered with
Bifidobacterium triple live capsules (Shanghai Shanyao Xinyi
Pharmaceutical Co., Ltd.; Guoyao Zhunzi approval no. S10950032).
Each capsule contained 210 mg powder, and the number of live
bacteria was >1.0×107 CFU. The capsule was dissolved
in warm water (<40°C), and half a capsule was administered
twice/day for 7 consecutive days.
Detection of indices of stress
response and intestinal permeability
At 1 day before treatment and 7 days after
treatment, 2 ml of venous blood was drawn from the neonates prior
to the intravenous infusion and breast feeding, and the serum was
isolated via centrifugation at 1,500 × g for 5 min at 25–30°C for
later use. Corticotropin-releasing factor (CRF) levels were
determined using ELISA (cat. no. DECO0324; Beijing Zhongke Quality
Inspection Biotechnology Co., Ltd.), cortisol using a
radioimmunoprecipitation assay (cat. no. 2114-500; Biovision,
Inc.), D-lactate and diamine oxidase (DAO) using an ultraviolet
spectrometer (UVS-99; Avans Biotechnology Inc.), procalcitonin
(PCT) using ECL (cat. no. 1705060; Bio-Rad Laboratories, Inc.), and
CRP using a protein analyzer (cat. no. HC01001319; Gerhardt GmbH).
All procedures were conducted in strict accordance with the kit
instructions, and each experiment was conducted in triplicate and
the results were averaged. Additionally, the mean daily milk intake
during 7 days of treatment, length of hospital stay (LOS) and total
parenteral nutrition duration were recorded.
Radioimmunoprecipitation assay was performed as
follows: A total of 10 µl cortisol blank serum and 10 µl serum
samples were added to two coating tubes respectively. Each tube was
added with 1.0 ml labelling buffer solution and mixed by low speed
vortex at 37°C for 46 min, following which excess liquid was
removed. 125Iodine-labeled cortisol in the remaining
liquid was then counted in the γ-counter for 1 min and cortisol
concentration was calculated using a standard cortisol curve at 1,
3, 10, 25 and 60 µg/dl with repetitions of the steps described
above. ECL was performed using a fully automated chemiluminescence
immunoassay analyzer (SMART 300; Chongqing Keysmile Biotechnology
Co., Ltd.)
Statistical analysis
SPSS software (version 17.0; SPSS, Inc.) was
utilized for the data analyses. Enumeration data were compared
using a χ2 test. Measurement data are presented as the
means ± SD and were compared using a Kolmogorov-Smirnov test. All
data was normally distributed and analyzed using at-test. The
intragroup comparisons before and after treatment were conducted
with a paired t-test. Repeated measures ANOVA with Bonferroni post
hoc test was used for multiple comparisons. All data were
calculated from three experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of general data
There were no significant differences identified in
gestational age, sex, weight, Apgar score, type of birth and
intrauterine hypoxia history between the observation and control
groups (P>0.05; Table I).
 | Table I.Comparison of the general data of the
observation and control groups. |
Table I.
Comparison of the general data of the
observation and control groups.
| Factors | Observation group
(n=40), n (%) | Control group (n=38),
n (%) | t or
χ2 | P-value |
|---|
| Sex |
|
| 1.251 | 0.364 |
| Male | 24 (60.00) | 18 (47.37) |
|
|
|
Female | 16 (40.00) | 20 (52.63) |
|
|
|
Gestational age (weeks) | 38.96±1.02 | 38.73±0.98 | 1.015 | 0.314 |
| Height
(cm) | 48.63±3.56 | 49.04±2.97 | 0.551 | 0.583 |
| Weight
(kg) | 3.86±0.38 | 3.77±0.34 | 1.100 | 0.275 |
| 1-min
Apgar score | 5.26±0.81 | 5.41±0.89 | 0.779 | 0.438 |
| 5-min
Apgar score | 6.41±1.26 | 6.58±1.12 | 0.629 | 0.532 |
| Type of birth |
|
| 0.425 | 0.607 |
| Natural
labor | 9 (22.50) | 11 (28.95) |
|
|
| Cesarean
section | 31 (77.50) | 27 (71.05) |
|
|
| Intrauterine
hypoxia |
|
| 0.167 | 0.815 |
| Yes | 26 (65.00) | 23 (60.53) |
|
|
| No | 14 (35.00) | 15 (39.47) |
|
|
Stress response of neonates before vs.
after treatment
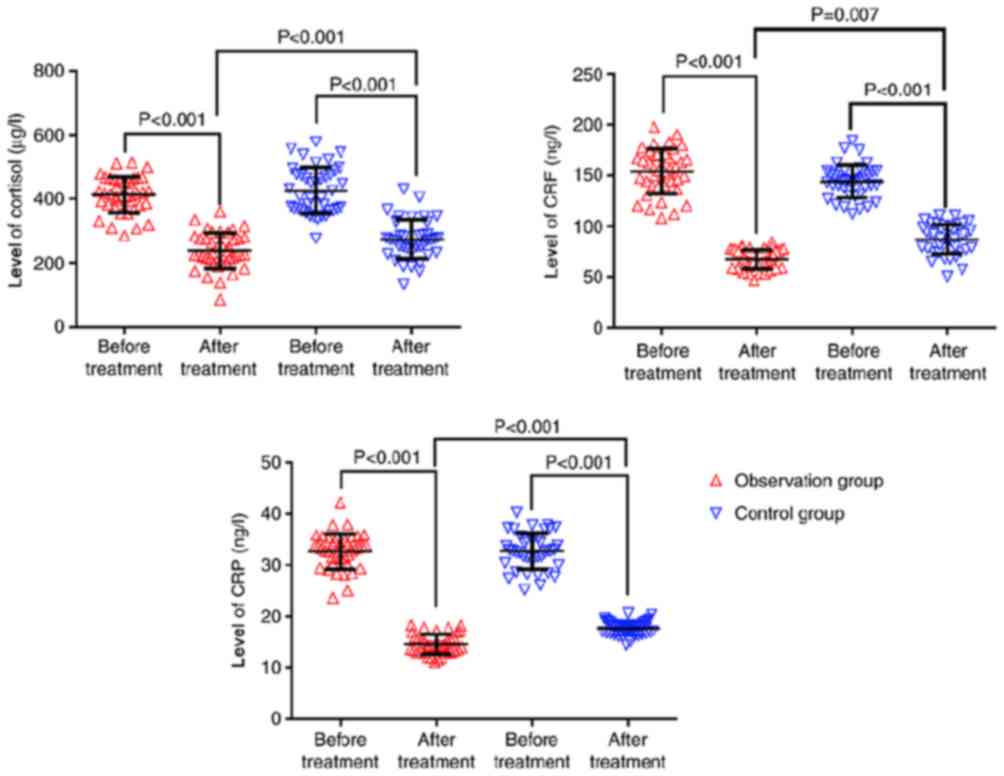
The cortisol, CRF and CRP levels were significantly
lower after treatment compared with before treatment in the two
groups (P<0.001). Before treatment, there were no significant
intergroup differences (P>0.05), but after treatment, the levels
in the observation group were all significantly lower than those in
the control group (P<0.05; Fig.
1; Table II).
 | Table II.Comparison of stress responses of
neonates before vs. after treatment in the observation and control
groups. |
Table II.
Comparison of stress responses of
neonates before vs. after treatment in the observation and control
groups.
| Group | Number (n) | Time point | Cortisol (µg/l) | CRF (ng/l) | CRP (ng/l) |
|---|
| Observation | 40 | Before treatment | 147.56±21.05 | 421.65±63.75 | 32.53±3.16 |
|
|
| After treatment |
68.63±11.58a |
235.82±42.32a |
14.64±1.65a |
|
|
| t | 20.78 | 15.36 | 31.74 |
|
|
| P-value | <0.001 | <0.001 | <0.001 |
| Control | 38 | Before treatment | 144.67±19.68 | 416.58±76.15 | 31.78±3.56 |
|
|
| After
treatment | 84.62±14.53 | 265.37±51.26 | 17.35±1.52 |
|
|
| t | 15.13 | 10.15 | 22.98 |
|
|
| P-value | <0.001 | <0.001 | <0.001 |
Intestinal permeability of neonates
before vs. after treatment
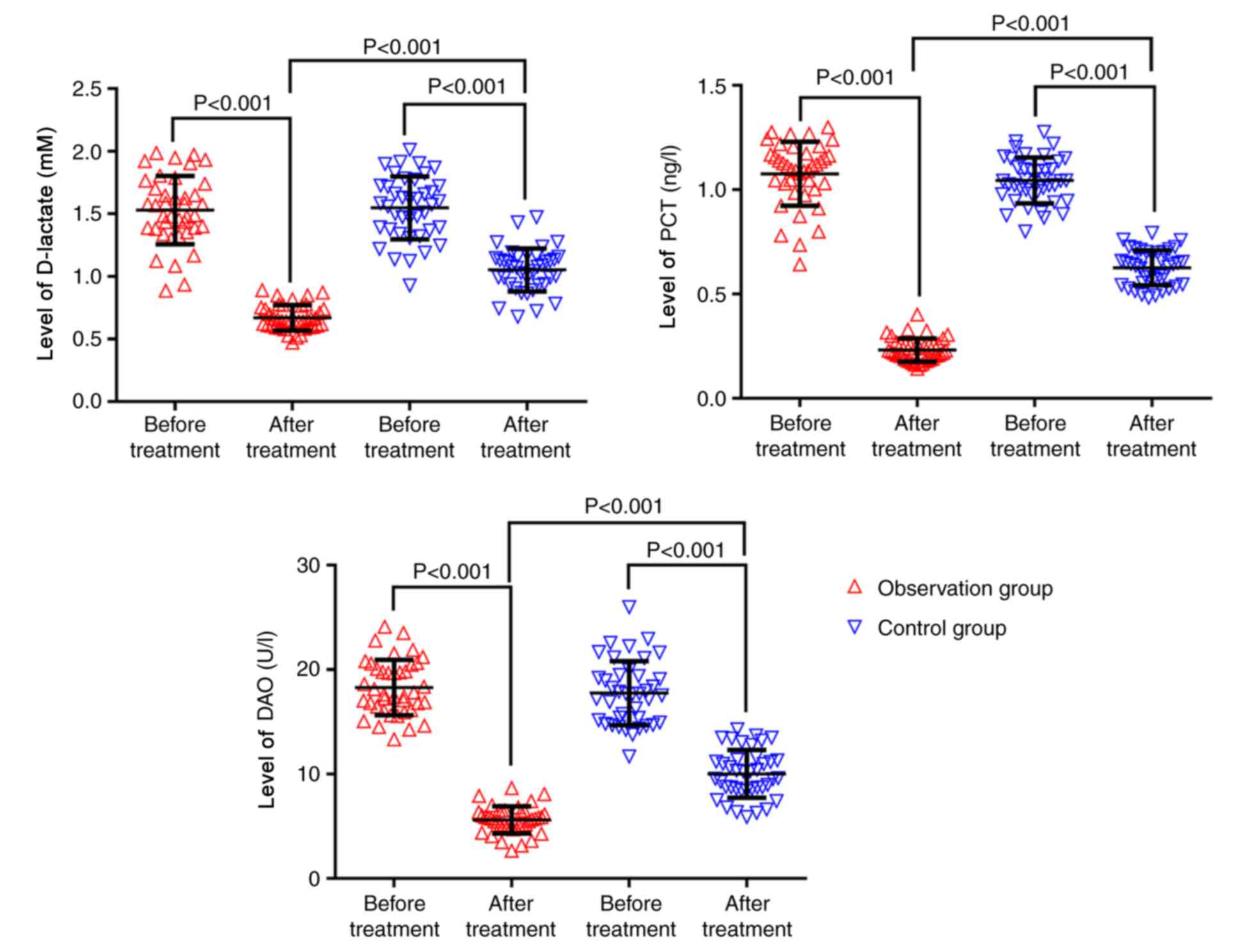
The D-lactate, PCT and DAO levels of the observation
and control groups before treatment were 1.59±0.28 and 1.57±0.26
mM, 1.08±0.15 and 1.05±0.13 ng/l, and 18.62±3.14 and 18.12±2.98
U/l, respectively. The D-lactate, PCT and DAO levels of the
observation and control groups after treatment were 0.68±0.12 and
1.09±0.18 mM, 0.24±0.05 and 0.62±0.08 ng/l, and 5.63±1.35 and
9.82±2.46 U/l, respectively. The D-lactate, PCT and DAO levels were
significantly lower after treatment compared with before treatment
in the two groups (P<0.001). Before treatment, there were no
significant intergroup differences (P>0.05); however, after
treatment, the levels in the observation group were all
significantly lower than those in the control group (P<0.001;
Fig. 2; Table III).
 | Table III.Comparison of intestinal permeability
of neonates before vs. after treatment in the observation and
control groups. |
Table III.
Comparison of intestinal permeability
of neonates before vs. after treatment in the observation and
control groups.
| Group | Number (n) | Time point | D-lactate (mM) | PCT (ng/l) | DAO (U/l) |
|---|
| Observation | 40 | Before
treatment | 1.59±0.28 | 1.08±0.15 | 18.62±3.14 |
|
|
| After
treatment |
0.68±0.12a |
0.24±0.05a |
5.63±1.35a |
|
|
| t | 18.89 | 33.60 | 24.04 |
|
|
| P-value | <0.001 | <0.001 | <0.001 |
| Control | 38 | Before
treatment | 1.57±0.26 | 1.05±0.13 | 18.12±2.98 |
|
|
| After
treatment | 1.09±0.18 | 0.62±0.08 | 9.82±2.46 |
|
|
| t | 9.433 | 17.48 | 13.24 |
|
|
| P-value | <0.001 | <0.001 | <0.001 |
Gastrointestinal function recovery of
neonates
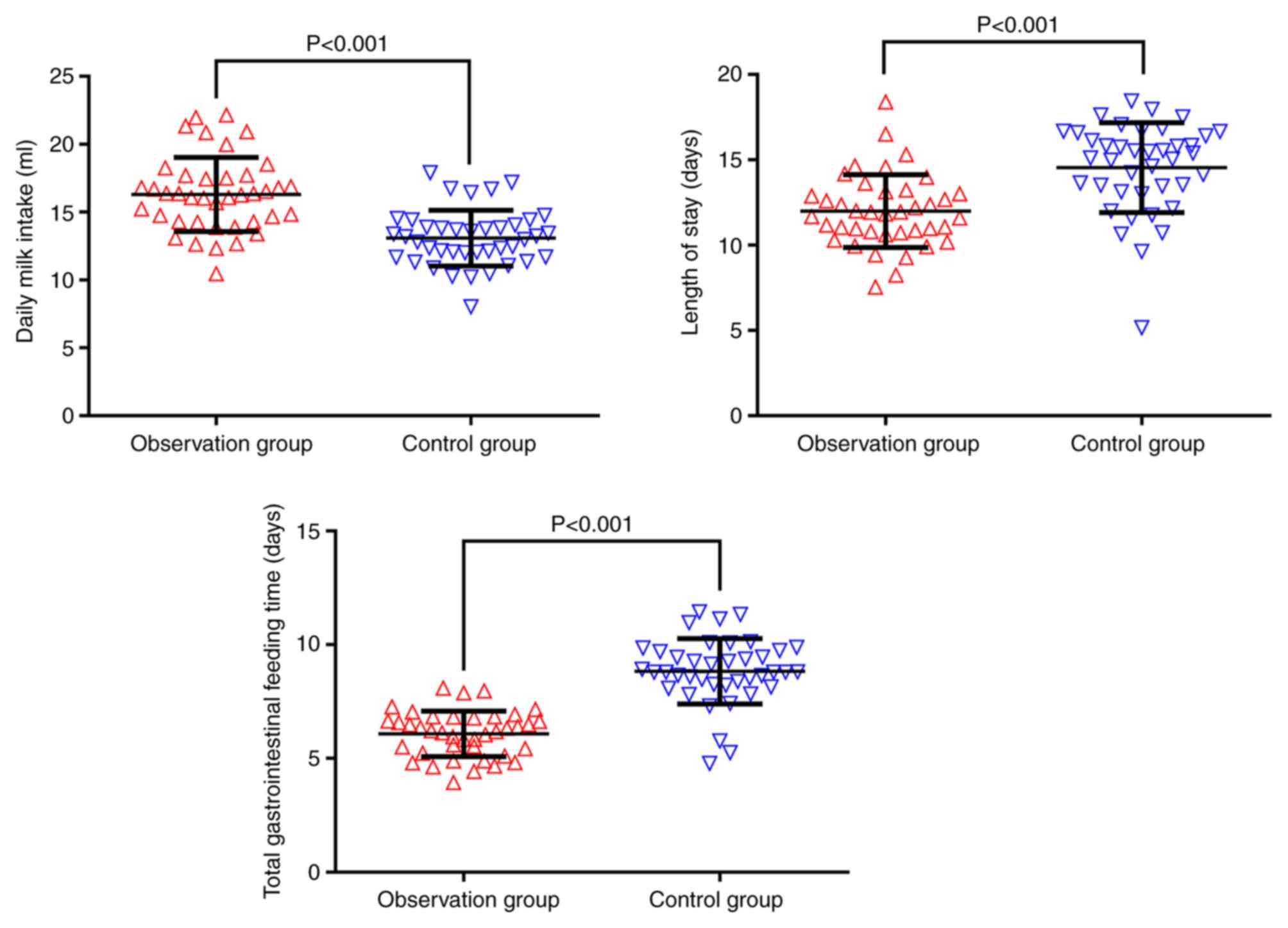
The mean daily milk intake was significantly higher
in the observation group (16.57±2.58 ml) than in the control group
(13.26±1.87 ml), while the mean LOS and total parenteral nutrition
duration of the observation group (12.31±2.02 and 6.21±1.26 days)
were significantly shorter than those of the control group
(14.86±2.58 and 8.86±1.78 days; P<0.001; Fig. 3; Table
IV).
 | Table IV.Comparison of gastrointestinal
function recovery of neonates before vs. after treatment in the
observation and control groups. |
Table IV.
Comparison of gastrointestinal
function recovery of neonates before vs. after treatment in the
observation and control groups.
| Group | Number (n) | Daily milk intake
(ml) | Length of stay in
hospital (days) | Total
gastrointestinal feeding time (days) |
|---|
| Observation | 40 | 16.57±2.58 | 12.31±2.02 | 6.21±1.26 |
| Control | 38 | 13.26±1.87 | 14.86±2.58 | 8.86±1.78 |
| T |
| 6.459 | 4.874 | 7.619 |
| P-value |
| <0.001 | <0.001 | <0.001 |
Infections during treatment
In the observation and control groups, there were 4
cases (10.0%) and 11 cases (28.95%) with infections during the
treatment. The occurrence of infection in the observation group was
significantly lower than that in the control group (P<0.05). In
the observation group, one case of umbilical inflammation and three
cases of bacterial pneumonia occurred. In the control group, two
cases of umbilical inflammation, one case of scleredema, one case
of impetigo and seven cases of bacterial pneumonia occurred
(Table V). No sepsis and other
infectious diseases, including bacterial meningitis, occurred in
either group. All infections were controlled after treatment with
the anti-infective regimen.
 | Table V.Comparison of the infections in the
observation and control groups. |
Table V.
Comparison of the infections in the
observation and control groups.
| Group | Number (n) | Umbilical
inflammation (n) | Bacterial pneumonia
(n) | Scleredema (n) | Impetigo (n) | Total infection, n
(%) |
|---|
| Observation | 40 | 1 | 3 | 0 | 0 | 4 (10.00) |
| Control | 38 | 2 | 7 | 1 | 1 | 11 (28.95) |
| t |
|
|
|
|
| 4.504 |
| P-value |
|
|
|
|
| 0.034 |
Discussion
Neonates with low Apgar scores are more susceptible
to hypoxia and ischemia, which cause organ damage and functional
anomalies (15). Due to the
vulnerability of the neonatal gastrointestinal tract mucosa this
can result in abdominal distension, milk regurgitation or even
necrotic colitis (16). The neonatal
gastrointestinal tract is developing, which makes it more
vulnerable to bacterial invasion via the gut due to decreased
amounts of bile acids and gastric acid, particularly in the
presence of mucosal damage (17). An
animal experiment revealed that probiotics can regulate intestinal
barrier function long after the stress responses (18). Additionally, a clinical trial
demonstrated that probiotics can improve epithelial barrier
function (19). Therefore, it is
rational to use probiotics in the treatment of stress responses and
intestinal permeability in cases of neonatal asphyxia.
The severity of neonatal asphyxia is associated with
the circulating cortisol level (20). CRF, a neuroendocrine peptide composed
of several amino acids, can regulate stress responses in humans and
animals (21). In neonatal blood,
CRP levels are regulated by multiple factors, including neonatal
asphyxia, intracranial infection, premature rupture of the fetal
membranes and marked alterations in humoral immune responses after
birth (22). Therefore, in the
present study, cortisol, CRF and CRP levels served as indicators of
the degree of the stress response.
Damage to the intestinal mucosa also affects the
microecological balance in the intestinal bacterial community due
to the production of large amounts of D-lactate which can be
rapidly delivered into the blood circulation due to increased
intestinal permeability (23).
Additionally, damage to the intestinal mucosa and an increase in
intestinal permeability enhance DAO activity (24). A study on PCT that mainly focused on
acute infections suggested that PCT levels are physiologically
increased following premature birth, primarily due to neonatal
asphyxia (25). Thus, D-lactate, PCT
and DAO were selected as indicators of intestinal permeability in
the present study.
In the present study, the levels of cortisol, CRF,
CRP, D-lactate, PCT and DAO in the observation and control groups
were significantly lower after treatment compared with before
treatment (P<0.001). Prior to treatment, intergroup comparisons
exhibited no statistically significant differences in the levels of
cortisol, CRF, CRP, D-lactate, PCT and DAO (P>0.05), whereas
after treatment, these levels in the observation group were all
significantly lower than those in the control group (P<0.05).
These results suggested that treatment in combination with
probiotics can mitigate the stress response and intestinal
permeability of term neonates with low Apgar scores.
Among the few studies reporting on asphyxia in term
neonates, some reported that probiotics can sustain the balance of
intestinal bacteria and promote maturation of the gastrointestinal
function, while reducing the incidence of feeding intolerance in
premature neonates; thus, probiotics have been widely used in the
treatment of gastrointestinal diseases in infants (26,27). In
the present study, the daily mean milk intake was significantly
higher in the observation group (16.57±2.58 ml) than in the control
group (13.26±1.87 ml; P<0.001), which suggested that probiotics
can facilitate the recovery of gastrointestinal function of term
neonates with low Apgar scores. Probiotics treatment could reduce
the stress response and accelerated the recovery of
gastrointestinal function. Probiotics interact with other anaerobic
bacteria to form a natural barrier, which can efficiently reduce or
even block the contact of intestinal mucosa with pathogenic
microorganisms (28). Additionally,
probiotics can induce the immune response, enhance cellular
immunity and mitigate stress responses (29,30).
In summary, the administration of probiotics in
combination with standard treatment could mitigate the stress
response and decreased the intestinal permeability of term neonates
with low Apgar scores to protect and facilitate gastrointestinal
function recovery. Therefore, the results of the present study
provided a theoretical basis for clinical treatment and contribute
to the wide application of probiotics in clinical practice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW conceived and designed the study and interpreted
the results of the experiments. JZ and YH contributed to the design
of the study and the interpretation of experimental results. JC
performed experiments, analyzed data, prepared figures and drafted
the manuscript. JW and YH approved final version of manuscript. YH
edited and revised manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Second Hospital of Lanzhou University, and the family of each
neonate provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koyama S, Kaga K, Sakata H, Iino Y and
Kodera K: Pathological findings in the temporal bone of newborn
infants with neonatal asphyxia. Acta Otolaryngol. 125:1028–1032.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nisar M, Sabir H and Ahad Z: Etiological
causes of respiratory distress syndrome in full term neonates
presenting within 24 hours of life. Pakistan Journal of Medical and
Health Sciences. 11:460–462. 2017.
|
|
3
|
Chen CC, Li LZ, Lu QS and Lang YL: Color
Doppler ultrasound evaluation of asphyxial neonatal left
ventricular function and its correlation with target organ damage.
J Hainan Med Univ. 23:147–150. 2017.(In Chinese).
|
|
4
|
Shankaran S, Laptook AR, Pappas A,
McDonald SA, Das A, Tyson JE, Poindexter BB, Schibler K, Bell EF,
Heyne RJ, et al: Effect of depth and duration of cooling on deaths
in the NICU among neonates with hypoxic ischemic encephalopathy: A
randomized clinical trial. JAMA. 312:2629–2639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seikku L, Gissler M, Andersson S, Rahkonen
P, Stefanovic V, Tikkanen M, Paavonen J and Rahkonen L: Asphyxia,
neurologic morbidity, and perinatal mortality in early-term and
postterm birth. Pediatrics. 137:e201533342016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roro EM, Sisay MM and Sibley LM:
Determinants of perinatal mortality among cohorts of pregnant women
in three districts of north showa zone, oromia region, ethiopia:
Community based nested case control study. BMC Public Health.
18:8882018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalili H, Nili F, Sheikh M, Hardani AK,
Shariat M and Nayeri F: Comparison of the four proposed apgar
scoring systems in the assessment of birth asphyxia and adverse
early neurologic outcomes. PLoS One. 10:e01221162015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cnattingius S, Norman M, Granath F,
Petersson G, Stephansson O and Frisell T: Apgar score components at
5 minutes: Risks and prediction of neonatal mortality. Paediatric
Perinat Epidemiol. 31:328–337. 2017. View Article : Google Scholar
|
|
9
|
Su Q, Zhang H, Zhang Y, Zhang H, Ding D,
Zeng J, Zhu Z and Li H: Maternal stress in gestation: Birth
outcomes and stress-related hormone response of the neonates.
Pediatr Neonatol. 56:376–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao A, Jianling L, Xia L, Yao L, Wang Y
and Yang H: The influences of DAO and SNGF in the treatment of
hypothermia for neonates after birth asphyxia. Chin J Primary Med
Pharm. 23:387–390. 2016.
|
|
11
|
Chalak LF: Inflammatory biomarkers of
birth asphyxia. Clin Perinatol. 43:501–510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
AlFaleh K and Anabrees J: Probiotics for
prevention of necrotizing enterocolitis in preterm infants. Evid
Based Child Health. 9:584–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nuñez IN, Perdigón G and Galdeano CM:
Chapter 6 - Immune System in Undernourished Host: Probiotics as
Strategy to Improve ImmunityNutrients in dairy and their
implications on health and disease. Watson RR, Collier RJ and
Preedy VR: Elsevier; Amsterdam: pp. 77–86. 2018
|
|
14
|
Persson M, Razaz N, Tedroff K, Joseph KS
and Cnattingius S: Five and 10 minute apgar scores and risks of
cerebral palsy and epilepsy: Population based cohort study in
Sweden. BMJ. 360:k2072018. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laptook AR, Shankaran S, Ambalavanan N,
Carlo WA, McDonald SA, Higgins RD and Das A; Hypothermia
Subcommittee of the NICHD Neonatal Research Network, : Outcome of
term infants using apgar scores at 10 minutes following
hypoxic-ischemic encephalopathy. Pediatrics. 124:1619–1626. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dupont C, Kalach N and Rousseau V:
Gastrointestinal Problems of the NewbornTextbook of Pediatric
Gastroenterology, Hepatology and Nutrition. Guandalini S, Dhawan A
and Branski D: Springer; Basel: pp. 41–52. 2016, View Article : Google Scholar
|
|
17
|
Neu J: Preterm infant nutrition, gut
bacteria, and necrotizing enterocolitis. Curr Opin Clin Nutr Metab
Care. 18:285–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Halpern MD and Denning PW: The role of
intestinal epithelial barrier function in the development of NEC.
Tissue Barriers. 3:e10007072015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alvarez CS, Badia J, Bosch M, Giménez R
and Baldomà L: Outer membrane vesicles and soluble factors released
by probiotic escherichia coli nissle 1917 and commensal ECOR63
enhance barrier function by regulating expression of tight junction
proteins in intestinal epithelial cells. Front Microbiol.
7:19812016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kovacs K, Szakmar E, Meder U, Cseko A,
Szabo AJ, Szabo M and Jermendy A: Serum cortisol levels in
asphyxiated infants with hypotension. Early Hum Dev. 120:40–45.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu RJ, Ota KT, Dutheil S, Duman RS and
Aghajanian GK: Ketamine strengthens CRF-activated amygdala inputs
to basal dendrites in mPFC layer V pyramidal cells in the prelimbic
but not infralimbic subregion, a key suppressor of stress
responses. Neuropsychopharmacology. 40:2066–2075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shrivastava AK, Singh HV, Raizada A and
Singh SK: C-reactive protein, inflammation and coronary heart
disease. Egypt Heart J. 67:89–97. 2015. View Article : Google Scholar
|
|
23
|
Ficek J, Wyskida K, Ficek R, Wajda J,
Klein D, Witkowicz J, Rotkegel S, Spiechowicz-Zatoń U,
Kocemba-Dyczek J, Ciepał J, et al: Relationship between plasma
levels of zonulin, bacterial lipopolysaccharides, D-lactate and
markers of inflammation in haemodialysis patients. Inter Urol
Nephrol. 49:717–725. 2017. View Article : Google Scholar
|
|
24
|
Meng Y, Zhang Y, Liu M, Huang YK, Zhang J,
Yao Q, Ling Zhao Y and Jing Xiong J: Evaluating intestinal
permeability by measuring plasma endotoxin and diamine oxidase in
children with acute lymphoblastic leukemia treated with high-dose
methotrexate. Anticancer Agents Med Chem. 16:387–392. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ochi F, Higaki T, Ohta M, Yamauchi T,
Tezuka M, Chisaka T, Moritani T, Tauchi H and Ishii E:
Procalcitonin as a marker of respiratory disorder in neonates.
Pediatr Int. 57:263–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alderliesten T, de Vries LS, Staats L, van
Haastert IC, Weeke L, Benders MJ, Koopman-Esseboom C and
Groenendaal F: MRI and spectroscopy in (near) term neonates with
perinatal asphyxia and therapeutic hypothermia. Arch Dis Child
Fetal Neonatal Ed. 102:F147–F152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amara AA and Shibl A: Role of probiotics
in health improvement, infection control and disease treatment and
management. Saudi Pharm J. 23:107–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Servin AL and Coconnier MH: Adhesion of
probiotic strains to the intestinal mucosa and interaction with
pathogens. Best Pract Res Clin Gastroenterol. 17:741–754. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dolpady J, Sorini C, Di Pietro C, Cosorich
I, Ferrarese R, Saita D, Clementi M, Canducci F and Falcone M: Oral
probiotic VSL#3 prevents autoimmune diabetes by modulating
microbiota and promoting indoleamine 2,3-Dioxygenase-Enriched
tolerogenic intestinal environment. J Diabetes Res.
2016:75694312016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dawood MAO, Koshio S, Ishikawa M,
El-Sabagh M, Esteban MA and Zaineldin AI: Probiotics as an
environment-friendly approach to enhance red sea bream, pagrus
major growth, immune response and oxidative status. Fish ShellfiSh
Immunol. 57:170–178. 2016. View Article : Google Scholar : PubMed/NCBI
|