Introduction
Adeno-associated viruses (AAV) are small (a diameter
of approximately 25 nm), non-encapsulated, icosahedral viruses.
They are classified as members of the Parvoviridae family,
the Dependovirus genus. AAV can replicate only in the
presence of helper viruses, such as adenovirus, herpes simplex
virus and human papilloma virus (1).
The contribution of genotoxic factors in the activation of AAV
replication is also indicated (2).
The AAV genome is single-stranded DNA (approximately 4.7 kb)
consisting mainly of two reading frames: the rep and cap genes, and
ITR flanking sequences. The expression cassette in recombinant
vectors, containing the promoter and the transgene, is cloned in
place of deleted rep and cap genes, between 145 nucleotides ITR
sequences (1). Due to the
non-pathogenic nature and occurrence of serotypes with defined
organ tropism, recombinant AAV vectors are increasingly used in
gene therapy trials (3). There
already are registered AAV-based drugs (3). The safety of rAAV vectors as well as
their presence in medicine result in a number of studies revealing
critical points in the pathway of gene transfer and intracellular
events involving rAAV (1,4). The role of the miRNA signature
(5) and the expression of AAV
membrane receptors (6–8) in the AAV cellular transmission are in
the course of documentation. Discovering cellular mechanisms of
rAAV transduction helps to understand the AAV biology and makes it
possible to design new vectors-synthetic AAV mosaic serotypes
(9), vectors with dsDNA (10), AAV chemo-conjugates (11). There is research indicating the
possibilities of optimizing the efficiency of rAAV transduction by
various physicochemical treatments. The increase of rAAV
transduction efficiency is observed as a result of hyperthermia
(12). Also, the use of proteasome
inhibitors and the degradation of proteins associated with
endoplasmic reticulum induce the same effect (13,14). The
possibility of physicochemical manipulation of transduction
efficiency significantly increases the use of bio-safe AAV vectors
in gene therapy.
The normal temperature of the human body, around
37°C, is a condition for maintaining homeostasis and is necessary
for the course of physiological processes. Thermoregulation is
crucial in the context of maintaining the continuity of human life,
and it is based on many well-defined and complex physiological
processes controlled by the function of the thermoregulation
center, the vasomotor system and the skin. Incorrect temperature
fluctuations, which go beyond the range of the menstrual cycle or
the aging of the body, cause changes in the functioning of relevant
cellular biomolecules, including protein denaturation and
irreversible DNA damage. Exceeding the thermoregulatory thresholds
results in disturbances in the essential function of
cardiovascular, nervous and respiratory systems (15–18).
Temperature modulation is also the basis for the development of new
biomaterials and therapeutic technologies (19). The synthesis of thermally sensitive
hydrogels allows the design of controlled drug release systems in
response to an external stimulus, such as temperature (20). Controlled temperature increase is
also the basis of oncological hyperthermia (21). Local or whole-body hyperthermia is
considered a complementary therapy in oncology, in combination with
chemotherapy or radiotherapy (21,22).
Cell profiling in terms of their temperature
sensitivity, e.g., by evaluating expression of heat shock proteins
(HSP) or transient receptor potential (TRP) channels, is important
for the design of new temperature-regulating drugs and medical
technologies, also in the field of gene therapy (23,24). The
temperature response of cells is closely related to the heat shock
protein family gene expression pattern. HSPs are classified based
on molecular weight and function (e.g., HSP27, HSP70, HSP90). HSP
biosynthesis is precisely regulated by the activity of
transcription factors (HSF) that recognize heat shock elements in
the promoter regions of genes. HSPs are involved in the folding,
maturation, functioning and degradation of many crucial proteins
(being chaperones they interact with many proteins). Therefore,
they perform regulatory functions in the fundamental biological
processes such as proliferation, apoptosis, drug resistance, stress
response (25–27). HSP signatures in cells are different,
there are significant differences in the constitutive expression of
HSP genes in tumor cells, which are associated with the
pathomechanisms of cancer development (28,29). In
ovarian cancers, the important role of HSP27, HSP70 and HSP90 is
stressed (29). It is documented
that HSP expression changes under stress conditions, and individual
HSPs can be expressed at different levels. The role of HSP90 in AAV
transduction to cells is emphasized, underlining functional
relationships between FKBP52 and HSP90 (12,27,30). The
phosphorylated form of the FKBP52 chaperone protein has been shown
to interact with the D-sequence of the ITRs in the AAV genome. It
inhibits the synthesis of the second viral DNA strand and thereby
leads to inefficient expression of the transgene from the rAAV
vector. The heat shock leads to the dephosphorylation of FKBP52,
stabilization of the FKBP52-HSP90 complex, which results in the
increase of rAAV transduction efficiency (12,30). It
seems that the transduction efficiency of rAAV depends on the cells
HSP signatures. The level of selected HSPs in the cells promotes an
increase the transduction efficiency with the use of AAV vectors.
It is worth considering that the determination of the HSP signature
in tumors may be helpful in planning efficient gene therapy
trials.
At present, gene therapy of ovarian cancers is based
primarily on the use of suppressor gene (e.g., WWOX) strategies,
suicide therapy (e.g., HVS-TK), anti-angiogenic strategy (e.g.,
VEGF) and a strategy directed towards multidrug resistance genes
(e.g., MDR1) or based on oncolytic virotherapy (31). Despite progress in the field of
ovarian gene therapy, there are too few clinical trials on oncology
patients and there is still a lack of efficient and safe gene
transfer systems. The aim of this study was to investigate the
effect of hyperthermia conditions (40 and 43°C) on the rAAV
transduction efficiency of ovarian cancers with various clinical
origins. Moreover, the purpose was to evaluate the expression of
representative genes involved in rAAV transmission with special
emphasis on heat shock protein genes. Notably, depending on the
origin of ovarian cancer cells, the increase of rAAV transduction
efficiency at elevated temperature was subsequently the cause of
the search for functional linkages between the high sensitivity of
ascites-derived ovarian cancer to rAAV, and for the expression of
representative genes for rAAV transmission and HSP.
Materials and methods
Cell lines
The ovarian cancer cell lines with a different
clinical origin (32,33): Caov-3 (ATCC® HTB-75),
NIH:OVCAR-3 (ATCC® HTB-161, American Type Culture
Collection) were cultured in the Dulbecco's Modified Eagle Medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and RPMI Medium-1640
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 20% FBS
and 0.6 mg insulin (Insulin solution from bovine pancreas;
Sigma-Aldrich; Merck KGaA)/500 ml medium, respectively. Cell line
AAV-293 (Agilent Technologies) derived from human embryonic kidney
cells was cultured in DMEM supplemented with 10% FBS. Human
fibroblasts CCD-18Co (ATCC® CRL-1459) were cultured in
Minimum Essential Media (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS. The media were also supplemented with 1%
Antibiotic-Antimycotic (Gibco; Thermo Fisher Scientific, Inc.), and
the cells were maintained at 37°C in a humidified 5% CO2
atmosphere. Mycoplasma test was performed in our laboratory, and
its shown no contamination. The analyzed cells were verified in the
International Cell Line Authentication Committee and ExPASy
Cellosaurus databases (34,35), in order to exclude their
contamination with other cell lines or their incorrect
identification.
rAAV transduction at hyperthermia
conditions
Cells were seeded at the density 1×105
for Caov-3, AAV-293 and 1.5×105 for NIH:OVCAR-3 per a 6
cm diameter dish in the appropriate medium and incubated for 24 h
in a normal culture condition. Just before transduction, cell
culture media were replaced with adequate ones (AAV-293,
Caov-3-DMEM; NIH:OVCAR-3-RPMI-1640 with insulin) heated up to 37,
40 or 43°C with FBS reduced to 2%. To transduce the ovarian cancer
cells, the recombinant AAV vector rAAV/DJ-cmv-eGFP (cat. no: 7101;
Vector Biolabs) was added to the new, heated media with the
multiplicity of infection (MOI) of 4×104 genome copies
or 0 genome copies (non-transduction control group). The vector
rAAV/DJ-cmv-GFP expresses eGFP gene under a control of cmv
promoter. To test the promoters' transcriptional activity, AAV-293
cells were transfected with rAAV/DJ-CAG-GFP (cat. no: 7078; Vector
Biolabs) at the same conditions and MOI. The vector rAAV/DJ-CAG-GFP
expresses eGFP gene under a control of CAG promoter. The cells were
incubated for 3 h in hyperthermia conditions (40 or 43°C) in a
humidified 5% CO2 atmosphere. Then the cells were moved
to 37°C. After 48 and 96 h of transduction, the cell culture media
were replaced with fresh 2% FBS media. Finally, the cells were
harvested on the 6th day after transduction.
Non-transduction cells were examined to test HSP
(Caov-3 and NIH:OVCAR-3) and AAV receptor (CCD-18Co, AAV-293,
Caov-3 and NIH:OVCAR-3) expression. They were harvested after 24 h
from exposure to hyperthermia conditions.
Transduction efficiency
measurement
The Countess II FL Automated Cell Counter
(Invitrogen; Thermo Fisher Scientific, Inc.) with EVOS Light
Cube-GFP (470/22 nm Excitation; 510/42 nm Emission) was used to
determine the percentages of Green Florescent Protein-positive
(GFP+) cells. The images of GFP-expressing cells were obtained
using an inverted fluorescence microscope (Olympus IX53; Olympus)
with the pE-300white (CoolLED) illumination system.
Pictures of non-transduced (in BF) and transduced (in FITC) cells
were taken at ×10 magnification. All measurements were performed on
the 6th day after transduction, before the cells harvesting for DNA
isolation to analyze the transduction efficiency by qPCR.
Quantitative (q)PCR for AAV genome
copy number determination
Total DNA was isolated using the High Pure Viral
Nucleic Acid kit (Roche Life Science) from AAV-293, Caov-3 and
NIH:OVCAR-3 cell lines. The amount of DNA was quantified by
260/280/230 nm absorbance measurements using spectrophotometer
Quawell Q5000 UV–Vis (Quawell). TaqMan assays for rAAV genome copy
number were developed using probe and primers for the ITR region.
The fluorescent probe (5′-CACTCCCTCTCTGCGCGCTCG-3′) featured 6-FAM
and TAMRA. The forward primer was 5′-GGAACCCCTAGTGATGGAGTT-3′ and
the reverse primer was 5′-CGGCCTCAGTGAGCGA-3′ (36). Total volume of qPCR reactions was 10
µl, contained 50 ng DNA and ran under the following conditions:
50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 sec and
60°C for 60 sec. qPCR was performed in StepOnePlus™ Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Absolute quantification analysis with the StepOne Software v2.3
(Thermo Fisher Scientific, Inc.) was performed to determine
transcripts numbers. Data were quantified using the standard curve
(37, 38). Briefly, standard curves were generated using serial
dilutions of plasmid pAAV-hrGFP (Agilent Technologies) used to
calculate the number of genome copies in the tested samples. The
rAAV genome copy number was normalized as viral genome copy number
per 50 µg of total genomic DNA.
Reverse transcription qPCR
To test gene expression, total RNA was isolated
(39) using the TRI Reagent
(Sigma-Aldrich; Merck KGaA). To examine the miRNA profile, RNA was
isolated with the used of the TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.). DNA-free™ DNA Removal Kit (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to remove the contaminating DNA
from RNA samples. Single-stranded cDNA was synthesized with the
High Capacity RNA-to-cDNA kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). All primers listed below are available on Thermo
Fisher Scientific website as appropriate TaqMan Assays (Thermo
Fisher Scientific Website; http://www.thermofisher.com/pl/en/home.html).
The expression of genes-encoding receptors for AAV
was examined with the use of the following TaqMan Assays (assay ID;
Thermo Fisher Scientific, Inc.): AAVR (Hs00967343_m1), HSPG1
(Hs01081432_m1), HSPG2 (Hs01078536_m1), and ACTB (Hs01060665_g1) as
endogenous control. The expressions were measured after 24 h from
exposure at 40 and 43°C for 3 h. The ovarian cancer cells, AAV-293
and CCD-18Co were used. CCD-18Co was selected as a reference sample
in ΔΔCq method (37).
The expression of miRNA responsible for silencing
AAVR, HSPG1 and HSPG2 was examined on the designed TaqMan Custom
MiRNA Array Card (TaqMan Low-Density Array-TLDA card; Thermo Fisher
Scientific, Inc.) for Caov-3, NIH:OVCAR-3 and AAV-293 cell lines
maintained in normal culture condition. The following human miRNA
were analyzed (assay ID, Thermo Fisher Scientific): miR-15b-5p
(000390), miR-133a-3p (002246), miR-195-5p (000494), miR-23a-3p
(000399), miR-23b-3p (000400), miR-27a-3p (000408), miR-30a-5p
(000417), miR-34a-5p (000426), miR-34c-5p (000428), miR-101-3p
(002253), and U6 snRNA (001973). Functional links between the
designated miRNAs and the AAVR, HSPG1 and HSPG2 genes were searched
in miRNA databases (40, 41).
The expression of HSP was examined on TaqMan Array
96-Well FAST Plate Human Heat Shock Proteins (cat. no. 4418733;
Applied Biosystems; Thermo Fisher Scientific, Inc.) in ovarian
cancer cells 24 h after exposure to hyperthermia condition. Results
for cells maintained at 37°C were selected as reference samples and
used for the comparison of constitutive levels of HSP in ovarian
cancer cell lines.
The expression of genes associated with the
molecular mechanisms of cancer was examined on TaqMan Array 96-Well
FAST Plate Human Molecular Mechanisms of Cancer (MMoC; cat. no.
4418806; Applied Biosystems; Thermo Fisher Scientific, Inc.) for
Caov-3 and NIH:OVCAR-3 maintained in normal culture condition.
In all gene expression tests total volumes of qPCR
reactions were 10 µl, contained 100 ng cDNA and ran under the
following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles of
95°C for 15 sec and 60°C for 60 sec. The FAM was used as
fluorophore and NFQ-MGB as a quencher (Thermo Fisher Scientific,
Inc.). Transcript levels of target genes were normalized to the
level of housekeeping genes encoding: β-actin (ACTB) for AAV
receptors, U6 snRNA for miRNA, β-glucuronidase (GUSB) and
hypoxanthine phosphoribosyltransferase 1 (HPRT1) for HSP and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for MMoC. All
reactions were performed according to manufacturer instructions in
the StepOnePlus™ Real-Time PCR System. Relative gene expression
levels were calculated using the ΔΔCq method (37) by Expression Suite Software v1.1
(Thermo Fisher Scientific, Inc.). The Morpheus matrix visualization
and analysis software was used to present results of miRNA and MMoC
expression as heat maps (in the form of 2−ΔCt)
(Morpheus-versatile matrix visualization and analysis software;
http://software.broadinstitute.org/morpheus).
Protein CA 125 measure
To measure the level of CA 125 marker, Caov-3,
NIH:OVCAR-3 and AAV-293 cells were harvested, washed with PBS and
lysed in lysis RIPA buffer [10 mM Tris-HCl (pH 7.5), 150 mM NaCl,
1% NP-40, 0,5% Sodium Deoxycholate, 0,1% SDS and protein inhibitor
cocktail (cat. no. P8340; Sigma-Aldrich; Merck KGaA)] for 30 min on
ice. The probes were centrifuged (20 min, 4°C, 12,500 rpm), and the
supernatants were collected for further tests. CA 125 was measured
in protein samples using solid phase, two-site sequential
chemiluminescent assays (CLIA) with paramagnetic microparticle
solid phase that was fully processed on an automated randomaccess
immunoassay analyzer LIAISON XL (DiaSorin, Saluggia, Italy). The
intra- and interassay CV for CA 125 ranged from 1.4–2.2 and
4.6–5.8%, respectively.
Cell adhesion
The adhesion potency of the tested cells was
determined using laminin coated plates (cat. no. 354404; 6-well
plates; BD BioCoat™ Laminin). The experiment was performed for
transduced and non-transduced cells incubated at a different
temperature (37, 40, 43°C). The examined cell lines on the 6th day
after transduction were trypsinized (Trypsin-EDTA (0.25%), phenol
red; Gibco; Thermo Fisher Scientific, Inc.), counted (in an
automatic cell counter) and seeded on laminin plates. From a 6 cm
diameter dish, the cells were transferred to 1 well in laminin
coated plate. A control was performed, i.e., cells were seeded on
6-well plates without laminin (Nunc; Thermo Fisher Scientific,
Inc.). After 4 h of incubation under standard culture conditions,
the attached cells were trypsinized and the number of viable cells
was counted. Subsequently, the percentage of adhered cells was
estimated due to laminin presence.
Invasion chamber assay
To verify how hyperthermia and transduction modify
cell invasion, invasion chamber assay was performed using a 24-well
cell culture insert 8 µm pore (cat. no. 354480; Corning Matrigel
Invasion Chamber; Corning). The examined cell lines on the 6th day
after transduction were trypsinized, cell suspensions in 2% FBS
media at a concentration of 1×105/ml for AAV-293, Caov-3
and 1.5×105/ml for NIH: OVCAR-3 were prepared. Cell
suspensions in volume 0.5 ml were added per well into the upper
chambers, the lower chambers were filled with 0.5 ml of complete
media. Cells which migrated to the lower chamber were counted after
24 and 48 h. The cells from the upper membrane were aspirated by
gentle pipetting. The GFP+ cells from the bottom side of the
Matrigel chamber were visualized with the fluorescence microscope
(with FITC) after 24 h. After 48 h of incubation, cells which
migrated to the bottom of the Matrigel surface were fixed with
methanol and stained with 0.5% crystal violet.
Statistical analysis
The transduction efficiency, receptor expression
(AAVR, HSPG1, HSPG2), cell adhesion and cell invasion results
expressed as mean and standard deviation. HSP results were
performed as mean and standard error. A one-way analysis of
variance (ANOVA, α=0.05) with a post-hoc Bonferroni (α=0.05) test
using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA) was
used to calculate the statistical significance of differences
between mean values of compared samples. P<0.05 was considered
to indicate a statistically significant difference.
Results
rAAV transduction of ovarian cancer
cells at hyperthermia conditions
The effect of elevated temperature on rAAV
transduction was investigated using ovarian carcinoma cell lines of
different clinical origin and pathomechanism of carcinogenesis. The
NIH:OVCAR-3 cell line was originally derived from the malignant
ascites of a patient with progressive adenocarcinoma of the ovary.
NIH:OVCAR-3 cells are resistant to cytostatics at concentrations
used in clinics and they are sensitive to hormonal therapy
(32). Caov-3 cells are derived from
the ovarian solid tumor (33). The
cells' functional variability was visualized through the analysis
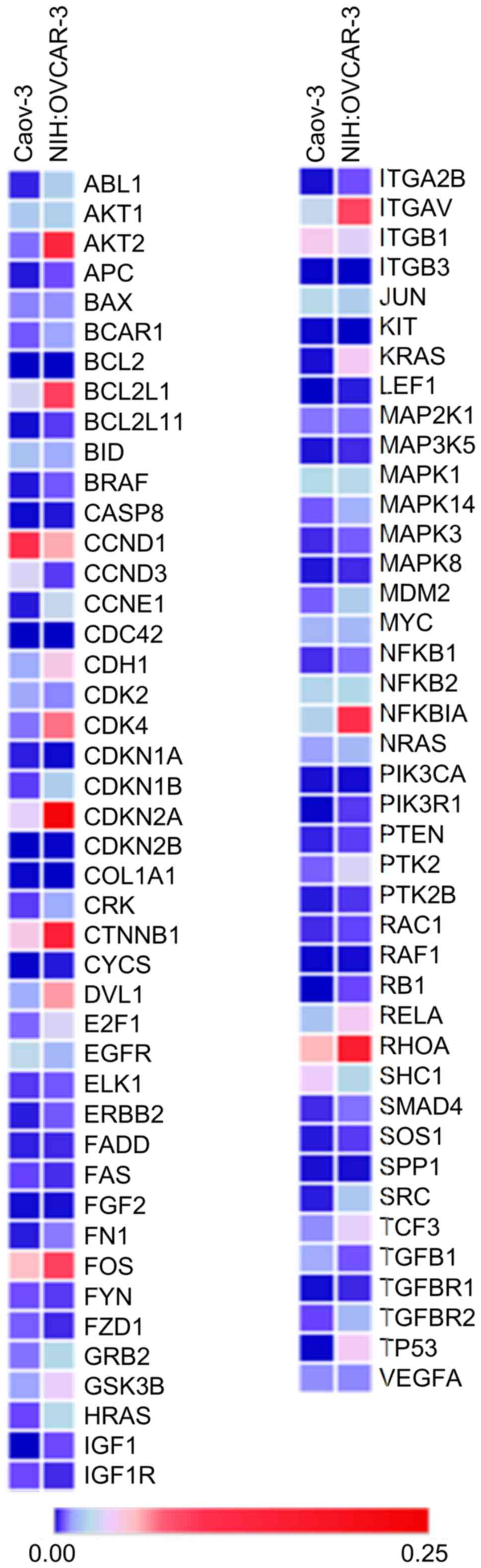
of the expression of Molecular Mechanisms of Cancer (MMoC) genes
that are essential for carcinogenesis. The MMoC gene expression
pattern of functionally differentiating ovarian cancer lines is
shown in Table I and Fig. 1. Ascites-derived NIH:OVCAR-3 cells
were characterized by a higher expression of almost all genes
involved in proliferation, cell cycle, cell differentiation,
metastasis, invasion, angiogenesis, apoptosis and signal
transduction processes, than the solid-derived Caov-3 cells
(Table I). Additionally, the
estimated ovarian cancer marker CA 125 confirmed the origin of
ovarian cancer cells, as well provided information about the
protein expression in the studied cells. CA 125 is a tumor marker,
which is mainly used in the diagnosis of ovarian cancer and
monitoring treatment. As shown in Table
II, the level of CA 125 for NIH:OVCAR-3 and Caov-3 was
>68,590.0 and 2,220.0 U/ml, respectively. In the case of control
line AAV-293, marker was determined on the level <0.3 U/ml. The
obtained results showed that ascites-derived NIH:OVCAR-3 cells were
characterized by increase expression of this cancer marker, which
may indicate a higher invasiveness and progress in carcinogenesis
of this line, compared to Caov-3 derived from a solid tumor.
 | Table I.Expression of representative genes
involved in cancer. |
Table I.
Expression of representative genes
involved in cancer.
| NIH:OVCAR-3 |
|---|
|
|---|
| Fold change
(2−ΔΔCq) | Proliferation, cell
cycle, cell differentiation | Metastasis,
invasion, angiogenesis | Apoptosis | Signal
transduction |
|---|
| 0.00 |
| CDC42, ITGB3,
COL1A1 | BCL2 | KIT |
| <0.50 | TGFB1 CCND3,
CDKN1A |
|
| FDZ1 |
| 0.51–1.00 | EGFR JUN, CCND1,
CDK2 | FYN, ITGB1, SPP1,
VEGFA | BID | FAS, IGF1R, MAP2K1,
PIK3CA, SHC1 |
| 1.01–1.50 | ELK1, FGF2 MYC |
| BAX, FADD | AKT1, MAPK1, NFKB2,
NRAS, RAC1 |
| 1.51–2.00 | FOS BCAR1,
PTEN |
|
| RAF1, MAPK8 |
| 2.01–2.50 |
| PTK2B |
| MAP3K5, MAPK14,
MAPK3, NFKB1, RELA, SMAD4, SOS1 |
| 2.51–3.00 | MDM2 | CRK, RHOA | BCL2L1, CASP8,
GSK3B | ERBB2, GRB2,
TGFBR2 |
| 3.01–4.00 | TCF3 CDH1,
E2F1 | CTNNB1, ITGAV |
| APC, BRAF,
TGFBR1 |
| 4.01–5.00 | DVL1 CDKN1B | FN1 | BCL2L11 | HRAS, NFKBIA,
PTK2 |
| 5.00–10.00 | ABL1, SRC CDK4,
CDKN2A | ITGA2B | CYCS |
|
| 10.01–15.00 | CCNE1 |
|
| AKT2, PIK3R1 |
| 15.01–50.00 | CDKN2B |
|
| KRAS |
| 50.01–300.00 | TP53 |
|
| LEF1 |
| >300.01 | RB1 |
|
| IGF1 |
 | Table II.CA 125 protein levels in AAV-293,
Caov-3 and NIH:OVCAR-3 cell lines. |
Table II.
CA 125 protein levels in AAV-293,
Caov-3 and NIH:OVCAR-3 cell lines.
| Cell lines | CA 125 (U/ml) |
|---|
| AAV-293 | <0.3 |
| Caov-3 |
2,220.0 |
| NIH:OVCAR-3 | >68,590.0 |
Transductions were carried out under conditions of
37, 40 and 43°C, defined as hyperthermia conditions (21). To determine the transcriptional
activity of the CAG and cmv promoters, the cells were transduced
with the rAAV/DJ mosaic vector with the GFP reporter gene under the
control of the hybrid CAG promoter (AAV-293) or the conventional
cmv promoter (Caov-3 and NIH:OVCAR-3). The transduction efficiency
was determined by direct counts of GFP+ cells and measuring genome
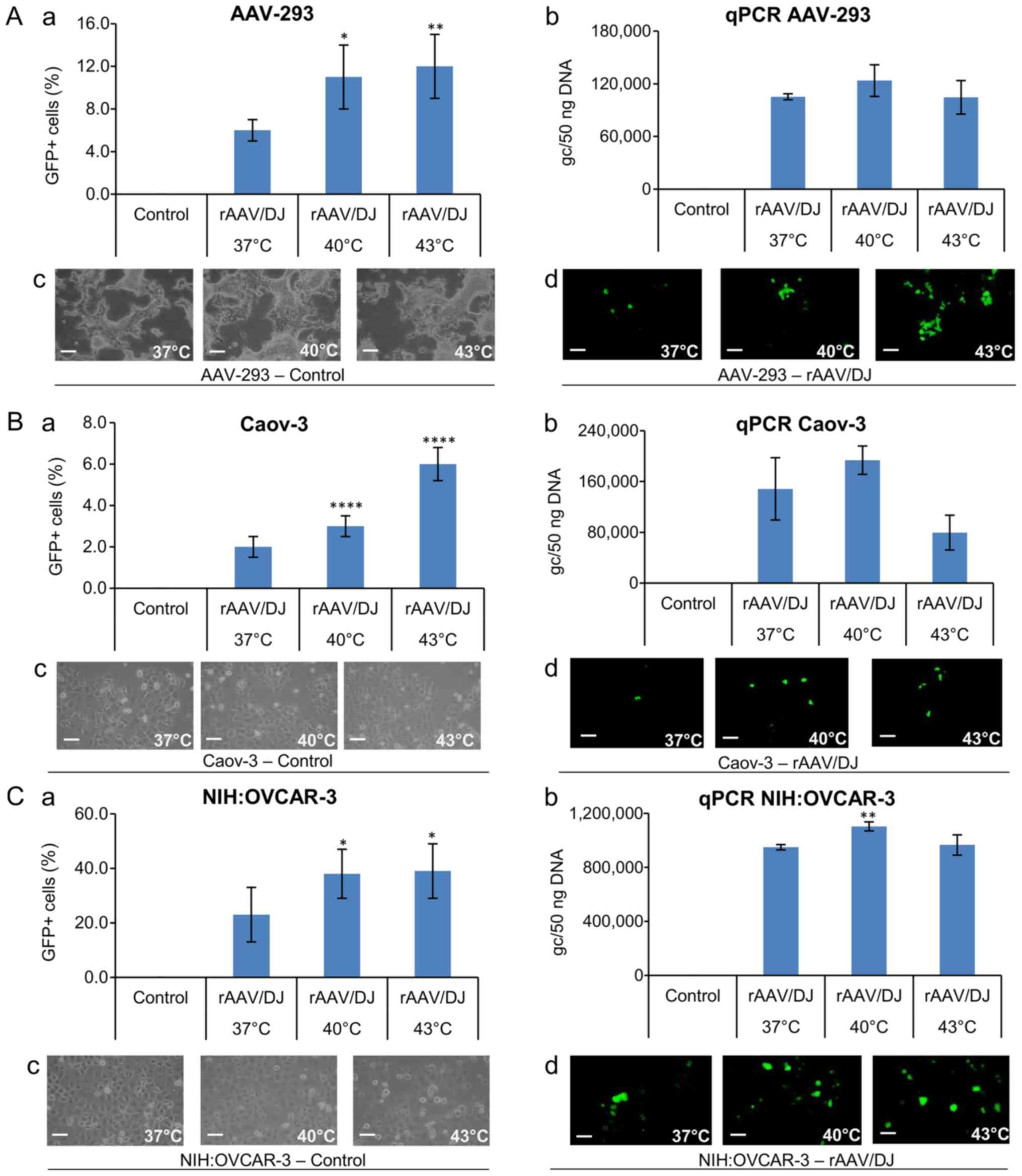
copies of rAAV in transduced cells with qPCR. As Fig. 2A shows, as the temperature elevates,
the transduction efficiency increases. Furthermore, there were
differences in the transduction efficiency between the lines. The
highest efficiency of transduction was demonstrated for NIH:OVCAR-3
cells. At 37°C, approximately 23% of cells were GFP+. In comparison
only 2% of Caov-3 cells, and 6% of AAV-293 cells under the same
conditions were transduced. The temperature effect was visible for
all lines. As the temperature raised, the percentage of GFP+ cells
increased significantly. Despite the lower level of transduction
efficiency, Caov-3 cells responded to temperature stimulation in
both 40 and 43°C. In 40°C, a 50% increase in GFP+ cells was
observed, and in 43°C, GFP+ cells showed an increase of 200%
compared to 37°C. The percentage of fluorescent NIH:OVCAR-3 cells
was significantly increased in 40°C (about 65% compared to normal
temperature; Fig. 2A).
The transduction efficiency was also determined by
the qPCR method (Fig. 2B). The
percentage of GFP+ cells corresponds to the number of rAAV genome
copies (gc) in all transduced cells. As shown in Fig. 2B, at 40°C a higher level of gc was
demonstrated than at 37°C, which reflects the increase in
transduction efficiency due to response to the rise in temperature.
Interestingly, at 43°C an increase in the number of GFP+ cells was
observed, with the gc number in all lines being lower than at 40°C
and even 37°C (Fig. 2B).
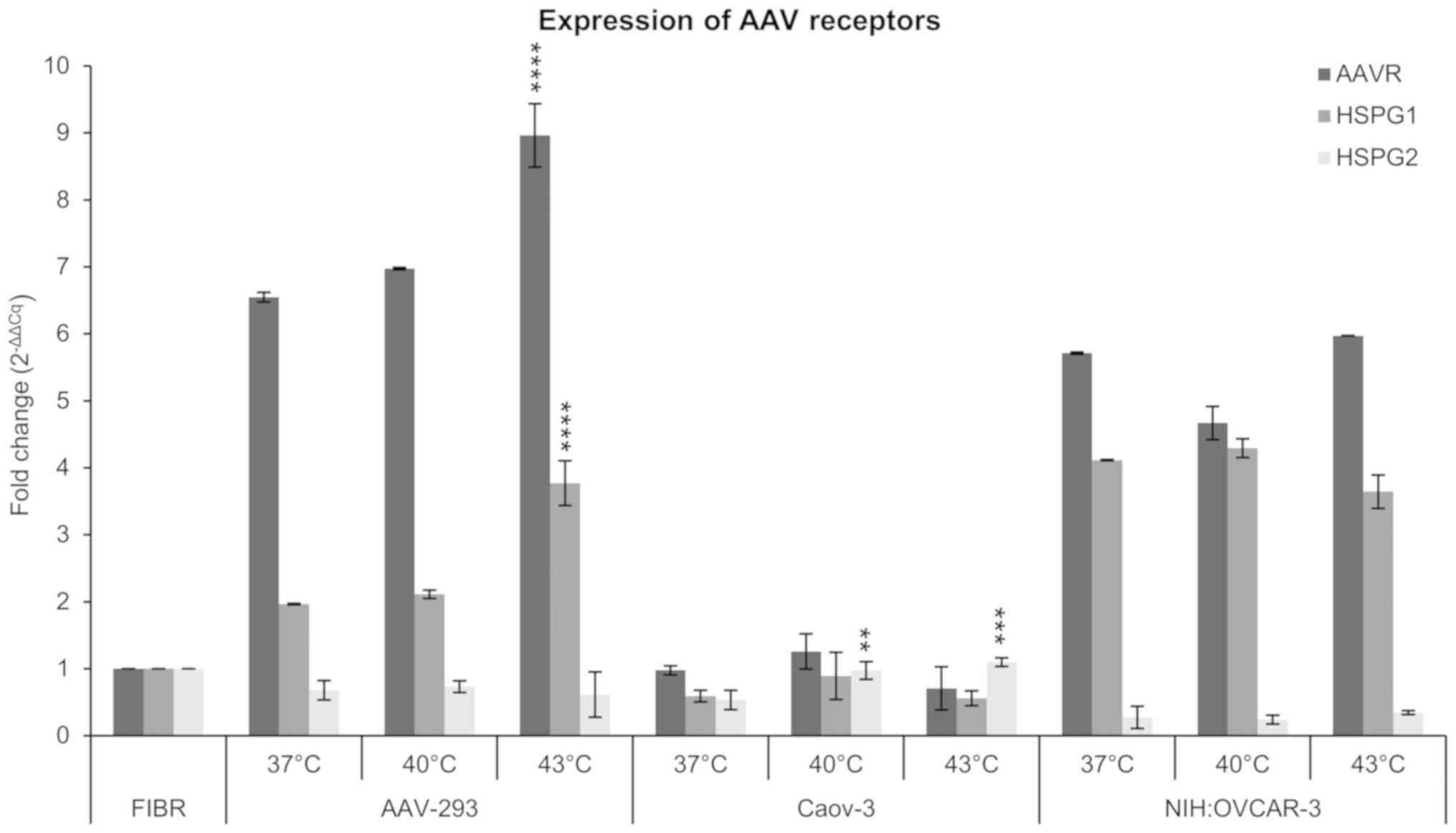
AAV receptor expression
The expression of crucial genes of the extracellular
rAAV transmission was analyzed in the study. The AAVR, HSPG1 and
HSPG2 genes were selected for evaluation. The qPCR method was
determined by normalizing the results to the level of ACTB gene
expression, the human fibroblasts (CCD-18Co cells) were used as
reference. As shown in Fig. 3, the
expression of the examined genes changes with increasing
temperature. Additionally, the expression pattern for AAVR, HSPG1
and HSPG2 genes was different in the lines tested. In NIH:OVCAR-3
cells, AAV receptor signature was revealed as a high expression of
AAVR and HSPG1, and as a low expression of HSPG2. On the other hand
in Caov-3 cells, the signature of selected genes occurred as a
comparable expression level of all three genes tested. However, the
expression of AAVR dominated in AAV-293 cells. Expression of
receptors in NIH:OVCAR-3 ovarian cancer cells was higher than in
Caov-3 cells at 37, 40, and 43°C, which corresponds with the higher
transduction efficiency in NIH:OVCAR-3 (Fig. 2). Caov-3 cells, despite lower
expression of AAV receptors, respond better to the increase of
temperature. The expression of AAVR, HSPG1 and HSPG2 at 40°C has
increased by 29, 51 and 82% respectively, compared to 37°C. In the
case of AAVR and HSPG1 after 43°C, the expression normalized to the
level observed in 37°C, but for HSPG2 the expression remained at a
level similar to 40°C. For NIH:OVCAR-3 cells, there was an increase
in AAVR expression in 43°C of about 5% and HSPG2 of about 33%, vs.
37°C. The most proportional increase in the expression of AAVR and
HSPG1 to the temperature elevation was observed for AAV-293 cells.
They responded with the increase of AAVR and HSPG1 by approx. 6, 7%
at 40°C, and 37 and 92% at 43°C, respectively. Interestingly,
despite an increase in the number of GFP+ cells (Fig. 2A), a lower level of receptor
expression as well as the rAAV copy number was observed in cells
exposed to 43°C (Fig. 2B).
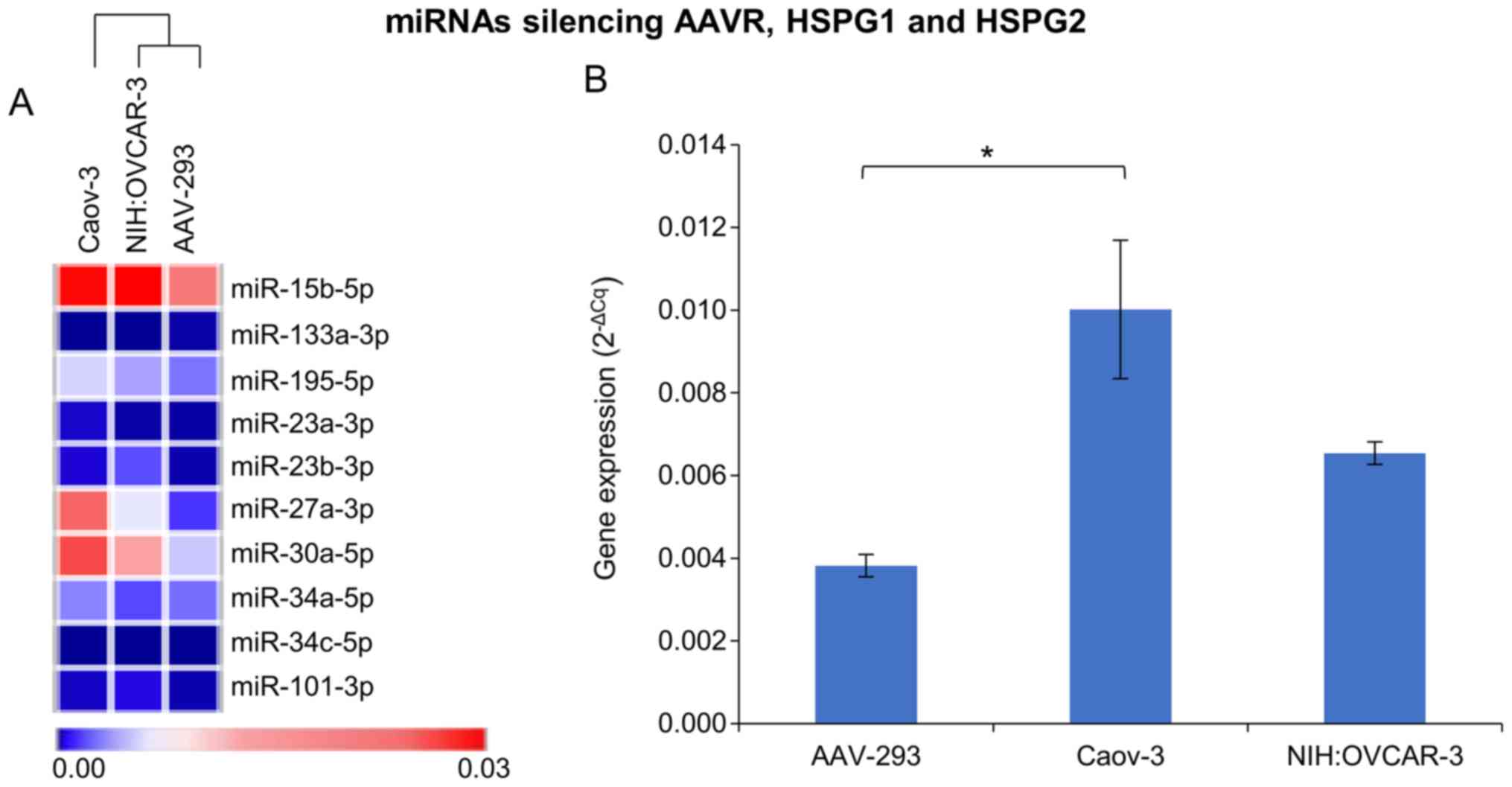
miRNA expression panel
During the search for mechanisms to increase the
efficiency of rAAV transduction in cells exposed to elevated
temperature, particular attention was paid to the expression of
selected miRNAs. MiRNAs involved in the silencing processes of rAAV
transmission were analyzed. Fig. 4B
shows the mean expression of silencing miRNAs in the cells tested
at 37°C and indicates that the level of miRNA corresponds to the
rAAV transduction efficiency. The mean values were calculated due
to underline the miRNA expression differences between studied cell
lines. The lowest level of miRNAs responsible for silencing AAV
transmission genes was observed for NIH:OVCAR-3. This ovarian
cancer cell line was transfected at the highest level. The level of
silencing miRNAs was about 35% higher in Caov-3 cells which
transduced almost 12 times less efficiently than NIH:OVCAR-3. In
the case of AAV-293 cells there was no functional coherence between
the miRNA level and the transduction efficiency. The level of miRNA
in AAV-293 cells was about 60% lower than in NIH:OVAR-3, although
the AAV-293 transduction efficiency (Fig. 2A) was lower than NIH:OVCAR-3.
HSP expression
The result of HSPs expression in NIH:OVCAR-3 and
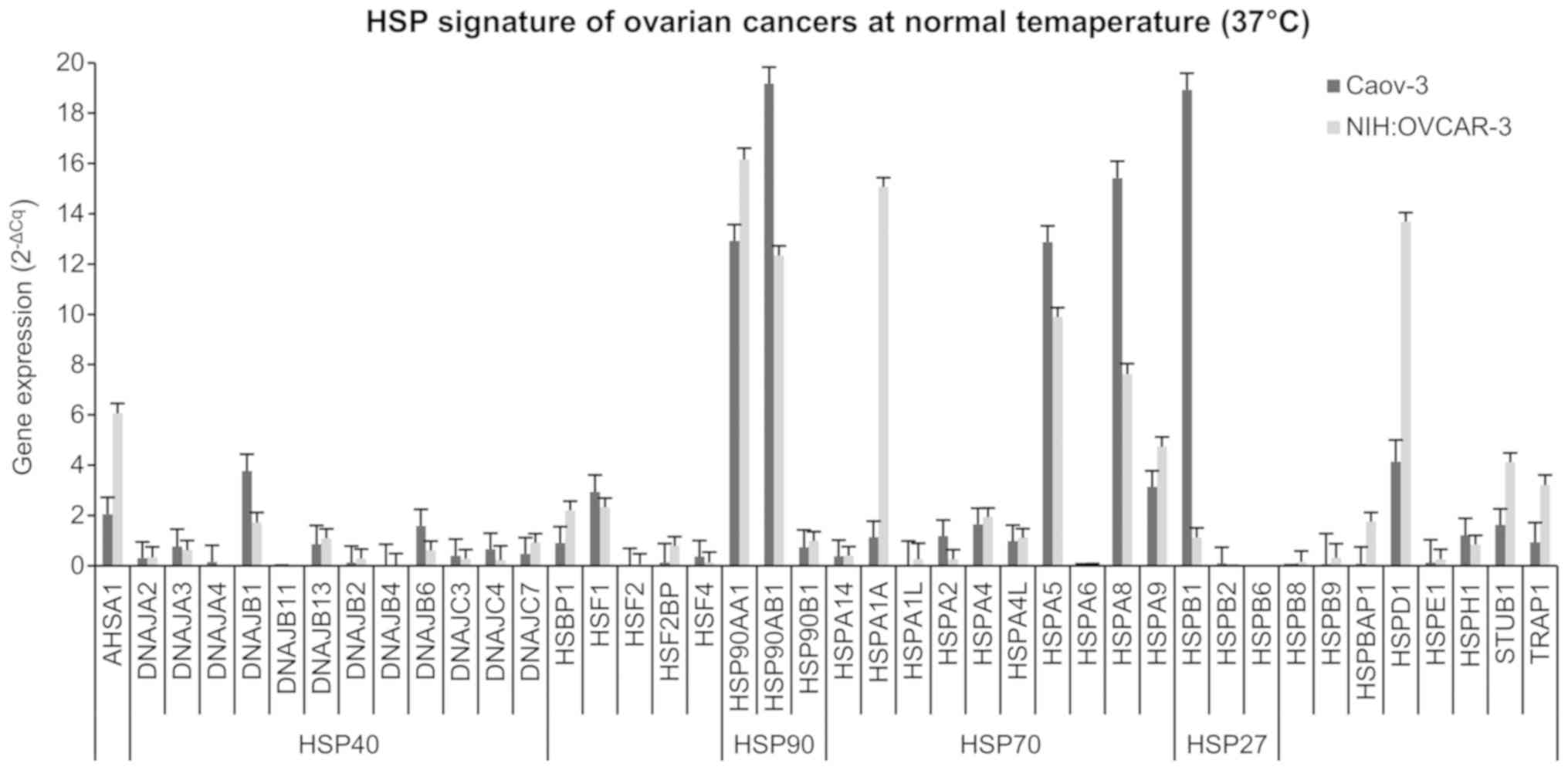
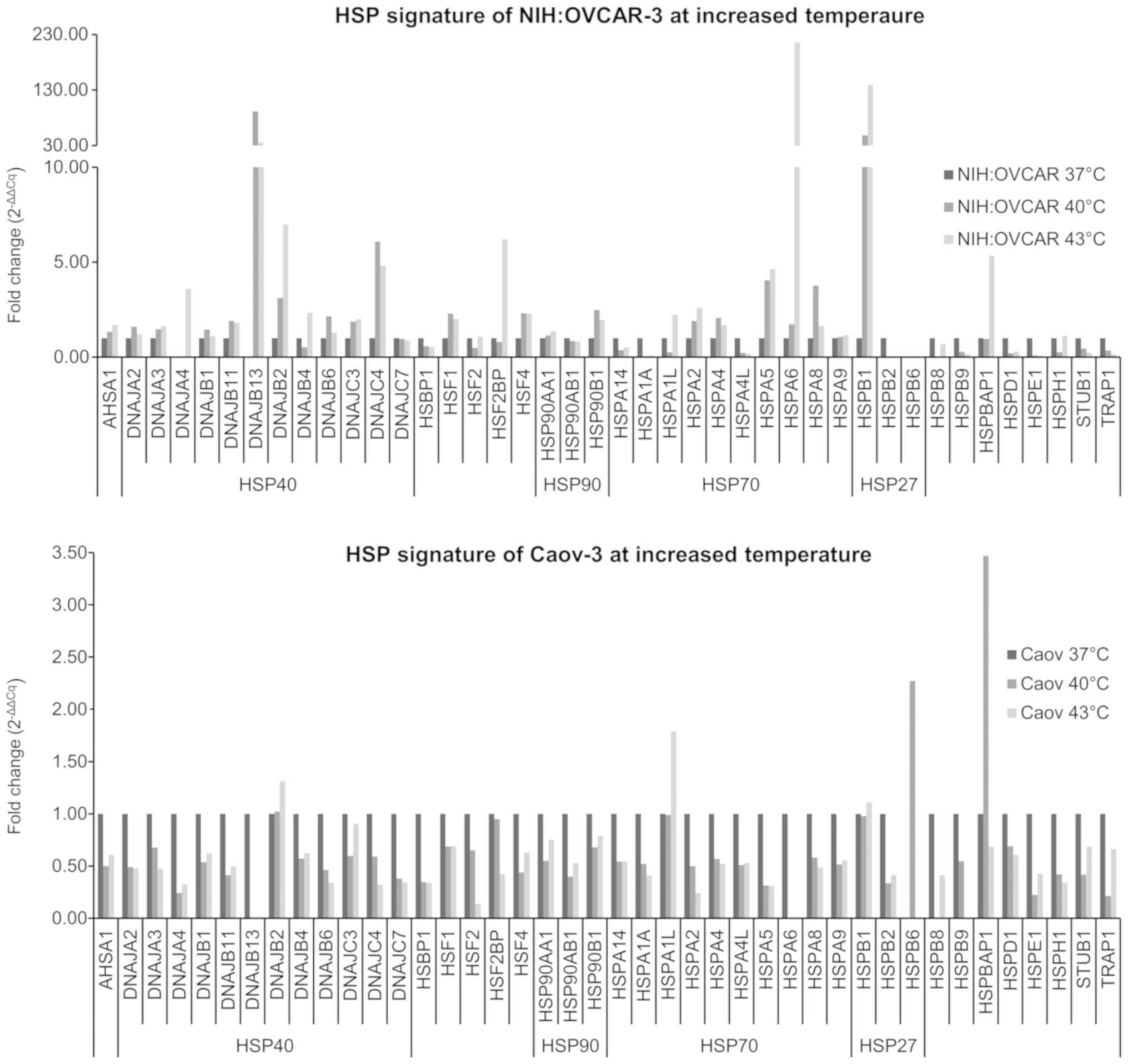
Caov-3 ovarian cancer cells are shown in Figs. 5 and 6. Fig. 5
shows the constitutive level of HSP expression in lines at 37°C,
expressed in 2−ΔCq form, while Fig. 6 shows changes in HSP level
(2−ΔΔCq, fold range) in cells exposed to elevated
temperature (40 and 43°C). As shown in Fig. 5, in NIH:OVCAR-3 22 out of 42 HSP
genes tested had higher expression than in Caov-3 cells.
NIH:OVCAR-3 cells compared to Caov-3 are characterized by higher
expression of the HSP40 family (DNAJA2, DNAJB13, DNAJB2, DNAJB4,
DNAJC7), HSP90 family (HSP90AA1, HSP90B1), and HSP70 family
(HSPA14, HSPA1A, HSPA1L, HSPA4, HSPA4L, HSPA6). NIH:OVCAR-3 cells
also had a higher expression of HSP than Caov-3: AHSA1, HSPB8,
HSPB9, HSPBAP1, HSPD1, HSPE1, STUB1, and TRAP1. Based on the
highest expression HSP (3–10× higher than in Caov-3), the form of
AHSA1+, HSP90AA1+, HSPA1A+, HSPD1+, STUB1+, and TRAP1+ can be
proposed as constitutive signature for NIH:OVCAR-3 cells. On the
other hand, the characteristic HSP signature for Caov-3 (3–10×
higher HSP levels than in NIH:OVCAR-3) is DNAJB1+, HSP90AB1+,
HSPA5+, HSPA8+, and HSPB1+. The obtained results (Fig. 5) may explain the differences between
the NIH:OVCAR-3 and Caov-3 cell lines in the transduction
efficiency (Fig. 2). Results
presented in Fig. 6 show that the
selected hyperthermia conditions caused the increase of selected
HSP expression. NIH:OVCAR-3 cells demonstrated an increase
expression at 40 and 43°C for AHSA1, DNAJA3, DNAJB2, DNAJC3,
HSP90AA1, HSPA2, HSPA5, HSPA6, and HSPB1 (Fig. 6). A particularly high (>10×)
increase in expression for DNAJB13, HSPA6, and HSPB1 was observed.
Caov-3 cells were much less responsive to hyperthermia through
increased HSP expression (Fig. 6).
Of the 42 HSP genes examined, only 5 genes showed increased
expression at elevated temperature (DNAJB2, HSPA1L, HSPB1, HSPB6,
HSPBAP1). In conclusion, the various ovarian cancer cells responded
significantly differently to both the constitutive HSP expression
level at 37°C and after the exposure cells to 40 and 43°C.
Temperature-dependent changes in HSP expression appear to be
helpful to understand the rAAV transduction efficiency increase in
cells exposed to elevated temperature.
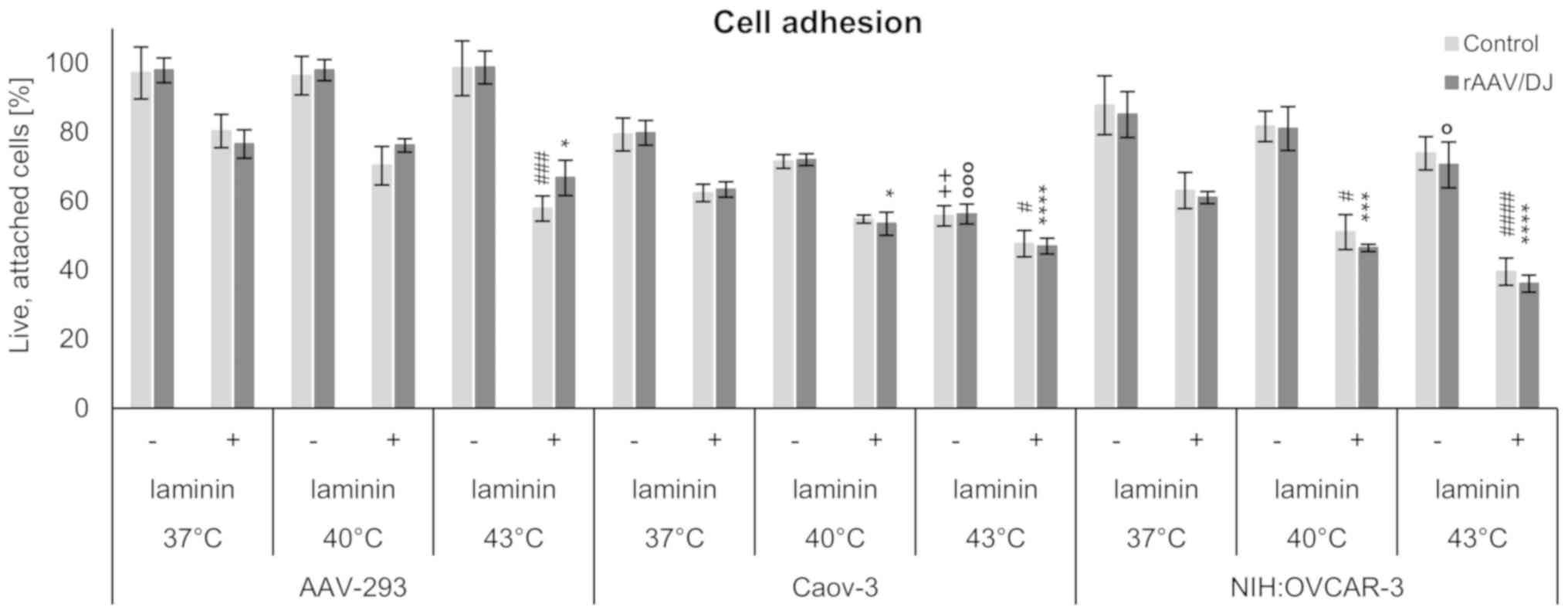
Cell adhesion
To investigate the influence of rAAV transduction
and hyperthermia on the biology of the studied cells, adhesion
assays were performed. The cells were plated on dishes covered with
laminin which is described as crucial protein for extracellular
matrix maintenance. Generally, as shown in Fig. 7, the greater number of cells attached
to the control plates (without laminin) was noted. The results
demonstrated that hyperthermia induced the decrease of the number
of adhered cells. In the case of AAV-293 line, in plate without
extracellular matrix protein were small differences in the
percentage of viable, adherent cells for each temperature. Whereas,
for the ovarian cancer cell lines a decrease in attached cells
exposed to hyperthermia was observed for both panels, with and
without laminin. Additionally, the NIH:OVCAR-3 cell line showed the
lowest adhesion potency after incubation at 43°C. The control
AAV-293 cells were characterized by a greater ability to adhere
compared to ovarian cancer cells. In all the tested cell lines no
effect of rAAV/DJ transduction for adhesion was observed.
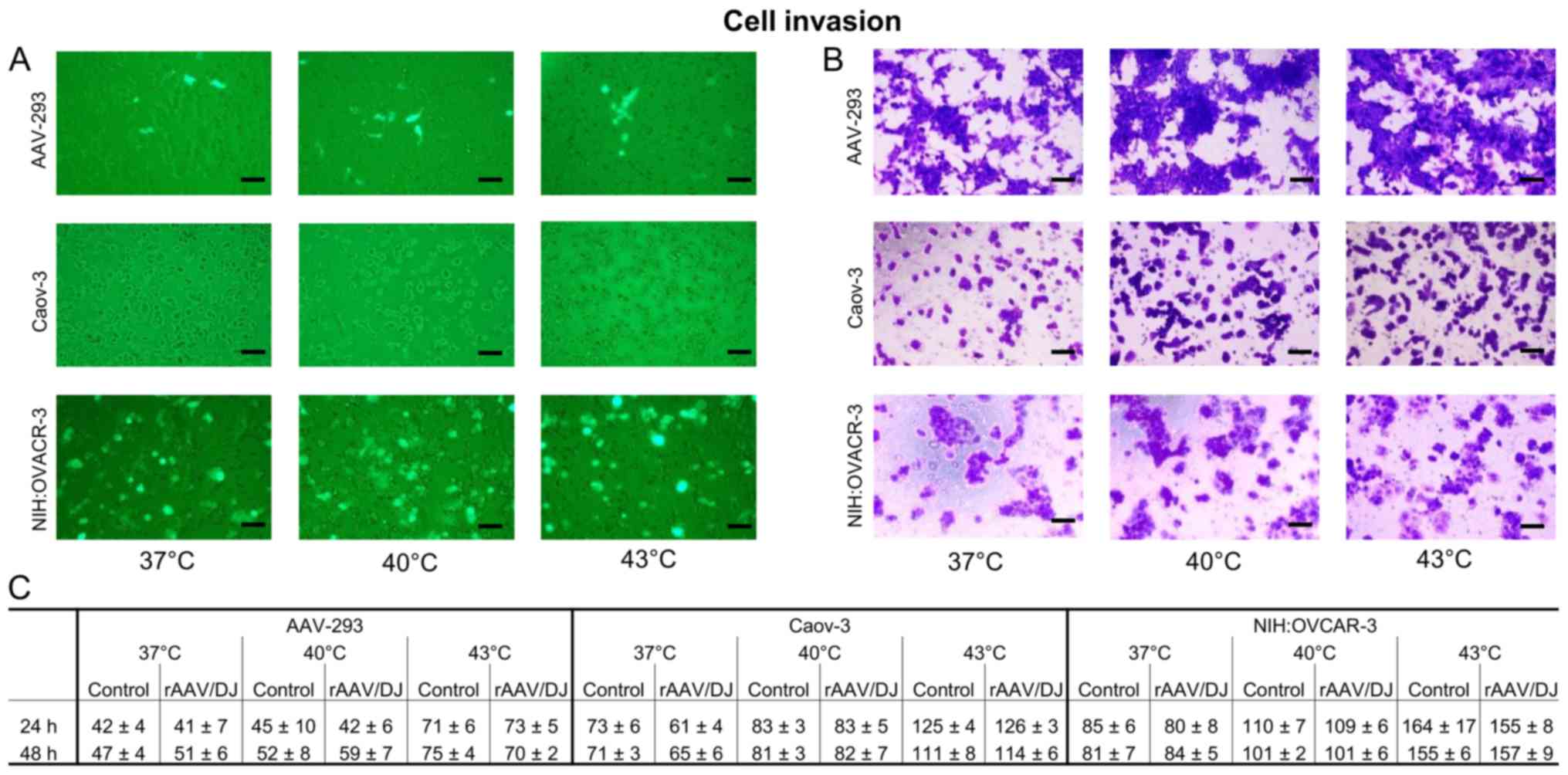
Invasion chamber assay
To determine how transduction and hyperthermia can
change the invasiveness od tested cells, Matrigel chamber assays
were performed. The invasive cells were counted, and crystal violet
stained. Results presented on Fig. 8
showed that hyperthermia increased the invasiveness of all tested
cells in both groups (transductants and non-transductants) in
temperature-dependent manner. The highest cell numbers in the lower
chambers were observed at 43°C. Moreover, the NIH:OVCAR-3 was
indicated as the most invasive cell line. The non-cancerous AAV-293
line revealed the lowest number of cells which pass through the
chamber membrane. Number of the cells migrated to the lower chamber
through the Matrigel, collected after 24 and 48 h were similar,
which may indicate significant changes in cellular physiology that
occurred after incubation at hyperthermia. The rAAV/DJ transduction
did not affect the invasive potential of the examined cells.
Discussion
As we read in one of the last issues of the
prestigious journal Science (42), gene therapy, thanks to translational
research, is strengthening its position in clinical trials. The
authors of the review, leaders in the field of gene therapy,
conclude that clinical trials, which started in the 1990 with
varying degrees of success, enabled that gene therapy to now play a
significant role in medicine thanks to advanced basic and
translational research. The authors of the Science article
emphasize the main, extremely crucial solutions in the field of DNA
vectorology, which form the basis for the development of gene
therapy. Moreover, researchers suggest further advances in gene
therapy will be determined by three approaches. These are
strategies based on rAAV vectorology, lenivirus vectors and gene
editing technologies (42). In each
of these strategies, intensive research is conducted to increase
the efficiency of transduction and in vivo selectivity, and
to reduce side effects. Research aimed at optimizing the dose of
used viral vectors is important. Having the tools to reduce the
doses of the vectors used, while maintaining high efficiency of
gene transfer, will lead to increase in the biosafety of viral
vectorology and consequently facilitate the introduction of viral
vectors to patients. The use of physicochemical solutions in gene
transfer methods is gaining attention. One of the approaches is the
use of physical factors, such as temperature, pH, light, sounds for
precise activation/deactivation of gene expression and to improve
the efficiency of transduction (19).
Recently observed interest in using temperature for
gene therapy purposes is partly due to the development of
innovative, oncological patient treatment methods based on
temperature modulation. The hyperthermia described in the
literature assumes local or comprehensive (whole body) heating of
tissues to produce a therapeutic effect. Hyperthermia is primarily
considered a therapeutic strategy complementing or supporting
classical protocols of oncological treatment (21). In addition to the direct cytotoxic
effect on cancer cells, the influence of hyperthermia to vascular
permeability and drug penetration is highlighted (19,21). In
the field of oncological hyperthermia, an interesting solution is
Hyperthermic Intraperitoneal Chemotherapy-HIPEC. The importance of
HIPEC in the treatment of intraperitoneal dissemination of tumors
originating from the gastrointestinal tract and the female genital
organs, including ovarian cancer, is emphasized (22,43,44).
Individual selected cytostatics (e.g., mitomycin C, doxorubicin,
cisplatin) administered to oncological patients by perfusion under
elevated temperature (hyperthermia conditions; e.g., 0.5–1.5 h,
41–43°C) (43) may significantly
increase the patients' lifetime (22,44). The
use of hyperthermia in clinics may be associated with some
complications. Generally, healthy tissues are not destroyed during
hyperthermia. The differences into tissue characteristics implicate
to the changes between heat distribution in patient's body. This
may result in burns, discomfort or pain. Complications associated
with the method of achieving high temperature of tissue, in the
case of perfusion techniques may appear tissue swelling, blood
clots, bleeding, and other damage in the perfusion area, however,
most of these side effects are temporary. Whole body hyperthermia
may cause more serious side effects, such as cardiological and
vascular disorders, but these are rare events. However, in
comparison with the results achieved by the combination of
chemotherapy and hyperthermia (significantly increase the patients'
lifetime), it is worth using this method of treatment in oncology
(22,43–46).
Increasing the temperature is usually accompanied by a change in
the pattern of HSP gene expression. Grimmig et al (26) emphasize the functional meaning of the
increase in HSP expression in the course of chemotherapy in
hyperthermia. Modern treatment of ovarian cancer is also based on
the participation of gene therapy (31).
The study attempts to determine the effect of
temperature on the transduction efficiency the ovarian cancer cells
of various clinical origin by substantial gene therapy
vectors-rAAV. Research done in the present study is a continuation
of the work carried out by our team aimed at introducing genes into
cancer cells (47,48). Some researchers are practicing
physicians and are directly involved in treating patients through
hyperthermia. As shown in Fig. 2A,
we reveal that increasing temperature from 37 to 40°C or even to
43°C leads to the increase of the ovarian cancer cell transduction
efficiency by the rAAV/DJ vectors. In order to check the
transcriptional activity of promoters, the rAAV/DJ mosaic vector
with the CAG hybrid promoter for AAV-293 cells was used in the
work, while the conventional cmv promoter was used for ovarian
cancer lines. In the case of AAV-293 (Fig. 2), 11% GFP+ cells correspond to
approx. 123000 gc, while extrapolating the transduction results for
the Caov-3, 11% of GFP+ cells would correspond to as much as 600000
gc. The obtained results indicate higher transcriptional activity
of CAG promoters, compared to cmv, which is consistent with results
in other studies (49,50). Although the used rAAV mosaic vector
is considered to have a wide range of tropism (51), in the present study, as shown in
Fig. 2A, substantial differences in
the transduction of tested cell lines by rAAV/DJ are evident. For
ascites-derived ovarian cells, the transduction efficiency in 37°C
was at 23%, and for solid-derived cancer cells-Caov-3 at only 2%.
In the context of gene therapy reasons, the differences between
cell transduction efficiency may have clinical importance and
define the necessary selectivity of drug delivery due to
development of the personalized treatment. In fact, the
transduction differs depending on the cell types. In the present
study, an universal vector (rAAV/DJ) was used, which is generally
characterized by good transduction efficiency, regardless of the
cells' origin. It is worth noting that in the gene therapy
protocols the different vectors are used. The examples of efficient
vectors for cells poorly transduced by rAAV are known (e.g.,
lentiviral vectors, adenoviral vectors). Our research is
innovative, and we decided to choose one vector, which will
transduce different cell lines. Differences between rAAV/DJ
transduction efficiency are not obstacle for the develop further
gene therapy and hyperthermia-based treatment, because gene therapy
engineering propose various types of effective vectors.
The use of elevated temperatures significantly
increased the efficiency of rAAV transduction in both lines. For
NIH:OVCAR-3, a 65% increase in transduction was observed at 40°C
and 70% at 43°C, while for Caov-3 cells, a 50 and a 200% increase,
respectively. The number of copies of the vector in the
transductants corresponded to the number of GFP+ cells, but only at
the temperatures of 37 and 40°C. Unexpectedly, the Caov-3 cells
exposed to 43°C were characterized with high number of GFP+ cells
and lower level of rAAV genome copies. This may suggest that
hyperthermia (at higher temperatures, like 43°C) activates the
intracellular mechanism of vector degradation in Caov-3. On the
other hand, the other lines appear to be more resistant to
potential temperature-stimulated degradation.
The stimulatory effect of the elevated temperature
on the performance of rAAV transduction was also demonstrated in
the work of other authors. In the publication of Zhong et al
(12) showed that 4 h exposure of
Hela cells to 42,5°C leads to about 6× increase in rAAV
transduction efficiency. In this study, 3 h stimulation was used at
40 and 43°C. The viability cells in response to heat shock was
determined at 95–98% (results not shown in the paper). The works of
Zhong et al (12) and Zhao
et al (52) it was revealed
that the increase in rAAV transduction efficiency at a higher
temperature is associated with the expression of selected HSP genes
and the existence of functional connections between HSP and FKBP52
protein. The authors of the works showed that phosphorylated FKBP52
interacts with D-sequences in inverted terminal repeats of
adeno-associated virus 2 genome, which in turn leads to inhibition
of second strand synthesis of AAV DNA and further to inefficient
expression of reporter transgenes, i.e. a decrease in transduction
efficiency. In this study, attention was paid to the expression of
a comprehensive panel of HSP (HSP expression plate) genes including
42 genes predominantly from the HSP40, HSP60, and HSP90 families.
The constitutive HSP levels in cells cultured under standard
conditions (37°C) as well as in cells exposed to 40 and 43°C for 3
h were examined. The study allowed us to define HSP expression
signature cells transduced with different levels of efficiency.
Differences in constitutive and temperature-stimulated HSP
expression between the cell lines were demonstrated in Figs. 5 and 6. It can be postulated that HSP specific
signatures may promote rAAV transduction. Efficiently transducing
NIH:OVCAR-3 cells are characterized by highly expressed HSP40
(DNAJA2, DNAJB13, DNAJB2, DNAJB4, DNAJC7), HSP90 (HSP90AA1,
HSP90B1), and HSP70 (HSPA14, HSPA1A, HSPA1L, HSPA4, HSPA4L, HSPA6).
Lower levels of HSP expression, mainly DNAJB1+, HSP90AB1+, HSPA5+,
HSPA8+, and HSPB1+, were observed in less-effectively transduced
Caov-3 cells.
In 2016, a study published in Nature was the
key to determine the concept of the cellular transmission of rAAV
(6). Pillay et al (6) pointed to the significant role of AAV
transmembrane protein (KIAA0319L), defined as AAVR, in the
transmission of rAAV. Subsequent team work expanded the knowledge
of AAV transmission with the participation of AAVR (7,8). This
study also analyzed the expression of crucial genes of the rAAV
transmission to cells. The AAVR, HSPG1 and HSPG2 genes were
selected for evaluation. Differences were found in the expression
pattern between the cell lines and the effect of elevated
temperature on the level of expression (Fig. 3). The obtained results correspond
with the literature data indicating that there are specific
patterns of rAAV transmission for various cells, which are
sometimes even, as recently reported by Dudek et al
(7), which are independent of the
expression of AAVR. The expression of the AAV transmission protein
genes was also associated with the miRNA pattern in transduced
cells. Based on the information available in miRNA databases and
using the proposed algorithms, functional links were found between
the designated miRNAs and the AAVR, HSPG1 and HSPG2 genes (40,41).
Based on preliminary studies, it was established that a high rAAV
transduction efficiency and high levels of AAVR, HSPG1 and HSPG2
expression (Fig. 3), occur together
with a lower level of miRNAs (Fig.
4) that silence the expression of AAV receptors and genes. On
the other hand, in the cells with a high level of miRNA silencing
(Caov-3 cells, Fig. 4), lower
transduction efficiency and a lower 3–5× expression level of the
tested receptors was observed. Moreover, considering our other
studies on miRNA profiling in tumors, it seems that the miRNA
signatures can be a valuable complement to the information panel,
which should be analyzed before attempting gene therapy. In studies
that are the subject of another work (based on TLDA cards that
allow the evaluation of the expression of more than 700 miRNA) we
have demonstrated that, for example, in breast cancer cells there
is a specific miRNA signature that is helpful in choosing a vector
for gene therapy trials. The clinical benefits of miRNA profiling
can also be found in the work of other authors (53,54).
To better understand the changes occurring in the
studied cells transduced at hyperthermia, experiments for adhesion
and invasion were performed. As shown in the Fig. 7, laminin reduces cell adhesion. The
results demonstrated that hyperthermia induced the decrease of the
number of adhered cells. Luchetti et al (55) showed that in cells of neuroblastoma
exposed to hyperthermia, a floating population of cells increased,
which had down-regulation expression of CD11a integrin molecule.
Perhaps the analogous mechanism occurred for ovarian cancer and
AAV-293 cells. Probably, the incubation at the increased
temperature can cause change of integrins expression in cells,
which in turn promoted their detachment. Furthermore, hyperthermia
increased the invasiveness of all tested cells (Fig. 8), which may suggest the relation to
HSPs essential for cell adhesion and invasion. For example, HSP27
is involved in the regulation of the cytoskeleton, which may affect
the migration of tumor cells (56).
Voll et al (57) showed that
HSP27 increased in prostate cancer cell motility and invasion.
Another instance can be over-expression of HSP70 enhances tumor
growth, cancer cell migration and metastasis. It is postulated that
the expression of HSP correlates with poor prognosis of treatment
(58). In our study the expression
of HSP27 in cells exposed to hyperthermia was increased in
NIH:OVCAR-3, interestingly this line after incubation at 43°C was
also characterized by the smallest percentage of cells adhered to
laminin. Furthermore, NIH:OVCAR-3 showed the greatest invasion
potency and had increased expression of HSP70, both constitutively
and after exposed to hyperthermia. On the other hand, in study Xie
et al (59) and Jin et
al (60) indicated that
hyperthermia could inhibit cancer cells invasion in vitro.
However, the methodology details in the mentioned publications were
different. It is worth underlining that the time of measurement can
be an important factor, it seems that the ability to
invasion/migration of cells measured directly after the exposure of
cells to the high temperatures is decreased. But after a longer
interval of time from hyperthermia (as documented in our study)
cell migration and invasion may achieve higher results than these
from original state. The time required to expression of HSP seems
to be a crucial factor in cancer invasiveness.
The study showed that at elevated temperature
ovarian cancer cells were more efficiently transduced with rAAV. It
was also indicated that temperature-dependent transduction is in
relation to the expression of the rAAV receptor genes and HSP
genes. Moreover, hyperthermia modified the invasiveness of the
studied cells. The obtained information can be helpful in the
design and implementation of effective protocols for ovarian cancer
gene therapy. Ovarian cancer is currently the primary cause of
death among women suffering from gynecological cancer. The classic
treatment protocols are not very effective. Among patients, there
is a low 5-year survival rate and a high percentage of
chemo-resistant cancer recurrence (31,61). The
need to develop new therapeutic solutions is extremely pressing.
Pre-clinical studies and weak effects of clinical trials of ovarian
cancer force the search for new solutions in the field of gene
transfer methods (31). In the
context of the results obtained in this study, it seems that the
hyperthermia strategy present in oncology clinics, as a method
supporting oncological treatment, may also be helpful in increasing
the effectiveness of gene therapy of ovarian cancer based on rAAV
vectors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Centre for Research and Development (grant no.
Strategmed1/233264/4/NCBR/2014; acronym, MentorEYE).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AB and MM conceived and designed the experiments of
the present study. AB, MD, MO, ŻS and OC performed the experiments.
AB, JJ and MM analyzed the data. AB and MM wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Naso MF, Tomkowicz B, Perry WL and Strohl
WR: Adeno-associated virus (AAV) as a vector for gene therapy.
BioDrugs. 31:317–334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yalkinoglu AO, Heilbronn R, Bürkle A,
Schlehofer JR and zur Hausen H: DNA Amplification of
adeno-associated virus as a response to cellular genotoxic stress.
Cancer Res. 48:3123–3129. 1988.PubMed/NCBI
|
|
3
|
Ginn SL, Amaya AK, Alexander IE, Edelstein
M and Abedi MR: Gene therapy clinical trials worldwide to 2017: An
update. J Gene Med. 20:e30152018. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berry GE and Asokan A: Cellular
transduction mechanisms of adeno-associated viral vectors. Curr
Opin Virol. 21:54–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stutika C, Mietzsch M, Gogol-Döring A,
Weger S, Sohn M, Chen W and Heilbronn R: Comprehensive small
RNA-seq of adeno-associated virus (AAV)-infected human cells
detects patterns of novel, non-coding AAV RNAs in the absence of
cellular miRNA regulation. PLoS One. 11:e01614542016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pillay S, Meyer NL, Puschnik AS, Davulcu
O, Diep J, Ishikawa Y, Jae LT, Wosen JE, Nagamine CM, Chapman MS
and Carette JE: An essential receptor for adeno-associated virus
infection. Nature. 530:108–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dudek AM, Pillay S, Puschnik AS, Nagamine
CM, Cheng F, Qiu J, Carette JE and Vandenberghe LH: An alternate
route for adeno-associated Virus (AAV) entry independent of AAV
receptor. J Virol. 92:e02213–e02217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pillay S and Carette JE: Host determinants
of adeno-associated viral vector entry. Curr Opin Virol.
24:124–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gigout L, Rebollo P, Clement N, Warrington
KH Jr, Muzyczka N, Linden RM and Weber T: Altering AAV tropism with
mosaic viral capsids. Mol Ther. 11:856–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCarty DM: Self-complementary AAV
vectors; advances and applications. Mol Ther. 16:1648–1656. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei F, McConnell KI, Yu TK and Suh J:
Conjugation of paclitaxel on adeno-associated virus (AAV)
nanoparticles for co-delivery of genes and drugs. Eur J Pharm Sci.
46:167–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong L, Qing K, Si Y, Chen L, Tan M and
Srivastava A: Heat-shock treatment-mediated increase in
transduction by recombinant adeno-associated virus 2 vectors is
independent of the cellular heat-shock protein 90. J Biol Chem.
279:12714–12723. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mitchell AM and Samulski RJ: Mechanistic
insights into the enhancement of adeno-associated virus
transduction by proteasome inhibitors. J Virol. 87:13035–13041.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berry GE and Asokan A: Chemical modulation
of endocytic sorting augments adeno-associated viral transduction.
J Biol Chem. 291:939–947. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morrison SF and Nakamura K: Central neural
pathways for thermoregulation. Front Biosci (Landmark Ed).
16:74–104. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romanovsky AA: Skin temperature: Its role
in thermoregulation. Act physiol (Oxf). 210:498–507. 2014.
View Article : Google Scholar
|
|
17
|
González-Alonso J: Human thermoregulation
and the cardiovascular system. Exp Physiol. 97:340–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walter EJ, Hanna-Jumma S, Carraretto M and
Forni L: The pathophysiological basis and consequences of fever.
Crit Care. 20:2002016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazzotta E, Tavano L and Muzzalupo R:
Thermo-sensitive vesicles in controlled drug delivery for
chemotherapy. Pharmaceutics. 10:E1502018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Zhang Y, Huang H, Zhang H, Hou L
and Zhang Z: Functionalized graphene oxide-based thermosensitive
hydrogel for near-infrared chemo-photothermal therapy on tumor. J
Biomater Appl. 30:1230–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hildebrandt B, Wust P, Ahlers O, Dieing A,
Sreenivasa G, Kerner T, Felix R and Riess H: The cellular and
molecular basis of hyperthermia. Crit Rev Oncol Hematol. 43:33–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jewell A, McMahon M and Khabele D: Heated
intraperitoneal chemotherapy in the management of advanced ovarian
cancer. Cancers (Basel). 10:E2962018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hantute-Ghesquier A, Haustrate A,
Prevarskaya N and Lehen'kyi V: TRPM family channels in cancer.
Pharmaceuticals (Basel). 11:582018. View Article : Google Scholar
|
|
24
|
Fels B, Bulk E, Pethő Z and Schwab A: The
role of TRP channels in the metastatic cascade. Pharmaceuticals
(Basel). 11:E482018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arrigo AP: Mammalian HspB1 (Hsp27) is a
molecular sensor linked to the physiology and environment of the
cell. Cell Stress Chaperones. 22:517–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grimmig T, Moll EM, Kloos K, Thumm R,
Moench R, Callies S, Kreckel J, Vetterlein M, Pelz J, Polat B, et
al: Upregulated heat shock proteins after hyperthermic chemotherapy
point to induced cell survival mechanisms in affected tumor cells
from peritoneal carcinomatosis. Cancer Growth Metastasis.
10:11790644177305592017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prodromou C: Mechanisms of Hsp90
regulation. Biochem J. 473:2439–2452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu Y, Tian Y, Wu Y and Cui S: Clinical
significance of heat shock proteins in gastric cancer following
hyperthermia stress: Indications for hyperthermic intraperitoneal
chemoperfusion therapy. Oncol Lett. 15:9385–9391. 2018.PubMed/NCBI
|
|
29
|
Stope MB, Koensgen D, Burchardt M, Concin
N, Zygmunt M and Mustea A: Jump in the fire-heat shock proteins and
their impact on ovarian cancer therapy. Crit Rev Oncol Hematol.
97:152–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qing K, Hansen J, Weigel-Kelley KA, Tan M,
Zhou S and Srivastava A: Adeno-Associated virus type 2-mediated
gene transfer: Role of cellular FKBP52 protein in transgene
expression. J Virol. 75:8968–8976. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Áyen Á, Jiménez Martínez Y, Marchal JA and
Boulaiz H: Recent progress in gene therapy for ovarian cancer. Int
J Mol Sci. 19:E19302018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hamilton TC, Young RC, McKoy WM,
Grotzinger KR, Green JA, Chu EW, Whang-Peng J, Rogan AM, Green WR
and Ozols RF: Characterization of a human ovarian carcinoma cell
line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer
Res. 43:5379–5389. 1983.PubMed/NCBI
|
|
33
|
Karlan BY, Jones J, Slamon DJ and Lagasse
LD: Glucocorticoids stabilize HER-2/neu messenger RNA in human
epithelial ovarian carcinoma cells. Gynecol Oncol. 53:70–77. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Capes-Davis A, Theodosopoulos G, Atkin I,
Drexler HG, Kohara A, Macleod RA, Masters JR, Nakamura Y, Reid YA,
Reddel RR and Freshney RI: Check your cultures! A list of
cross-contaminated or misidentified cell lines. Int J Cancer.
127:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bairoch A: The cellosaurus, a cell-line
knowledge resource. J Biomol Tech. 29:25–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aurnhammer C, Haase M, Muether N, Hausl M,
Rauschhuber C, Huber I, Nitschko H, Busch U, Sing A, Ehrhardt A and
Baiker A: Universal real-time PCR for the detection and
quantification of adeno-associated virus serotype 2-derived
inverted terminal repeat sequences. Hum Gene Ther Methods.
23:18–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bustin SA: A-Z of quantitative
PCRInternational University Line; La Jolla, CA: 2004
|
|
39
|
Chomczynski P and Sacchi N: Single step
method of RNA isolation by acid guanidinium
thyocyanate-phenolcholoroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Research. 43((Database Issue)): D146–D152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: A resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46(D1): D296–D302.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dunbar CE, High KA, Joung JK, Kohn DB,
Ozawa K and Sadelain M: Gene therapy comes of age. Science.
359:eaan46722018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Turaga K, Levine E, Barone R, Sticca R,
Petrelli N, Lambert L, Nash G, Morse M, Adbel-Misih R, Alexander
HR, et al: Consensus guidelines from The American Society of
Peritoneal Surface Malignancies on standardizing the delivery of
hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal
cancer patients in the United States. Ann Surg Oncol. 21:1501–1505.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rutkowski P, Śpiewankiewicz B, Herman K,
Jastrzębski T, Kładny J, Kojs Z, Krzakowski M, Polkowski W, Wyrwicz
L, Wysocki P, et al: Polish clinical practice guideline on
hyperthermic intraperitoneal chemotherapy (HIPEC) with
cytoreductive surgery in peritoneal melignancy treatment. Curr
Gynecol Oncol. 12:86–97. 2014. View Article : Google Scholar
|
|
45
|
van der Zee J: Heating the patient: A
promising approach? Ann Oncol. 13:1173–1184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wust P, Hildebrandt B, Sreenivasa G, Rau
B, Gellermann J, Riess H, Felix R and Schlag PM: Hyperthermia in
combined treatment of cancer. Lancet Oncol. 3:487–497. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Orzechowska M, Fabijańska M, Ochocki J and
Małecki M: Anticancer activity of a trans-platinum(II) complex of
3-aminoflavone to ovarian cancer cells. Ginekol Pol. 88:68–74.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grecka E, Statkiewicz M, Gorska A,
Biernacka M, Grygorowicz MA, Masnyk M, Chmielewski M, Gawarecka K,
Chojnacki T, Swiezewska E and Malecki M: Prenyl ammonium salts-new
carriers for gene delivery: A B16-F10 mouse melanoma model. PLoS
One. 11:e01536332016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kosuga M, Enosawa S, Li XK, Suzuki S,
Matsuo N, Yamada M, Roy-Chowdhury J, Koiwai O and Okuyama T:
Strong, long-term transgene expression in rat liver using chicken
beta-actin promoter associated with cytomegalovirus immediate-early
enhancer (CAG promoter). Cell Transplant. 9:675–680. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Damdindorj L, Karnan S, Ota A, Takahashi
M, Konishi Y, Hossain E, Hosokawa Y and Konishi H: Assessment of
the long-term transcriptional activity of a 550-bp-long human
β-actin promoter region. Plasmid. 68:195–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lerch TF, O'Donnell JK, Meyer NL, Xie Q,
Taylor KA, Stagg SM and Chapman MS: Structure of AAV-DJ, a
retargeted gene therapy vector: Cryo-electron microscopy at 4.5 Å
resolution. Structure. 20:1310–1320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao W, Zhong L, Wu J, Chen L, Qing K,
Weigel-Kelley KA, Larsen SH, Shou W, Warrington KH Jr and
Srivastava A: Role of cellular FKBP52 protein in intracellular
trafficking of recombinant adeno-associated virus 2 vectors.
Virology. 353:283–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ganju A, Khan S, Hafeez BB, Behrman SW,
Yallapu MM, Chauhan SC and Jaggi M: miRNA nanotherapeutics for
cancer. Drug Discov Today. 22:424–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luchetti F, Mannello F, Canonico B,
Battistelli M, Burattini S, Falcieri E and Papa S: Integrin and
cytoskeleton behaviour in human neuroblastoma cells during
hyperthermia-related apoptosis. Apoptosis. 9:635–648. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang B, Xie F, Aziz AUR, Shao S, Li W,
Deng S, Liao X and Liu B: Heat shock protein 27 phosphorylation
regulates tumor cell migration under shear stress. Biomolecules.
9:E502019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Voll EA, Ogden IM, Pavese JM, Huang X, Xu
L, Jovanovic BD and Bergan RC: Heat shock protein 27 regulates
human prostate cancer cell motility and metastatic progression.
Oncotarget. 5:2648–2663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Calderwood SK, Khaleque MA, Sawyer DB and
Ciocca DR: Heat shock proteins in cancer: Chaperones of
tumorigenesis. Treands Biochem Sci. 31:164–172. 2006. View Article : Google Scholar
|
|
59
|
Xie X, Shao X, Gao F, Jin H, Zhou J, Du L,
Zhang Y, Ouyang W, Wang X, Zhao L, et al: Effect of hyperthermia on
invasion ability and TGF-β1 expression of breast carcinoma MCF-7
cells. Oncol Rep. 25:1573–1579. 2011.PubMed/NCBI
|
|
60
|
Jin H, Xie X, Hu B, Gao F, Zhou J, Zhang
Y, Du L, Wang X, Zhao L, Zhang X, et al: Hyperthermia inhibits the
proliferation and invasive ability of mouse malignant melanoma
through TGF-β(1). Oncol Rep. 29:725–734. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Narod SA: Personalised medicine and
population health: Breast and ovarian cancer. Hum Genet.
137:769–778. 2018. View Article : Google Scholar : PubMed/NCBI
|