Introduction
Thyroid hormones have profound effects on the heart
and cardiovascular system (1,2).
Cardiopulmonary bypass (CPB) leads to a ‘euthyroid-sick’ state
(3). This low triiodothyronine
(T3) state manifests as low circulating levels of
T3 with hemodynamic abnormalities (4). There is a growing body of experimental
data that suggests that T3 supplementation improves
hemodynamic parameters after ischemic injury in animal models of
CPB (5,6) and in isolated heart studies (7,8).
Previously, studies using experimental models of ischemia
reperfusion (I/R) revealed that T3 administration
improved the recovery of post-ischemic cardiac performance and
reduced the pro-fibrotic process that leads to adverse cardiac
remodeling (2,9–11).
Cytosolic calcium (Ca2+) overload plays a
major role in the development of myocardial injury during I/R. The
sarcoplasmic reticulum (SR) is critical in Ca2+ uptake
via Ca2+-ATPase (SERCA2a) and Ca2+ release
via the Ca2+ release channels (ryanodine receptors;
RyRs). Human cardiomyocyte calcium handling and phenotype are
strongly influenced by T3. ‘Low T3 syndrome’
is observed in the progression of I/R injury (12). It is possible that myocardial
protection induced by T3 supplementation involves the
protection of SR function. The effects of T3
supplementation on myocardial contractility may be attributed to
their effects on intracellular Ca2+ homeostasis. The
authors have previously reported a marked Ca2+ overload
in the mitochondrial compartment during I/R (13). The ability of T3
supplementation to improve cardiac function, induce the activation
of sarcolemma and SERCA2a, and decrease cytosolic (Ca2+)
accumulation have been reported previously (13). However, the mechanisms that underlie
these cardioprotective effects of thyroid hormone remain largely
unknown. In the present study, it was hypothesized that the cardiac
protection of T3 supplementation against I/R injury is
associated with the preservation of Ca2+-cycling
proteins and high-energy phosphate contents.
Materials and methods
Animals
Adult male Sprague-Dawley rats (n=50; weight,
350–425 g) were obtained from the Animal Center of Soochow
University (Suzhou, China). Rats were fed a standard diet and were
housed at 25°C with 60% humidity under a 12 h light-dark cycle. All
rats were allowed access to food and water ad libitum for
one week before experiments. The protocol was reviewed and approved
by the Institutional Animal Care and Use Committee of Nanjing
University.
Langendorff isolated heart preparation
and measurements
The methods employed have been previously described
(13). Briefly, rats were
anesthetized and decapitated when unresponsive to noxious
stimulation. The hearts were excised and perfused in the
Langendorff mode at a perfusion pressure equivalent to 80 mmHg.
Perfusate and bath temperatures were maintained at 37.2±0.1°C using
a thermostatically controlled water circulator (Lauda E100; Lauda
Dr R Wobser GmbH & Co., KG). Left ventricular pressure (LVP)
and coronary flow (CF) were measured at a constant temperature and
perfusion pressure (100 mmHg). Coronary inflow and coronary venous
Na+, K+, Ca2+ and pH were measured
offline with an intermittently self-calibrating analyzer system
(Radiometer Copenhagen ABL 505; Radiometer, Ltd.). Coronary outflow
(coronary sinus) O2 tension was also measured online
continuously with a Clark-type O2 electrode (203B;
Instech Laboratories, Inc.). Myocardial O2 consumption
(MVO2) was calculated as [(coronary flow/g heart weight)
× (arterial pO2-venous pO2)] × 24 µl
O2/ml at 760 mmHg; and cardiac work efficiency was
calculated as [systolic-diastolic LVP × HR]/MVO2. At the
end of the experiments, the hearts were freeze-clamped and stored
at −80°C until subsequent use in western blot assays.
Experimental group establishment
Animals were randomly divided into 5 groups. The
untreated sham (non-ischemic) group (Sham, n=10) was perfused for
225 min and after 15 min the equilibration/stabilization of
functional parameters were employed; hearts were not subjected to
ischemia. Ischemia groups underwent 15 min of stabilization and 60
min perfusion with or without T3 administration at three
different doses (10, 25 and 50 nM), followed by 30 min ischemia and
120 min reperfusion [n=10 each for the control (CTL),
T3−10, T3−25 and T3−50 groups].
The range of doses was selected according to a previous study
(14). A three-way stopcock, located
immediately above the aortic cannula, allowed the induction of
global, no-flow ischemia.
Western blot assays for SR and
sarcomlemmal Ca2+-cycling and -regulating proteins
Western blot assays were conducted as previously
described (15). Briefly, aliquots
of homogenate samples (20 µg protein/lane) were solubilized in
Laemmli sample buffer (cat. no. S3401; Sigma-Aldrich; Merck KGaA)
and fractionated by SDS-PAGE using a 4–20% gel. After transfer to
nitrocellulose membranes, membranes were blocked with 5% nonfat
milk for 1.5 h at room temperature in phosphate-buffered saline and
probed with primary antibodies against RyR2 (cat. no. MA3-916;
1:2,000,), SERCA2a (cat. no. 2A7-A1; 1:2,000), Phospholamban (cat.
no. 2D12; PLB; 1:1,000), and sarcolemmal Ca2+-adenosine
triphosphatase (PMCA; cat. no. 5F10; 1:1,000). The above antibodies
were purchased from Affinity Bioreagents, Inc. The sodium-calcium
exchanger (cat. no. ab3516P; NCX; 1:1,000) was obtained from
Sigma-Aldrich; Merck KGaA. RyR-ser2809 and PLB-Thr17 were purchased
from Badrilla., Ltd., (1:1,000). Secondary antibodies (m-lgGk
BP-HRP, cat. no. sc-516102, Santa Cruz Biotechnology; 1:20,000)
were conjugated to horseradish peroxidase. Immunoreactive bands
were visualized by enhanced chemiluminescence (SuperSignal™ west
pico PLUS chemilumiluminescent substrate, cat. no. 34577; Pierce;
Thermo Fisher Scientific, Inc.). The amount of protein was
determined by densitometry using Kodak 1D software (version 3.4.5;
Sigma-Aldrich; Thermo Fisher Scientific, Inc.) and normalized to
protein load. Positive (purified proteins) and negative (blocking
peptide or blot without primary antibodies) controls were used to
establish the specificity of the protein signals.
Biochemical analysis
At the end of reperfusion, the hearts were
freeze-clamped with aluminum tongs pre-cooled with liquid nitrogen
as described previously (16), to
measure myocardial ATP and creatine phosphate (CP) levels. Briefly,
frozen ventricles were pulverized and mixed with 0.3 M
HClO4 and 0.25 mM EDTA under liquid nitrogen cooling.
The extract was centrifuged at 8,000 × g for 15 min at 4°C and the
resulting supernatant was sampled to measure myocardial ATP and CP
using the high pressure liquid chromatography method as previously
described (16). Myocardial CP was
converted to ATP via the creatine kinase enzymatic reaction.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis used SPSS 20.0 (IBM Corp.). A
two-way analysis of variance was used to assess the overall
difference between groups. Student Newman Keuls' post hoc test was
used for multiple comparisons between the different groups. All
experiments were repeated three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cardiac performance
Table I summarizes
the changes in the indices for coronary flow, heart rate,
MVO2 and cardiac efficiency in the Sham, CTL,
T3−10, T3−25 and T3−50 groups
before 30 min ischemia, and at 120 min of reperfusion. The heart
rates of all the groups were similar prior to ischemia, but
decreased after I/R. There was no significant difference among the
different groups. Coronary flow was much lower than baseline
throughout reperfusion in each of the ischemia groups, but it was
increased in all of the T3 treated groups (returned to
55% in T3−10, 64% in T3−25 and 67% in
T3−50) compared with the CTL group (47%) throughout
reperfusion. After 120 min of reperfusion, MVO2 and
cardiac efficiency were increased in the T3
administrated groups compared with in the CTL group, but remained
lower than the baseline in all of the ischemic groups. Both
parameters of MVO2 and cardiac efficiency in the
T3−25 and T3−50 groups were significantly
increased compared with those in the CTL group (P<0.05; Table I).
 | Table I.Cardiac effects in Sham, CTL, T3 10
nM, T3 25 nM and T3 50 nM before and after ischemia
reperfusion. |
Table I.
Cardiac effects in Sham, CTL, T3 10
nM, T3 25 nM and T3 50 nM before and after ischemia
reperfusion.
| Group | Sham | CTL | T3 10
nM | T3 25
nM | T3 50
nM |
|---|
| HR (beat/min) |
|
Baseline | 266±14 | 269±15 | 262±10 | 265±16 | 271±18 |
|
Reperfusion 120′ | 261±16 | 241±24 | 240±21 | 245±14 | 248±19 |
| CF (ml/min) |
|
Baseline | 12.2±0.8 | 11.9±0.8 | 12±0.7 | 12.2±0.9 | 11.9±0.7 |
|
Reperfusion 120′ | 11.8±1.0 |
5.6±0.6a,b |
6.6±0.5a,b |
7.9±0.6a–c |
8.0±0.7a–c |
| MVO2
(mmHg) |
|
Baseline | 115±8 | 112±11 | 111±10 | 112±12 | 115±10 |
|
Reperfusion 120′ | 112±11 | 58±7a–c | 69±7a,b | 73±8a–c | 75±7a–c |
| Cardiac efficiency
(mmHg·beat·0.1 µl O2·g−1) |
|
Baseline | 16.5±1.3 | 16.6±1.5 | 16.2±1.6 | 16.3±1.1 | 16.5±1.4 |
|
Reperfusion 120′ | 15.6±1.2 |
6.3±0.7a,b |
7.2±0.8a,b |
8.6±0.8a–c |
8.7±0.8a–c |
Left ventricular developed pressure (LVDP) was
decreased after I/R compared with the baseline in each group. LVDP
was increased in the T3 treated groups compared with in
the CTL group upon reperfusion. The improvement in LVDP was
significantly different in the T3−25 and
T3−50 groups compared with the CTLgroup (P<0.05).
There was no significant difference in the T3−10 group
(Fig. 1A). Hearts subjected to
global ischemia for 30 min showed a marked increase in LVDP.
However, a significant reduction in LVEDP was observed with
T3 25 or 50 nM treatment (P<0.05; Fig. 1B). Cardiac contractility +dP/dt and
relaxation -dP/dt (Fig. 1C and D)
were reduced during ischemia in all groups. Upon reperfusion,
contractility increased but still remained lower than that recorded
before ischemia throughout reperfusion in each group. T3
administration at doses of 25 and 50 nM significantly improved
contractile recovery in I/R injury hearts (P<0.05). This was
evidenced by a greater recovery in LVDP and +dP/dt and -dP/dt,
respectively, when compared with the pre-ischemic values.
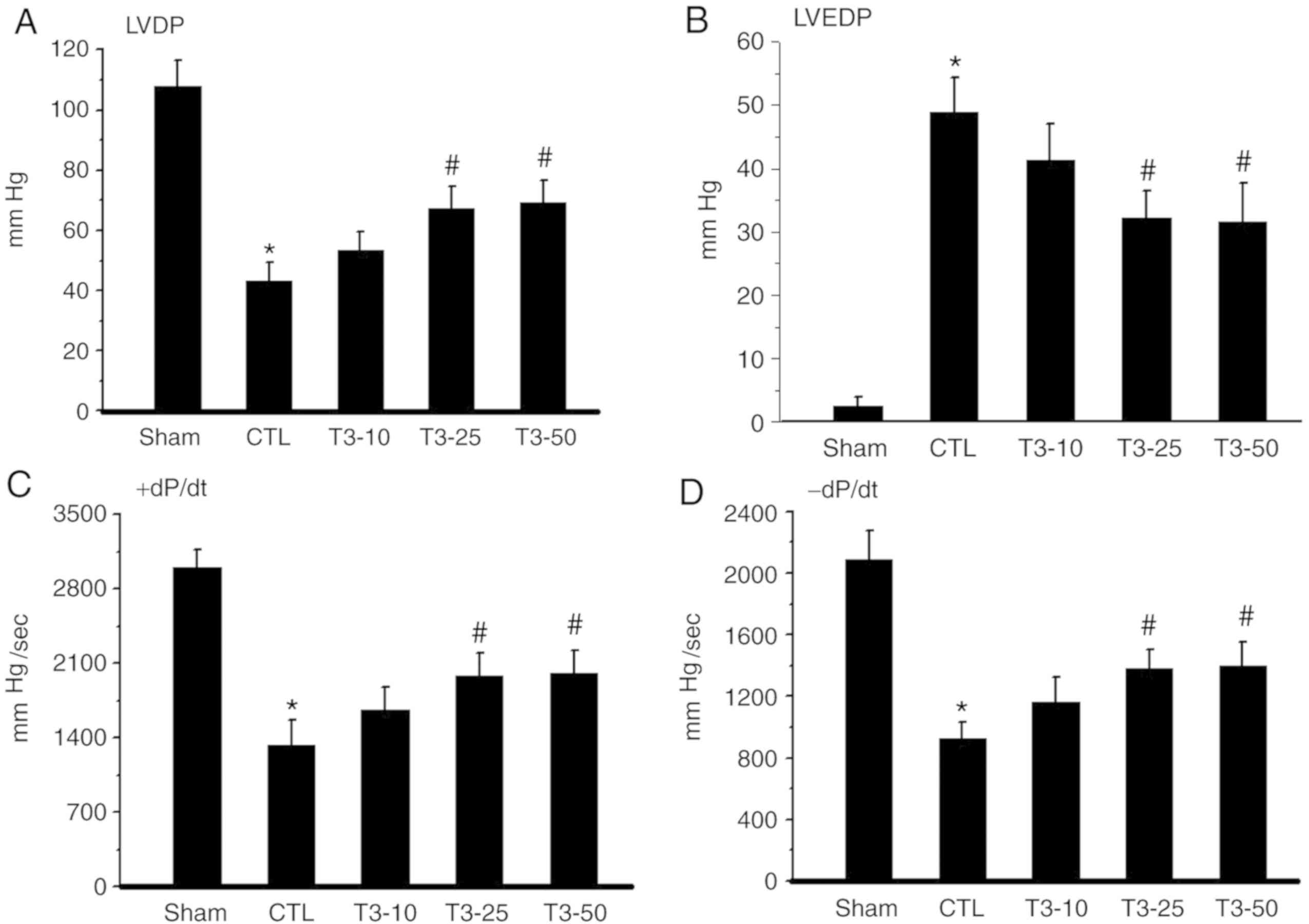 | Figure 1.LVDP, LVEDP, +dP/dt and -dP/dt after
30 min ischemia and 120 min reperfusion. (A) LVDP values in the
Sham, CTL, T3−10, T3-25 and T3−50 groups. (B)
LVEDP values in Sham, CTL, T3−10, T3−25 and
T3−50 groups. Cardiac contractility (C) +dP/dt and
relaxation (D) -dP/dt values in Sham, CTL, T3−10,
T3−25 and T3−50 groups. Values are presented
as the mean ± standard deviation. *P<0.05 vs. the Sham group;
#P<0.05 vs. the CTL group. LVDP, left ventricular
developed pressure; LVEDP, left ventricular end-diastolic pressure;
dP/dt, rate of ventricular pressure development; CTL, ischemia
control group; Sham group, non-ischemic group; T3−10,
T3 10 nM; T3−25, T3 25 nM;
T3−50, T3 50 nM; T3,
Triiodothyronine. |
Effect of T3
supplementation on the SR Ca2+ regulatory proteins
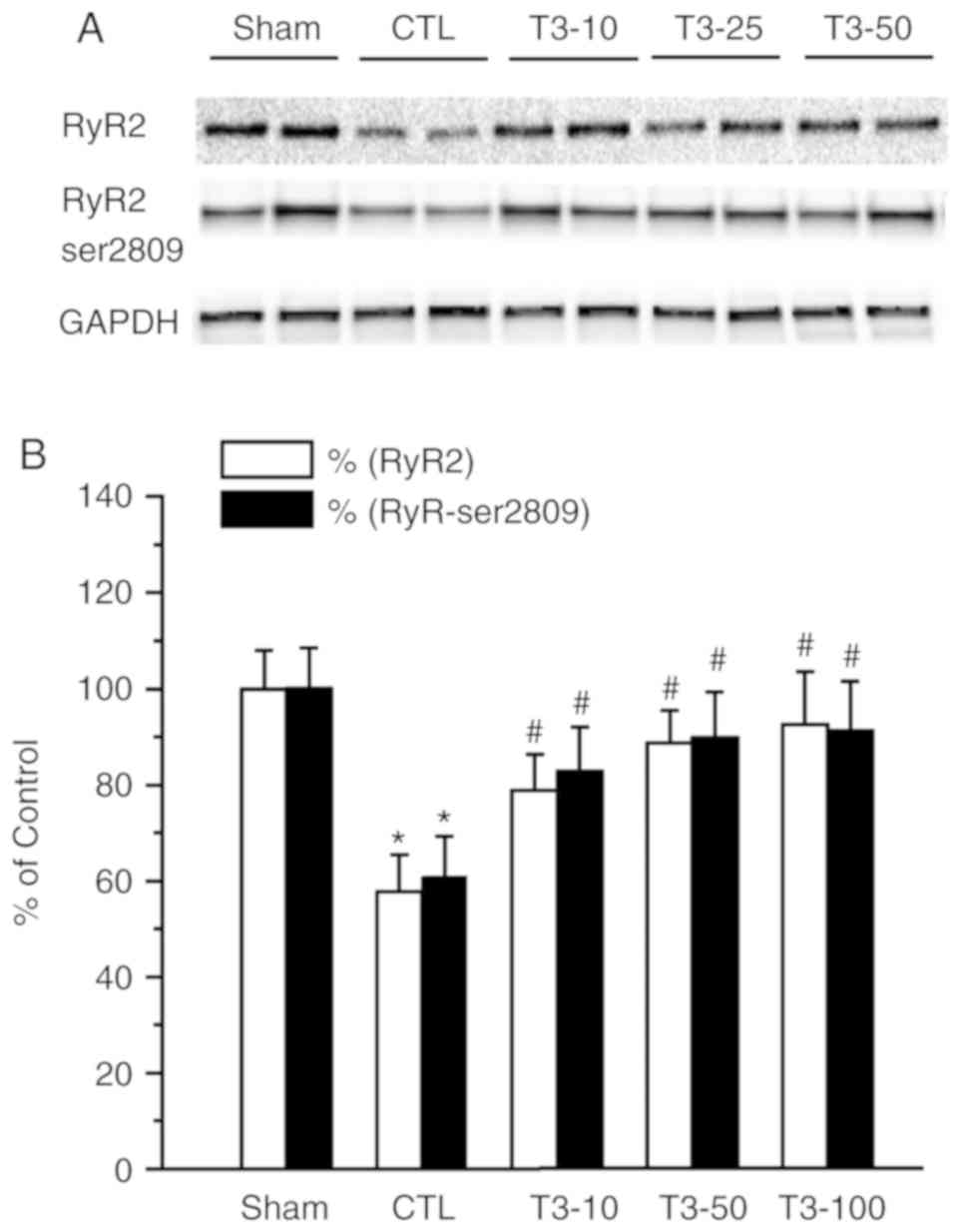
There was a significant reduction in the density of
RyR2 and the phosphorylation status of RyR2 at S2809 in the CTL
group (P<0.05). Treatment with T3 in I/R hearts
significantly prevented the attenuation of RyR2 density and
Ser-2809 (P<0.05 Fig. 2).
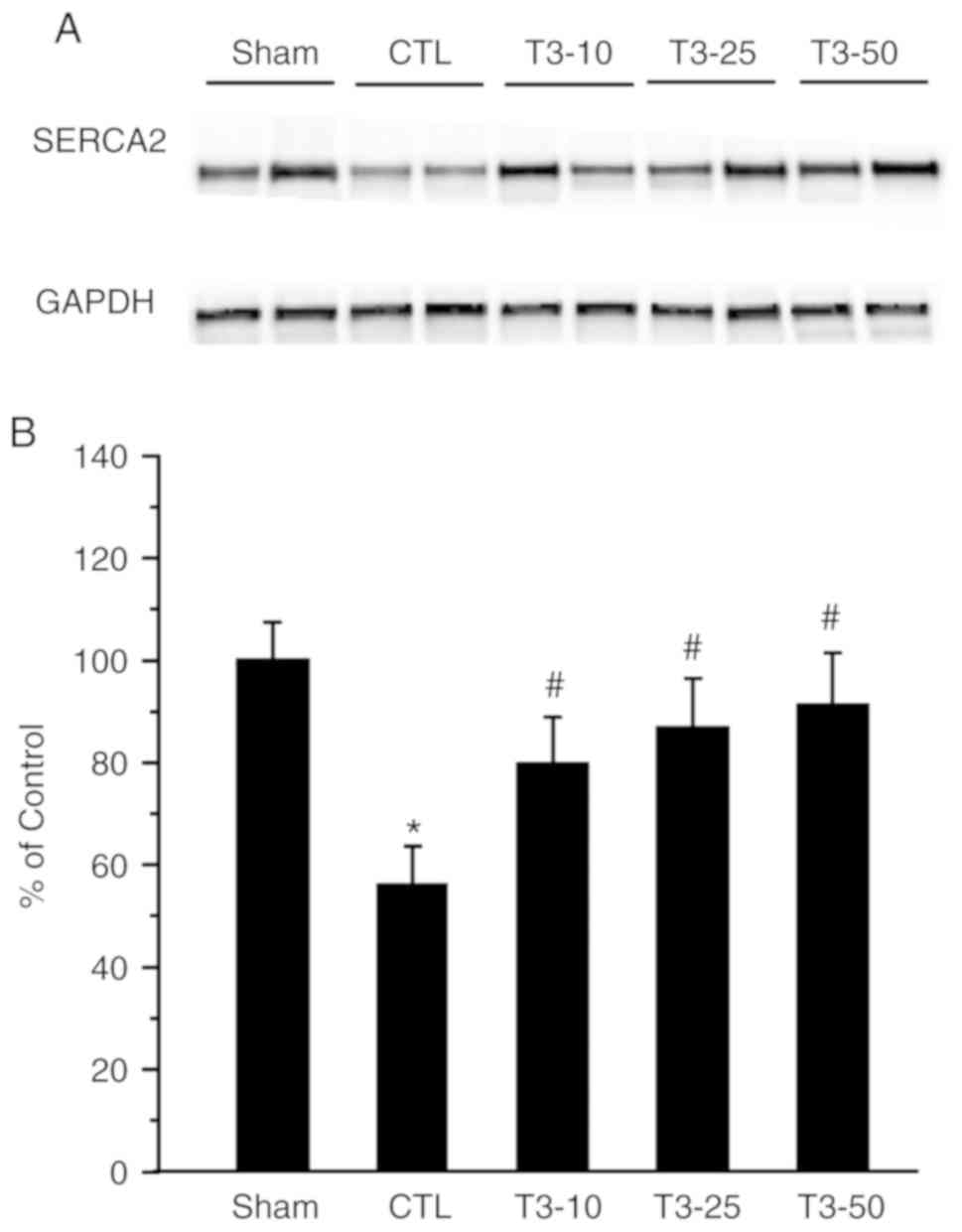
Fig. 3 presents the protein density
of SERCA2a in the control and I/R hearts that were treated with or
without T3 supplementation. Compared with the
non-ischemic values, SERCA2a expression decreased significantly
after I/R injury (P<0.05).
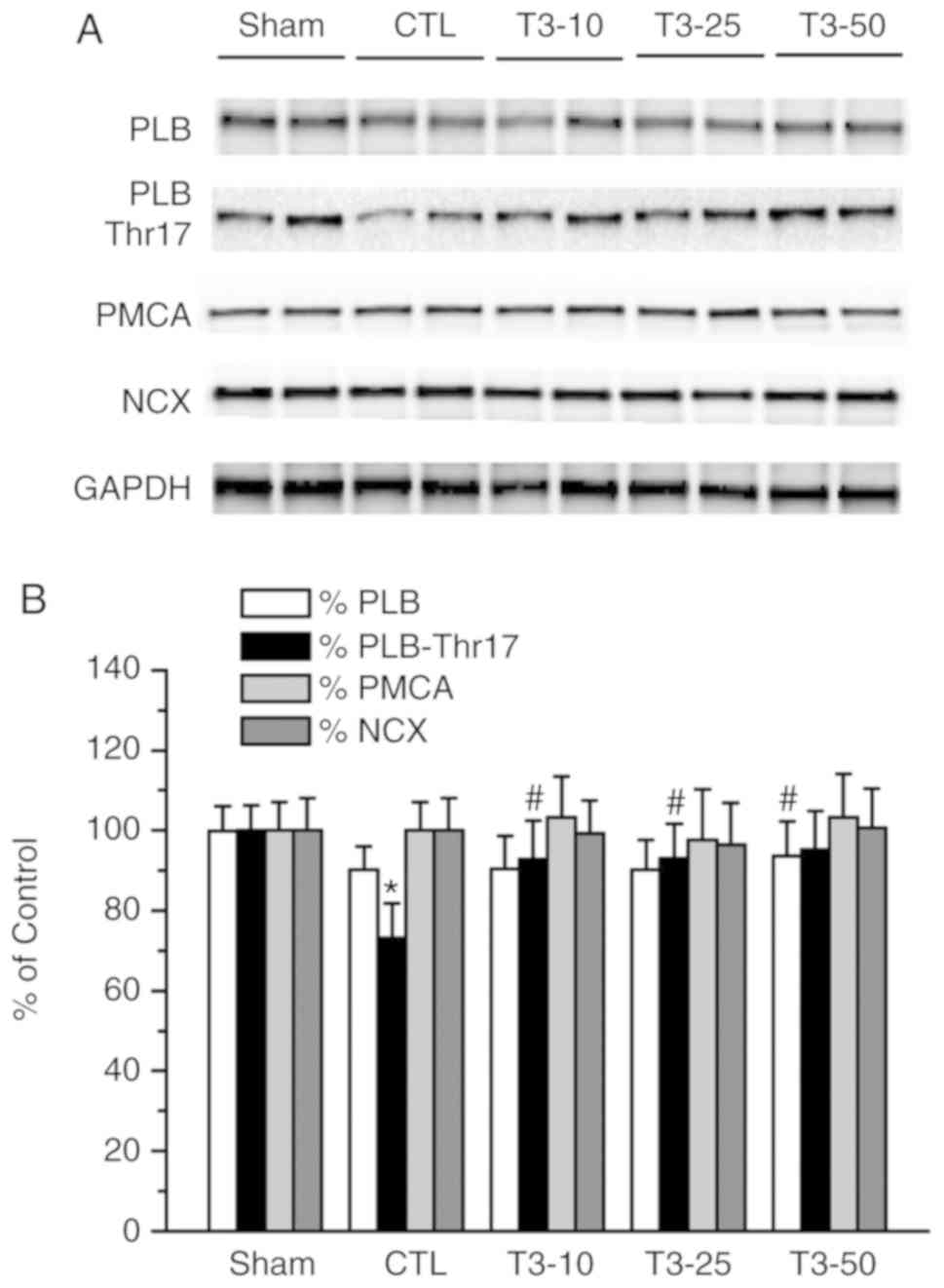
Effect of T3
supplementation on sarcomlemmal Ca2+- regulatory
protein
The density of PLB was lower in all the ischemia
groups; however, there was no significant difference when compared
with the Sham group (Fig. 4). The
phosphorylation of PLB at Thr-17 significantly decreased after 30
min of ischemia compared with the non-ischemia group (Sham group;
P<0.05). T3 treatment at all three doses before
ischemia attenuated this decrease and a significant difference was
detected in all T3 dose groups compared with the ISC
group (P<0.05). The immunoblots of the protein expression of
sarcolemmal PMCA and NCX revealed that there were no changes in
PMCA and NCX after I/R in the CTL and T3 supplementation
groups.
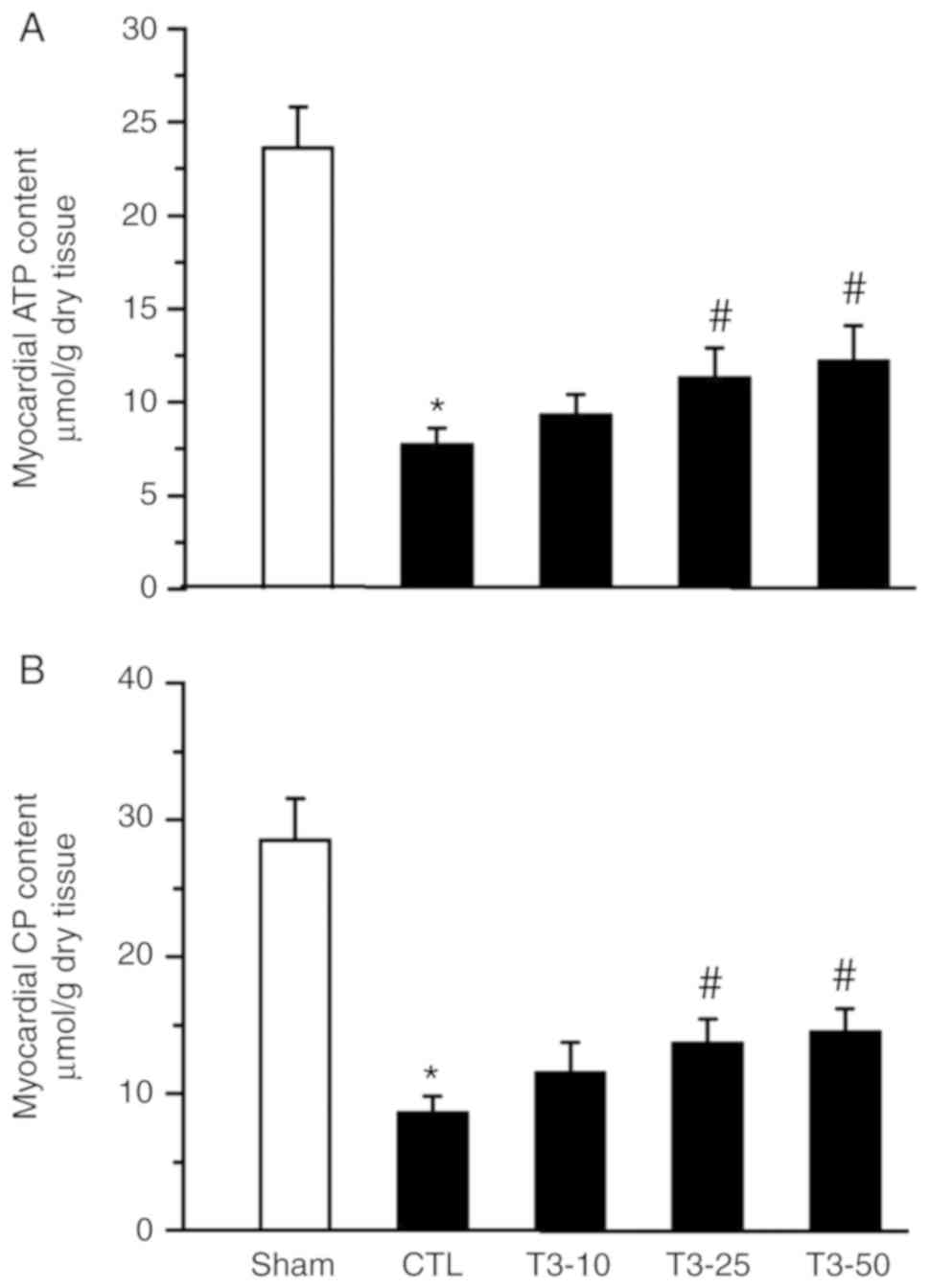
ATP and CP content
The ATP and CP content of transmural sections taken
from the left ventricular free wall at the end of reperfusion were
determined. In the CTL and T3 groups, the ATP contents
were 6.7±0.9, 9.3±1.1, 11.3±1.6 and 12.2±1.9 µmol/g, respectively;
the CP contents were 8.6±1.2, 11.5±2.3, 13.7±1.8 and 14.5±1.7
µmol/g at the end of reperfusion, respectively. The contents of ATP
and CP were better preserved in the T3 (10, 25 and 50
nM) treated groups compared with in the CTL group (Fig. 5).
Discussion
A number of studies have confirmed that a
cardiopulmonary bypass affects thyroid hormone metabolism to reduce
circulating free T3, which leads to a ‘euthyroid-sick’
state. It is not clear how acute stress-induced hypothyroidism
affects the myocardium pathophysiological change; however, previous
studies (4,17) have suggested that T3 is a
positive inotrope and acts by improving the ‘aerobic capacity’ of
the myocardium after ischemia. This then leads to an enhanced
availability of myocardial high-energy phosphates for contractile
work (4). The results of the present
study demonstrated that the recovery of myocardial function was
improved after ischemia by treatment with T3 (at 10, 25
and 50 nM). The supplementation of thyroid hormone improved the
recovery of cardiac function and persisted throughout the
reperfusion interval. This is consistent with data reported by
Klemperer et al (18), in
which acute administration of T3 to post-ischemic hearts
improved the preload recruitable stroke work area, but had no
effect on the preload stroke work area of the non-ischemic hearts.
In the present study, T3 at 10 nM, ten times the
physiological dose, produced a measurable effect. T3 at
25 nM had a greater effect on post-ischemic recovery, but there was
no additional recovery at 50 nM. T3 had a similar
salutary effect on the recovery of the diastolic chamber, with a
direct relationship between the dose of T3 required and
the severity of the injury. The observation that T3 (50
nM) did not make additional improvements is important. It suggests
the need to further define the effective therapeutic range for
T3 supplementation.
T3 supplementation could improve cardiac
functions and limit infarct size against I/R injury (10). Previous studies have clearly shown
that in addition to binding to nuclear-localized receptors,
T3 can also activate signaling processes at the plasma
membrane, in mitochondria or within the cytosol by targeting
T3 receptors or other proteins (2,19).
Although the heart may be capable of synthesizing 1 mg of protein
per g of heart tissue per h (20),
the rapid post-ischemic recovery of cardiac function evident in the
present study suggests that the site of action targeted by
T3 is more likely to be in the plasma membrane or
cytosol. There is also strong evidence that the accumulation of
cytosolic Ca2+ during I/R is highly correlated with the
severity of injury (13). The
present study using isolated hearts revealed a marked improvement
in cardiac function by T3 supplementation before I/R,
which is consistent with previous studies (2,8,21). As the inhibition of calpain prevented
the I/R-induced degradation of key SR Ca2+ cycling
proteins (such as RyR2 and SERCA2a) and improved contractile
function (22), the present study
measured the protein contents of the Ca2+ release
channel, Ca2+ uptake and other
Ca2+-regulating proteins. The results demonstrated that
an I/R-induced depression in cardiac performance was associated
with a downregulation of the major SR Ca2+-cycling
proteins. This downregulation could be attenuated by T3
supplementation before ischemia.
RYR2 is a protein found primarily in cardiac muscle.
The RYR2 protein functions as the major component of a calcium
channel located in the SR that supplies ions to the cardiac muscle
during systole to initiate contraction (23). In the present study, T3
supplementation improved cardiac function recovery in I/R hearts
and was accompanied with the preservation of RyR2 protein content.
To date, it has been demonstrated that Ser-2809 is one of the main
sites of phosphorylation on RYR2. Phosphorylation of RYR2 by
CaMKII, or protein kinase A (PKA), at Ser-2809 induced channel
function changes in vitro. These changes include an increase
in the probability of being open (24). In the present study a low level of
Ser-2809 phosphorylation was observed in the CTL group, but marked
Ser-2809 phosphorylation was expressed following supplementation
with T3. The results suggest that T3 may
facilitate the phosphorylation of RYR2 by CaMKII or PKA.
SERCA2 in cardiac muscle plays an important role in
the muscle's overall contractility status. Improved cardiac SR
function by T3 supplementation against I/R injury may
partly contribute to a higher level of the protein content of major
SR Ca2+ uptake proteins including SERCA2a and PLB, a 52
amino acid phosphoprotein. Unphosphorylated PLB inhibits SERCA2a,
but the phosphorylation of PLB prevents the inhibition of SERCA2a
at either Ser-16 by PKA or Thr-17 by CaMKII, thereby increasing
SERCA2a activity and the rate of Ca2+ uptake by the SR
(25). The present results showed
that the protein level of PLB was decreased in I/R hearts and
SERCA2a levels were reduced even more. Therefore it is in agreement
with a previous study in which the PLB/SERCA2a interaction
controlled the calcium content of the SR and ultimately controlled
cardiac contractility (25). A
decrease in the phosphorylation of PLB, along with an increase in
the PLB/SERCA2a ratio was observed (data not shown) in the CTL
group, suggesting the strong inhibitory effect of PLB on SERCA2a in
I/R hearts. Phosphorylation of the PKA substrate PLB is a critical
determinant of Ca2+ re-entry into the SR and is
coordinated by CaMKII and PKA. In the present study, PLB
phosphorylation on Thr-17 was decreased during I/R. PLB
phosphorylation was downregulated in I/R hearts, suggesting that
the rate of Ca2+ uptake by the SR decreased due to the
inhibition of SERCA2a. The present results demonstrated that
T3 supplementation improved PLB phosphorylation at
Thr-17 in I/R hearts. T3 supplementation increased the
PLB/SERCA2a ratio by valid preservation of SERCA2a. The
phosphorylation of PLB by T3 reduced the inhibition of
SERCA2a and therefore enhanced SR Ca2+ uptake in I/R
hearts. T3 supplementation inhibited ATP-dependent
Ca2+ uptake in isolated cardiac SR vesicles (26). There is evidence that alterations in
SR Ca2+ cycling function are components of the impaired
SR Ca2+ uptake, Ca2+ release and the content
of Ca2+-cycling proteins (23,27); the
beneficial effect of T3 supplementation on SR
Ca2+ cycling function may contribute to the attenuation
of I/R-induced changes in SR function and protein content.
Cytosolic Ca2+ regulates several cellular
processes and its concentration is, in turn, finely regulated by
various channels, pumps and exchangers. The NCX and the PMCA pump
are two concurrent mechanisms for Ca2+ extrusion from
the cell. There is a very large transmembrane electrochemical
Ca2+ gradient driving the entry of the ion into cells,
yet it is very important that they maintain low concentrations of
Ca2+ for appropriate cell signaling. Thus, it is
necessary for cells on pumps to remove Ca2+. PMCA and
NCX together are the main regulators of intracellular
Ca2+ concentrations (25). In this manner, intracellular
Ca2+ and SR Ca2+ content are regulated and
maintained. The present results revealed no alterations in PMCA and
NCX contents after I/R. Furthermore, T3 supplementation
did not affect the content of PMCA or NCX.
The results obtained from measuring myocardial ATP
and CP in the present study suggested that T3 induces
inotropic effects via the rapid replacement and maintenance of high
energy phosphate stores within the myocardium. The administration
of T3 led to the return of normal mitochondrial
function, reactivation of the tricarboxylic acid cycle and full
aerobic metabolism; tissue lactate levels were reduced and high
energy phosphate stores were more rapidly replaced (4). T3 may lead to both the
increased synthesis of high energy stores and increased utilization
of such stores, which results in improved myocardial function. This
hypothesis is also supported by previous studies (2,4).
Sterling et al (28)
demonstrated the rapidly increased synthesis of ATP production
secondary to mitochondrial stimulation in both euthyroid and
hypothyroid rats treated with T3.
In conclusion, the results of the present study
demonstrated that T3 supplementation improves left
ventricular function after I/R in isolated rat hearts by preserving
major Ca2+ cycling proteins. Rapid cardiac functional
improvements by T3 supplementation suggested that these
effects may be mediated through T3 binding at the plasma
membrane or SR rather than at the nuclear level. Increased
synthesis of myocardial high energy phosphate ATP and CP are
attributed to the improvement of myocardial function.
T3-induced preservation of calcium cycling proteins
potentially involves the direct inhibition of protease activities.
However, the mechanisms underlying these effects require further
elucidation.
Acknowledgements
The authors would like to thank Dr Chen Wang
(Department of Anesthesiology, The Affiliated Suzhou Science and
Technology Town Hospital of Nanjing Medical University) for his
technical assistance in the study and for reading the
manuscript.
Funding
The present study was supported by the Suzhou
‘Kejiaoxinwei’ program (grant no. KJXW2015062), the Xiangcheng
Science and Technology Program (grants nos. XJ201657 and 201704),
the Suzhou Science and Technology Bureau (grant no. SS201756), and
the Suzhou Gaoxin District Science and Technology Plan (grant no.
2017Z004).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceived and designed the experiments: LF, LL and
JA. Performed the experiments: LF, ZX, JL, LH and SQ. Analyzed the
data: LF, LL and JA. Contributed reagents/materials/analysis tools:
LF, ZX, JL, LH and SQ. Wrote the paper: LF, LL and JA. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Nanjing Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
T3
|
triiodothyronine
|
|
I/R
|
ischemic reperfusion
|
|
RyR2
|
Ca2+-release channels
|
|
SERCA2a
|
Ca2+-adenosine
triphosphatase
|
|
PLB
|
phospholamban
|
|
PMCA
|
sarcolemmal Ca2+-adenosine
triphosphatase
|
|
NCX
|
sodium-calcium exchanger
|
|
CPB
|
cardiopulmonary bypass
|
|
Ca2+-
|
calcium
|
|
SR
|
sarcoplasmic reticulum
|
|
RyRs
|
ryanodine receptors
|
|
LVP
|
left ventricular pressure
|
|
CF
|
coronary flow
|
|
MVO2
|
myocardial O2
consumption
|
|
ATP
|
adenosine triphosphate
|
|
CP
|
creatine phosphate
|
|
LVDP
|
Left ventricular developed
pressure
|
|
CaMKII
|
calmodulin-dependent kinase II
|
|
PKA
|
protein kinase A
|
References
|
1
|
Li L, Guo CY, Yang J, Jia EZ, Zhu TB, Wang
LS, Cao KJ, Ma WZ and Yang ZJ: Negative association between free
triiodothyronine level and international normalized ratio in
euthyroid subjects with acute myocardial infarction. Acta Pharmacol
Sin. 32:1351–1356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ragone MI, Bonazzola P, Colareda GA and
Consolini AE: Cardioprotective effect of hyperthyroidism on the
stunned rat heart during ischaemia-reperfusion: Energetics and role
of mitochondria. Exp Physiol. 100:680–697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holland FW II, Brown PS Jr and Clark RE:
Acute severe postischemic myocardial depression reversed by
triiodothyronine. Ann Thorac Surg. 54:301–305. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Novitzky D and Cooper DK: Thyroid hormone
and the stunned myocardium. J Endocrinol. 223:R1–R8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Velissaris T, Tang AT, Wood PJ, Hett DA
and Ohri SK: Thyroid function during coronary surgery with and
without cardiopulmonary bypass. Eur J Cardiothorac Surg.
36:148–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Priest JR, Slee A, Olson AK, Ledee D,
Morrish F and Portman MA: Triiodothyronine supplementation and
cytokines during cardiopulmonary bypass in infants and children. J
Thorac Cardiovasc Surg. 144:938–943.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forini F, Lionetti V, Ardehali H, Pucci A,
Cecchetti F, Ghanefar M, Nicolini G, Ichikawa Y, Nannipieri M,
Recchia FA and Iervasi G: Early long-term L-T3 replacement rescues
mitochondria and prevents ischemic cardiac remodelling in rats. J
Cell Mol Med. 15:514–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pingitore A, Chen Y, Gerdes AM and Iervasi
G: Acute myocardial infarction and thyroid function: New
pathophysiological and therapeutic perspectives. Ann Med.
44:745–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forini F, Kusmic C, Nicolini G, Mariani L,
Zucchi R, Matteucci M, Iervasi G and Pitto L: Triiodothyronine
prevents cardiac ischemia/reperfusion mitochondrial impairment and
cell loss by regulating miR30a/p53 axis. Endocrinology.
155:4581–4590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicolini G, Forini F, Kusmic C, Pitto L,
Mariani L and Iervasi G: Early and short-term triiodothyronine
supplementation prevents adverse postischemic cardiac remodeling:
Role of transforming growth factor-β1 and antifibrotic miRNA
signaling. Mol Med. 21:900–911. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bi W, Jia J, Pang R, Nie C, Han J, Ding Z,
Liu B, Sheng R, Xu J and Zhang J: Thyroid hormone postconditioning
protects hearts from ischemia/reperfusion through reinforcing
mitophagy. Biomed Pharmacother. 118:1092202019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forini F, Paolicchi A, Pizzorusso T, Ratto
GM, Saviozzi M, Vanini V and Iervasi G: 3,5,3′-Triiodothyronine
deprivation affects phenotype and intracellular [Ca2+]i of human
cardiomyocytes in culture. Cardiovasc Res. 51:322–330. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
An J, Rhodes SS, Jiang MT, Bosnjak ZJ,
Tian M and Stowe DF: Anesthetic preconditioning enhances Ca2+
handling and mechanical and metabolic function elicited by Na+-Ca2+
exchange inhibition in isolated hearts. Anesthesiology.
105:541–549. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pantos C, Mourouzis I, Saranteas T, Clavé
G, Ligeret H, Noack-Fraissignes P, Renard PY, Massonneau M,
Perimenis P, Spanou D, et al: Thyroid hormone improves
postischaemic recovery of function while limiting apoptosis: A new
therapeutic approach to support hemodynamics in the setting of
ischaemia-reperfusion? Basic Res Cardiol. 104:69–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An J, Bosnjak ZJ and Jiang MT: Myocardial
protection by isoflurane preconditioning preserves Ca2+ cycling
proteins independent of sarcolemmal and mitochondrial KATP
channels. Anesth Analg. 105:1207–1213. 2007.table of contents.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasamori J, Abe Y, Marunouchi T, Manome Y,
Uchibori T and Tanonaka K: Effects of 2-octynyladenosine (YT-146)
on mitochondrial function in ischemic/reperfused rat hearts. Biol
Pharm Bull. 38:1946–1953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ranasinghe AM, Quinn DW, Pagano D, Edwards
N, Faroqui M, Graham TR, Keogh BE, Mascaro J, Riddington DW, Rooney
SJ, et al: Glucose-insulin-potassium and tri-iodothyronine
individually improve hemodynamic performance and are associated
with reduced troponin I release after on-pump coronary artery
bypass grafting. Circulation. 114 (1 Suppl):I245–I250. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klemperer JD, Zelano J, Helm RE, Berman K,
Ojamaa K, Klein I, Isom OW and Krieger K: Triiodothyronine improves
left ventricular function without oxygen wasting effects after
global hypothermic ischemia. J Thorac Cardiovasc Surg. 109:457–465.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dyke CM, Yeh T Jr, Lehman JD, Abd-Elfattah
A, Ding M, Wechsler AS and Salter DR: Triiodothyronine-enhanced
left ventricular function after ischemic injury. Ann Thorac Surg.
52:14–19. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Umeda PK, Darling DS, Kennedy JM, Jakovcic
S and Zak R: Control of myosin heavy chain expression in cardiac
hypertrophy. Am J Cardiol. 59:49A–55A. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Accorroni A, Saponaro F and Zucchi R:
Tissue thyroid hormones and thyronamines. Heart Fail Rev.
21:373–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh RB, Chohan PK, Dhalla NS and
Netticadan T: The sarcoplasmic reticulum proteins are targets for
calpain action in the ischemic-reperfused heart. J Mol Cell
Cardiol. 37:101–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eisner DA, Caldwell JL, Kistamás K and
Trafford AW: Calcium and excitation-contraction coupling in the
heart. Circ Res. 121:181–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mourouzis I, Mantzouratou P, Galanopoulos
G, Kostakou E, Roukounakis N, Kokkinos AD, Cokkinos DV and Pantos
C: Dose-dependent effects of thyroid hormone on post-ischemic
cardiac performance: Potential involvement of Akt and ERK
signalings. Mol Cell Biochem. 363:235–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
del Monte F, Harding SE, Dec GW, Gwathmey
JK and Hajjar RJ: Targeting phospholamban by gene transfer in human
heart failure. Circulation. 105:904–907. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pantos C, Mourouzis I, Saranteas T, Brozou
V, Galanopoulos G, Kostopanagiotou G and Cokkinos DV: Acute T3
treatment protects the heart against ischemia-reperfusion injury
via TRalpha1 receptor. Mol Cell Biochem. 353:235–241. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Temsah RM, Kawabata K, Chapman D and
Dhalla NS: Preconditioning prevents alterations in cardiac SR gene
expression due to ischemia-reperfusion. Am J Physiol Heart Circ
Physiol. 282:H1461–H1466. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sterling K, Brenner MA and Sakurada T:
Rapid effect of triiodothyronine on the mitochondrial pathway in
rat liver in vivo. Science. 210:340–342. 1980. View Article : Google Scholar : PubMed/NCBI
|