Introduction
Vertebral artery dissecting aneurysm (VADA) is a
rare condition accounting for 3% of intracranial aneurysms. VADA
may result in subarachnoid hemorrhage and ischemic stroke (1). However, the incidence may be
underestimated due to a high rate of mortality during the early
stages as a result of mortality from severe hemorrhaging. It is
necessary to treat a ruptured VADA as soon as possible due to the
high risk of rebleeding and mortality. Craniotomy remains a
challenge for neurosurgeons. Endovascular treatment is a suitable
alternative option for treating patients with VADA. It is difficult
to treat VADA with a coil, and additional assistive technologies
are required. The stent-assisted coiling technique extends the
usage of endovascular treatment to VADA. Endovascular treatment
includes destruction (parent artery occlusion) and reconstruction
techniques (stent implantation with or without coiling) (2–5).
However, there is currently controversy regarding the preferred
treatment option. Therefore, it is necessary to determine whether
endovascular treatment results in favorable outcomes in patients
with VADA, and which endovascular modalities is optimal for each
specific subtype of VADA. In the present study, data from 47
patients with 50 VADAs were collected and the clinical and
angiographic outcomes were analyzed.
Materials and methods
Patient population
A total of 47 patients with 50 VADAs undergoing
endovascular treatment between January 2012 and March 2018 at
Yijishan Hospital, Wannan Medical College (Wuhu, China) were
included in the present study. Following approval from the
institutional review board, the clinical and radiological materials
were collected. The inclusion criterion was that VADAs were
confirmed by digital subtraction angiography (DSA). The exclusion
criterion was that VADAs originated from the extracranial vessel or
involved the basilar artery. Hunt-Hess grading was used to evaluate
the condition of the patients on admission. The clinical data are
presented in Table I. The cohort was
comprised of 31 males and 16 females with a mean age of 54.57±10.27
years (age range, 28–75 years). A total of 22 patients (46.8%)
presented with hypertension, 28 patients were admitted to hospital
due to subarachnoid hemorrhage, 14 had ischemic events and 5 had
other irrelevant presentations. In addition, 15 patients were
admitted to hospital with a Hunt-Hess grade of 4 or 5 and 13 with a
Hunt-Hess grade of 1–3.
 | Table I.Characteristics of the patients with
vertebral artery dissecting aneurysms (n=47). |
Table I.
Characteristics of the patients with
vertebral artery dissecting aneurysms (n=47).
| Characteristics | N (%) |
|---|
| Gender |
|
| Male | 31 |
|
Female | 16 |
| Age (years) |
|
| Mean | 54.57±10.27 |
|
Median | 54 |
|
Range | 28–75 |
| Hypertension | 22 (46.8) |
| Hunt-Hess
grading |
|
| 1–3 | 13 (27.7) |
| 4–5 | 15 (31.9) |
| Initial
presentation |
|
| SAH | 28 (59.6) |
| Ischemic
events | 14 (29.8) |
|
Other | 5 (10.6) |
Radiological characteristics
Subarachnoid hemorrhage was confirmed by CT
scanning. All VADAs were confirmed by DSA with or without CTA or
MRA. They were defined as dominant vertebral arteries when there
was no contralateral vertebral artery or the ipsilateral vertebral
artery was significantly larger compared with the contralateral
one.
Among the 47 cases, three patients had bilateral
VADAs. The radiological characteristics of the aneurysms are
presented in Table II. There were
28 ruptured aneurysms and 22 unruptured ones; furthermore, 25
aneurysms were located on the left vertebral artery and 25 on the
right one. A total of 13 aneurysms were located at the dominant
vertebral artery and 37 at the non-dominant vertebral artery.
According to the association between the aneurysms and the
posterior inferior cerebellar artery (PICA), the aneurysms were
divided into three types: Proximal, distal and with involvement of
the PICA. Based on this classification, 11 aneurysms were proximal
to the PICA, 27 were distal to the PICA and 12 aneurysms had
involvement with the PICA.
 | Table II.Radiological characteristics of
aneurysms. |
Table II.
Radiological characteristics of
aneurysms.
| Characteristics | N (%) |
|---|
| Total | 50 |
| Ruptured | 28 (56) |
| Unruptured | 22 (44) |
| Left/right | 25/25 |
| Blood supply to
basilar artery |
|
|
Dominant | 13 (26) |
|
Non-dominant | 37 (74) |
| Location |
|
|
Proximal | 11 (22) |
|
Distal | 27 (54) |
|
Involvement | 12 (24) |
Treatment
The anti-platelet drugs (aspirin 100 mg/day and
clopidogrel 75 mg/day) were administered for 3 days prior to the
procedure for patients with unruptured aneurysms. All patients with
ruptured aneurysms received endovascular treatment within 24 h to
reduce the risk of rebleeding, except one patient who initially
refused endovascular treatment. A loading dose of anti-platelet
drugs (aspirin and clopidogrel, 300 mg each) was administered to
patients with ruptured aneurysms who were to undergo stent
deployment by oral or nasal feeding. The anti-platelet drugs
(aspirin 100 mg/day and clopidogrel 75 mg/day) were administered
for 6 weeks post-operatively, followed by aspirin alone.
The treatment strategy was formulated by experts
according to the patients' clinical presentation, whether the
aneurysm had ruptured, involvement of the dominant or non-dominant
vertebral artery, and association between the aneurysms and the
PICA. Parent artery occlusion was performed when the vertebral
artery involved was non-dominant and the PICA was not involved.
Stent implantation with or without coiling was performed in
patients with unruptured aneurysms and in patients with ruptured
aneurysms when the PICA was involved or the aneurysm was located on
the dominant vertebral artery.
All procedures were performed under general
anesthesia. A 6F sheath was placed into the right femoral artery
and a 6F guiding catheter was placed into the ipsilateral vertebral
artery. In certain instances, a 5F or 6F sheath was placed into the
bilateral femoral artery when a bilateral vertebral artery approach
was required. The stent-delivery-catheter was navigated into the
basilar artery on the roadmap. A suitable stent was selected
according to the length of the vessel involved. The stent should at
least cover the proximal and distal part of a lesioned vessel. The
diameter of a stent should be larger than that of the parent artery
in order to obtain stability and adherence to the vessel. An
enterprise or LVIS stent was used to reconstruct the parent artery.
For patients undergoing parent artery occlusion, two
micro-catheters were placed into the lesioned area in order to
obtain dense embolization. For patients with bilateral aneurysms,
the responsible aneurysm was estimated according to the bleeding
characteristics and aneurysmal figuration. The responsible aneurysm
was first treated and the other aneurysm was treated at a second
stage.
Follow-up
Follow-up by DSA was scheduled to be performed 3
months after the procedure, as well as 9 and 21 months after the
operation. The immediate angiographic outcomes were divided into
complete occlusion and incomplete occlusion. The angiographic
outcomes at follow-up were classified as follows: Complete
regression (no contrast filling), incomplete regression (no change
in coil configuration, obliteration grade or contrast filling) and
recurrence (aneurysm recurrence evident due to aneurysm growth or
coil compaction). The clinical outcomes were evaluated by using the
Glasgow Outcome Scale (GOS). Favorable outcomes were defined as GOS
4 or 5 and poor outcomes were defined as GOS 1–3.
Statistical analysis
Statistical analysis was performed using SPSS for
Windows v.17.0 (IBM Corp.). The Chi-squared test was used to
compare the occlusion rate between parent artery occlusion and
stent implantation with or without coiling. The favorable outcome
rate between ruptured aneurysms and unruptured aneurysms was also
compared using the chi-squared test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Treatment and peri-operative
morbidities
The aneurysms were treated as follows: 18 were
treated by parent artery occlusion, 11 by stent implantation (1
aneurysm by single LVIS, 7 by double enterprise and 3 by triple
enterprise) and 21 by stent-assisted coiling (7 aneurysms by single
enterprise, 12 by double enterprise and 2 by triple enterprise).
There were three patients with bilateral VADAs. Of these three
patients, one patient with unruptured bilateral VADAs underwent
double stent implantation in stages. The second patient with
ischemic stroke underwent stent-assisted coiling and another
aneurysm was treated by parent artery occlusion at the second
stage. For the third patient with subarachnoid hemorrhage, the
major aneurysm was subjected to stent-assisted coiling and another
aneurysm underwent stent-assisted coiling during the second stage.
Complete occlusion was achieved in 33 aneurysms and incomplete
occlusion in 17 aneurysms. All aneurysms treated by parent artery
occlusion gained complete occlusion. Complete occlusion was
achieved in 46.9% (15/32) of aneurysms by stent implantation with
or without coiling. There was a significant difference in the
complete occlusion rate between aneurysms treated by parent artery
occlusion and stent implantation with or without coiling
(P<0.001).
There was no evidence of intra-operative rupture in
any of the procedures. Intra-operative thrombosis occurred in one
patient treated by stent-assisted coiling; this patient developed
symptoms of cerebellar infarction. No ischemic symptoms were
observed in the patients treated by parent artery occlusion.
External ventricular drainage was performed in 8 patients with
ruptured aneurysms. Post-operative bleeding occurred in two
patients treated by stent-assisted coiling. Post-operative frontal
hematoma occurred in one patient due to a puncture. Another patient
received craniotomy due to subdural hematoma. No post-operative
bleeding occurred in patients treated by parent artery occlusion.
Peri-operative morbidity occurred in 10.7% (3/28) of patients with
a ruptured aneurysm. There was no peri-operative morbidity in
patients with unruptured aneurysms (Table III).
 | Table III.Treatment and morbidities. |
Table III.
Treatment and morbidities.
|
Treatment/outcomes | N (%) |
|---|
| Treatment |
|
| Parent
artery occlusion | 18 (36) |
| Stent
implantation | 11 (22) |
|
Stent-assisted coiling | 21 (42) |
| Outcomes |
|
| Complete
occlusion | 33 (66) |
|
Incomplete occlusion | 17 (34) |
| Complete
occlusion rate after parent artery occlusion | 18/18
(100)a |
| Complete
occlusion rate after stent placement | 15/32
(46.9)a |
|
Perioperative morbidity for
ruptured aneurysms | 3/28 (10.7) |
|
Perioperative morbidity for
unruptured aneurysms | 0 (0) |
Ventriculo-peritoneal shunt was performed in 4
patients due to delayed hydrocephalus. Post-operative frontal
hematoma occurred in one patient treated by stent-assisted coiling
three days after ventriculo-peritoneal shunt, which caused an
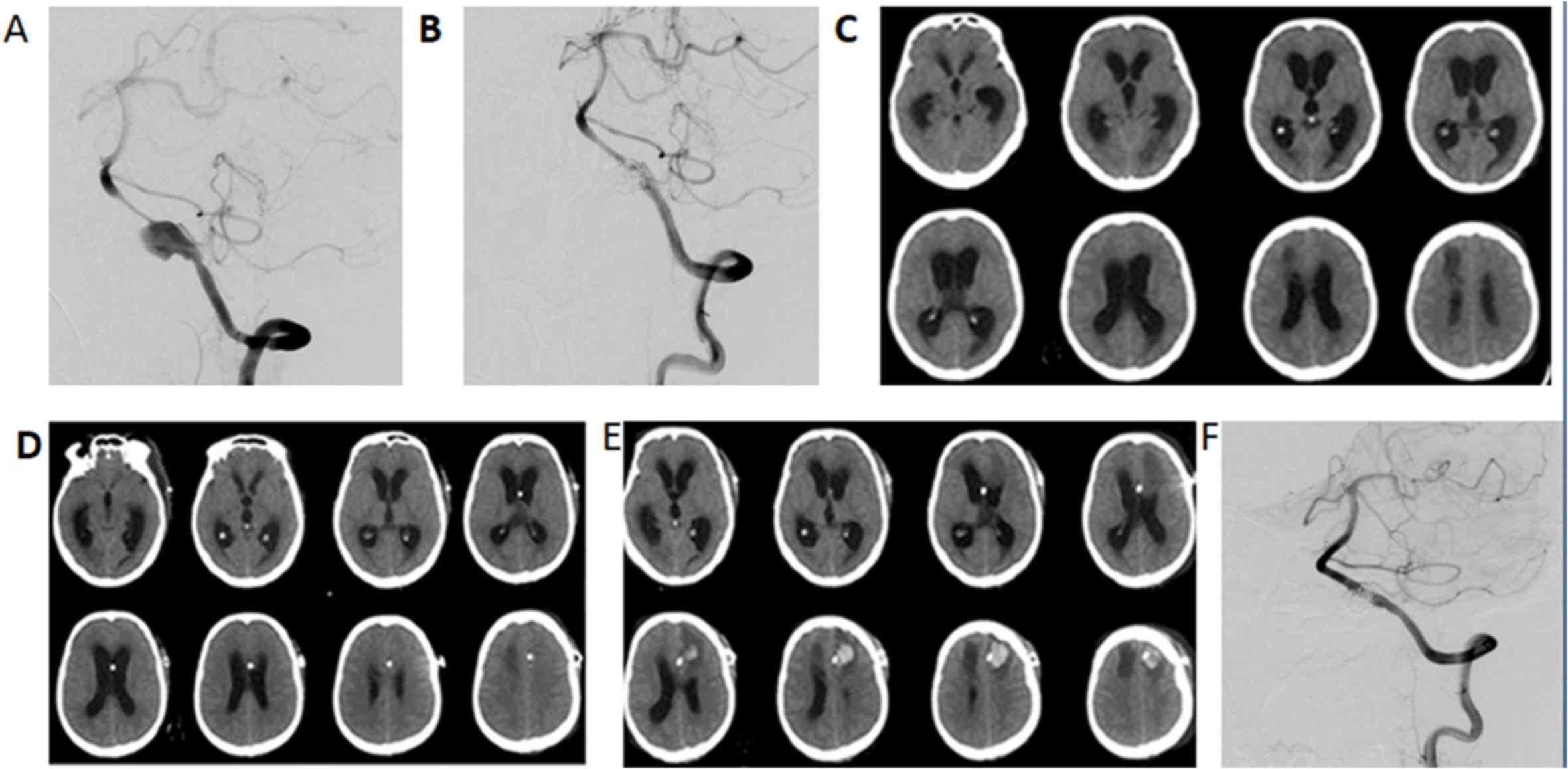
obstruction of the shunt (Fig. 1).
Ventriculo-peritoneal shunt was performed at the contralateral
side.
Angiographic outcomes
In total, 31 patients (34 aneurysms) were followed
up for 11.97±12.96 months (range, 3–52 months) by DSA. Of these, 4
aneurysms in 4 patients (11.8%) presented with recurrence. The
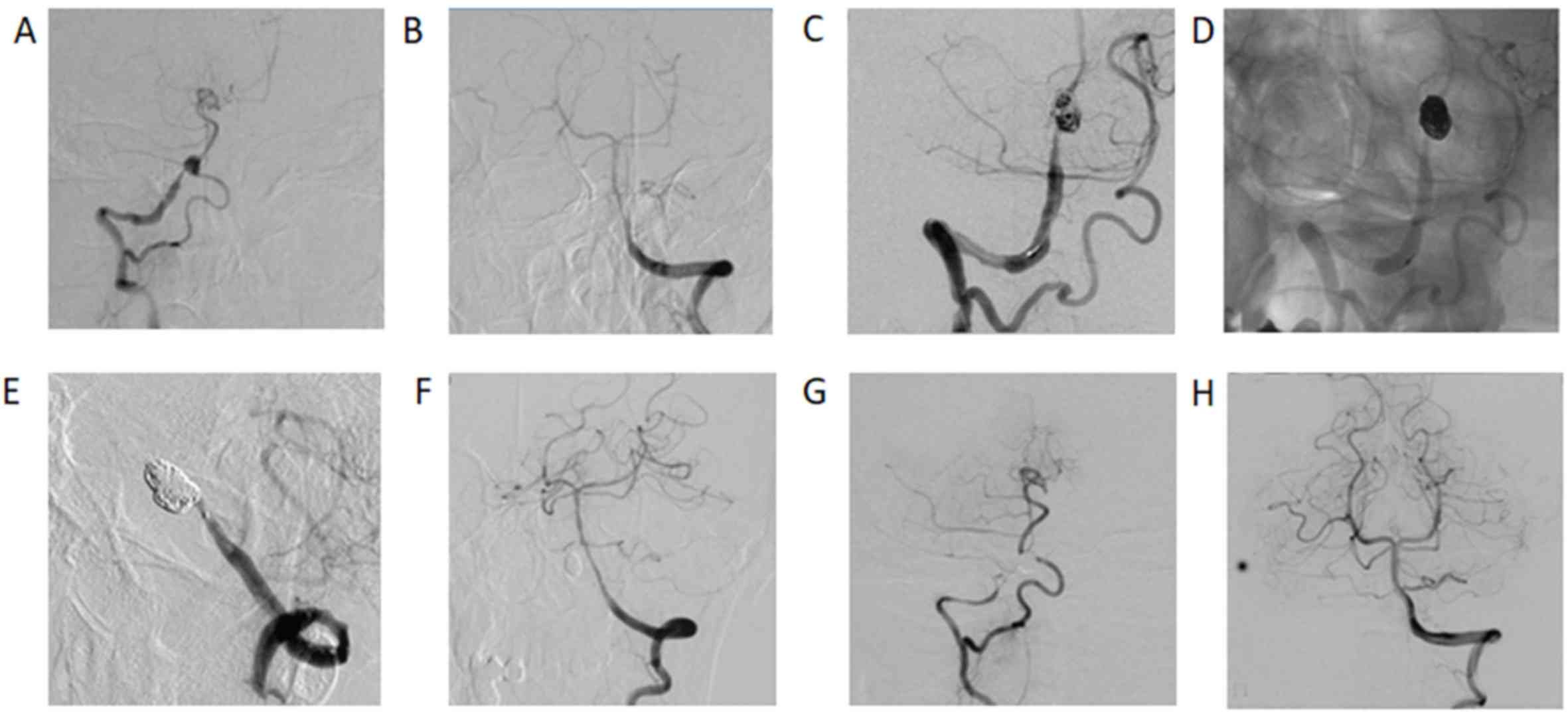
recurrence rate was 8.3% (1/12) in aneurysms treated by parent
artery occlusion (Fig. 2) and 13.6%
(3/22) in aneurysms treated by stent implantation with or without
coiling (Table IV). In 6 patients
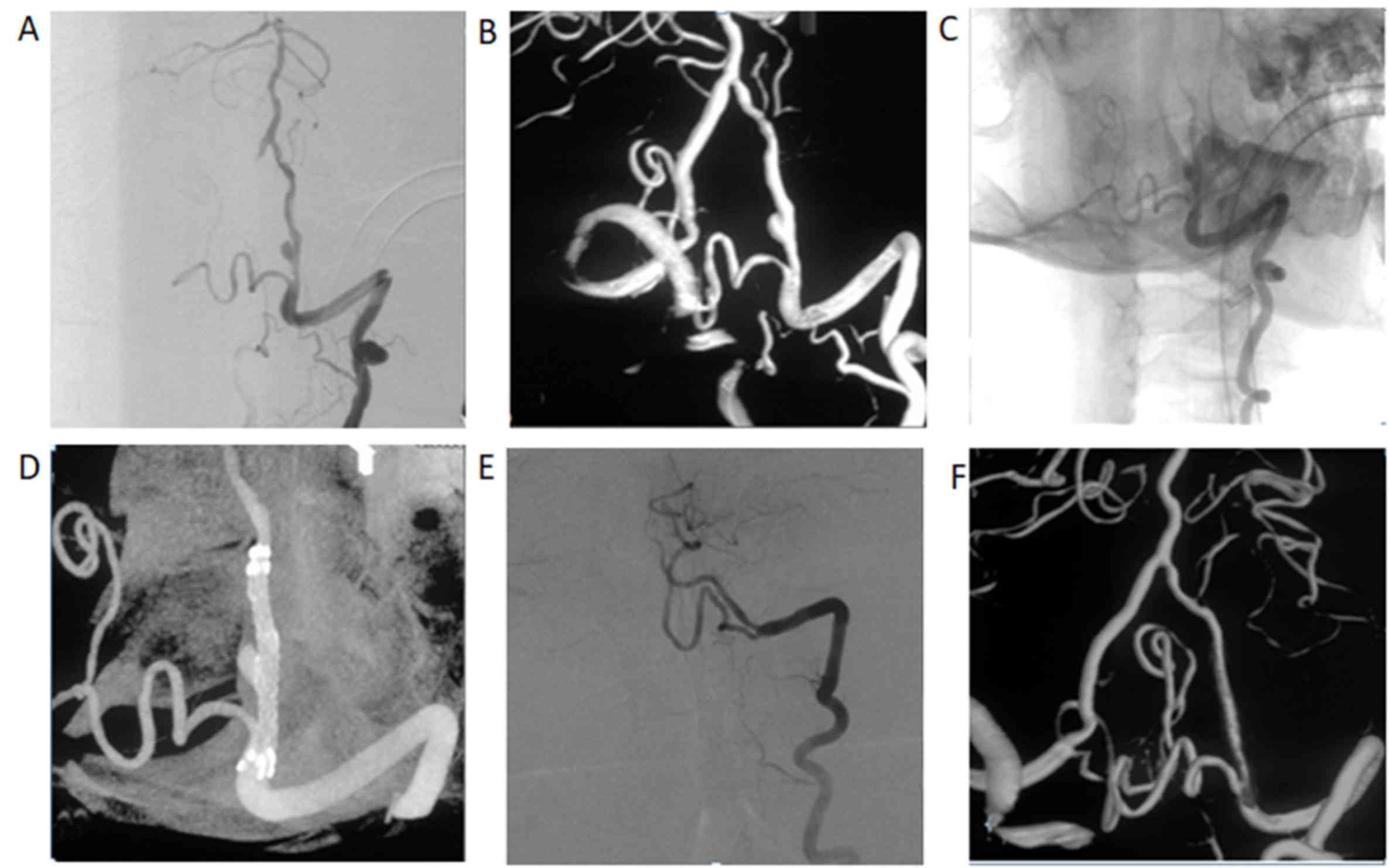
initially treated by stent implantation, four aneurysms obtained
incomplete regression and two achieved complete occlusion (Fig. 3). Recurrence occurred in 3 of the 16
aneurysms treated by stent-assisted coiling.
 | Table IV.Angiographic and clinical
outcomes. |
Table IV.
Angiographic and clinical
outcomes.
| Outcomes | N (%) |
|---|
| Angiographic
outcomes |
|
| Recurrence rate after
parent artery occlusion | 1/12 (8.3) |
| Recurrence rate after
stent | 3/22 (13.6) |
| Clinical outcomes at
discharge |
|
| GOS
1 | 3/47 (6.4) |
| GOS
2 | 2/47 (4.3) |
| GOS
3 | 5/47 (10.6) |
| GOS
4 | 4/47 (8.5) |
| GOS
5 | 33/47 (70.2) |
| Favorable outcomes
for ruptured aneurysms | 18/28
(64.3)a |
| Favorable outcomes
for unruptured aneurysms | 19/19
(100)a |
| Clinical outcomes at
follow-up |
|
| GOS
3 | 3 (7.7) |
| GOS
4 | 3 (7.7) |
| GOS
5 | 33 (84.6) |
Clinical outcomes
The mortality rate was 6.4% (3/47) in the patients
prior to discharge (Table IV).
These deaths all occurred in patients with a Hunt-Hess grade of 5
on admission. The outcome at discharge was GOS grade 2 in 2
patients, GOS grade 3 in 5 patients, GOS grade 4 in 4 patients and
GOS grade 5 in 33 patients. Favorable outcomes were achieved in
78.7% (37/47) of the entire cohort. Among the 15 patients with a
Hunt-Hess grade of 4 or 5 on admission, the outcome at discharge
was GOS grade 1 in 3 patients, GOS grade 2 in 2 patients, GOS grade
3 in 5 patients, GOS grade 4 in 2 patients and GOS grade 5 in 3
patients. Favorable outcomes were achieved in 64.3% (18/28) of the
patients with ruptured aneurysms on admission. All 19 patients with
unruptured aneurysms on admission obtained favorable outcomes.
There was a significant difference in favorable outcomes between
patients with ruptured aneurysms and unruptured aneurysms
(P=0.003).
A total of 3 patients had died prior to discharge
and 5 patients (10.6%) were lost to follow-up; the remaining 39
patients were followed up for 14.56±14.91 months (range, 3–63
months) by telephone and second admission. GOS grade 3 was obtained
in 3 patients, GOS grade 4 in 3 patients and GOS grade 5 in 33
patients at the end of the follow-up. Favorable outcomes were
achieved in 92.3% (36/39) of the patients. The favorable outcome
rate was 72.7% (8/11) in patients with a Hunt-Hess grade of 4 or 5
on admission and 100% (28/28) inpatients with unruptured aneurysms
and a Hunt-Hess grade of 1–3 on admission.
Discussion
VADA usually occurs in adult patients aged 40–50
years (1). The major manifestations
are subarachnoid hemorrhage and ischemic events. The diagnosis is
based on CTA, MRA or DSA. DSA is currently the golden standard for
the diagnosis of VADA. The rate of rebleeding and mortality is high
in patients with ruptured VADA (6–9). Yamada
et al (7) reported on 24
conservatively treated patients with ruptured VADA. Recurrent
hemorrhage occurred in 58% of the patients and 46% of those cases
died due to massive rebleeding. Timely treatment may reduce the
rate of mortality and morbidity resulting from rebleeding.
Therefore, the majority of the patients in the present study
underwent endovascular treatment during the first 24 h. The
treatment for patients with an unruptured VADA remains
controversial. According to certain clinicians, conservative
treatment may achieve good outcomes (9). Guan et al (10) suggested that patients with enlarged
VADA aneurysms and those with progressive ischemia following
medical treatment should be treated using an endovascular approach.
Naito et al (11) indicated
that patients with VADA with relatively large or growing dilations
should receive endovascular treatment. An unruptured VADA should be
treated due to a higher mortality rate following rupture,
particularly for aneurysms with irregular figuration or
morphological changes, multiple aneurysms or when combined with
hypertension.
The primary treatment strategy consists of surgery
and endovascular embolization. Ligation of the lesioned artery and
blood reconstruction may be performed by surgery (vertebral artery
or occipital artery-PICA bypass) (12,13).
However, it may be difficult to perform surgical treatment due to a
deep location, adjacent brain stem and posterior cranial nerves.
Endovascular embolization is the preferred strategy for VADA due to
its safety and efficiency, which includes reconstruction and
destruction techniques (1,14). Destruction technique refers to parent
artery occlusion. The reconstruction technique consists of stent
implantation with or without coiling and flow-diverting stent.
The destruction technique is the most effective
method to prevent aneurysm rupture and rebleeding (2,3). The
immediate obliteration rate is high. The obliteration rate was 100%
in the patients included in the present study, which was similar to
that in other studies. Madaelil et al (3) reported on 12 patients with VADA
involving distal vertebral artery (n=10) or PICA (n=2) treated by
parent artery occlusion. Only one patient suffered from ischemic
stroke and one patient died prior to discharge. There was no
recurrence at follow-up and 10 patients (83%) achieved favorable
outcomes. A meta-analysis by Guan et al (10) demonstrated that the immediate
obliteration rate was higher in patients treated with parent artery
occlusion compared with that in patients treated with
stent-assisted coiling. However, there was no significant
difference in clinical outcomes and recurrence rate between them in
the long-term follow-up. The efficacy of parent artery occlusion
comes at the expense of a lesioned vertebral artery. Parent artery
occlusion may result in severe complications when the lesioned
artery is dominant compared with the contralateral side or if the
PICA is involved. Parent artery occlusion should be performed when
the basilar artery and the PICA are able to maintain a sufficient
blood supply (15). There was no
evidence of brain infarction in the 18 patients treated by parent
artery occlusion in the present study. The rate of favorable
outcome for patients with ruptured aneurysms in the present study
was 64.3%, which appeared to be worse than that reported in
previous studies (5,16). However, this may be due to high
Hunt-Hess grades in the cohort of the present study. Patients with
a Hunt-Hess grade of 4 or 5 accounted for 53.6% (15/28) of cases
with ruptured aneurysms.
The aim of stent implantation with or without
coiling is to keep the vertebral artery unobstructed and reduce the
pressure of blood flow on the vessel wall by maintaining the flow
direction. The coil facilitates the repair of dissection by
thrombosis. Multiple stents may increase the metal coverage. Zhao
et al (5) reported on 57
patients with VADA treated by stent-assisted coiling. The
peri-operative morbidity rate was 5% (3/57) and the overall
mortality rate was 5% (3/57). After an average of 27 months of
angiographic follow-up, 42 of the 54 patients obtained complete
occlusion, 7 exhibited improved results and 5 experienced
recurrence. They indicated that immediate incomplete occlusion was
an independent risk factor for recurrence. After a mean follow-up
of 62 months, 47 patients obtained favorable outcomes (mRS, 0–1),
and high age (56.90±10.88 years) and worse Hun-Hess grading were
associated with poor outcome (5).
Chung et al (4) reported on 8 patients with 9 VADAs
(including 1 ruptured and 8 unruptured) treated with triple stent
implantation. A total of 8 unruptured VADAs returned to normal
after 3 months of angiographic follow-up and 1 patient suffered
from recurrence and received double stent implantation. Finally, 7
patients obtained favorable outcomes after a median clinical
follow-up of 22.6 months. Flow-diversion, including pipeline
embolization device, has higher metal coverage. An increasing
number of studies have been reporting on the use of flow-diversion
in the treatment of VADA. Narata et al (15) suggested that flow-diversion modifies
aneurysmal hemodynamics and accelerates thrombosis.
However, the use of stents may increase the risk of
an ischemic event during the acute phase (16). The use of anti-platelet drugs may
increase the rate of rebleeding, particularly in patients with an
incomplete occlusion. It may result in post-operative hematoma due
to puncture, particularly in patients with acute hydrocephalus
requiring external drainage. In the present study, 8 patients
received external ventricular drainage. Although heparin was
neutralized by protamine, one patient exhibited subdural hematoma
and another patient suffered from frontal hematoma due to puncture.
All hematoma occurred in patients treated with stent-assisted
coiling. Ventriculo-peritoneal shunt was performed in 4 patients
due to delayed hydrocephalus. Post-operative frontal hematoma
occurred in one patient treated with stent-assisted coiling three
days after shunting. Ventriculo-peritoneal shunt was performed on
the contralateral side.
There are certain limitations to the present study.
It was a retrospective and non-randomized study. The number of
patients and duration of follow-up were also limited. Certain
patients were lost to follow-up due to unfavorable outcomes or loss
of contact information.
In conclusion, endovascular treatment of VADA is an
effective and safe method. Stent implantation with or without
coiling is suitable for unruptured VADA, PICA involvement and
dominant vertebral artery. In cases with a ruptured VADA, parent
artery occlusion may be preferred, as the obliteration rate is
higher. It may decrease the probability of post-operative
rebleeding and reduce the requirement for anti-platelet drugs,
which increases the probability of post-operative rebleeding,
particularly in patients with acute hydrocephalus requiring
external drainage.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572486).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ was involved in drafting the manuscript, revising
it critically for important intellectual content and analysing the
data; HW made substantial contributions to acquisition of data, or
analysis and interpretation of data; JL made substantial
contributions to conception, design and analysis. ZZ made
substantial contributions to analysis and revising it
critically.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
Yijishan Hospital, Wanan Medical College (Wuhu, China). Written
informed consent was obtained from the patients or their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VADA
|
vertebral artery dissecting
aneurysm
|
|
DSA
|
digital subtraction angiography
|
|
PICA
|
posterior inferior cerebellar
artery
|
|
GOS
|
Glasgow Outcome Scale
|
References
|
1
|
Su W, Gou S, Ni S, Li G, Liu Y, Zhu S and
Li X: Management of ruptured and unruptured intracranial vertebral
artery dissecting aneurysms. J Clin Neurosci. 18:1639–1644. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Urasyanandana K, Withayasuk P, Songsaeng
D, Aurboonyawat T, Chankaew E and Churojana A: Ruptured
intracranial vertebral artery dissecting aneurysms: An evaluation
of prognostic factors of treatment outcome. Interv Neuroradio.
23:240–248. 2017. View Article : Google Scholar
|
|
3
|
Madaelil TP, Wallace AN, Chatterjee AN,
Zipfel GJ, Dacey RG Jr, Cross DT III, Moran CJ and Derdeyn CP:
Endovascular parent vessel sacrifice in ruptured dissecting
vertebral and posterior inferior cerebellar artery aneurysms:
Clinical outcomes and review of the literature. J Neurointerv Surg.
8:796–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung Y, Lee SH, Choi SK, Kim BJ, Lee KM
and Kim EJ: Triple stent therapy for the treatment of vertebral
dissecting aneurysms: Efficacy and safety. World Neurosurg.
99:79–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao KJ, Fang YB, Huang QH, Xu Y, Hong B,
Li Q, Liu JM, Zhao WY and Deng BQ: Reconstructive treatment of
ruptured intracranial spontaneous vertebral artery dissection
aneurysms: Long-term results and predictors of unfavorable
outcomes. PLoS One. 8:e671692013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin SC, Kwon DH, Choi CG, Ahn JS and Kwun
BD: Endovascular strategies for vertebrobasilar dissecting
aneurysms. AJNR Am J Neuroradiol. 30:1518–1523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamada I, Kitahara T, Kurata A, Fujii K
and Miyasaka Y: Intracranial vertebral artery dissection with
subarachnoid hemorrhage: Clinical characteristics and outcomes in
conservatively treated patients. J Neurosurg. 101:25–30. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizutani T, Aruga T, Kirino T, Miki Y,
Saito I and Tsuchida T: Recurrent subarachnoid hemorrhage from
untreated ruptured vertebrobasilar dissecting aneurysms.
Neurosurgery. 36:905–923. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arnold M, Bousser M, Fahrni G, Fischer U,
Georgiadis D, Gandjour J, Benninger D, Sturzenegger M, Mattle HP
and Baumgartner RW: Vertebral artery dissection: Presenting
findings and predictors of outcome. Stroke. 37:2499–2503. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guan J, Li G, Kong X, He C, Long J, Qin H,
Zhang H and Wang R: Endovascular treatment for ruptured and
unruptured vertebral artery dissecting aneurysms: A meta-analysis.
J Neurointerv Surg. 9:558–563. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naito I, Iwai T and Sasaki T: Management
of intracranial vertebral artery dissections initially presenting
without subarachnoid hemorrhage. Neurosurgery. 51:937–938. 2002.
View Article : Google Scholar
|
|
12
|
Czabanka M, Ali M, Schmiedek P, Vajkoczy P
and Lawton MT: Vertebral artery-posterior inferior cerebellar
artery bypass using a radial artery graft for hemorrhagic
dissecting vertebral artery aneurysms: Surgical technique and
report of 2 cases. J Neurosurg. 114:1074–1079. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park W, Ahn JS, Park JC, Kwun BD and Kim
CJ: Occipital artery-posterior inferior cerebellar artery bypass
for the treatment of aneurysms arising from the vertebral artery
and its branches. World Neurosurg. 82:714–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han J, Lim DJ, Ha SK, Choi JI, Jin SW and
Kim CJ: Endovascular treatment of symptomatic vertebral artery
dissecting aneurysms. J Cerebrovasc Endovasc Neurosurg. 18:201–207.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Narata AP, Yilmaz H, Schaller K, Lovblad
KO and Pereira VM: Flow-diverting stent for ruptured intracranial
dissecting aneurysm of vertebral artery. Neurosurgery. 70:982–989.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bechan RS, Sprengers ME, Majoie CB, Peluso
JP, Sluzewski M and van Rooij WJ: Stent-Assisted coil embolization
of intracranial aneurysms: Complications in acutely ruptured versus
unruptured aneurysms. AJNR Am J Neuroradiol. 37:502–507. 2016.
View Article : Google Scholar : PubMed/NCBI
|