Introduction
Due to continuous progress in the construction and
mining industries, and the development of the transportation
industry, the number of accidental spinal cord injuries (SCIs)
caused by crashes or car accidents has increased in recent years
(1,2). SCI often results in spasticity and
dysfunction under the injured spinal cord segment, with
characteristic high morbidity and mortality (3). Furthermore, due to the nature of their
occupation, young adults >40 years if age are at high-risk
(4). SCI treatment is challenging
due to its high cost and invasiveness. SCI not only leads to
physical and psychological damage to the patient, but also causes a
notable economic burden (5,6). SCI can be classified as primary or
secondary in which primary injury can lead to local tissue damage,
ischemia and hypoxia, inflammatory mediator release and
pathological changes. Secondary lesions are more severe, and result
from the cascade-amplification effects of primary injury. Secondary
lesions can result in damage to residual neural pathways and
further loss of function, but are both controllable and reversible
(7,8).
With advancements in medical treatment technology,
the emergence of surgical methods and drugs has shown initial
success in SCI treatment. Rehabilitation interventions cannot be
neglected and are considered to promote spinal cord remodeling
(9,10). Different drugs are used to relieve
pain in patients with SCI (11);
methylprednisolone attenuates the peroxidation of membrane lipids
and post-traumatic inflammation, and has consistently been
associated with improved neurobehavioral outcome in preclinical
studies (12). High-frequency
electrotherapy, a non-invasive and inexpensive technique is also
widely used for physical therapy to treat pain in SCI patients.
Additionally, transcutaneous electrical nerve stimulation is the
most commonly used electrotherapy method to relieve pain (13). However, the effect of
methylprednisolone combined with high-frequency electrotherapeutic
treatment on SCI and its associated mechanisms is yet to be
elucidated. Therefore, the present study established a rat SCI
model to analyze the impact and possible mechanisms of
methylprednisolone treatment combined with high-frequency
electrotherapy.
Materials and methods
Experimental animals
Healthy, specific pathogen free (SPF) grade male
Wistar rats (2 months old; 250±20 g) were purchased from the
experimental animal center and maintained in the SPF Xi'an Medical
University Animal Experimental Center. The animals were maintained
at 21±1°C, at a relative humidity of 50–70% and a 12 h day/night
cycle. All procedures were approved by the Animal Ethics Committee
of The First Affiliated Hospital of Xi'an Medical University.
Reagents and instruments
Pentobarbital sodium was purchased from Zhpharma
Ltd. PVDF membranes were purchased from Pall Life Sciences. Western
blotting-related chemical reagents were purchased from the Beyotime
Institute of Biotechnology and enhanced chemiluminescence (ECL)
reagents were purchased from GE Healthcare. Rabbit anti-BDNF and
rabbit anti-NF-κB antibodies, as well as sheep anti-rabbit
horseradish peroxidase (HRP)-labeled IgG secondary antibodies were
purchased from Abcam, Inc. Methylprednisolone was purchased from
Sigma-Aldrich (Merck KGaA). Tumor necrosis factor (TNF)-α and IL-2
ELISA kits were purchased from R&D Systems, Inc., and the
Caspase 3 Activity Assay kit was purchased from Cell Signaling
Technology, Inc. Microsurgical instruments were purchased from
Suzhou Medical Instrument Factory. The RNA extraction kit and
reverse transcription kit were purchased from Applied Biosystems
(Thermo Fisher Scientific, Inc.). The Multi-Parameter Monitor Small
Animal Physiology Monitor was purchased from Shanghai Yuken
Instrument Company, and the Amp PCR System 2400 DNA Amplification
System was purchased from PerkinElmer, Inc. The Imark microplate
reader was purchased from BD Biosciences. Key-point evoked
potential meters, electromyographs and Magpro magnetic stimulators
were purchased from Dantec Dynamics. The UWM-02 Ultrashort Wave
Therapy Instrument was purchased from Marubeni Corporation.
Animal grouping and treatment
The rats were randomly divided into four groups:
SCI; methylprednisolone (300 mg/kg); high-frequency electrotherapy
(treated with microwave irradiation from 7 cm at 10 w for 10 min
per day); and combination (treated with electrotherapy combined
with 300 mg/kg methylprednisolone). N=10 for each group.
SCI modeling
According to current literature, the rat SCI model
was established using the modified ALLEN struck method (14). Following anesthetization using an
intraperitoneal injection of 30 mg/kg sodium pentobarbital, the
rats were immobilized on the operating table. The vertebral plates
and spinous processes of the thoracic vertebrae (T9-T11) were
removed. The center was set at the T10 spinal cord and a circular
area with a diameter of approximately 4 mm was determined as the
lesion area. According to the physiological curvature of the dorsal
spinal cord of rats, pre-bent plastic flexion pads of 3 mm in
length, 2 mm in width, and 1 mm thick were prepared. The pads were
placed outside of the spinal cord in the T10 area. Using the
modified ALLEN's striking device, the center was set in the median
posterior of the spinal cord. The striker rod moves freely through
the sleeve at a height of 5 cm and directly hits the plastic
gasket. Signs of successful modeling include the body and lower
extremities retracting and flapping, in additional to tail swing.
The surgical incision was closed and antibiotics
(Baytril®; Bayer AG; 4 mg/kg subcutaneous) were
routinely administered.
Collection of spinal cord tissues
Spinal cord tissues were collected as previously
described (15). The rats were
sacrificed using sodium pentobarbital to effect, and transcardially
perfused with 0.1 M phosphate-buffered saline (PBS, pH 7.4)
followed by 4% paraformaldehyde (in PBS). To maintain consistency,
each spinal cord was rostrally transected at the T6 spinal root,
and a 3 cm segment of spinal cord was immediately dissected and
fixed in paraformaldehyde for 2 h at 4°C, followed by the relevant
analysis.
Basso, Beattie and Bresnahan (BBB)
scoring
On the 20th day after surgery, the BBB score was
measured to evaluate the recovery ability of the joints and lower
limbs. The score was 0–21 points; 0 points indicated no visible
hind limb movement, and 21 points indicated continuous palmar
movement, continuous coordination gait, continuous toe grip, the
parallel position of the active paw to the body, continued trunk
stability and tail tilt. A higher BBB score indicates improved
recovery (12).
SEP and MEP measurement
At 20 days post-surgery, evoked potentials and
electromyography were used to detect SEP and MEP. The rats were
anesthetized and stabilized at the right iliac and the Achilles
tendon. A DC square wave pulse current with a wave width of 0.2
msec and a frequency of 2 Hz was selected. The test time was 20
msec, and a superposition range of ±200 msec was recorded. The
current waveform P1 and an incubation period of N1 were recorded.
The main electrode was placed in the muscle belly of the right
gastrocnemius and the action potential was recorded using a
magnetic stimulator with a test time of 0.2 msec.
Sample collection
On the 20th day after surgery, a total of 5 ml blood
was collected via the tail vein. The blood was centrifuged at 1,200
× g rpm for 15 min, and the serum was collected and stored at
−80°C.
ELISA
TNF-α and IL-2 expression levels were detected by
ELISA according to the manufacturer's protocol. A total of 50 µl
diluted standard or sample was added in triplicate to a 96-well
plate and incubated at 37°C for 1 h. After washing five times, 50
µl enzyme-labeled reagent was added to each well and incubated at
37°C for 30 min. The plate was subsequently treated with 50 µl each
of color agent A and B at 37°C for 10 min. Finally, 50 µl stop
solution was added and the absorption at 450 nm was recorded using
a microplate reader. The concentration of the sample was calculated
and linear regression was compared with the absorbance value of the
standard.
Caspase 3 activity
Caspase 3 activity in spinal cord tissue was
determined using the Caspase 3 Activity Assay kit according to the
manufacturer's protocol. Briefly, the cells were enzymatically
digested and centrifuged at 600 × g for 5 min (4°C). The cells were
subsequently treated with RIPA lysis buffer (Thermo Fisher
Scientific, Inc.) on ice for 15 min and centrifuged at 20,000 × g
for 5 min (4°C). Absorbance was detected at 405 nm following the
addition of 2 mM Ac-DEVD-pNA to each test sample.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the tissue samples
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
and reverse transcribed using a High-Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.) per the
manufacturer's protocol. The primers for qPCR were designed by
Primer Premier 6.0 (Premier Biosoft International) and synthetized
by Invitrogen; Thermo Fisher Scientific, Inc. (Table I). qPCR was performed using SYBR
Green (Thermo Fisher Scientific, Inc.) and the thermocycling
conditions were as follows: 35 cycles at 92°C for 30 sec, 58°C for
40 sec, and 72°C for 35 sec. The 2−DDCq method (16) was used to calculate relative
expression levels in reference to GAPDH.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|
| GAPDH |
GGAGTCAACGGATTTGGTCGTAT |
AGCCTTCTCCATGGTGGTGAAGAC |
| BDNF |
CACTCCGACCCCGCCCGCCG |
TCCACTATCTTCCCCTTTTA |
| NF-κB | AATTGCCCCGGCAT | TCCCGTAACCGCGTA |
Western blotting
The sample tissues were lysed using RIPA buffer and
quantified using a bicinchoninic acid assay. The proteins (40
µg/lane) were separated by SDS-PAGE using a 10% gel, and
transferred to a PVDF membrane at 160 mA for 1.5 h. The membrane
was blocked with 5% skim milk at room temperature for 2 h, and
subsequently incubated with primary antibodies against BDNF
(1:1,000; cat. no. ab226843), NF-κB (1:1,500; cat. no. ab16502) and
β-actin (1:2,000; cat. no. ab8227) at 4°C overnight. After washing
three times with PBS-Tween, the membrane was incubated with a
secondary horseradish peroxidase-conjugated antibody (1:5,000; cat.
no. ab6721) at room temperature for 30 min. The proteins were then
visualized using ECL reagent. Each experiment was repeated four
times and protein expression was quantified using Quantity One
software (version 4.6.8; Bio-Rad Laboratories, Inc.).
Statistical analysis
All data analysis was performed using SPSS 19.0
software (IBM Corp.). The data are presented as the mean ± standard
deviation and compared by one-way ANOVA and Newman-Keuls multiple
comparisons analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
The impact of methylprednisolone
combined with high frequency electrotherapy on SCI rats
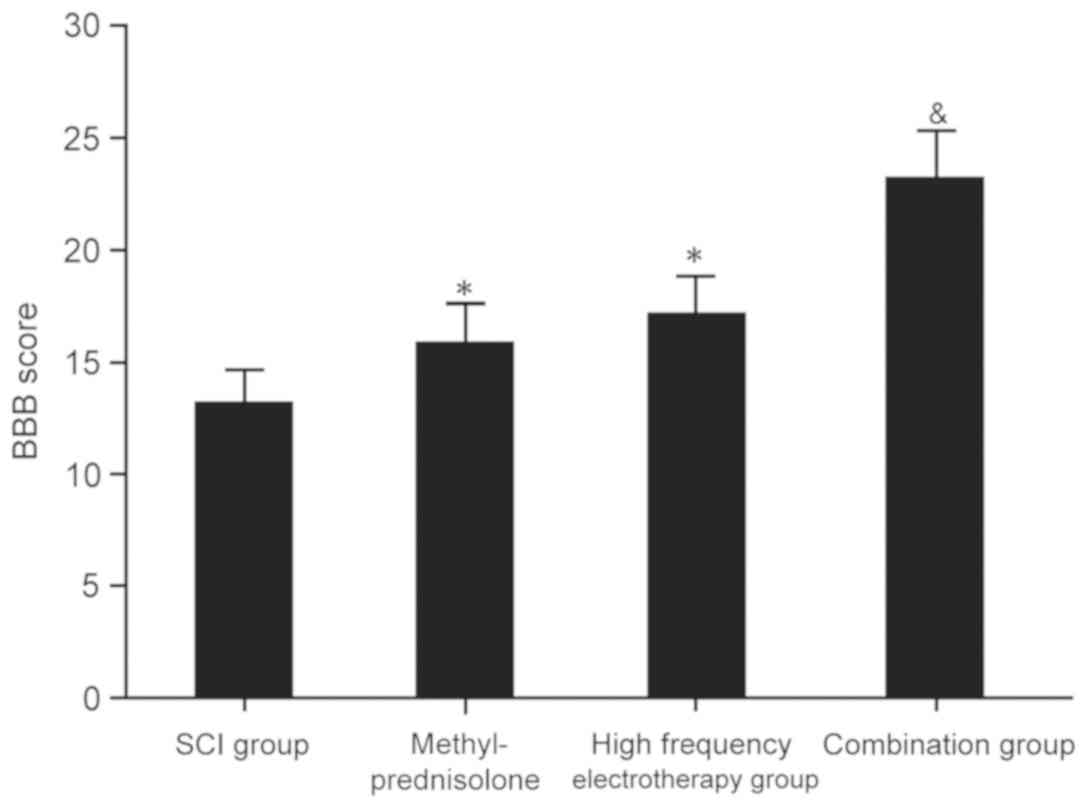
BBB score analysis was performed to evaluate the
impact of methylprednisolone combined with high frequency
electrotherapy on SCI rats. All treatment groups exhibited
significantly elevated BBB scores compared with the untreated, SCI
group (P<0.05). However, the combination group exhibited more
significant effects on SCI (P<0.05; Fig. 1). This suggested that
methylprednisolone combined with high frequency electrotherapy
improved the pathological process of SCI and promoted recovery.
The influence of methylprednisolone
combined with high frequency electrotherapy on the SEPs and MEPs of
SCI rats
SEPs and MEPs were analyzed to assess the influence
of methylprednisolone combined with high frequency electrotherapy
on SCI rats. All treatment groups displayed obviously improved SEPs
and MEPs compared with the SCI group (P<0.05), though the
combination group exhibited the most significant effects on SCI
(P<0.05; Table II). These
results indicated that methylprednisolone combined with high
frequency electrotherapy alleviated the pathological effects of SCI
and promoted recovery.
 | Table II.Influence of methylprednisolone
combined with high frequency electrotherapy. |
Table II.
Influence of methylprednisolone
combined with high frequency electrotherapy.
|
| SEP | MEP |
|---|
|
|
|
|
|---|
| Group | P1 incubation period,
ms | N1 incubation period,
ms | Incubation period,
ms |
|---|
| SCI | 42.12±2.24 | 67.92±5.21 | 14.81±1.47 |
| High-frequency
electrotherapy |
34.17±3.36a |
51.35±3.24a |
9.53±0.38a |
| Combination |
21.36±1.47b |
43.92±5.48b |
6.35±0.28b |
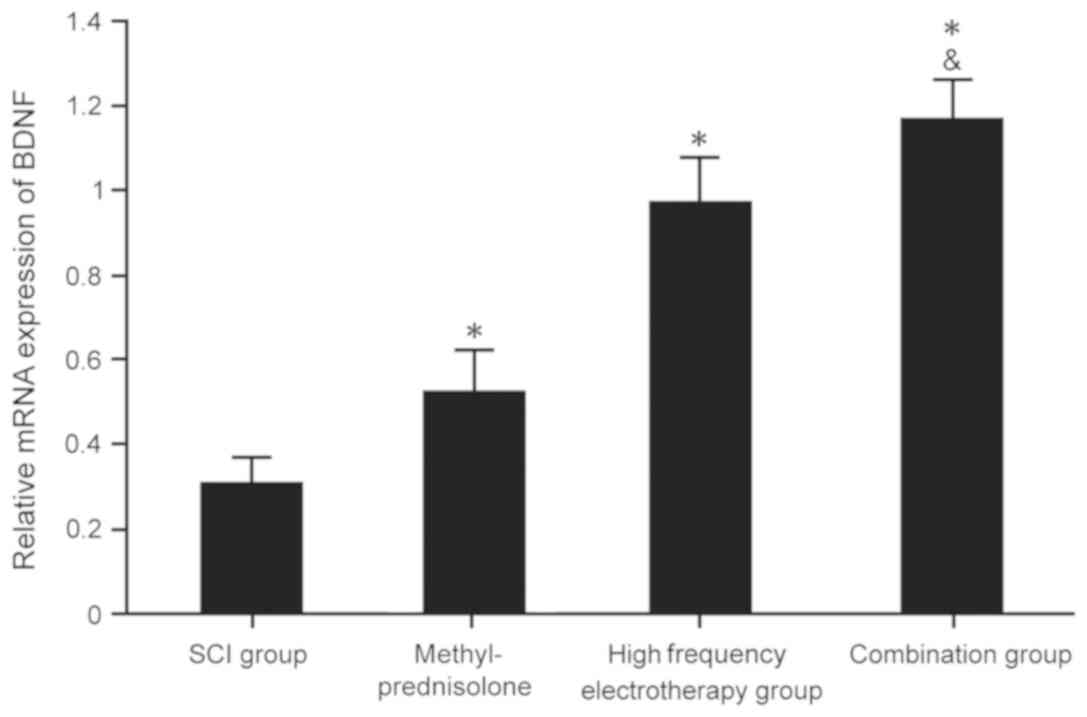
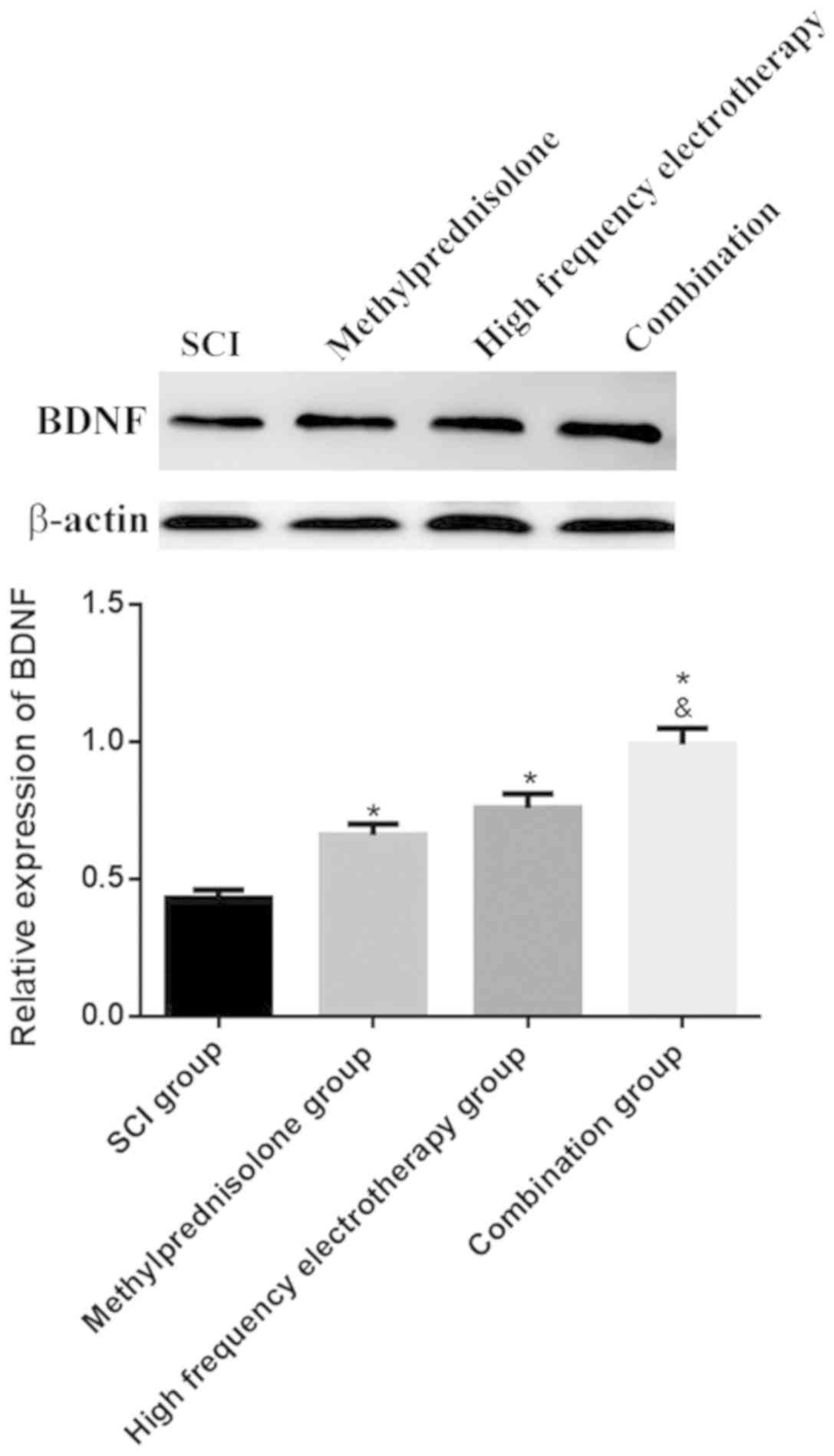
BDNF mRNA and protein expression
levels in spinal cord tissues
RT-qPCR and western blotting were used to determine
BDNF mRNA and protein expressions levels in the spinal cord tissues
of rats. All treatment groups presented with markedly increased
BDNF mRNA and protein expression levels compared with the SCI group
(P<0.05). The combination group exhibited more significant
effects on SCI (P<0.05) (Fig. 2
and 3).
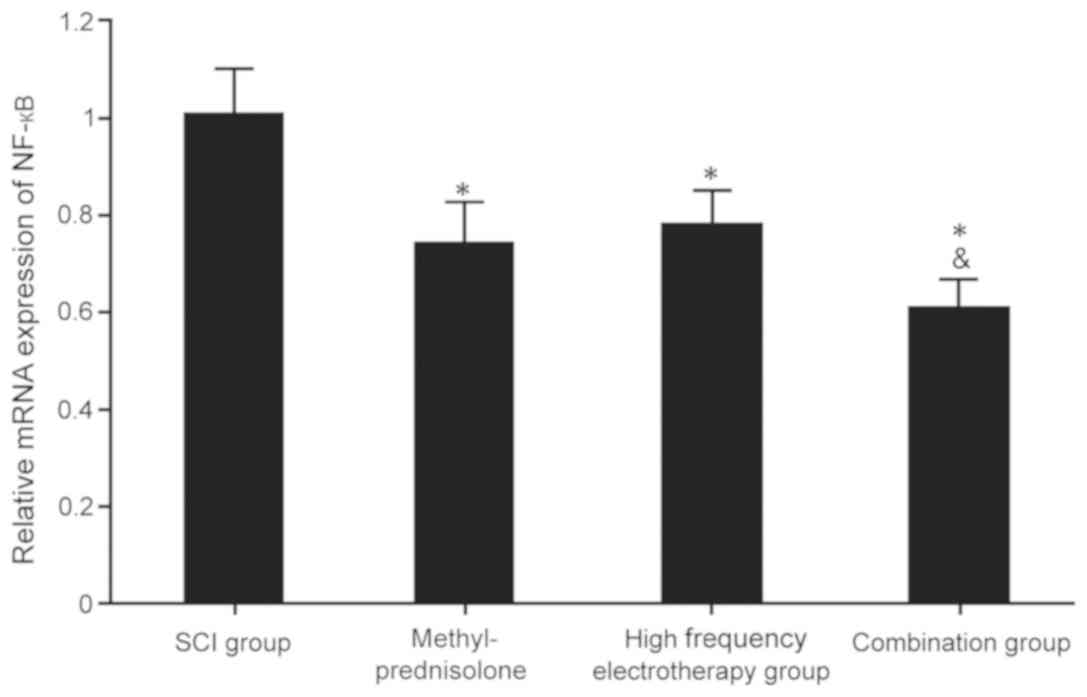
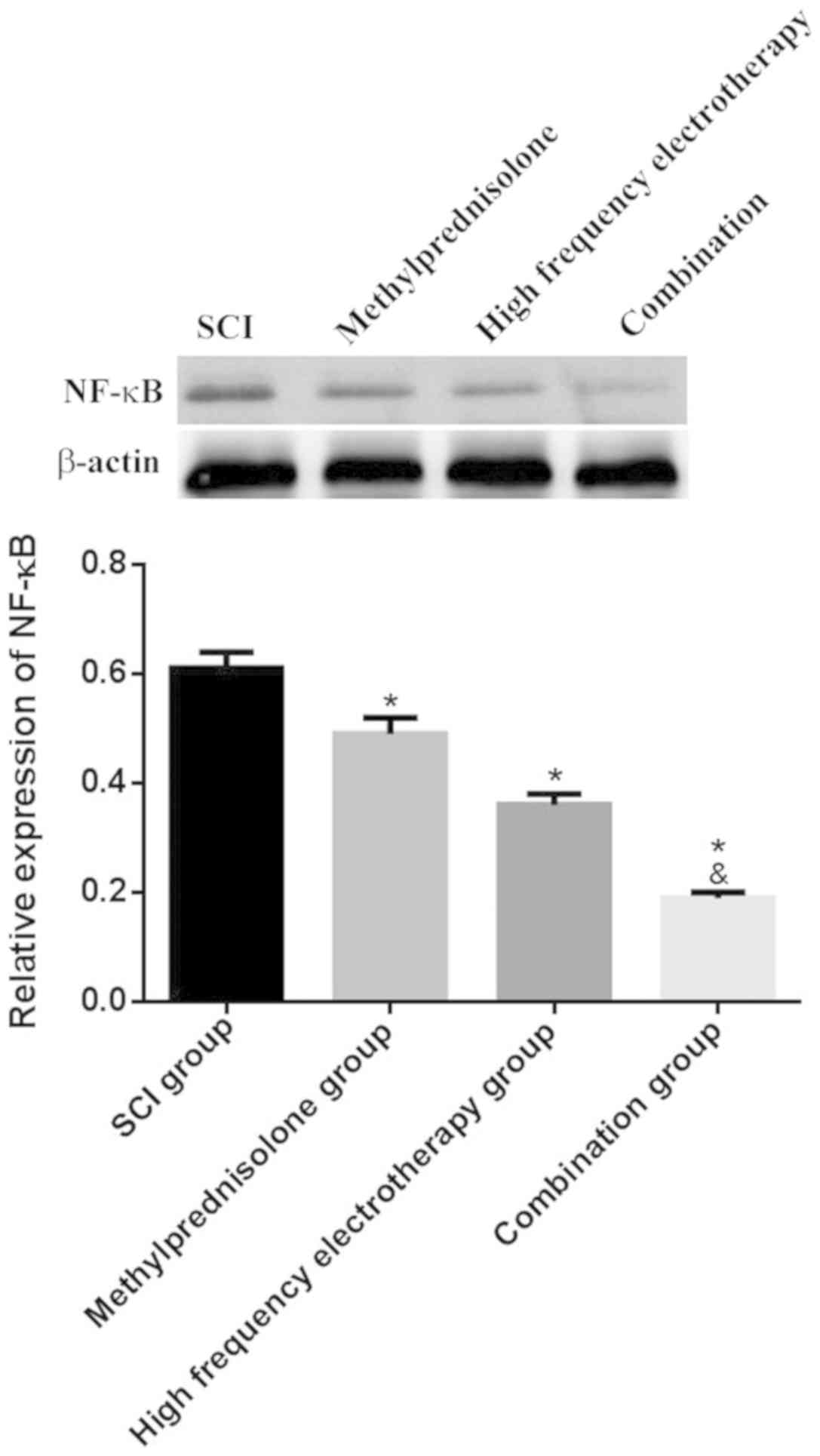
NF-κB mRNA and protein expression
levels in spinal cord tissues
RT-qPCR and western blot analysis were used to
evaluate the expression levels of NF-κB mRNA and protein in rat
spinal cord tissues. The treatment groups showed considerably
reduced expression levels of NF-κB mRNA and protein compared with
the untreated, SCI group (P<0.05), and the combination group
exhibited the most significant effects on SCI (P<0.05; Fig. 4 and 5).
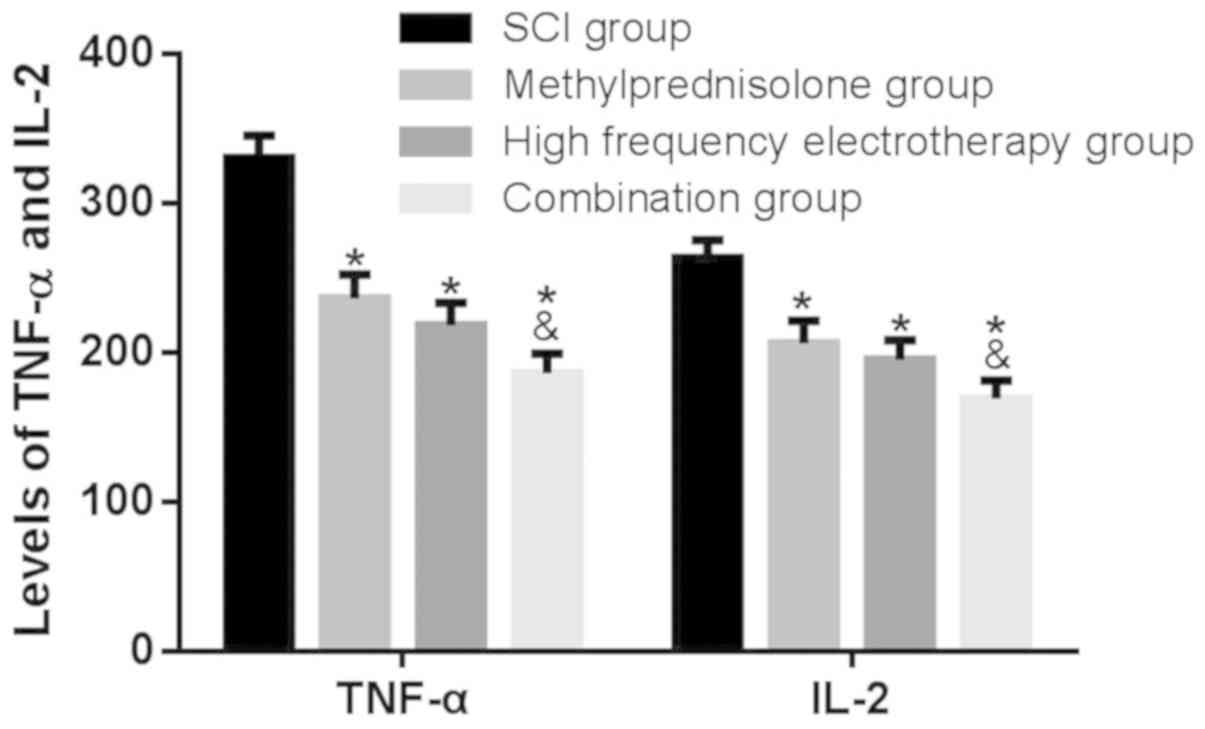
The effect of methylprednisolone
combined with high frequency electrotherapy on serum TNF-α and IL-2
expression levels in SCI rats
The effects of methylprednisolone combined with high
frequency electrotherapy on serum TNF-α and IL-2 expression level
were assessed by ELISA. The treatment groups revealed a reduction
in serum TNF-α and IL-2 expression levels compared with the SCI
group (P<0.05); furthermore, the combination group exhibited a
more significant effect on SCI (P<0.05; Fig. 6) revealing that methylprednisolone
combined with high frequency electrotherapy inhibited cytokine
release, alleviating inflammatory damage.
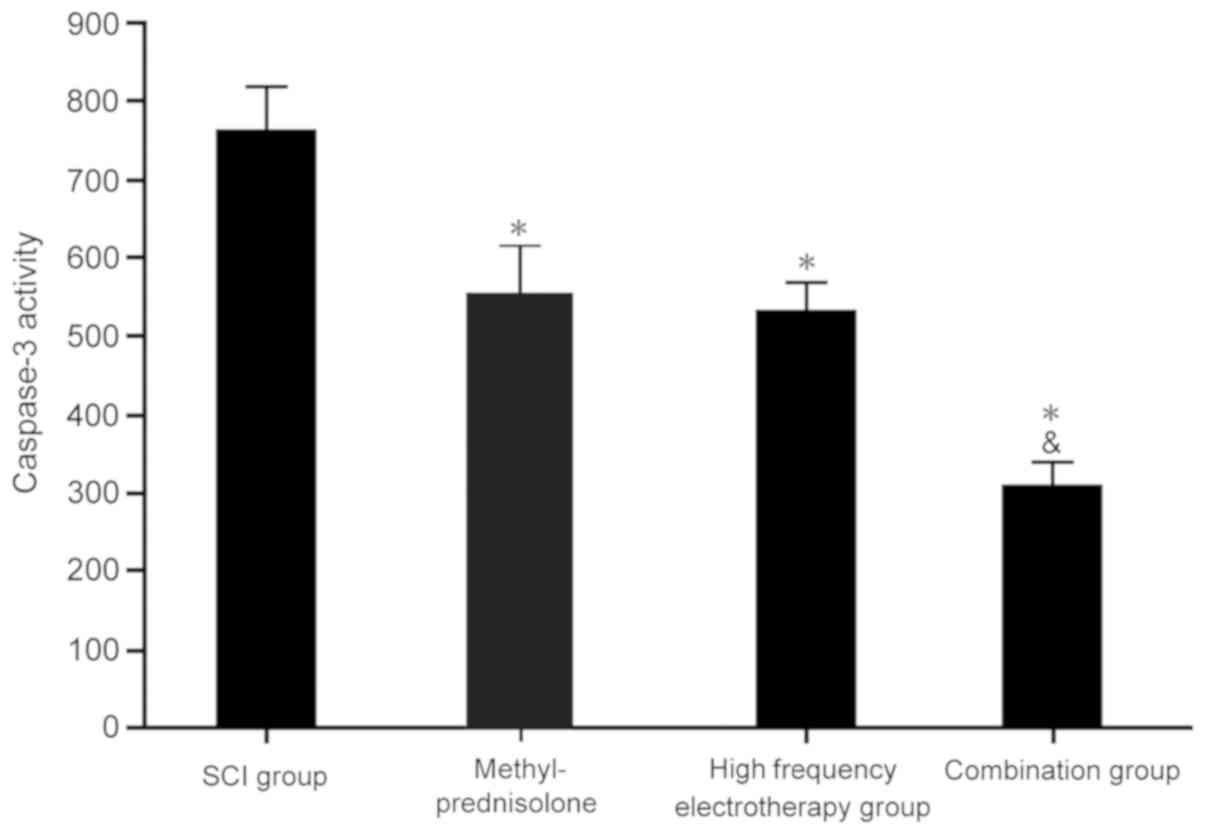
The impact of methylprednisolone
combined with high frequency electrotherapy on caspase 3
activity
Caspase 3 activity was detected in the spinal cord
tissues. Each treatment group displayed significantly decreased
caspase 3 activity compared with the SCI group (P<0.05). The
combination group exhibited more significant effects on SCI
(P<0.05; Fig. 7), which
collectively indicated that methylprednisolone combined with high
frequency electrotherapy suppressed caspase 3 activity to regulate
apoptosis.
Discussion
With the rapid development of science and
technology, the use of physics at a clinical level is able to
significantly promote spinal nerve regeneration and improve the
local microenvironment of the spinal cord. It has been confirmed
that high-frequency microwave treatment is beneficial to
nutritional axons, promoting regeneration at sites of peripheral
nerve injury, accelerating tissue recovery and reducing
pathological injury (13,14). Conversely, the adrenal cortical
hormone methylprednisolone has a strong anti-inflammatory effect
that is conducive to recovery in the early stages of SCI (15,17).
Therefore, the present study aimed to investigate the effect of
combined treatment with methylprednisolone and physical
rehabilitation on SCI and its associated mechanisms.
The BBB scoring method is widely used to evaluate
the degree of SCI (12), and SEPs
and MEPs can effectively analyze the functional recovery degree of
SCI. The present study revealed significantly increased BBB scores
in the methylprednisolone, high-frequency electrotherapy and
combination groups, in additional to elevated SEPs and MEPs.
However, methylprednisolone combined with high-frequency treatment
exhibited a greater significant influence on BBB score, SEPs and
MEPs in SCI rats. These results suggested that high-frequency
electrotherapy may effectively improve SCI, which may be associated
with high-frequency electrotherapy waves promoting the movement,
stretching, migration and swinging of membrane lipids and membrane
proteins, strengthening metabolism and accelerate neuronal changes
in the spinal cord (18,19). Analysis of inflammatory factors
revealed that both singular treatments were able to reduce serum
TNF-α and IL-2 expression levels in SCI rats, but that
methylprednisolone treatment combined with high-frequency
electrotherapy resulted in a more significant improvement, which
may be associated with the marked inhibition of inflammation caused
by methylprednisolone (15,17). The combined effects of
methylprednisolone effectively boosted the healing effects of high
frequency electrotherapy on SCI.
Neurotrophin family member BDNF promotes the growth
and survival of sensory and motor nerves, thus serves an important
role in nerve regeneration (20,21).
Activation of NF-κB during SCI promotes neutrophil accumulation and
macrophage adhesion, leading to the release of a large number of
oxygen free radicals; this promotes the secretion of inflammatory
cytokines such as TNF-α and IL-2, and subsequently compromises the
integrity of spinal cord tissue by inducing vascular endothelial
deterioration, increasing edema and necrosis of the spinal cord
tissue, and promoting secondary SCI (22,23).
Caspase 3 is a critical protease in apoptosis and is also the
common downstream target of each apoptotic pathway (24). In the present study, the
methylprednisolone, high-frequency electrotherapy and combination
groups exhibited significantly increased expression levels of BDNF
mRNA and protein, reduced NF-κB mRNA and protein expression levels,
and a reduction in caspase 3 activity. The combination group
exhibited the greatest effects on SCI.
The present study aimed to analyze the mechanisms
and subsequent effects of methylprednisolone treatment combined
with rehabilitation high-frequency electrotherapy on SCI, and to
further verify the relevant roles and mechanisms observed in
clinical trials. In conclusion, methylprednisolone combined with
high frequency electrotherapy was able to improve SCI, possibly by
increasing BDNF and decreasing NF-κB expression levels, in addition
to suppressing the release of inflammatory factors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL, CL, WW, LL and QZ performed the experiments and
analyzed the data. YO designed the study and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Animal Ethics
Committee of The First Affiliated Hospital of Xi'an Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burkovskiy I, Zhou J and Lehmann C:
Experimental cannabinoid 2 receptor inhibition in CNS
injury-induced immunodeficiency syndrome. Microcirculation.
23:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheah M, Andrews MR, Chew DJ, Moloney EB,
Verhaagen J, Fässler R and Fawcett JW: Expression of an activated
integrin promotes long-distance sensory axon regeneration in the
spinal cord. J Neurosci. 36:7283–7297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang H, Zhang JC, Yang M, Li HF, Zhang JP,
Zhang FX, Wang QY, Wang RR and Liu J: Perfusion of gastrodin in
abdominal aorta for alleviating spinal cord ischemia reperfusion
injury. Asian Pac J Trop Med. 9:688–693. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qin W, Li X, Peng Y, Harlow LM, Ren Y, Wu
Y, Li J, Qin Y, Sun J, Zheng S, et al: Sclerostin antibody
preserves the morphology and structure of osteocytes and blocks the
severe skeletal deterioration after motor-complete spinal cord
injury in rats. J Bone Miner Res. 31:14822016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rao SN and Pearse DD: Pearse, regulating
axonal responses to injury: The intersection between signaling
pathways involved in axon myelination and the inhibition of axon
regeneration. Front Mol Neurosci. 9:332016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harsha KJ and Parameswaran K: Permanent
spinal cord injury during lumbar spinal anesthesia: A report of two
cases. Neurol India. 64:808–811. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nunnerley J, Gupta S, Snell D and King M:
Training wheelchair navigation in immersive virtual environments
for patients with spinal cord injury-end-user input to design an
effective system. Disabil Rehabil Assist Technol. 12:417–423. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sachdeva R, Farrell K, McMullen MK, Twiss
JL and Houle JD: Dynamic changes in local protein synthetic
machinery in regenerating central nervous system axons after spinal
cord injury. Neural Plast. 2016:40872542016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haik MN, Alburquerque-Sendín F, Moreira
RF, Pires ED and Camargo PR: Effectiveness of physical therapy
treatment of clearly defined subacromial pain: A systematic review
of randomised controlled trials. Br J Sports Med. 50:1124–1134.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Page MJ, Green S, Mrocki MA, Surace SJ,
Deitch J, McBain B, Lyttle N and Buchbinder R: Electrotherapy
modalities for rotator cuff disease. Cochrane Database Syst Rev
CD012225. 2016. View Article : Google Scholar
|
|
11
|
D'Angelo R, Morreale A, Donadio V, Boriani
S, Maraldi N, Plazzi G and Liguori R: Neuropathic pain following
spinal cord injury: What we know about mechanisms, assessment and
management. Eur Rev Med Pharmacol Sci. 17:3257–3261.
2013.PubMed/NCBI
|
|
12
|
Braughler JM and Hall ED: Effects of
multi-dose methylprednisolone sodium succinate administration on
injured cat spinal cord neurofilament degradation and energy
metabolism. J Neurosurg. 61:290–295. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bi X, Lv H, Chen BL, Li X and Wang XQ:
Effects of transcutaneous electrical nerve stimulation on pain in
patients with spinal cord injury: A randomized controlled trial. J
Phys Ther Sci. 27:23–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Phillips AA, Matin N, Frias B, Zheng MM,
Jia M, West C, Dorrance AM, Laher I and Krassioukov AV: Rigid and
remodelled: Cerebrovascular structure and function after
experimental high-thoracic spinal cord transection. J Physiol.
594:1677–1688. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rabchevsky AG, Fugaccia I, Sullivan PG,
Blades DA and Scheff SW: Efficacy of methylprednisolone therapy for
the injured rat spinal cord. J Neurosci Res. 68:7–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hextrum S and Bennett S: A Critical
examination of subgroup analyses: The national acute spinal cord
injury studies and beyond. Front Neurol. 9:112018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abnoosian A and Maguire G: Case report of
an interaction of a vagal nerve stimulation system with a microwave
current from a body fat analyzer. Ann Clin Psychiatry. 20:229–230.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
von Bary C, Mazzitelli D, Voss B, Kübler
F, Schmeller ML, Ndrepepa G and Zrenner B: Evaluation of epicardial
microwave lesions in the pig model using an electroanatomic mapping
system. J Interv Card Electrophysiol. 22:5–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Man L, Lv X, Du XD, Yin G, Zhu X, Zhang Y,
Soares JC, Yang XN, Chen X and Zhang XY: Cognitive impairments and
low BDNF serum levels in first-episode drug-naive patients with
schizophrenia. Psychiatry Res. 263:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sapkota S and Dixon RA: A network of
genetic effects on non-demented cognitive aging: Alzheimer's
genetic risk (CLU + CR1 + PICALM) intensifies cognitive aging
genetic risk (COMT + BDNF) selectively for APOe4 carriers. J
Alzheimers Dis. 62:887–900. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen BH, Park JH, Lee TK, Song M, Kim H,
Lee JC, Kim YM, Lee CH, Hwang IK, Kang IJ, et al: Melatonin
attenuates scopolamine-induced cognitive impairment via protecting
against demyelination through BDNF-TrkB signaling in the mouse
dentate gyrus. Chem Biol Interact. 285:8–13. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanada M, Tsutsumi K, Arima H, Shinjo R,
Sugiura Y, Imagama S, Ishiguro N and Matsuyama Y: Evaluation of the
effect of tranilast on rats with spinal cord injury. J Neurol Sci.
346:209–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu YH, Liu GH, Mei JJ and Wang J: The
preventive effects of hyperoside on lung cancer in vitro by
inducing apoptosis and inhibiting proliferation through caspase-3
and P53 signaling pathway. Biomed Pharmacother. 83:381–391. 2016.
View Article : Google Scholar : PubMed/NCBI
|