Introduction
Suppurative hidradenitis (SH) is a chronic,
relapsing, inflammatory condition, usually with insidious onset,
affecting typically flexural areas such as the axillary, inguinal
and anogenital region, with a variable clinical presentation,
inflamed nodules, suppurating sinus tracts, scarring and secondary
contracture (1,2).
Genetic, immunological and environmental factors
(infectious agents, smoking, adipose tissue, friction) favor hair
follicle occlusion, accumulation of cellular debris and cyst
formation, followed by follicular rupture, inflammation and chronic
suppuration (3). When the deep part
of the hair follicle is damaged, it will result in open comedones,
frequently grouped and interconnecting (4,5).
Reepithelization of the follicles will form sinus tracts and areas
of confluent nodular lesions will heal with inter-communicating
sinuses and fistulas, punctate, acneiform, atrophic or hypertrophic
scars and fibrotic bridges (2,4).
Pyoderma gangrenosum (PG) is a neutrophilic
dermatosis, an inflammatory and ulcerative disorder of the skin
with rapid and painful onset of inflammatory papules, pustules,
vesicles or nodules that form erosions or ulcers that heal with
atrophic, cribriform scars, commonly localized on lower extremities
and trunk (6). Genetic and
immunological factors (dysregulation of the innate immune system,
neutrophil dysfunction) induce perifollicular inflammation and
intradermal abscess and later epidermal and superficial dermal
necrosis with an underlying mixed inflammatory cell infiltrate,
with or without vasculitis (7).
Despite clinical differences, PG and SH seem to
share similar pathogenic pathway (8). Understanding the inflammatory process
plays an essential role in cutaneous pathology, in both
inflammatory diseases and tumorigenesis (9,10). HS,
PG and cystic acne can be present in the same patient as part of
pyoderma gangrenosum, acne and suppurative hydradenitis (PASH) an
autoinflammatory syndrome characterized by neutrophilic
inflammation, mediated by IL-1β, controlled by NALP3 inflammasome
pathway (8).
Case report
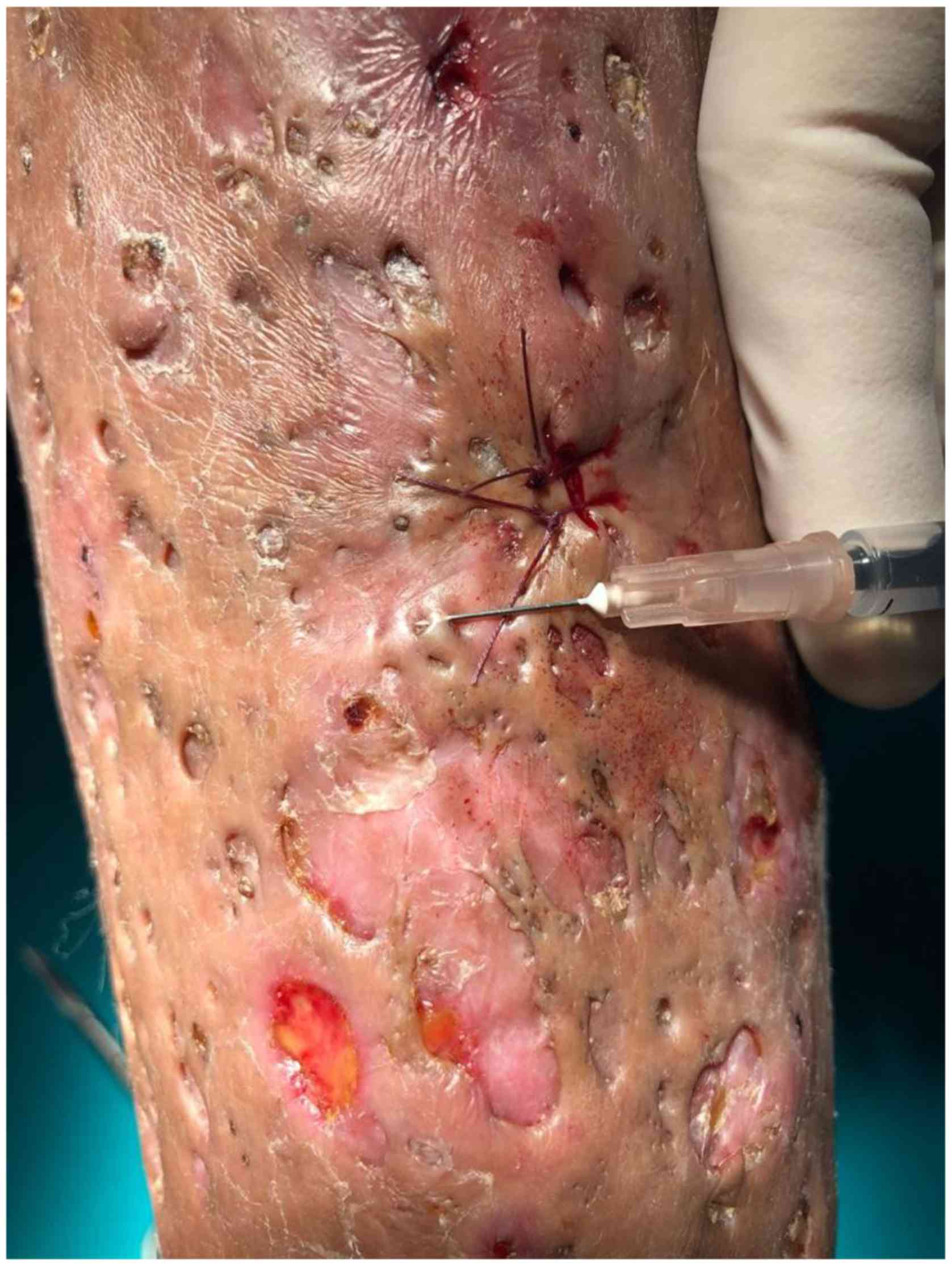
We report a case of a 53-year-old male patient,
normal weight, 16.5-pack-year smoker, referred to the Dermatology
Department for a large scarring plaque of ~20x8 cm on his left
anterior calf, with cribriform openings, multiple fibrous bridges,
open comedones, double-ended pseudo-comedones on the surface and
several painful ulcers of 0.5-2 cm in diameter, with undefined,
violaceous borders and seropurulent, sanguinous discharge (Fig. 1). Consent for publication was
obtained from the patient.
The lesion first appeared 2 months prior to
consultation, after a traumatic injury, with a very painful onset
of an inflammatory plaque with pustules on the surface that rapidly
progressed (24-48 h) to form ulcers. The patient self treated with
2 courses of a 10-day oral ampicillin with no signs of improvement.
Therefore he had been hospitalized in the Infectious Diseases
Department. Laboratory workup revealed increase of inflammatory
markers and serology for hepatitis infections, HIV and syphilis,
fungal culture and PCR for BK were all negative. Bacterial cultures
from multiple sites showed growth of Escherichia coli,
Enteroccocus faecalis and Peptophilus spp. Lower limb
X-ray showed heterogeneous soft tissue swelling. Treatment was
undertaken with ceftriaxone and vancomycin and switched to
amoxicillin/clavulanic acid and vancomycin, as the antimicrobial
susceptibility tests showed resistance to ceftriaxone, for 20 days,
with clinical improvement. Biopsy specimen from the border of the
leg ulcer revealed neutrophilic inflammation and areas of necrotic
and fibrotic connective tissue consistent with ulcerative PG and
the patient was referred to the dermatology clinic.
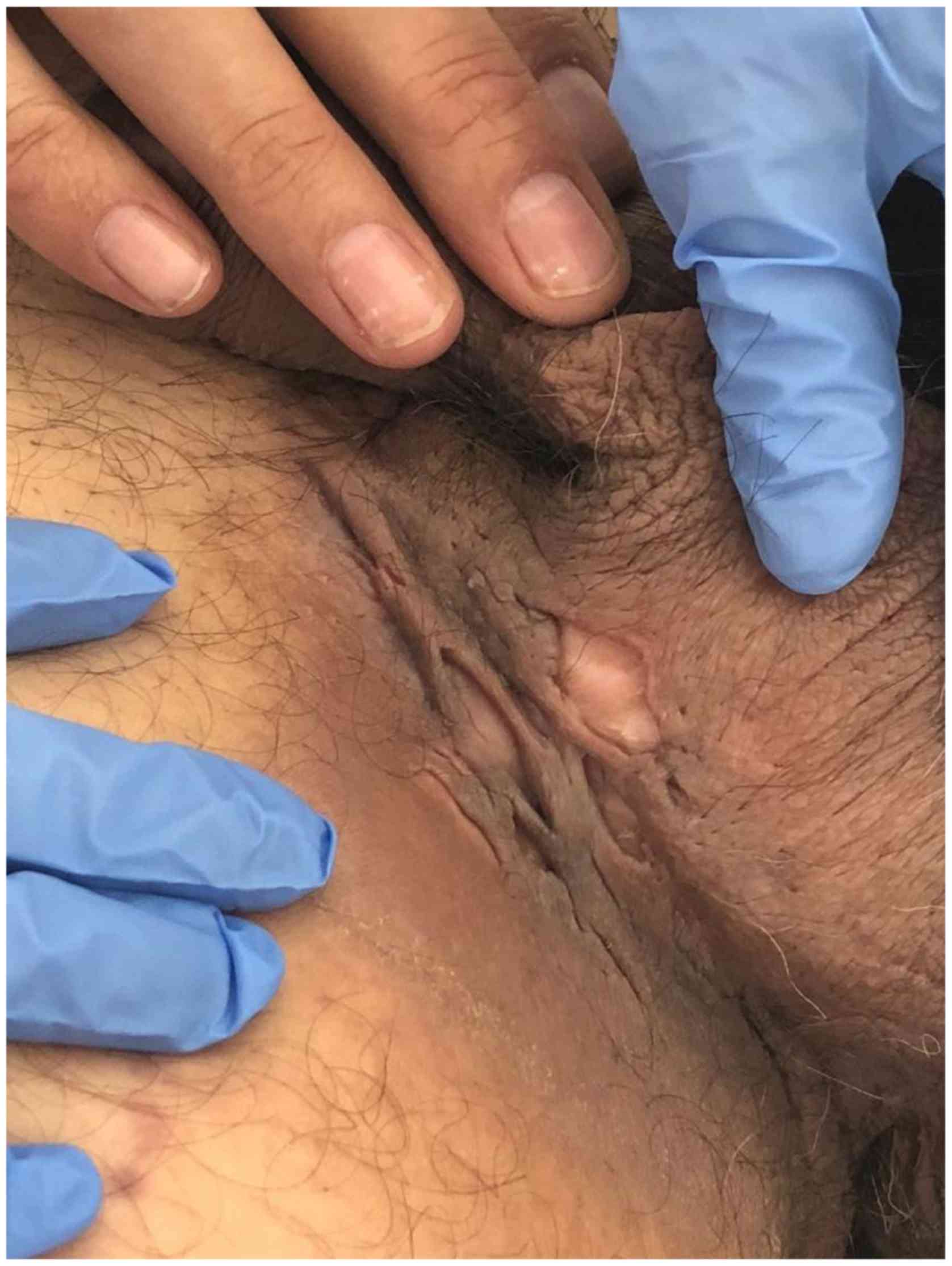
Physical examination also revealed a bilateral,
inguinal-scrotal area, fibrotic scar tissue, depressed scars,
hyperpigmentation and open comedones, compatible with the diagnosis
of HS (Fig. 2). The patient
underwent surgery for relapsing left and right inguinal abscess in
2014 and 2016. No acne scars were identified on the face.
Laboratory workup revealed decrease of inflammatory markers and
increased liver enzymes, that normalized rapidly in the following
days (probably drug induced). No pathergy sign. Medical
investigations did not reveal signs of inflammatory bowel disease,
hematologic malignancy or arthritis.
Results
Due to the atypical healing pattern, with the
predominance of comedo-like openings, double-ended pseudocomedones
and bridging fibrosis, a new biopsy was taken which showed
fistulous cystic tract associated with fibrotic tissue. These
aspects are most commonly met in HS, but also in primary infection,
ruptured epidermal cyst, pilonidal sinus, acne vulgaris,
Favre-Racouchot disease, syringoma, tricoepithelioma, nevus
comedonicus or vegetative PG (6,11).
Multiplicity, no evidence of microorganisms on special stains and
clinical correlation were suggestive for the diagnosis of late
stage of suppurative hidradenitis.
Systemic treatment with Sulfasalazine 500 mg once
daily for one week, then increasing the dose weekly, until 2 g/day
and topical potent steroids controlled the inflammation
satisfactorily, few small ulcers still continuing to appear
occasionally, but without accompanying pain.
Discussion
We had 3 questions regarding the lesion from the
leg: Is this a typical PG but with atypical healing pattern? Is
this an ectopic HS in a male patient with typical HS lesion in
inguinal area? Is this a lesion with elements of both PG and
HS?
The abrupt onset of the painful lesion, after local
trauma, with pustule that rapidly form ulceration, with violaceous
border, together with the pathological aspect and the lack of
criteria for other causes of ulceration are all suggestive for PG.
But regarding the presence of double-ended pseudo-comedones, Revuz
and Jemec (12), considered them a
typical sign of HS, formed after reepithelisation of 2 adjacent
destroyed follicles (13). Boer and
Jemec (14) studied pseudo-comedo
formation in HS and showed that they appear secondary to hair
follicle damage. Although inflammation in PG starts
perifollicularly, healing with pseudo-comedones is not a feature
described in PG. Sinus tract formation can be consistent with PG,
but the vegetative subtype and this was not the case (6). Although not mentioned in the text, in
one of the four cases of PASH presented by Join-Lambert et
al (15), as seen in the
pictures, the lesions of ulcerative PG healed with open
comedones.
There are few studies of cases of chronic cutaneous
lupus erythematosus healing with pseudo-comedones and we can
hypothesize that foliculocentric inflammation is responsible also
in these situations for the pseudo-comedones formation, but still,
it rarely happens (16).
Regarding the second question, the scarring aspect
of the plaque on the leg, in a patient known with HS in
inghinoscrotal area, was suggestive for ectopic HS, as well as the
aspect of the second biopsy, as in the case described by Rondags
et al (17), but the rapid
onset of the lesions is not compatible with the diagnosis of
HS.
So we appreciate that the lesion on the leg had
features of both PG and HS, proving the link between the two
diseases, probably through mechanisms not yet elucidated.
The differential diagnosis issues we highlighted in
the presented case also permitted us to speculate that the
association between HS, PG and acne in syndromes such as PASH may
have a deeper understanding. Does the association of the three
diseases constitute a syndrome or is there a single disease in
which one, two or all three manifestations may be observed in the
same patient?
We consider that the study of comedogenesis remains
one of the essential objectives for elucidating the mechanisms
involved in diseases such as pyoderma gangrenosum, supurative
hydradenitis and acne. Interestingly, as an easily clinically
recognizable mark by adolescents and cosmetics experts, for
dermatologists and researchers, the comedo is still an incomplete
elucidated mystery regarding the biological events involved in its
formation. The complexity of the comedo's significance also arises
from the different roles that it seems to play in various diseases:
in acne, microcomedo formation is the essential element for the
development of acne lesions; in HS the comedo is formed in the
active phases of the disease when the deep part of the follicle is
affected; in diseases such as PG, comedo may be an aspect of
healing phases, through poorly understood mechanisms, probably also
as a consequence of follicular destruction.
Acknowledgements
Not applicable.
Funding
This study is supported by grants of Ministery of
Research and Innovation, CNCS-UEFISCDI, project number
PN-III-P4-ID-PCE-2016-0641, within PNCDI III and CCCDI-UEFISCDI,
project number 61PCCDI⁄2018 PN-III-P1-1.2-PCCDI-2017-0341, within
PNCDI-III.
Availability of data and materials
All patient data are mentioned in the article and
are available from the corresponding author on resonable
request.
Authors' contributions
GT, RIN, ITN, AH, MB and AB made substantial
contributions to the conception and the acquisition of data for the
study. LN, CGP, SAZ and RTA made substantial contributions to the
analysis and interpretation of data for the work. All the authors
revised critically for important intellectual content, approved the
final version to be published and agreed to be accountable for all
aspects of the study in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Patient consent to participate was obtained.
Patient consent for publication
Patient consent for the publication of the images
was obtained before publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kurzen H, Kurokawa I, Jemec GBE, Emtestam
L, Sellheyer K, Giamarellos-Bourboulis EJ, Nagy I, Bechara FG,
Sartorius K, Lapins J, et al: What causes hidradenitis suppurativa?
Exp Dermatol. 17:455–472. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ingram JR: Hidradenitis suppurativa:
Pathogenesis, clinical features, and diagnosis. UpToDate 2018 (Jan
1, 2018). Available from: https://www.uptodate.com/contents/hidradenitis-suppurativa-pathogenesis-clinical-features-and-diagnosis.
|
|
3
|
von Laffert M, Stadie V, Wohlrab J and
Marsch WC: Hidradenitis suppurativa/acne inversa: Bilocated
epithelial hyperplasia with very different sequelae. Br J Dermatol.
164:367–371. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Habif T: Clinical Dermatology: A Color
Guide to Diagnosis and Therapy. Mosby, New York, NY, 2004.
|
|
5
|
Desai N, van der Zee HH and Jemec GB:
Hidradenitis Suppurativa. In: Rook's Textbook of Dermatology.
Griffiths CE, Barker J, Bleiker T, Chalmers R, Creamer D (eds). 9th
edition. Wiley Blackwell. (pp90.1-90.66)2016.
|
|
6
|
Schadt C: Pyoderma Gangrenosum:
Pathogenesis, Clinical Features, and Diagnosis. 2018. Available
from: https://www.uptodate.com/contents/pyoderma-gangrenosum-pathogenesis-clinical-features-and-diagnosis#H603259.
|
|
7
|
Ahronowitz I, Harp J and Shinkai K:
Etiology and management of pyoderma gangrenosum: A comprehensive
review. Am J Clin Dermatol. 13:191–211. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Braun-Falco M, Kovnerystyy O, Lohse P and
Ruzicka T: Pyoderma gangrenosum, acne, and suppurative hidradenitis
(PASH) - a new autoinflammatory syndrome distinct from PAPA
syndrome. J Am Acad Dermatol. 66:409–415. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Antohe M, Nedelcu R, Nichita L, Popp C,
Cioplea M, Brinzea A, Hodorogea A, Calinescu A, Balaban M, Ion DA,
et al: Tumor infiltrating lymphocytes: The regulator of melanoma
evolution (Review). Oncol Lett. 17:4155–4161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cioplea M, Caruntu C, Zurac S, Bastian A,
Sticlaru L, Cioroianu A, Boda D, Jugulete G, Nichita L and Popp C:
Dendritic cell distribution in mycosis fungoides vs. inflammatory
dermatosis and other T-cell skin lymphoma. Oncol Lett.
17:4055–4059. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Calonje E, Brenn T and Lazar A:
Neutrophilic and eosinophilic dermatoses. In: McKee's Pathology of
the Skin. 4th edition. McKee PH (ed). Elsevier Limited.
p657:2012.
|
|
12
|
Revuz JE and Jemec GB: Diagnosing
hidradenitis suppurativa. Dermatol Clin. 34:1–5. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Margesson LJ and Danby FW: Hidradenitis
suppurativa. Best Pract Res Clin Obstet Gynaecol. 28:1013–1027.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Boer J and Jemec GB: Mechanical stress and
the development of pseudo-comedones and tunnels in Hidradenitis
suppurativa/Acne inversa. Exp Dermatol. 25:396–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Join-Lambert O, Duchatelet S, Delage M,
Miskinyte S, Coignard H, Lemarchand N, Alemy-Carreau M, Lortholary
O, Nassif X, Hovnanian A, et al: Remission of refractory pyoderma
gangrenosum, severe acne, and hidradenitis suppurativa (PASH)
syndrome using targeted antibiotic therapy in 4 patients. J Am Acad
Dermatol. 73 (Suppl 1):S66–S69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Farias DF, Gondim RM, Redighieri IP,
Muller H and Petri V: Comedonic lupus: a rare presentation of
discoid lupus erythematosus. An Bras Dermatol. 86 (Suppl
4):S89–S91. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rondags A, Diercks GF, Werker PMN, Jonkman
MF and Horváth B: Ectopic hidradenitis suppurativa on the dorsal
foot of a road maker. JAAD Case Rep. 3:429–431. 2017.PubMed/NCBI View Article : Google Scholar
|