Introduction
Ulcerative colitis (UC) is a refractory, relapsing
and potentially malignant inflammatory disease, and is one of the
main types of inflammatory bowel disease (IBD) (1). In recent years, the incidence of UC in
China has risen, mostly in young adults, exhibiting regional
differences and genetic tendencies, and has become subject to an
increasing amount of research in the field of gastroenterology
(2,3). Dextran sodium sulfate (DSS) is widely
used to induce UC in model mice for study (4). The symptoms in mice with DSS-induced UC
are very similar to those of human UC, suggesting that it is an
ideal model for study. These symptoms include disruption to the
epithelial cell barrier, colitis induced by altered DNA
replication, inhibition of the growth of epithelial cells,
induction of macrophage activation, increased release of cytokines
and disruption to the balance of gut microflora (5). The disease has become a target of
research into new therapies, due to the fact it causes recurrent
attacks, has a poor therapeutic response, leads to prolonged
illness and has the possibility of developing into cancer (6,7). The
main goals in the clinical treatment of UC are to improve
interactions between the symbiotic mucosal flora and the intestinal
mucosa, to prevent the activation of lymphocytes, to induce the
production of regulatory T cells, to inhibit the inflammatory
response and concomitant production of inflammatory cytokines, and
to repair damaged mucosa. Mesalamine (MES) is currently the
first-choice drug for UC, as it can be used to both cause and
maintain the remission of UC (8–10).
Meta-analyses indicated that the effective rate and remission rate
of UC patients treated with MES was better than that in a placebo
group (11,12). However, although only a small amount
of MES reaches the colon, it often produces adverse reactions, such
as abdominal pain, diarrhea and nephrotoxicity (13–15).
Research is therefore focused on developing a high-efficiency and
low-toxicity treatment. In treatment of UC, the new application of
old drugs may play a role in future clinical practice (5,16).
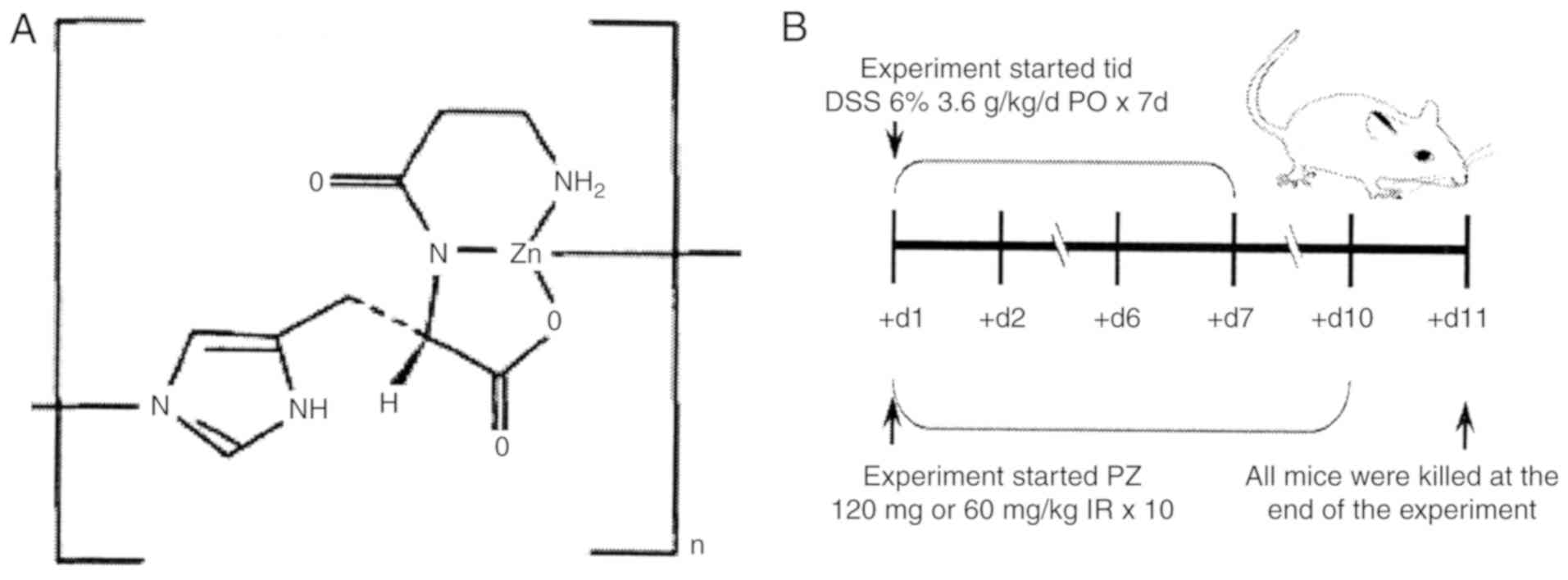
PZ is a chelate compound of zinc and L-carnosine,
and has been used in the treatment of gastric ulcers in Japan since
1994 (17). Researchers have found
that it has a beneficial effect in various experimentally induced
models of colitis in mice (18). The
mechanism of action of PZ in the treatment of UC may involve
induction of heat shock protein (HSP) production, inhibition of the
inflammatory response, anti-oxidation and cell membrane
stabilization (19,20). In the present study DSS was used to
induce a UC model in mice. These mice were then used to explore the
mechanisms of the therapeutic effect of PZ on UC induced by DSS,
and its influence on inflammatory AKT signaling.
Materials and methods
Experimental drugs
PZ was obtained from the Jilin Broadwell
Pharmaceutical Co. Ltd. in 5 g bags, batch number 25-160512
(Fig. 1). A pH 6.8 PZ balanced salt
solution was prepared, and mice received PZ via enema, at a dose of
120 mg/kg body weight or 60 mg/kg body weight. As a positive
control drug, MES was obtained (Sunflower Pharmaceutical Group of
Jiamusi Luling Pharmaceutical Co., Ltd.) as enteric-coated tablets,
containing 0.25 g raw material/tablet (batch no. 170306). The mice
were given 300 mg/kg MES by enema. MES was ground into a powder
using a pestle and mortar and dissolved in 0.9% aseptic sodium
chloride solution.
DSS was obtained from the Shanghai Yisheng
Biotechnology Co., Ltd. (lot no. D97990), as a white or grayish
white powder, mice received DSS dissolved in H2O via
intragastric administration of 0.2 ml/10 g body weight. three times
per day (Table I).
 | Table I.Mouse experimental grouping and means
of drug administration. |
Table I.
Mouse experimental grouping and means
of drug administration.
| Group | Drug doses
administered | Drug administration
modes | Duration of drug
administration |
|---|
| Vehicle control | 0 MES/PZ | N/A | N/A |
|
| 0 DES | N/A | N/A |
| UC only | 0 MES/PZ | N/A | N/A |
|
| 3.6 g/kg/day DSS | PO | Three times/day for 7
days |
| UC + MES | 300 mg/kg MES | IR | Once a day for 10
days |
|
| 3.6 g/kg/day
DSS | PO | Three times/day for
7 days |
| UC + PZ L | 60 mg/kg PZ | IR | Once a day for 10
days |
|
| 3.6 g/kg/day
DSS | PO | Three times/day for
7 days |
| UC + PZ H | 120 mg/kg PZ | IR | Once a day for 10
days |
|
| 3.6 g/kg/day
DSS | PO | Three times/day for
7 days |
Experimental animals
Male inbred Institute of Cancer Research (ICR) mice
of specific pathogen free grade (age 6–8 weeks; weight, 17–19 g; 8
mice per group leading to a total of 40 mice) were obtained from
the National Institute for Food and Drug Control in China
[experimental animal quality certificate no. SCXK (Beijing, China)
2014-0013]. Food and water was available ad libitum
throughout the experiment. The mice were kept at 20–25±3°C at a
relative humidity of 40–60% and on a 12 h light/dark cycle.
UC model induction and sample
collection
ICR mice were randomly divided into five groups of 8
mice per group (Table I). The groups
were vehicle control (intragastric H2O), UC model
control, UC + MES, UC + low-dose (L) PZ and UC + high-dose (H) PZ.
The UC model mice were intragastrically administered DSS at 3.6
g/kg/day, three times per day for 7 consecutive days, while the
vehicle control mice received the equivalent dose of
H2O. In the UC + MES group, 300 mg/kg MES was
intrarectally administered once per day for 10 days. PZ was
intrarectally administered once per day for 10 days, at 120 mg/kg
in PZ H group, and at 60 mg/kg in the PZ L group. The vehicle group
received the equivalent dose of vehicle by the same method and at
the same time intervals. The experimental animals were observed
daily for 10 days (Fig. 1B).
Approximately 24 h after the last treatment administration, and
following CO2 anesthesia, the animals were killed by
cervical dislocation. After the blood (~0.5 ml) was taken from the
fundus vein cluster of each mouse, the serum was collected by
centrifugation for 5 min, at 1,788.8 × g and 4°C. The serum was
then stored at −80°C. Serum levels of NF-κB and TNF-α were
determined within 3 days of collection.
Animal hemogram analysis
At the end of the experiment, blood (~0.1 ml) was
taken from the fundus vein cluster of each mouse put into 20 units
of heparin (Shanghai Biochemical Co., Ltd.) and the hemogram was
measured using an automatic blood analyzer (Sysmex Hematology
Analyzer KX-21; Hitachi, Ltd.) within 2 h.
Diarrhea scoring
The diarrhea of mice was observed during the
experimental period and the scores were recorded and confirmed as
follows: 0, no diarrhea; 1, mild diarrhea (perianal staining); 2,
moderate diarrhea (hind legs, upper and lower abdomen staining) and
3, severe diarrhea (hind legs and whole abdomen stained, or the
mouse had persistent defecation). Observers were not blinded, but
maintained objectivity.
Colon tissue fixation and hematoxylin
and eosin (H&E) staining
The colonic tissue of each mouse was fixed with
neutral 10% formalin at room temperature for 48 h, embedded in
paraffin and then sectioned with a microtome to obtain 4–5 µm-thick
paraffin sections. Dewaxed sections were then stained with H&E,
as previously described (21), and
the tissue morphological changes were visualized by light
microscopy using an Olympus PM-6 microscope (Olympus
Corporation).
Mouse ELISA
NF-κB (cat. no. DG30647M) and TNF-α (cat. no.
DG30048M) ELISA kits were supplied by Beijing Dongge Biotech Co.,
Ltd. and ELISAs were performed following the manufacturer's
instructions. The kits were single-step sandwich ELISAs. Serum
samples, standard samples and horseradish peroxidase (HRP)-labeled
antibodies were successively added into the wells of a 96-well
plate. After incubation and thorough washing, TMB chromogenic
substrate was added into each well, which was converted into a blue
compound by reduction with the HRP attached to the antibody. This
color change allowed for quantitative deduction of the protein
concentration in the samples. Optical density was determined using
a Tecan Infinite F50 microplate reader (Tecan Group Ltd.) to
measure the absorbance at a wavelength of 450 nm, and was used to
calculate the concentration of each group of samples.
Western blot analysis of the
expression of AKT, p-AKT and HSP70 in mouse colonic tissue
A segment of the colon of each mouse (from the
ileocecal valve to a fixed site before the beginning of the rectum)
was added to cell lysis solution [prepared using 50 mM Tris base,
150 mM NaCl and 0.1% SDS (0.303 g Tris base, 0.4383 g NaCl, 0.05 g
SDS and 40 ml H2O, using HCl to adjust pH to 8.0 in a 50
ml volume)] and the supernatant was collected after centrifugation
at 16,770 × g for 10 min, at 4°C. The total protein concentration
in each sample was determined using a Bradford protein assay. A
total of 10 µg of protein was loaded into each lane of a 10%
SDS-PAGE gel. After SDS-PAGE the proteins were transferred onto a
PVDF membrane. The membrane was blocked with 5% skim milk powder in
TBST (0.1% Tween) for 1 h at 25°C. Cells were then incubated with a
1:300 dilution of primary antibody (cat. no. AB26297; Abbkine
Scientific Co., Ltd.) or a 1:1,000 dilution of loading control
antibody (cat. no. AC21215; Abbkine Scientific Co., Ltd.) overnight
at 4°C. The membranes were washed before they were incubated with a
1:3,000 dilution of HRP-conjugated secondary antibody (cat. no.
ZB-2305; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) for 1 h
at room temperature. Blots were then visualized using an ECL kit
(Beijing Roby Biotechnology Co., Ltd.; RBU 117-100). The
densitometry was calculated using ImageJ (version no. 20150116;
National Institutes of Health).
Statistical analysis
Data (from 8 samples per group) are expressed as the
mean ± SD. Statistical significance was evaluated using comparisons
between groups analyzed by one-way ANOVA followed by the least
significant difference post hoc test. All statistical analyses were
performed with SPSS 17.0 (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
After 3 days of administration of DSS, the body
weight of the mice increased and there was a significant weight
difference between the DSS-treated mice and the normal control
group mice (P<0.01; Table II).
After administration of DSS, diarrhea occurred and weight gain
slowed down. DSS was found to induce diarrhea in mice after
intragastric administration, and PZ inhibited this DSS-induced
diarrhea. Compared with the DSS model group, there was significant
reduction in diarrhea in the PZ L group (P<0.05) and diarrhea
was mild in the PZ H group (P<0.01; Table III).
 | Table II.Effect of PZ on mouse body weight in
DSS-induced UC. |
Table II.
Effect of PZ on mouse body weight in
DSS-induced UC.
| Group | Pre-experiment
weight (g) | DSS day 3 weight
(g) | DSS day 6 weight
(g) | DSS day 9 weight
(g) |
|---|
| Vehicle
control | 23.8±1.0 | 29.4±1.8 | 32.5±1.8 | 34.1±1.8 |
| UC only | 23.1±0.9 |
25.1±1.1a |
26.6±1.4a |
27.1±1.8a |
| UC + MES | 23.0±1.2 |
25.6±3.0a |
26.4±1.7a |
26.5±1.9a |
| UC + PZ L | 23.0±1.0 |
25.3±2.6a |
26.0±1.8a |
27.2±1.3a |
| UC + PZ H | 23.2±1.0 |
24.2±2.5a |
26.0±1.5a |
27.0±1.9a |
 | Table III.Effect of PZ diarrhea recovery in
dextran sodium sulfate-induced UC mice. |
Table III.
Effect of PZ diarrhea recovery in
dextran sodium sulfate-induced UC mice.
| Group | Individual mouse
diarrhea scores | Mean diarrhea
score |
|---|
| Vehicle
control | 0, 0, 0, 0, 0, 0,
0, 0 | 0 |
| UC only | 1, 1, 1, 1, 1, 1,
1, 2 | 1.125±0.353 |
| UC + MES | 1, 1, 1, 0, 0, 0,
0, 0 |
0.375±0.517a |
| UC + PZ L | 1, 1, 1, 1, 1, 0,
0, 0 |
0.625±0.517b |
| UC + PZ H | 1, 1, 1, 0, 0, 0,
0, 0 |
0.375±0.517a |
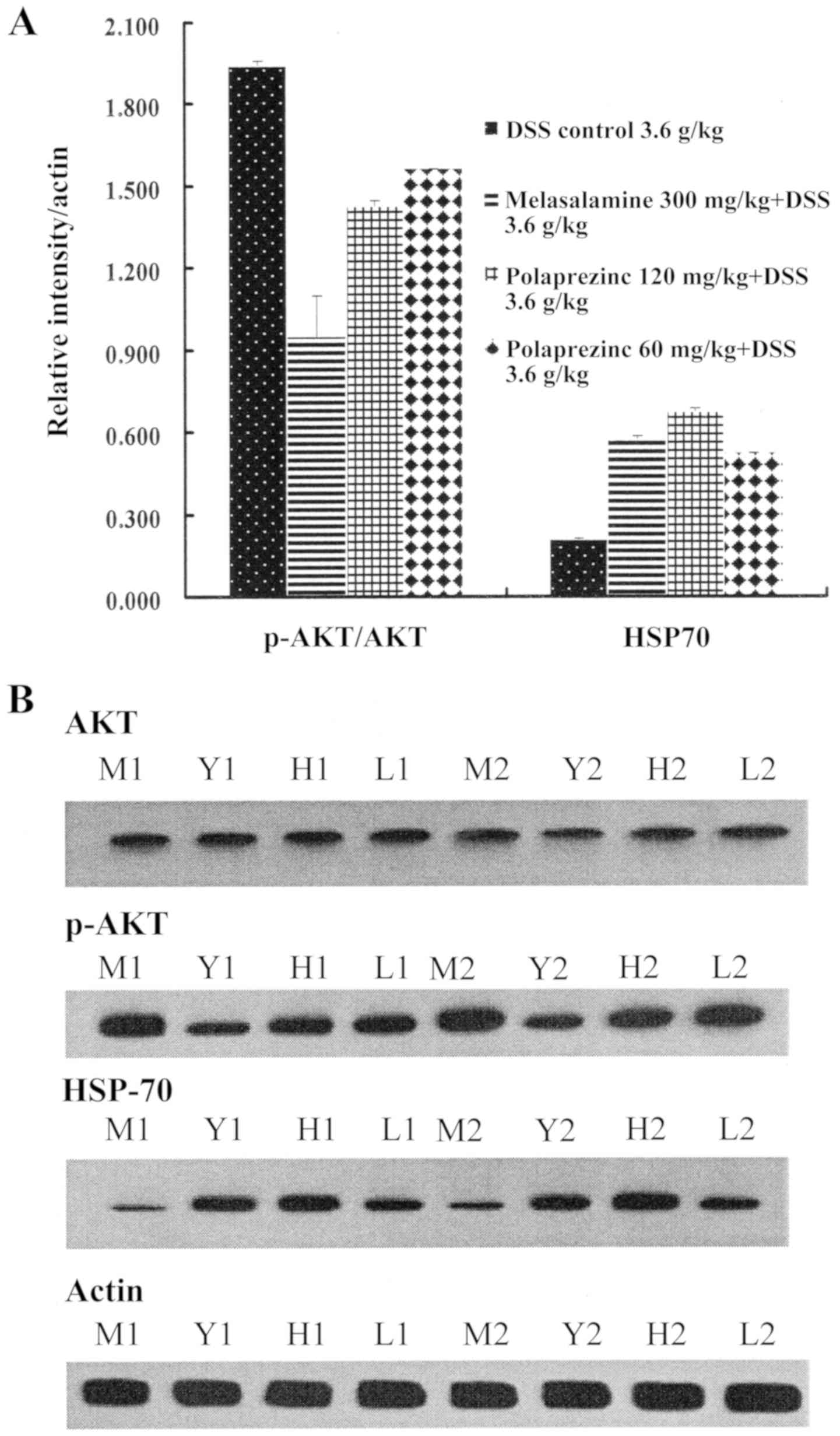
PZ reduced the release of the inflammatory factors
TNF-α and NF-κB in mice from the DSS-induced UC model, compared
with that in mice from the DSS control group (P<0.05). High dose
PZ increased HSP70 protein expression and inhibited the
phosphorylation of AKT, an important mediator of inflammatory
signaling, compared with UC model controls, though these changes
were not statistically significant (Table IV and Fig. 2).
 | Table IV.Effect of PZ on levels of TNF-α,
NF-κB, HSP70 and AKT phosphorylation in dextran sodium
sulfate-induced UC mice. |
Table IV.
Effect of PZ on levels of TNF-α,
NF-κB, HSP70 and AKT phosphorylation in dextran sodium
sulfate-induced UC mice.
| Group | TNF-α (pg/ml) | NF-κB (pg/ml) | p-AKT/AKT
ratio | HSP70 (grey
value) |
|---|
| Vehicle
control | 60.28±8.27 | 900.33±133.54 | Not tested | Not
tested |
| UC only |
84.82±7.40a |
1262.88±169.01a | 1.941±0.016 | 0.484±0.013 |
| UC + MES |
64.81±10.22b |
974.12±123.35b | 0.948±0.151 | 1.335±0.020 |
| UC + PZ L |
74.81±10.31a,c |
1070.38±168.44d | 1.560±0.002 | 1.219±0.003 |
| UC + PZ H |
62.38±8.70b |
826.80±116.88b | 1.427±0.019 | 1.577±0.015 |
A hemogram of mice with DSS-induced UC showed that
the symptoms and pathology were similar to those in humans with UC
(19), and the inflammation was
accompanied by an increase in the count of leukocytes and
neutrophils in peripheral blood, which was similar to that observed
in humans with UC. The results also showed that the white blood
cell and neutrophil % counts were increased in mice from the
DSS-induced UC model group compared with the vehicle control group,
and decreased after treatment with PZ (Table V).
 | Table V.Effect of polaprezinc on the recovery
of the blood hemogram in mice with UC induced by dextran sodium
sulfate. |
Table V.
Effect of polaprezinc on the recovery
of the blood hemogram in mice with UC induced by dextran sodium
sulfate.
| Group | Total WBC
(×109/l) | Neutrophil
granulocyte (%) |
|---|
| Vehicle
control | 5.70±0.53 | 23.33±1.94 |
| UC only |
8.32±2.42a |
34.96±9.90a |
| UC + MES | 7.86±1.05 | 31.36±11.08 |
| UC + PZ L | 7.20±1.50 | 27.54±10.86 |
| UC + PZ H | 6.73±1.14 |
19.4±6.50b |
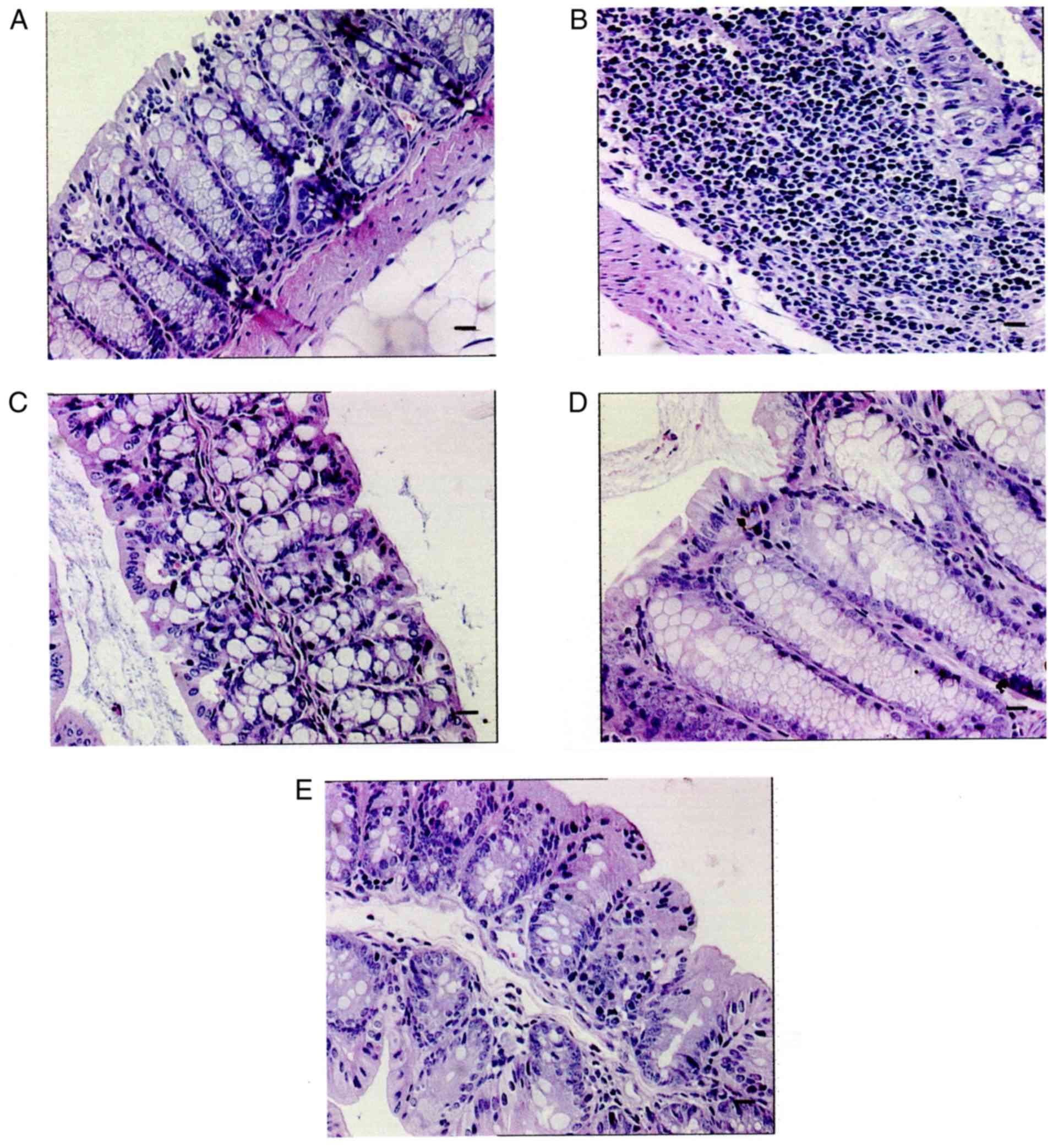
Histopathological observations in mice from the
DSS-induced UC model revealed that their colonic villi were
irregular and that they had an increased number of inflammatory
cells and mucosal damage in comparison with the vehicle control
group. The continuity of their intestinal mucosa was disrupted and
a large number of inflammatory cells were found to have
infiltrated, as the arrow shows. By contrast, observations of the
mice from the PZ-treated group revealed that their villi were
intact and that they only showed mild colonic mucosal inflammation
(Fig. 3).
Discussion
UC is a chronic recurrent form of IBD, characterized
by diffuse inflammation and ulceration. The main goals of the
clinical treatment of UC are to improve the interaction between the
mucosal symbiotic bacteria and intestinal mucosa, prevent the
activation of lymphocytes, induce production of regulatory T cells,
inhibit inflammatory reactions and concomitant production of
inflammatory cytokines and repair the damaged mucosa. The cascade
of mucosal inflammation in UC, and the imbalance between
proinflammatory cytokines, including interleukin (IL)-1, IL-6, IL-8
and tumor necrosis factor-α, and anti-inflammatory cytokines are
important linked events in the pathogenesis of UC.
PZ is a chelate compound composed of L-carnosine and
zinc, which has been widely used in Japan since 1994 for the
treatment of gastric ulcers. PZ stimulates mucus secretion and
antioxidant production, stabilizes cell membranes, induces the
production of HSPs and heme oxygenases, and can protect against
gastric mucosal ulcers induced by various agents, as well as
promoting healing. In recent years, researchers have found that PZ
has a therapeutic effect on various experimentally-induced colitis
models in mice (18,19). The mechanism of action of PZ may
involve the induction of HSPs, inhibition of the inflammatory
process, antioxidant functions and cell membrane stabilization
(18,22–26). It
has been reported that intrarectal administration of PZ can
effectively protect against subserous injection-induced colitis in
rats, and has a beneficial effect on ulcers during the healing
stage (27,28)
DSS-induced acute colitis is a chemically-induced
model of UC. The model is simple and practical, and has many
similarities with human UC, thus it is widely used in UC research
(29).
NF-κB is a universal transcription factor, which is
involved in the regulation of many pro-inflammatory factors and
plays an important role in the pathogenesis of IBD. The classic
NF-κB activation pathway is related to the rapid production of a
large number of pro-inflammatory mediators, such as cyclo-oxygenase
2, IL-1β and IL-6, in acute inflammation in patients with IBD
(30). Among the many inflammatory
cytokines, TNF-α, IL-1β, IL-6 and IL-8 play a major role in
inflammation (31). TNF-α is one of
the most abundant early inflammatory mediators and can activate
neutrophils and lymphocytes, promote neutrophil migration to the
inflammatory region of the colon mucosa, increase the permeability
of vascular endothelial cells, regulate tissue metabolic activity,
promote the synthesis and release of other pro-inflammatory
cytokines and immunomodulators and lead to enterocyte apoptosis.
TNF-α also promotes platelet activating factor release, which
accelerates thrombosis, leading to mucosal microcirculation
disturbance and extensive damage to the colonic mucosa (8,32). It
has been reported that an increase in TNF-α levels in UC patient
serum is related to exacerbation of the condition (33).
HSPs are a family of highly conserved and
ubiquitously expressed proteins. During infection, ischemia and
other physiological stress conditions, HSP expression has a
protective role. HSPs are key anti-inflammatory molecules, which
play a role in preventing a physiological reaction (34). It has been reported that the
expression of HSP in a colitis model was significantly increased
after treatment with drugs (35,36).
MES is a compound used in the treatment of UC, which
has a beneficial effect, MES inhibits the function of various
inflammatory mediators, such as leukotriene and prostaglandin, to
suppresses intestinal inflammatory reactions, but widespread use in
clinical practice is limited due to the potential for the
development of drug dependence, long-term drug resistance and
economic pressure on patients due to its high cost (37).
The results of the present study suggested that PZ
may significantly improve the symptoms of DSS-induced UC in mice.
PZ treatment appeared to reduce colon tissue hyperemia, edema and
inflammatory responses. As these effects are related to the
anti-inflammatory activity of PZ, it can be hypothesized that the
mechanism mediating these effects involves the inhibition of
inflammatory signaling via phosphorylated AKT and an increased HSP
level. The present study was limited by the small sample size and
the fact that only animal experiments could be carried out. Further
research into the effect of PZ on UC should be carried out in the
clinic.
The incidence of UC in China has been increasing
year on year (38,39). However, the emergence of new drugs,
new dosage forms and new therapies based on the clinical
manifestation of patients should allow for the identification of
individual treatment protocols, which will be helpful to improve or
cure the symptoms and intestinal lesions of patients with UC, and
make the treatment of UC more economical and effective.
Acknowledgements
The authors thank Dr. Ziqiang Zhang of the Pathology
Department, Chinese Academy of Medical Sciences and Peking Union
Medical College for assistance with paraffin sectioning and H&E
staining.
Funding
This study was supported by Jilin Province Broadwell
Pharmaceutical Co., Ltd. (Changchun, China; grant no. 2017-08-14)
and the Technology Innovation Fund for Enterprises (grant no.
2017-001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and WX performed molecular and animal
experiments, arranged the data and revised the article; ML, ZL and
WX analyzed and interpreted the data; JL performed molecular and
animal experiments; ZL, ML designed the concept and wrote the
manuscript; XL and TL participated in the molecular experiments.
All of the authors have approved the final manuscript for
publication.
Ethics approval and consent to
participate
The animal use protocol in this study went through
an Animal Experimental Ethical Inspection process and was reviewed
and approved by the Animal Care & Welfare Committee of the
Chinese Academy of Medical Sciences-Peking Union Medical
College.
Patient consent for publication
Not applicable.
Competing interests
One of the authors has received research grants from
Jilin Province Broadwell Pharmaceutical Co., Ltd. The authors
declare that they have no other competing interests.
References
|
1
|
Moura FA, de Andrade KQ, Dos Santos JCF,
Araújo ORP and Goulart MOF: Antioxidant therapy for treatment of
inflammatory bowel disease: Does it work? Redox Biol. 6:617–639.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suh JH and Saba JD:
Sphingosine-1-phosphate in inflammatory bowel disease and
colitis-associated colon cancer: The fat's in the fire. Transl
Cancer Res. 4:469–483. 2015.PubMed/NCBI
|
|
3
|
Yang QF, Chen BL, Zhang QS, Zhu ZH, Hu B,
He Y, Gao X, Wang YM, Hu PJ, Chen MH and Zeng ZR: Contribution of
MDR1 gene polymorphisms on IBD predisposition and response to
glucocorticoids in IBD in a Chinese population. J Dig Dis.
16:22–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eichele DD and Kharbanda KK: Dextran
sodium sulfate colitis murine model: An indispensable tool for
advancing our understanding of inflammatory bowel diseases
pathogenesis. World J Gastroenterol. 23:6016–6029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang ZL, Fan HY, Yang MY, Zhang ZK and
Liu K: Therapeutic effect of a hydroxynaphthoquinone fraction on
dextran sulfate sodium-induced ulcerative colitis. World J
Gastroenterol. 20:15310–15318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Däbritz J, Gerner P, Enninger A, Classen M
and Radke M: Inflammatory bowel disease in childhood and
adolescence. Dtsch Arztebl Int. 114:331–338. 2017.PubMed/NCBI
|
|
7
|
Balfe A, Lennon G, Lavelle A, Docherty NG,
Coffey JC, Sheahan K, Winter DC and O'Connell PR: Isolation and
gene expression profiling of intestinal epithelial cells: Crypt
isolation by calcium chelation from in vivo samples. Clin Exp
Gastroenterol. 11:29–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biasi F, Leonarduzzi G, Oteiza PI and Poli
G: Inflammatory bowel disease: Mechanisms, redox considerations,
and therapeutic targets. Antioxid Redox Signal. 19:1711–1747. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yarlas A, D'Haens G, Willian MK and Teynor
M: Health-related quality of life and work-related outcomes for
patients with mild-to-moderate ulcerative colitis and remission
status following short-term and long-term treatment with
multimatrix mesalamine: A prospective, open-label study. Inflamm
Bowel Dis. 24:450–463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oliveira L and Cohen RD: Maintaining
remission in ulcerative colitis-role of once daily extended-release
mesalamine. Drug Des Devel Ther. 5:111–116. 2011.PubMed/NCBI
|
|
11
|
Ming LR, Wu Bin W and Tang Yao T: Safety
and effectiveness of mesalazine in the treatment of ulcerative
colitis: A systematic review. China Pharm. 21:4201–4204. 2010.
|
|
12
|
Algaba A, Guerra I, García García de
Paredes A, Hernández Tejero M, Ferre C, Bonillo D, Aguilera L,
López-Sanromán A and Bermejo F: What is the real-life maintenance
mesalazine dose in ulcerative colitis? Rev Esp Enferm Dig.
109:114–121. 2017.PubMed/NCBI
|
|
13
|
Ransford RA and Langman MJ: Sulphasalazine
and mesalazine: Serious adverse reactions re-evaluated on the basis
of suspected adverse reaction reports to the Committee on Safety of
Medicines. Gut. 51:536–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimodate Y, Takanashi K, Waga E, Fujita
T, Katsuki S and Nomura M: Exacerbation of bloody diarrhea as a
side effect of mesalamine treatment of active ulcerative colitis.
Case Rep Gastroenterol. 5:159–165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sehgal P, Colombel JF, Aboubakr A and
Narula N: Systematic review: Safety of mesalazine in ulcerative
colitis. Aliment Pharmacol Ther. 47:1597–1609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaughn BP and Moss AC: Novel treatment
options for ulcerative colitis. Clin Investig (Lond). 3:1057–1069.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takei M: Development of polaprezinc
research. Yakugaku Zasshi. 132:271–277. 2012.(In Japanese).
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itagaki M, Saruta M, Saijo H, Mitobe J,
Arihiro S, Matsuoka M, Kato T, Ikegami M, Tajiri H and Scand J:
Efficacy of zinc-carnosine chelate compound, Polaprezinc, enemas in
patients with ulcerative colitis. Scand J Gastroenterol. 49:164–72.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ko JK and Leung CC: Ginger extract and
polaprezinc exert gastroprotective actions by anti-oxidant and
growth factor modulating effects in rats. J Gastroenterol Hepatol.
25:1861–1868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakae K and Yanagisawa H: Oral treatment
of pressure ulcers with polaprezinc (zinc L-carnosine complex):
8-week open-label trial. Biol Trace Elem Res. 158:280–288. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chassaing B, Aitken JD, Malleshappa M and
Vijay-Kumar M: Dextran sulfate sodium (DSS)-induced colitis in
mice. Curr Protoc Immunol. 104:Unit 15.25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Okamura S, Kudo T, Masuo T and
Mori M: Calcineurin inhibition by polaprezinc in rats with
experimentally-induced colitis. Life Sci. 88:432–439. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsukura T and Tanaka H: Applicability of
zinc complex of L-carnosine for medical use. Biochemistry (Mosc).
65:817–823. 2000.PubMed/NCBI
|
|
25
|
Ohkawara T, Nishihira J, Nagashima R,
Takeda H and Asaka M: Polaprezinc protects human colon cells from
oxidative injury induced by hydrogen peroxide: Relevant to
cytoprotective heat shock proteins. World J Gastroenterol.
12:6178–6181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Itagaki M, Saruta M, Saijo H, Mitobe J,
Arihiro S, Matsuoka M, Kato T, Ikegami M and Tajiri H: Efficacy of
zinc-carnosine chelate compound, Polaprezinc, enemas in patients
with ulcerative colitis. Scand J Gastroenterol. 49:164–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong Z, Du L, Xu X, Yang Y, Wang H, Qu A,
Qu X and Wang C: Aberrant expression of circulating Th17, Th1 and
Tc1 cells in patients with active and inactive ulcerative colitis.
Int J Mol Med. 31:989–997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murakami-Nakayama M, Tsubota M, Hiruma S,
Sekiguchi F, Matsuyama K, Kimura T, Moriyama M and Kawabata A:
Polaprezinc attenuates cyclophosphamide-induced cystitis and
related bladder pain in mice. J Pharmacol Sci. 127:223–228. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McDaniel DK, Eden K, Ringel VM and Allen
IC: Emerging roles for noncanonical NF-κB signaling in the
modulation of inflammatory bowel disease pathobiology. Inflamm
Bowel Dis. 22:2265–2279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kruis W, Kiudelis G and Racz I: Once daily
versus three times daily mesalazine granules in active ulcerative
colitis: A doube-blind, double-dummy, randomized, non-inferiority
trial. Gut. 58:233–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanchez-Munoz F, Dominguez-Lopez A and
Yamamoto-Furusho JK: Role of cytokines in inflammatory bowel
disease. World J Gastroenterol. 14:4280–4288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Li J, Meng Y, Cao M and Wang J:
Treatment effects of jinlingzi powder and its extractive components
on gastric ulcer induced by acetic acid in rats. Evid Based
Complement Alternat Med. 2019:73658412019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fausel R and Afzali A: Biologics in the
management of ulcerative colitis-comparative safety and efficacy of
TNF-α antagonists. Ther Clin Risk Manag. 11:63–73. 2015.PubMed/NCBI
|
|
34
|
Arrigo P: Pathology-dependent effects
linked to small heat shock proteins expression: An Update.
Scientifica (Cairo). 2012:1856412012.PubMed/NCBI
|
|
35
|
Gupta R, Chaudhary AR, Shah BN, Jadhav AV,
Zambad SP, Gupta RC, Deshpande S, Chauthaiwale V and Dutt C:
Therapeutic treatment with a novel hypoxia-inducible factor
hydroxylase inhibitor (TRC160334) ameliorates murine colitis. Clin
Exp Gastroenterol. 7:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moghadamtousi SZ, Rouhollahi E, Karimian
H, Fadaeinasab M, Abdulla MA and Kadir HA: Gastroprotective
activity of Annona muricata leaves against ethanol-induced gastric
injury in rats via Hsp70/Bax involvement. Drug Des Devel Ther.
8:2099–2110. 2014.PubMed/NCBI
|
|
37
|
Ono K, Nimura S, Hideshima Y, Nabeshima K
and Nakashima M: Orally administered sodium 4-phenylbutyrate
suppresses the development of dextran sulfate sodium-induced
colitis in mice. Exp Ther Med. 14:5485–5490. 2017.PubMed/NCBI
|
|
38
|
Yu Q, Mao R, Lian L, Ng SC, Zhang S, Chen
Z, Zhang Y, Qiu Y, Chen B, He Y, et al: Surgical management of
inflammatory bowel disease in China: A systematic review of two
decades. Intest Res. 14:322–332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhai H, Liu A, Huang W, Liu X, Feng S, Wu
J, Yao Y, Wang C, Li Q, Hao Q, et al: Increasing rate of
inflammatory bowel disease: A 12-year retrospective study in
NingXia, China. BMC Gastroenterol. 16:22016. View Article : Google Scholar : PubMed/NCBI
|