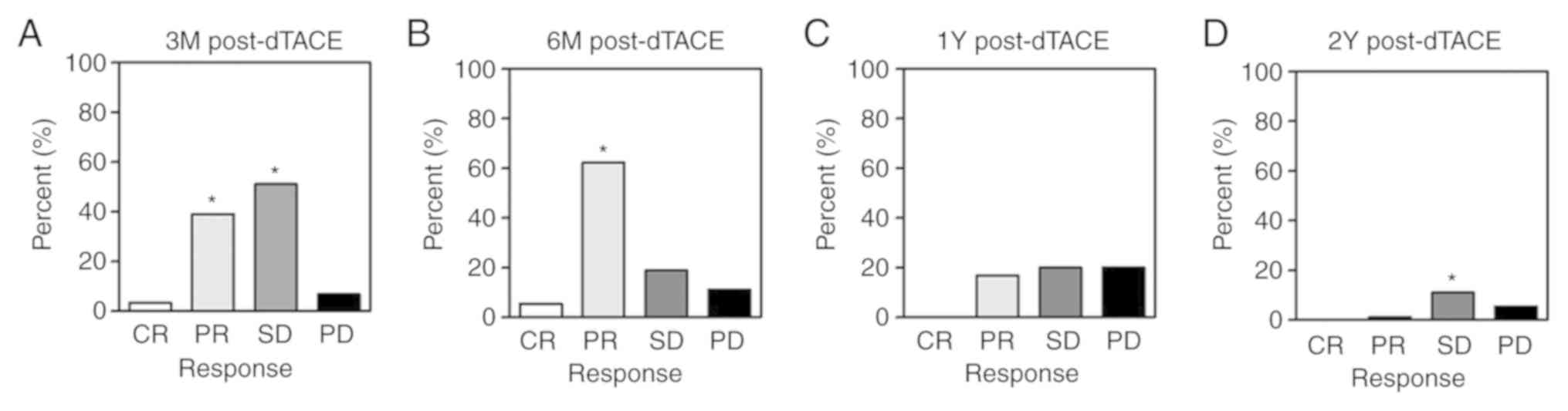
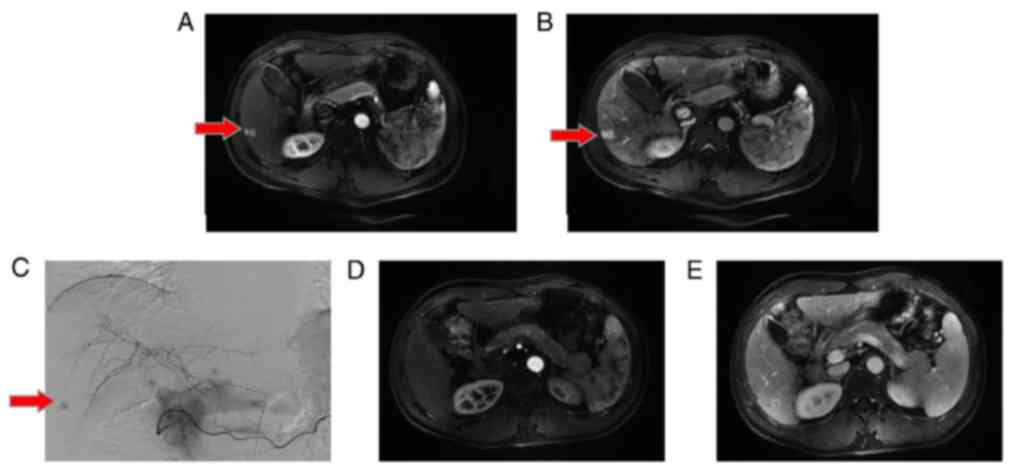
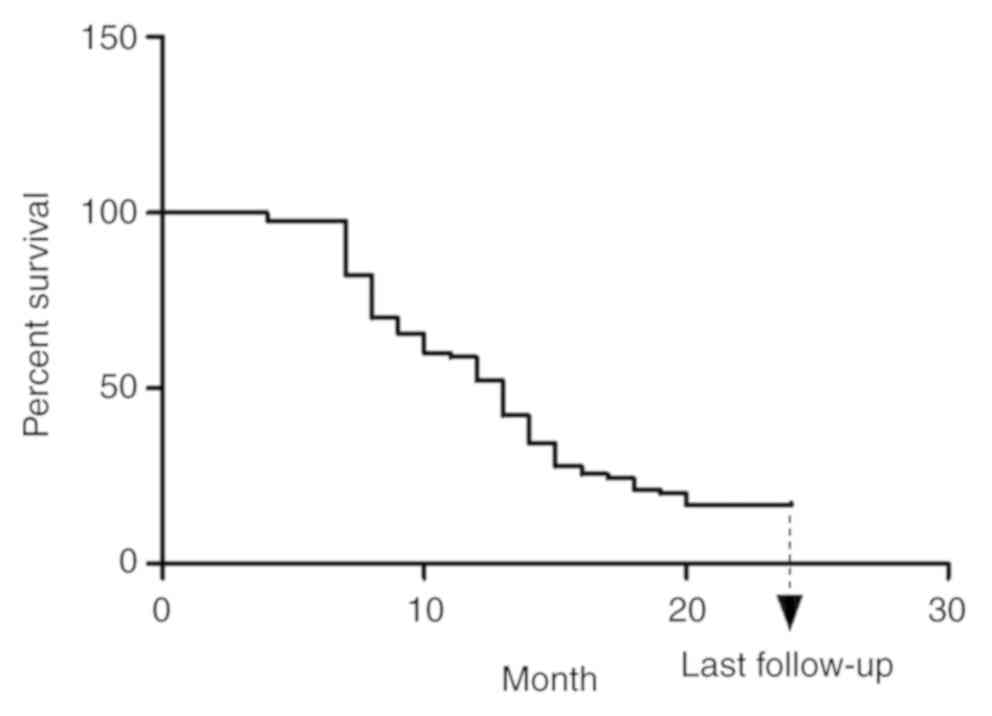
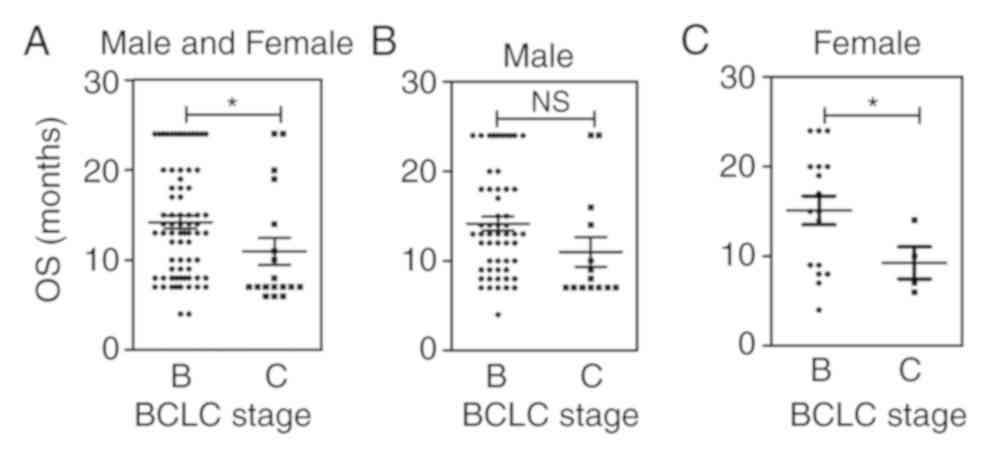
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T,
Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et
al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2016:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 4:1553–1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al: World Gastroenterology Organisation Guideline.
Hepatocellular carcinoma (HCC): A global perspective. J
Gastrointestin Liver Dis. 19:311–317. 2010.PubMed/NCBI
|
|
4
|
Bertino G, Demma S, Ardiri A, Proiti M,
Gruttadauria S, Toro A, Malaguarnera G, Bertino N, Malaguarnera M,
Malaguarnera M and Di Carlo I: Hepatocellular carcinoma: Novel
molecular targets in carcinogenesis for future therapies. Biomed
Res Int. 2014:2036932014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen F, Wang H, Zhu J, Zhao R, Xue P,
Zhang Q, Bud Nelson M, Qu W, Feng B and Pi J: Camptothecin
suppresses NRF2-ARE activity and sensitizes hepatocellular
carcinoma cells to anticancer drugs. Br J Cancer. 117:1495–1506.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiefer MV, Albert M, McNally M, Robertson
M, Sun W, Fraker D, Olthoff K, Christians K, Pappas S, Rilling W
and Soulen MC: Chemoembolization of intrahepatic cholangiocarcinoma
with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl
alcohol: A 2-center study. Cancer. 117:1498–1505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venturini M, Sallemi C, Agostini G, Marra
P, Cereda S, Reni M, Aldrighetti L, De Cobelli F and Del Maschio A:
Chemoembolization with drug eluting beads preloaded with irinotecan
(DEBIRI) vs. doxorubicin (DEBDOX) as a second line treatment for
liver metastases from cholangiocarcinoma: A preliminary study. Br J
Radiol. 89:201602472016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gusani NJ, Balaa FK, Steel JL, Geller DA,
Marsh JW, Zajko AB, Carr BI and Gamblin TC: Treatment of
unresectable cholangiocarcinoma with gemcitabine-based
transcatheter arterial chemoembolization (TACE): A
single-institution experience. J Gastrointest Surg. 12:129–137.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuhlmann JB, Euringer W, Spangenberg HC,
Breidert M, Blum HE, Harder J and Fischer R: Treatment of
unresectable cholangiocarcinoma: Conventional transarterial
chemoembolization compared with drug eluting bead-transarterial
chemoembolization and systemic chemotherapy. Eur J Gastroenterol
Hepatol. 24:437–443. 2012.PubMed/NCBI
|
|
11
|
Lencioni R: Loco-regional treatment of
hepatocellular carcinoma. Hepatology. 52:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rostas JW, Tam AL, Sato T, Scoggins CR,
McMasters KM and Martin RCG II: Health-related quality of life
during trans-arterial chemoembolization with drug-eluting beads
loaded with doxorubicin (DEBDOX) for unresectable hepatic
metastases from ocular melanoma. Am J Surg. 214:884–890. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Namur J, Citron SJ, Sellers MT, Dupuis MH,
Wassef M, Manfait M and Laurent A: Embolization of hepatocellular
carcinoma with drug-eluting beads: Doxorubicin tissue concentration
and distribution in patient liver explants. J Hepatol.
55:1332–1338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Delhaye C, Hendlisz A and Vouche M: Update
on transarterial approaches to locoregional treatment in
hepatocellular carcinoma. Curr Opin Oncol. 31:339–345. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varela M, Real MI, Burrel M, Forner A,
Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM and
Bruix J: Chemoembolization of hepatocellular carcinoma with drug
eluting beads: Efficacy and doxorubicin pharmacokinetics. J
Hepatol. 46:474–481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lammer J, Malagari K, Vogl T, Pilleul F,
Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S,
et al: Prospective randomized study of doxorubicin-eluting-bead
embolization in the treatment of hepatocellular carcinoma: Results
of the PRECISION V study. Cardiovasc Intervent Radiol. 33:41–52.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Golfieri R, Giampalma E, Renzulli M, Cioni
R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R,
Gasparini D, et al: Randomised controlled trial of
doxorubicin-eluting beads vs conventional chemoembolisation for
hepatocellular carcinoma. Br J Cancer. 111:255–264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan YS, He Q, Jin Y and Yao F:
Development of CalliSpheres® embolic microshperes.
Zhonghua Gan Zang Bing Za Zhi. 24:549–551. 2016.(In Chinese).
PubMed/NCBI
|
|
19
|
Zhang S, Huang C, Li Z, Yang Y, Bao T,
Chen H, Zou Y and Song L: Comparison of pharmacokinetics and drug
release in tissues after transarterial chemoembolization with
doxorubicin using diverse lipodol emulsions and CalliSpheres Beads
in rabbit livers. Drug Delivery. 24:1011–1017. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou GH, Han J, Sun JH, Zhang YL, Zhou TY,
Nie CH, Zhu TY, Chen SQ, Wang BQ, Yu ZN, et al: Efficacy and safety
profile of drug-eluting beads transarterial chemoembolization by
CalliSpheres® beads in Chinese hepatocellular carcinoma
patients. BMC Cancer. 18:6442018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Xu J, Zhang W, Chen J, Zhou X, Li Z
and Han X: Safety and efficacy of drug-eluting bead transarterial
chemoembolization combined with apatinib in patients with advanced
hepatocellular carcinoma. Acad Radiol. Jul 31–2019.doi:
10.1016/j.acra.2019.07.003 (Epub ahead of print). View Article : Google Scholar
|
|
22
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hinrichs JB, Hasdemir DB, Nordlohne M,
Schweitzer N, Wacker F, Vogel A, Kirstein MM, Marquardt S and Rodt
T: Health-related quality of life in patients with hepatocellular
carcinoma treated with initial transarterial chemoembolization.
Cardiovasc Intervent Radiol. 40:1559–1566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aliberti C, Carandina R, Sarti D,
Pizzirani E, Ramondo G, Mulazzani L, Mattioli GM and Fiorentini G:
Chemoembolization with Drug-eluting microspheres loaded with
doxorubicin for the treatment of cholangiocarcinoma. Anticancer
Res. 37:1859–1863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song J, Guijie L, Zhuqian Z and Niuwei:
Interventional chemoembolization with CalliSpheres-loaded
microspheres for the treatment of advanced hepatocellular
carcinoma. Clin J Inter Rad (Electrionic edition). 5:174–178.
2017.(In Chinese).
|
|
26
|
Kishore S, Friedman T and Madoff DC:
Update on embolization therapies for hepatocellular carcinoma. Curr
Oncol Rep. 19:402017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Purcell Y, Sartoris R, Paradis V, Vilgrain
V and Ronot M: Influence of pretreatment tumor growth rate on
objective response of hepatocellular carcinoma treated with
transarterial chemoembolization. J Gastroenterol Hepatol. Aug
1–2019.doi: 10.1111/jgh.14816 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK,
Seo YS, Yim HJ, Yeon JE and Byun KS: Hepatic resection compared to
chemoembolization in intermediate to advanced stage hepatocellular
carcinoma: A Meta-analysis of High-quality studies. Hepatology.
68:977–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang YK, Yen CL, Shiu SI, Lee SW, Chang
PY, Yeh HZ and Lee TY: Transcatheter arterial chemoembolization
after stopping sorafenib therapy for advanced hepatocellular
carcinoma. PLoS One. 12:e01889992017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poon RT, Tso WK, Pang RW, Ng KK, Woo R,
Tai KS and Fan ST: A phase I/II trial of chemoembolization for
hepatocellular carcinoma using a novel intra-arterial drug-eluting
bead. Clin Gastroenterol Hepatol. 5:1100–1108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai Z, Qin Y, Zhu G, Zhao G, Deng G, Guo J
and He S: Efficacy and safety of epirubicin applied in
transcatheter arterial chemoembolization for hepatocellular
carcinoma: A meta-analysis. J Cancer Res Ther. 14:133–138. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yi PS, Huang M, Zhang M, Xu L and Xu MQ:
Comparison of transarterial chemoembolization combined with
radiofrequency ablation therapy versus surgical resection for early
hepatocellular carcinoma. Am Surg. 84:282–288. 2018.PubMed/NCBI
|
|
35
|
Firouznia K, Ghanaati H, Alavian SM,
Azadeh P, Nasiri Toosi M, Haj Mirzaian A, Najafi S, Shakiba M and
Jalali AH: Transcatheter arterial chemoembolization therapy for
patients with unresectable hepatocellular carcinoma. Hepat Mon.
14:e257922014. View Article : Google Scholar : PubMed/NCBI
|