Introduction
Venous thromboembolism (VTE) is a clinically common
vascular disease, including deep vein thrombosis (DVT) and
pulmonary embolism (1,2). DVT refers to thrombosis in deep veins,
including, but not limited to, femoral veins, iliac veins and
intramuscular veins. According to the site of occurrence, DVT can
be divided into upper limb DVT and lower limb DVT, with lower limb
DVT being more common (3). The
typical clinical features of patients with DVT are lower limb
muscle soreness, swelling and tenderness, but there is a lack of
specific symptoms (4). On average,
~50% of DVT patients have no typical clinical manifestation, which
may lead to missed diagnosis and misdiagnosis, and therefore
increase the difficulty of treating DVT (2,5).
Additionally, post-thrombotic syndrome (PTS) occurs in 20–50% of
patients with DVT, even after the appropriate treatment (6). PTS is the most common long-term
complication in patients with DVT. The development of PTS is also
associated with an increased risk of the recurrence of VTE,
severely affecting the quality of life of patients and increasing
the economic burden of families and society (7–9).
Therefore, timely and accurate diagnosis of DVT, as well as
effective interventions, are of importance for alleviating the
suffering of patients and even saving lives.
Currently, the mechanism of action behind the
pathogenesis of DVT remains unclear, hindering its prevention and
treatment (10). It is now widely
considered that vascular endothelial cells, the
coagulation/anticoagulation system, the fibrinolysis/antifibrin
system, platelets, changes in blood rheology, inflammatory factors
and other factors are all involved in the pathophysiology behind
DVT (11). At present, vascular wall
injury, changes in blood flow and abnormal blood components are
considered to be the three most notable factors behind thrombosis
(12). Among them, blood vessel wall
injury mainly constitutes damage to vascular endothelial cells.
Vascular endothelial cell damage includes apoptosis, which is the
most important cause of venous thrombosis (13,14).
Previous studies have confirmed that damage to vascular endothelial
cells are closely associated with the development of DVT, and the
apoptosis of vascular endothelial cells reduces the levels of
active substances and impairs their multiple defense functions in
blood vessels, as well as reducing the stability of the
anticoagulation-fibrinolytic system, thus increasing the risk of
thrombus formation (14–17).
MicroRNAs (miRNAs) are endogenous non-coding
single-stranded small-molecule RNAs, found in eukaryotic cells,
that are ~22 nucleotides in length (18,19).
miRNAs regulate gene expression at the post-transcriptional level
mainly by binding to the 3′-untranslated region (3′-UTR) of target
mRNAs, inhibiting translation or degradation (18). miRNAs are widely involved in the
regulation of physiological and pathological processes in various
cells and tissues, and are involved with cell differentiation,
proliferation, apoptosis, and the development of tissues and organs
(20–22). In recent years, increasing evidence
has indicated that miRNAs play an important role in the development
of DVT (23–27). miRNA (miR)-195-5p has been studied in
several cancer types including breast cancer (28), non-small cell lung cancer (29), cervical carcinoma (30) and human endometrial carcinoma
(31). miR-195-5p has also been
found to regulate hair follicle inductivity of dermal papilla cells
by suppressing the activation of the Wnt/β-Catenin signaling
pathway (32). Furthermore, it has
been suggested that both peripheral blood and urinary miR-195-5p
may be a potential biomarker for membranous nephropathy (33). All of these observations indicated
that miR-195-5p is expressed differently under different
pathophysiological conditions and that miR-195-5p plays a very
important role in regulating cell growth. Additionally, miR-195-5p
has been proven to promote pulmonary arterial smooth muscle cell
proliferation and migration in pulmonary arterial hypertension
(34). Additionally, higher
expression of miR-195-5p inhibits angiogenesis in preeclampsia
(35). A recent study reported that
miR-195 is upregulated in the blood of DVT patients (36); however, to the best of our knowledge,
the expression and role of miR-195-5p in DVT remain unclear. Bcl-2,
the founding member of the Bcl-2 protein family, has anti-apoptotic
activity, and the anti-apoptotic function of Bcl-2 is mediated by
its effects on intracellular Ca2+ homeostasis and
dynamics (37). It was found by
bioinformatics analysis that miR-195-5p binds to the 3′-UTR of
Bcl-2, suggesting a direct interaction between miR-195-5p and
Bcl-2. Therefore, it was hypothesized that miR-195-5p may be
involved in DVT development by regulating vascular endothelial cell
apoptosis.
Therefore, the aim of the present study was to
investigate the expression of miR-195-5p in DVT patients, and to
explore whether miR-195-5p is involved in the development of DVT by
regulating the apoptosis of vascular endothelial cells.
Materials and methods
Clinical samples
This present study was approved by the ethics review
committee of the Gansu Provincial Hospital of TCM, and all patients
provided their written informed consent. Peripheral blood (5
ml/subject) was collected from 15 patients with DVT and from 15
healthy volunteers. All DVT patients were confirmed by color
Doppler ultrasonography (38). The
Doppler ultrasonographic criteria for DVT were as follows: No flow
signal; direct clot visualization; the absence of spontaneous flow;
and the absence of respiration-modulated phasicity of the evaluated
veins. The criterion for diagnosis of DVT was a filling defect of
the venous lumen in more than two observations, following the
American College of Chest Physicians Evidence-Based Clinical
Practice Guidelines of diagnosis for DVT (39). The exclusion criteria for the study
were as follows: Ongoing anticoagulation treatment for >3
months; pregnancy; duration of symptoms for more than 1 year; and
previous thrombosis within the last year.
Cell culture
HUVECs were obtained from the American Type Culture
Collection. Cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and maintained at 37°C with 5%
CO2.
Dual luciferase reporter assay
The binding sites between miR-195-5p and Bcl-2 were
predicted using TargetScan bioinformatics software (version 7.1;
www.targetscan.org/vert_71). To confirm
predictions, dual luciferase reporter assays were performed. The
wild-type (WT)-Bcl-2 and mutant (MUT)-Bcl-2 3′-UTRs of Bcl-2 were
cloned into a pmiR-RB-Report™ dual luciferase reporter gene plasmid
vector (Guangzhou RiboBio Co., Ltd.) according to the
manufacturer's instructions. HUVECs were co-transfected with
WT-Bcl-2 or MUT-Bcl-2 and miR-195-5p mimic or mimic control by
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. A total of 48 h after cell
transfection, the luciferase activity was analyzed using the
dual-luciferase assay system (Promega Corporation). Luciferase
activity was normalized to the Renilla luciferase activity
in the current study.
Cell transfection
Inhibitor control (chemically modified RNA single
strand), miR-195-5p inhibitor (chemically modified RNA single
strand), mimic control and miR-195-5p mimic were purchased from
Guangzhou RiboBio Co., Ltd. HUVECs were plated into 6-well plates
at a density of 1×106 cells/well and cultured at 37°C
with 5% CO2 for 24 h. Then, 100 nM miR-195-5p inhibitor
(5′-GCCAAUAUUUCUGUGCUGCUA-3′), 100 nM inhibitor control
(5′-CAGUACUUUUGUGUAGUACAA-3′), 50 nM miR-195-5p mimic
(5′-UAGCAGCACAGAAAUAUUGGC-3′), 50 nM mimic control
(5′-UUCUCCGAACGUGUCACGUTT-3′), 1 µg Bcl-2 CRISPR Activation Plasmid
(Bcl-2 plasmid; cat no. sc400025-ACT; Santa Cruz Biotechnology,
Inc.), 1 µg control CRISPR Activation Plasmid (control plasmid; cat
no. sc-437275; Santa Cruz Biotechnology, Inc.), or 50 nM miR-195-5p
mimic + 1 µg Bcl-2 plasmid was transfected into HUVECs by using
Lipofectamine 2000, according to the manufacturer's protocols. A
total of 48 h after cell transfection, reverse
transcription-quantitative PCR (RT-qPCR) was performed to assess
the transfection efficiency.
MTT assay
MTT assays were performed to detect the cell
viability. Briefly, 48 h after cell transfection, HUVECs were
seeded into a 96-well plate (2×104 cells/well). Then, 10
µl MTT reagent (Beyotime Institute of Biotechnology) was added to
each well and the cells were further incubated for 4 h at 37°C,
following which 150 µl DMSO was used to dissolve the purple
formazan crystals. Finally, absorbance at a wavelength of 490 nm
was measured using an automatic enzyme-linked immune detector. Cell
viability was calculated using the following formula: Cell
viability=optical density (OD) of treated cells/OD (control) ×100%.
Tests were repeated three times.
Cell apoptosis assay
After transfection for 48 h, the apoptosis of HUVECs
was analyzed by using the Annexin V-FITC/propidium iodide apoptosis
detection kit [cat no. 70-AP101-100; Hangzhou Multi Sciences
(Lianke) Biotech Co., Ltd.] in line with the manufacturer's
instructions. BD FACSCalibur™ flow cytometer with Cell Quest
software (version 5.1; BD Biosciences), was used to detect the cell
apoptosis rate. Each experiment was repeated three times.
RT-qPCR
To extract the total RNA from blood samples and
cells, TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used according to the manufacturer's
instructions. Total RNA was reverse transcribed into cDNAs using
the miScript Reverse Transcription kit (Qiagen GmbH). The
temperature protocol for the reverse transcription reaction was as
follows: 25°C for 5 min, 42°C for 60 min and 80°C for 2 min. For
qPCR analysis, the QuantiFast SYBR Green PCR kit (Qiagen GmbH) was
used. Amplification conditions were as follows: 95°C for 10 min;
and 35 cycles of 95°C for 15 sec and 55°C for 40 sec. GAPDH was
used as the internal control for Bcl-2 and Bax mRNA expression, and
U6 was used as the internal control for miR-195-5p expression.
Primer sequences for PCR were as follows: GAPDH, forward
5-′CTTTGGTATCGTGGAAGGACTC-3′, reverse 5-′GTAGAGGCAGGGATGATGTTCT-3′;
U6, forward 5-′GCTTCGGCAGCACATATACTAAAAT-3′, reverse
5-′CGCTTCACGAATTTGCGTGTCAT-3′; miR-195-5p forward
5-′GGGGTAGCAGCACAGAAAT-3′, reverse 5-′TCCAGTGCGTGTCGTGGA-3′; Bcl2
forward 5′-GCCCTGTGGATGACTGAGTA-3′, reverse,
5′-GGCCGTACAGTTCCACAAAG-3′; and Bax, forward
5′-TGGCAGCTGACATGTTTTCTGAC-3′, reverse 5′-TCACCCAACCACCCTGGTCTT-3′.
Relative gene expression was calculated by using the
2−ΔΔCq method (40).
Western blotting
Proteins from cells or blood samples were extracted
using the RIPA lysis buffer (Beyotime Institute of Biotechnology).
Protein concentration was quantified using Bicinchoninic Acid
Protein Assay kit. Proteins (30 µg/lane) were separated by using
SDS-PAGE on a 12% gel, and then transferred onto the PVDF
membranes. Membranes were then blocked with 5% skim milk at room
temperature for 1.5 h and incubated with primary antibodies
overnight at 4°C: Bcl-2 (cat no. 4223; 1:1,000; Cell Signaling
Technology, Inc.), Bax (cat no. 5023; 1:1,000; Cell Signaling
Technology, Inc.), and β-actin (cat no. 4970; 1:1,000; Cell
Signaling Technology, Inc.). The membranes were finally incubated
with the horseradish peroxidase-conjugated anti-rabbit IgG
secondary antibody (cat no. 7074; dilution ratio: 1:5,000; Cell
Signaling Technology, Inc.) at room temperature for 2 h. To
visualize the protein bands, the ECL detection system (Thermo
Fisher Scientific, Inc.) was used. Protein bands were quantified
using. β-actin was used as a reference protein for each experiment
and each experiment was repeated three times. Densitometric
analyzes were performed using ImageJ software (version 1.38X;
National Institutes of Health).
Statistical analysis
Data were analyzed using SPSS 18.0 software (SPSS,
Inc.), and are presented as the mean ± SD. Comparisons between
groups were made by Students t-test or one-way ANOVA with Tukey's
post hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
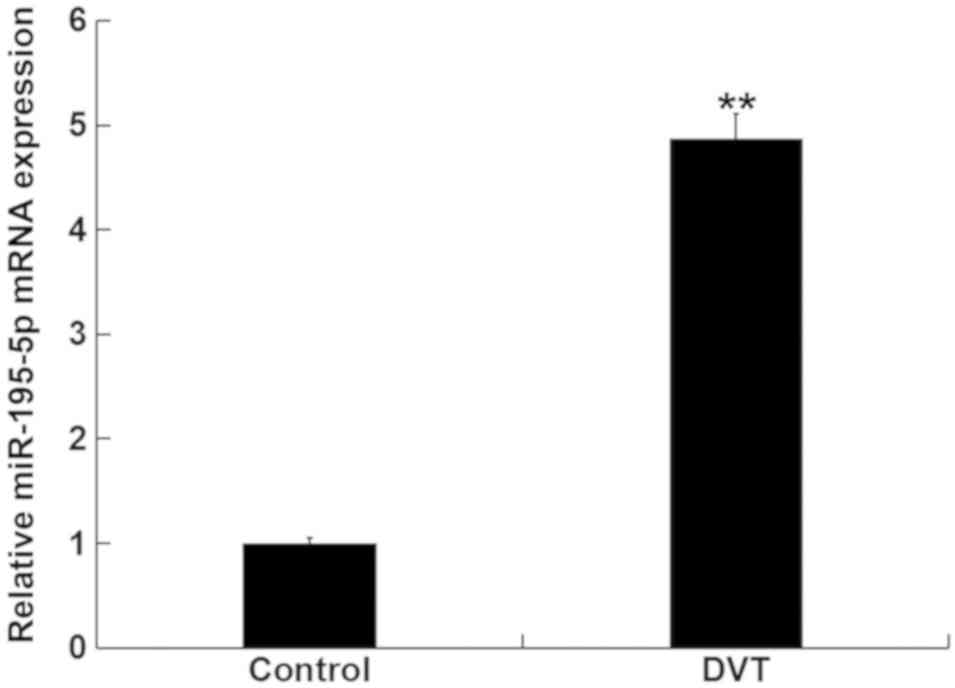
miR-195-5p is upregulated in patients
with DVT
Levels of miR-195-5p in the peripheral blood were
first analyzed in samples from 15 DVT patients and in 15
volunteers, using RT-qPCR. Levels of miR-195-5p in the peripheral
blood from DVT patients was significantly higher than those in the
peripheral blood from the healthy volunteers (Fig. 1). These data indicated that
miR-195-5p is involved in DVT progression.
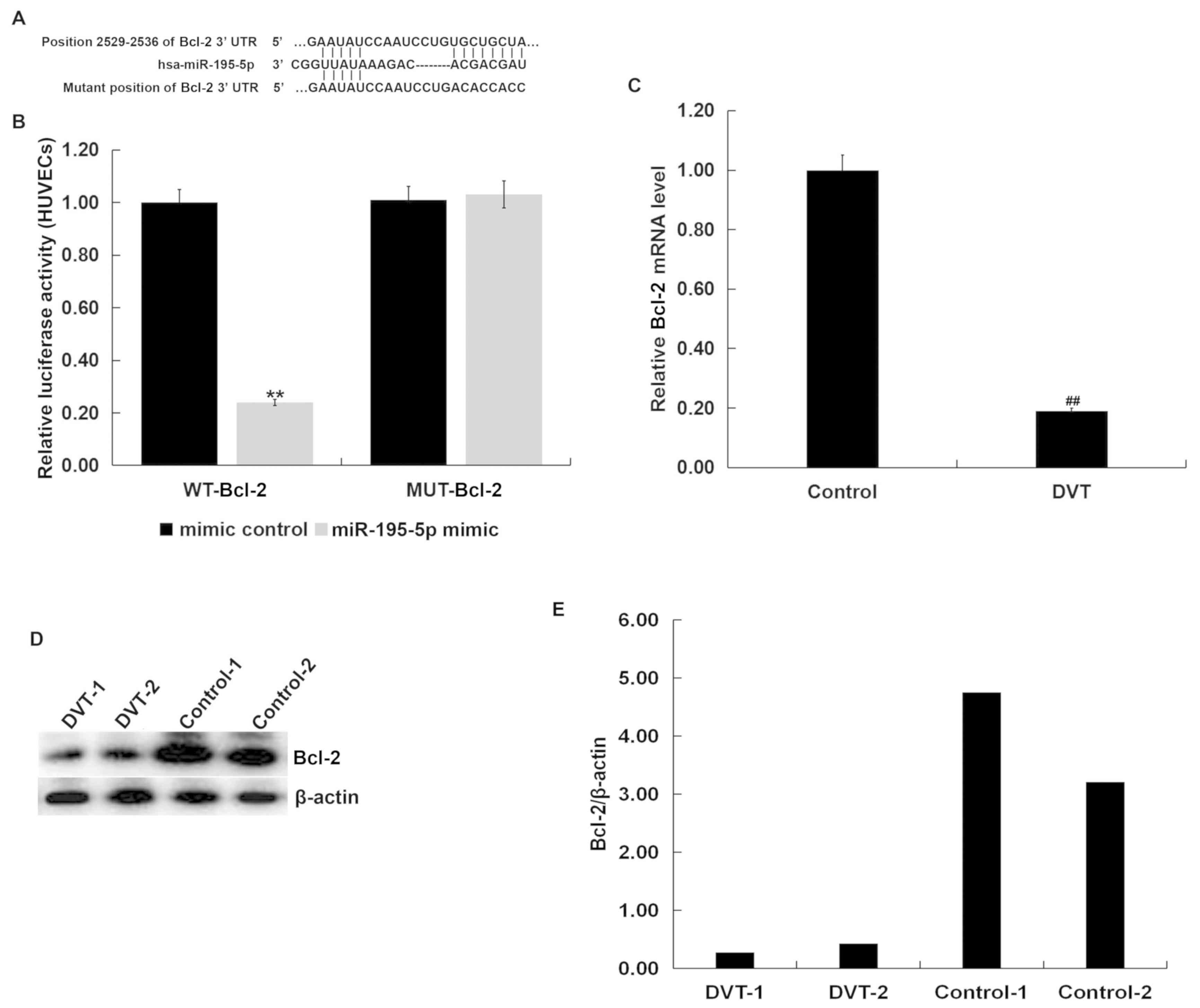
Bcl-2 is a direct target of
miR-195-5p
Results from TargetScan bioinformatics software
indicated that miR-195-5p has hundreds of potential target genes,
including Bcl-2 (Fig. 2A). Bcl-2,
the founding member of the Bcl-2 protein family, is a widely known
anti-apoptotic gene (37). Apoptosis
plays a crucial role in the pathophysiology of venous thrombosis
(13,14), and Bcl-2 has been identified to
participate in the development of thrombosis (41). Therefore, it was hypothesized that
miR-195-5p may play an important role in the development of DVT by
affecting the apoptosis of vascular endothelial cells by regulating
the expression of Bcl-2. Thus, Bcl-2 was chosen for further study,
and the dual luciferase reporter assay supported the prediction
(Fig. 2B). The results showed that
Bcl-2 was a direct target of miR-195-5p.
Furthermore, the mRNA levels of Bcl-2 in the
peripheral blood from DVT patients was significantly lower than
that in the peripheral blood from the healthy volunteers (Fig. 2C). Randomly selected blood samples
from 2 DVT patients and 2 healthy volunteers were tested for Bcl-2
protein expression. The protein level of Bcl-2 in the peripheral
blood from DVT patients was lower than that in the peripheral blood
from the healthy volunteers (Fig. 2D and
E). As Bcl-2 is a well-known anti-apoptotic gene, it was
hypothesized that miR-195-5p may be involved in the development and
progression of DVT by regulating the apoptosis of vascular
endothelial cells.
miR-195-5p downregulation promotes
cell viability and inhibits apoptosis in HUVECs
To investigate the effect of miR-195-5p
downregulation on the proliferation and apoptosis of HUVECs,
control inhibitor or miR-195-5p inhibitor was transfected into
HUVECs for 48 h. Compared with the inhibitor control group,
miR-195-5p inhibitor significantly inhibited miR-195-5p expression
in HUVECs (Fig. 3A). miR-195-5p
inhibitor significantly enhanced the cell viability and inhibited
the apoptosis of HUVECs (Fig. 3B and
C). Additonally, compared with the inhibitor control group, the
mRNA level of Bcl-2 was significantly enhanced, while Bax mRNA
expression was reduced (Fig. 3D and
E). Compared with the inhibitor control group, Bcl-2 protein
levels appeared raised, while Bax protein expression levels
appeared reduced in HUVECs transfected with miR-195-5p inhibitor
(Fig. 3F).
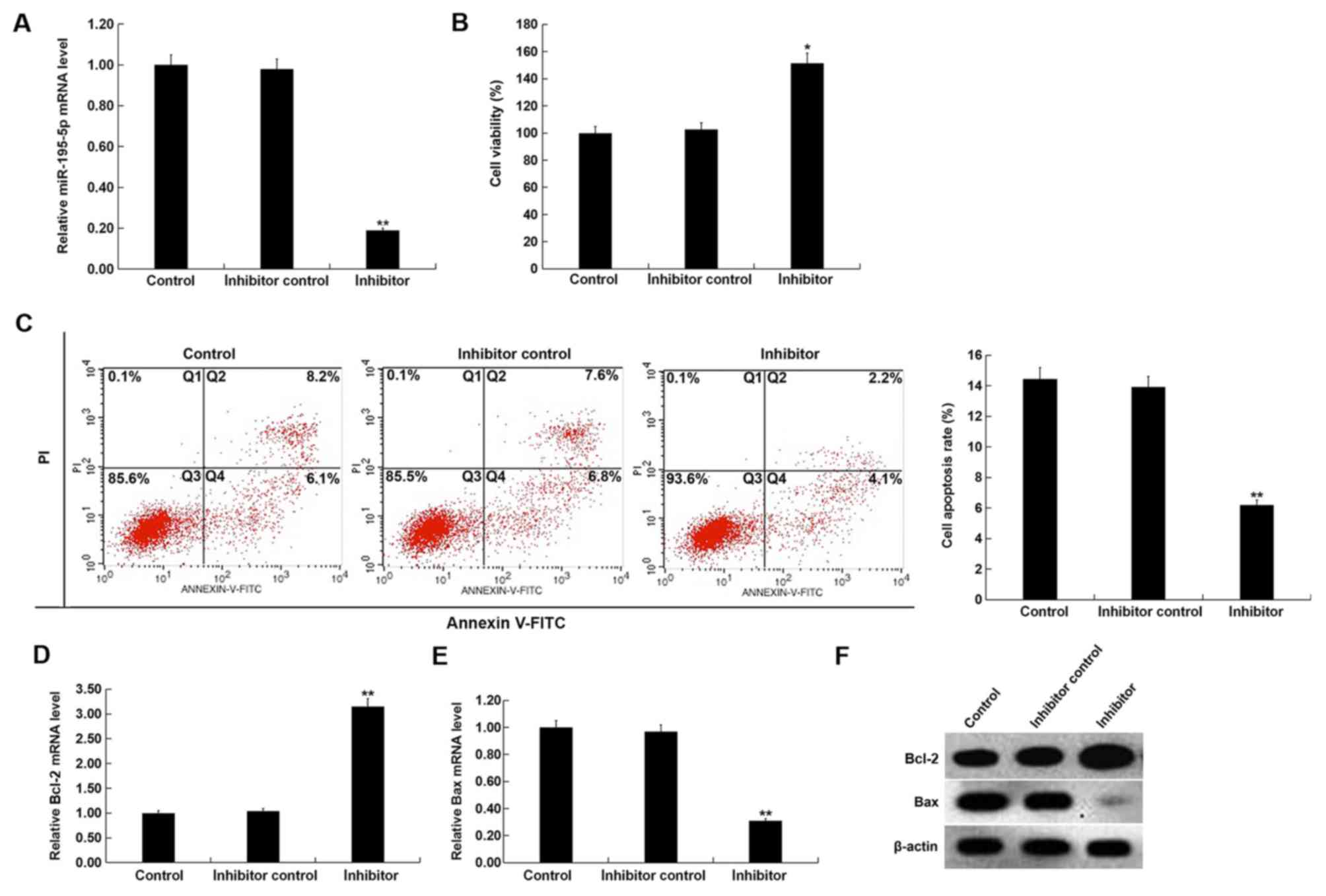 | Figure 3.Effect of miR-195-5p downregulation
on HUVECs. (A) After transfection with inhibitor control or
miR-195-5p inhibitor for 48 h, the level of miR-195-5p in HUVECs
was detected using RT-qPCR. (B) After transfection with inhibitor
control or miR-195-5p inhibitor for 48 h, the cell viability of
HUVECs was detected using MTT assay. (C) After transfection with
inhibitor control or miR-195-5p inhibitor for 48 h, cell apoptosis
of HUVECs was detected using flow cytometry, and the cell apoptosis
rate (Q2 + Q4) was calculated and presented. After transfection
with inhibitor control or miR-195-5p inhibitor for 48 h, the mRNA
level of (D) Bcl-2 and (E) Bax in HUVECs was detected using
RT-qPCR. (F) After transfection with inhibitor control or
miR-195-5p inhibitor for 48 h, the protein level of Bcl-2 and Bax
in HUVECs was detected using western blotting. Control, HUVECs
without any treatment; inhibitor control, HUVECs transfected with
inhibitor control for 48 h; inhibitor, HUVECs were transfected with
miR-195-5p inhibitor for 48 h. Data are presented as the mean ± SD.
*P<0.05, **P<0.01 vs. inhibitor control. HUVECs, human
umbilical vein endothelial cells; miR, microRNA; PI, propidium
iodide; Q, quadrant; RT-qPCR, reverse transcription-quantitative
PCR. |
miR-195-5p upregulation inhibits cell
viability and induces apoptosis in HUVECs
To investigate the effect of miR-195-5p upregulation
on the proliferation and apoptosis of HUVECs, HUVECs were
transfected with miR-195-5p mimic, mimic control, Bcl-2-plasmid,
control-plasmid, or miR-195-5p mimic + Bcl-2-plasmid, for 48 h.
Compared with the mimic control group, miR-195-5p mimic
significantly raised miR-195-5p expression in HUVECs (Fig. 4A). Transfection with the
Bcl-2-plasmid significantly increased the mRNA expression of Bcl-2
in HUVECs (Fig. 4B). Similarly, the
Bcl-2-plasmid appeared to markedly raise the protein expression of
Bcl-2 (Fig. 4C). miR-195-5p mimic
significantly inhibited the cell viability and induced the
apoptosis of HUVECs, although these effects were reversed by
Bcl-2-plasmid (Fig. 4D and E).
Additionally, compared with the mimic control group, miR-195-5p
mimic appeared to greatly reduce the protein levels of Bcl-2, while
also appearing to increase Bax protein expression levels (Fig. 4F). These changes appeared to reverse
when HUVECs were also transfected with the Bcl-2-plasmid. Compared
with the mimic control group, miR-195-5p mimic significantly
reduced the mRNA levels of Bcl-2, while Bax mRNA expression
increased (Fig. 4G and H), and these
changes were reversed when the Bcl-2-plasmid was co-transfected
with miR-195-5p mimic.
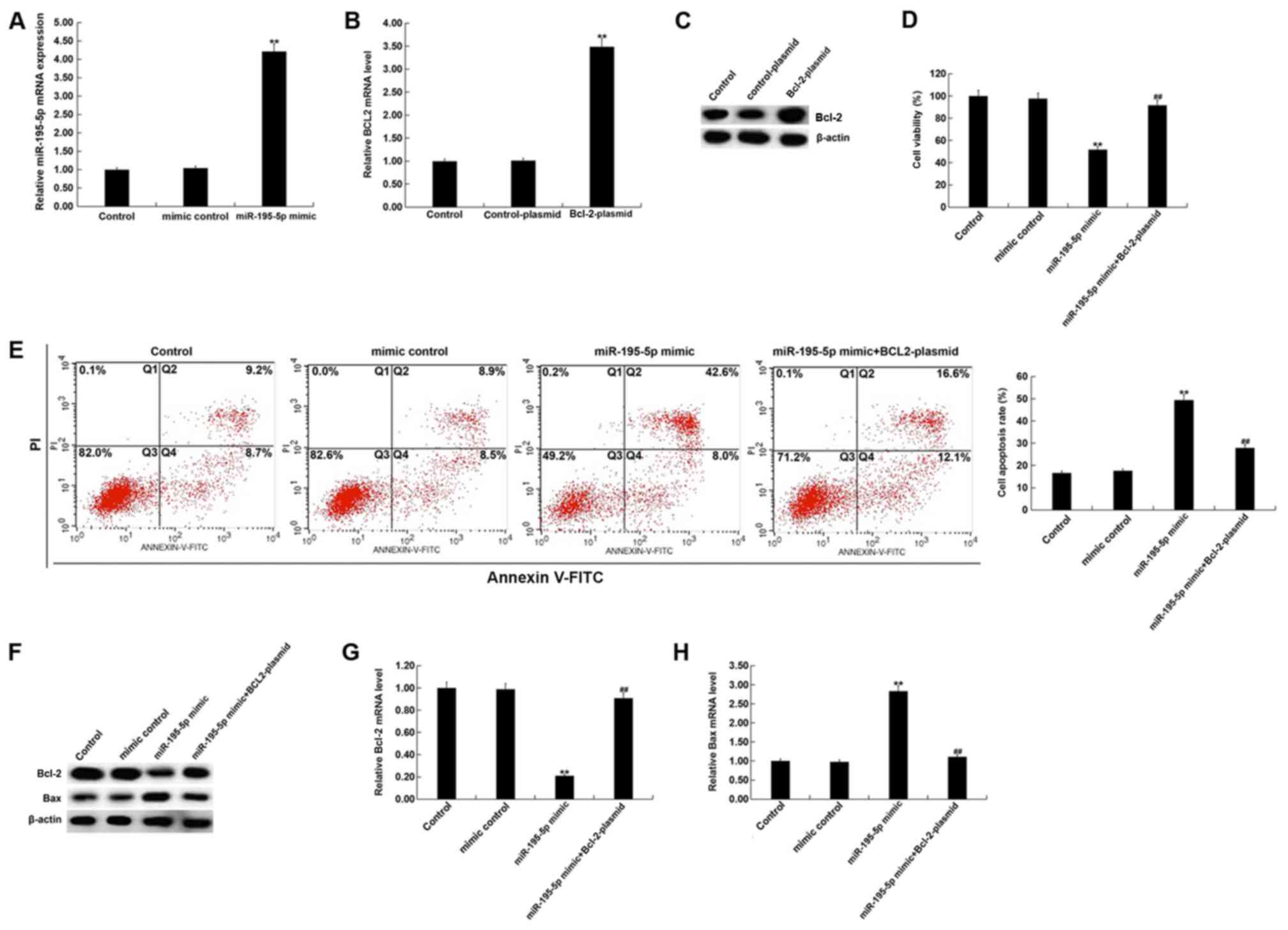 | Figure 4.Effect of miR-195-5p upregulation on
HUVECs. (A) After transfection with mimic control or miR-195-5p
mimic for 48 h, the level of miR-195-5p in HUVECs was detected
using RT-qPCR. After transfection with control-plasmid or
Bcl-2-plasmid for 48 h, the mRNA and protein level of Bcl-2 in
HUVECs was detected using (B) RT-qPCR and (C) western blotting. (D)
After transfection with mimic control, miR-195-5p mimic or
miR-195-5p mimic + Bcl-2-plasmid for 48 h, the cell viability of
HUVECs was detected using MTT assay. (E) After transfection with
mimic control, miR-195-5p mimic or miR-195-5p mimic + Bcl-2-plasmid
for 48 h, the cell apoptosis of HUVECs was detected using flow
cytometry, and the cell apoptosis rate (Q2 + Q4) was calculated and
presented. (F) After transfection with mimic control, miR-195-5p
mimic or miR-195-5p mimic + Bcl-2-plasmid for 48 h, the protein
level of Bcl-2 and Bax in HUVECs was detected using western
blotting. After transfection with mimic control, miR-195-5p mimic
or miR-195-5p mimic+Bcl-2-plasmid for 48 h, the mRNA level of (G)
Bcl-2 and (H) Bax in HUVECs was detected using RT-qPCR. Control,
HUVECs without any treatment; mimic control, HUVECs transfected
with mimic control for 48 h; miR-195-5p mimic, HUVECs transfected
with miR-195-5p mimic for 48 h; control-plasmid, HUVECs transfected
with control-plasmid for 48 h; Bcl-2-plasmid, HUVECs transfected
with Bcl-2-plasmid for 48 h; miR-195-5p mimic+Bcl-2-plasmid, HUVECs
co-transfected with miR-195-5p mimic + Bcl-2-plasmid for 48 h. Data
are presented as the mean ± SD. **P<0.01 vs. mimic control or
control-plasmid; ##P<0.01 vs. miR-195-5p mimic.
HUVECs, human umbilical vein endothelial cells; miR, microRNA; PI,
propidium iodide; Q, quadrant; RT-qPCR, reverse
transcription-quantitative PCR. |
Discussion
DVT is a clinically common peripheral vascular
lesion (1,2). Current clinical treatments for DVT
include anti-coagulation, thrombolysis and surgical thrombectomy.
However, all of the aforementioned methods have the disadvantages
of PTS, low long-term patency rate, and easy recurrence. Therefore,
developing more effective treatments for DVT is an important area
of research for vascular surgeons. There is increasing evidence
indicating that miRNAs, as micro-regulators, play an important role
in regulating angiogenic signaling pathways (36,42).
miR-195-5p has been discovered to play important
roles in various cancer types, including cervical carcinoma, human
endometrial carcinoma, colorectal cancer, melanoma and osteosarcoma
by regulating cell proliferation and apoptosis (30,31,43–45). A
recent study also reported the inhibitory role of miR-195 in
angiogenesis in human prostate cancer (46). Furthermore, Sandrim et al
(35) revealed an anti-angiogenic
role for miR-195-5p in endothelial cells. miR-195 is upregulated in
the blood of DVT patients (36).
However, the expression and role of miR-195-5p in DVT remain
unclear. This present study investigated the expression of
miR-195-5p in DVT patients, and explored whether miR-195-5p was
involved in the pathophysiology of DVT by regulating the apoptosis
of vascular endothelial cells.
Firstly, miR-195-5p levels were detected in the
blood of DVT patients and healthy controls using RT-qPCR. The
results indicated that compared with the healthy controls,
miR-195-5p was significantly upregulated in the peripheral blood of
DVT patients, indicating that miR-195-5p has a role in the
development of DVT. Bcl-2, a well-known anti-apoptotic gene, was
identified as a direct target of miR-195-5p, which also appeared to
be downregulated in the peripheral blood of DVT patients. Thus, it
was hypothesized that miR-195-5p may be involved in the development
and progression of DVT by regulating apoptosis in vascular
endothelial cells. It has to be noted, however, that a limitation
of this present study is that only two DVT samples were
analyzed.
Subsequently, the role of miR-195-5p in the
regulation of cell viability and apoptosis of vascular endothelial
cells was investigated. The findings suggested that miR-195-5p
inhibition significantly promoted the cell viability and inhibited
the apoptosis of HUVECs, while miR-195-5p upregulation
significantly inhibited cell viability and increased the apoptosis
of HUVECs. Additionally, miR-195-5p upregulation significantly
inhibited Bcl-2 expression and induced Bax expression in HUVECs,
while miR-195-5p inhibition demonstrated the opposite effects. It
is worth mentioning that all the effects of miR-195-5p mimic on
HUVECs were significantly reduced by Bcl-2 overexpression.
Taken together, the present study indicated that
miR-195-5p was significantly upregulated in DVT patients, and that
it may be involved in the development of DVT by regulating the
apoptosis of vascular endothelial cells. It was further identified
that Bcl-2 was a direct target of miR-195-5p, and that Bcl-2 was
downregulated in the peripheral blood of DVT patients. Thus, it is
thought that the downregulation of Bcl-2 in the peripheral blood of
DVT patients was associated with the upregulation of miR-195-5p.
Moreover, the data of this present study indicated that
downregulation of miR-195-5p significantly promoted the cell
viability and inhibited the apoptosis of HUVECs. Raised miR-195-5p
expression inhibited cell viability and induced apoptosis in
HUVECs, changes that were reversed by raised Bcl-2 expression.
These data indicated that miR-195-5p was involved in the
development of DVT by regulating the apoptosis of vascular
endothelial cells. Therefore, miR-195-5p may be a potential DVT
biomarker and therapeutic target. However, this study is only a
preliminary study of miR-195-5p in DVT and as such, contains
limitations. The sample size of this study was based on previous
studies (21–23). A power analysis should be performed
for future studies to determine the effective sample volume.
Furthermore, a ‘mimic + control plasmid group’ was not set up in
this study. Finally, in vivo experiments are also required
to further this research.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLJ contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. CXW, YJOY and DDZ contributed to data collection and
statistical analysis.
Ethics approval and consent to
participate
This study was approved by the Ethics Review
Committee of the Gansu Provincial Hospital of TCM, and all patients
have provided their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sakuma M, Nakamura M, Yamada N, Ota S,
Shirato K, Nakano T, Ito M and Kobayashi T: Venous thromboembolism:
Deep vein thrombosis with pulmonary embolism, deep vein thrombosis
alone and pulmonary embolism alone. Circ J. 73:305–309. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang KL, Yap ES, Goto S, Zhang S, Siu CW
and Chiang CE: The diagnosis and treatment of venous
thromboembolism in asian patients. Thromb J. 16:42018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hattab Y, Küng S, Fasanya A, Ma K, Singh
AC and DuMont T: Deep venous thrombosis of the upper and lower
extremity. Crit Care Nurs Q. 40:230–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hollenhorst MA and Battinelli EM:
Thrombosis, Hypercoagulable states and anticoagulants. Prim Care.
43:619–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Xia H, Wang Y, Chen L, Li S,
Hussein IA, Wu Y, Shang Y, Yao S and Du R: The rate of missed
diagnosis of lower-limb DVT by ultrasound amounts to 50% or so in
patients without symptoms of DVT: A meta-analysis. Medicine
(Baltimore). 98:e171032019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rabinovich A and Kahn SR: The
postthrombotic syndrome: Current evidence and future challenges. J
Thromb Haemost. 15:230–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heit JA, Spencer FA and White RH: The
epidemiology of venous thromboembolism. J Thromb Thrombolysis.
41:3–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kahn SR: The post-thrombotic syndrome.
Hematology Am Soc Hematol Educ Program. 2016:413–418. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stain M, Schonauer V, Minar E, Bialonczyk
C, Hirschl M, Weltermann A, Kyrle PA and Eichinger S: The
post-thrombotic syndrome: Risk factors and impact on the course of
thrombotic disease. J Thromb Haemost. 3:2671–2676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robert-Ebadi H and Righini M: Management
of distal deep vein thrombosis. Thromb Res. 149:48–55. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olaf M and Cooney R: Deep venous
thrombosis. Emerg Med Clin North Am. 35:743–770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mammen EF: Pathogenesis of venous
thrombosis. Chest. 102 (Suppl):640S–644S. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirwan CC, McCollum CN, McDowell G and
Byrne GJ: Investigation of proposed mechanisms of
chemotherapy-induced venous thromboembolism: Endothelial cell
activation and procoagulant release due to apoptosis. Clin Appl
Thromb Hemost. 21:420–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Wang J, Wang B, Yang J, Gong Z,
Zhao X, Zhang C and Du K: MiR-126 inhibits vascular endothelial
cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol.
95:365–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shyy JY and Chien S: Role of integrins in
endothelial mechanosensing of shear stress. Circ Res. 91:769–775.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bombeli T, Karsan A, Tait JF and Harlan
JM: Apoptotic vascular endothelial cells become procoagulant.
Blood. 89:2429–2442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mo J, Huang H, He F, et al: Influence of
apoptosis signal pathway in traumatic deep vein thrombosis. J
Kunming Med Univ. 28:5–7. 2007.
|
|
18
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2017. View Article : Google Scholar
|
|
21
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ten Cate H: MicroRNA and venous
thrombosis. Thromb Haemost. 116:2052016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Sundquist K, Elf JL, Strandberg K,
Svensson PJ, Hedelius A, Palmer K, Memon AA, Sundquist J and Zöller
B: Diagnostic potential of plasma microRNA signatures in patients
with deep-vein thrombosis. Thromb Haemost. 116:328–336. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Zhu X, Du X, Xu A, Yuan X, Zhan Y,
Liu M and Wang S: MiR-150 promotes angiogensis and proliferation of
endothelial progenitor cells in deep venous thrombosis by targeting
SRCIN1. Microvasc Res. 123:35–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kong L, Hu N, Du X, Wang W, Chen H, Li W,
Wei S, Zhuang H, Li X and Li C: Upregulation of miR-483-3p
contributes to endothelial progenitor cells dysfunction in deepvein
thrombosis patients via SRF. J Transl Med. 14:232016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin J, Liang H, Shi D, Dai J, Xu Z, Chen
D, Chen X and Jiang Q: A panel of microRNAs as a new biomarkers for
the detection of deep vein thrombosis. J Thromb Thrombolysis.
39:215–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang R, Xing L, Zheng X, Sun Y, Wang X and
Chen J: The circRNA circAGFG1 acts as a sponge of miR-195-5p to
promote triple-negative breast cancer progression through
regulating CCNE1 expression. Mol Cancer. 18:42019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng J, Xu T, Chen F and Zhang Y:
MiRNA-195-5p functions as a tumor suppressor and a predictive of
poor prognosis in non-small cell lung cancer by directly targeting
CIAPIN1. Pathol Oncol Res. 25:1181–1190. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li M, Ren CX, Zhang JM, Xin XY, Hua T and
Wang HB and Wang HB: The Effects of miR-195-5p/MMP14 on
proliferation and invasion of cervical carcinoma cells through TNF
signaling pathway based on bioinformatics analysis of microarray
profiling. Cell Physiol Biochem. 50:1398–1413. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong F, Ma J, Yang H, Yang D, Wang C and
Ma X: Long non-coding RNA PVT1 promotes malignancy in human
endometrial carcinoma cells through negative regulation of
miR-195-5p. Biochim Biophys Acta Mol Cell Res. Jul 19–2018.doi:
10.1016/j.bbamcr.2018.07.008 (Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu N, Huang K, Liu Y, Zhang H, Lin E,
Zeng Y, Li H, Xu Y, Cai B, Yuan Y, et al: miR-195-5p regulates hair
follicle inductivity of dermal papilla cells by suppressing
Wnt/β-catenin activation. Biomed Res Int. 2018:49243562018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou G, Zhang X, Wang W, Zhang W, Wang H
and Xin G: Both peripheral blood and urinary miR-195-5p,
miR-192-3p, miR-328-5p and their target genes PPM1A, RAB1A and
BRSK1 may be potential biomarkers for membranous nephropathy. Med
Sci Monit. 25:1903–1916. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeng Z, Yao J, Li Y, Xue Y, Zou Y, Shu Z
and Jiao Z: Anti-apoptosis endothelial cell-secreted
microRNA-195-5p promotes pulmonary arterial smooth muscle cell
proliferation and migration in pulmonary arterial hypertension. J
Cell Biochem. 119:2144–2155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sandrim VC, Dias MC, Bovolato AL,
Tanus-Santos JE, Deffune E and Cavalli RC: Plasma from
pre-eclamptic patients induces the expression of the
anti-angiogenic miR-195-5p in endothelial cells. J Cell Mol Med.
20:1198–1200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Latronico MV, Catalucci D and Condorelli
G: Emerging role of microRNAs in cardiovascular biology. Circ Res.
101:1225–1236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hirata H, Lopes GS, Jurkiewicz A,
Garcez-do-Carmo L and Smaili SS: Bcl-2 modulates endoplasmic
reticulum and mitochondrial calcium stores in PC12 cells. Neurochem
Res. 37:238–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Naz R, Naz S, Mehboob M, Achakzai A and
Khalid GH: Diagnostic yield of color Doppler ultrasonography in
deep vein thrombosis. J Coll Physicians Surg Pak. 15:276–279.
2005.PubMed/NCBI
|
|
39
|
Bates SM, Jaeschke R, Stevens SM, Goodacre
S, Wells PS, Stevenson MD, Kearon C, Schunemann HJ, Crowther M,
Pauker SG, et al: Diagnosis of DVT: Antithrombotic therapy and
prevention of thrombosis, 9th ed: American college of chest
physicians evidence-based clinical practice guidelines. Chest. 141
(2 Suppl):e351S–e418S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang H, Meng Z, Zhang C, Zhang P and Wang
Q: Establishing a new rat model of central venous sinus thrombosis
and analyzing its pathophysiological and apoptotic changes. J
Neurosci Methods. 203:130–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cordes KR and Srivastava D: MicroRNA
regulation of cardiovascular development. Circ Res. 104:724–732.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin Y, Wang M, Hu H, Huang Q, Chen Y and
Wang G: Overcoming stemness and chemoresistance in colorectal
cancer through miR-195-5p-modulated inhibition of notch signaling.
Int J Biol Macromol. 117:445–453. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chai L, Kang XJ, Sun ZZ, Zeng MF, Yu SR,
Ding Y, Liang JQ, Li TT and Zhao J: MiR-497-5p, miR-195-5p and
miR-455-3p function as tumor suppressors by targeting hTERT in
melanoma A375 cells. Cancer Manag Res. 10:989–1003. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang C, Wu K, Wang S and Wei G: Long
non-coding RNA XIST promotes osteosarcoma progression by targeting
YAP via miR-195-5p. J Cell Biochem. 119:5646–5656. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cai C, He H, Duan X, Wu W, Mai Z, Zhang T,
Fan J, Deng T, Zhong W, Liu Y, et al: miR-195 inhibits cell
proliferation and angiogenesis in human prostate cancer by
downregulating PRR11 expression. Oncol Rep. 39:1658–1670.
2018.PubMed/NCBI
|