Introduction
Gastric cancer (GC) is one of the leading causes of
mortality worldwide, particularly in Asian countries such as China
(1). Over the past three decades,
the incidence of gastric cancer has gradually declined due to
improved treatment (2). However, the
mortality rate of gastric cancer remains high and its burden on the
patients remains substantial (3,4). It was
reported that the 5 year survival rate of patients with GC treated
at an early stage was 90–95% in Japan from 1970 to 2000; however,
the overall survival rate of late stage GC is less than 20%
(5,6). Despite advances in GC treatment, a
substantial number of patients exhibit local recurrence or distant
metastasis, the underlying molecular mechanism of which remains
unclear (7). Cisplatin and
fluoropyrimidine-based chemotherapy, in combination with
trastuzumab, is widely used to treat patients with stage IV GC that
are human epidermal growth factor receptor 2-positive and who are
eligible to receive chemotherapy (8). Recently, it has been demonstrated that
biological therapies may be novel treatments of GC (9). For instance, overexpressed miR-200b
inhibited the proliferation and migration of GC cells (10). The current study therefore aimed to
assess the impact of micro (mi)RNAs (miRs) on the development of
GC.
miRNAs are a group of small non-coding RNAs that are
comprised of approximately 22–24 nucleotides (11). miRNAs specifically bind to the 3′
untranslated region (3′-UTR) of target mRNAs and as a result,
promote mRNA degradation and alter protein expression (12). In addition, miRNA expression profiles
in certain types of cancer serve primary roles in various human
biological processes, including migration, cellular metabolism,
cell proliferation, apoptosis and epithelial-mesenchymal transition
(13). Previous studies have
revealed that the dysregulation of certain miRNAs in
Helicobacter pylori-infected gastric mucosa and GC,
including miR-17-5p/20a, miR-106b and miR-93, induce the
development and progression of cancer, involving cell
proliferation, migration, invasion and apoptosis (14–17).
Furthermore, other findings have indicated that miR-627, miR-629
and miR-652 are highly expressed in patients with GC (7). In addition, human pancreatic cancer
progression was induced by miR-629 via FOXO3 targeting (18).
Forkhead box O3 (FOXO3) is a transcription factor
belonging to the FOXO family, the effects of which have been
assessed in a wide variety of cellular processes, including
proliferation, cell cycle arrest, cell death and metabolism
(19,20). A previous study has indicated that
FOXO3A promoted GC cell migration and invasion via the induction of
cathepsin L (21). Additionally,
FOXO3 exhibits tumor suppressive effects on GC, which might be a
promising therapy in clinic (22).
However, the impact of miR-629 on FOXO3 in GC has not yet been
fully elucidated. Based on previous studies (18,21), the
current study hypothesized that miR-629 may target FOXO3 to affect
GC cell activity.
The expression of miR-629 and FOXO3 in GC, and the
impact of miR-629 on FOXO3 expression and associated cell activity,
including cell proliferation and cell apoptosis, were assessed in
the current study. The results demonstrated that miR-629 may serve
as a therapeutic target for GC and that the effects of miR-629
indicate that it may serve as a novel molecular target for GC
diagnosis and treatment.
Materials and methods
Patients and tissues
A total of 44 patients with GC recruited into the
present study were hospitalized in The First Hospital of
Shijiazhuang City (Shijiazhuang City, China) from June 2016 to
August 2017. The patients (15 females and 29 males) aged between 36
and 67 years old. None of the patients had undergone surgical
treatment, radiotherapy or chemotherapy prior to enrollment. Tumor
and paracarcinoma tissues (obtained from each patient 5 cm away
from the edge of tumor) were stored in 10% formalin at room
temperature. The paracarcinoma tissues were used as the control
group. The current study was approved by the Ethics Committee of
The First Hospital of Shijiazhuang City and all patients provided
their written informed consent.
Cell line and plasmids
The SGC-7901 cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA) and maintained
in RPMI-1640 medium containing 10% fetal bovine serum (both Thermo
Fisher Scientific, Inc., Waltham, MA, USA). All cells were cultured
in a humidified incubator at 37°C in 5% CO2 for 24 h.
The miR-629 inhibitor (5′-GCUGGGCUUACGUUGGAGAAC-3′), negative
control (5′-TAACACGTCTATACGCCCA-3′), FOXO3 3′UTR wild-type (WT) and
FOXO3 3′UTR-mutant (MUT) were constructed by OriGene Technologies,
Inc. (Rockville, MD, USA).
miRNA transfection
SGC-7901 cells were seeded in 12-well plates
(2×105 cells/well) with RPMI-1640 medium and incubated
at 37°C with 5% CO2. Once cells reached a confluence of
60–70%, transfection was performed. miR-629 inhibitor or miR-NC
inhibitor (50 nmol/l) was transfected into cells using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) for
6 h according to the manufacturer's protocol. Subsequently,
transfected cells were cultured in RPMI-1640 medium containing 10%
fetal bovine serum in a humidified incubator at 37°C with 5%
CO2 for 48 h. At 48-h post transfection, cells were used
for the subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from GC and paracarcinoma tissues,
and SGC-7901 cells using the TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, RNA
concentration was determined using Nanodrop 2000 (Thermo Fisher
Scientific, Inc.). Takara Taq polymerase (Takara Bio, Inc., Otsu,
Japan) was used for DNA amplification. cDNA was synthesized using
the PrimeScript RT reagent kit (Takara Biotechnology, Co., Ltd.,
Dalian, China) at 37°C for 15 min. qPCR was performed using the
SYBR Premix EX Taq™ kit (Takara Bio, Inc.). The thermocycling
conditions were as follows: Initial denaturation at 95°C for 30
sec, followed by 40 cycles at 95°C for 30 sec, 60°C for 30 sec and
72°C for 30 sec, and then a final extension at 72°C for another 10
min. The expression of miR-629 was normalized to the endogenous
expression of U6 and the relative expression of protein mRNA was
normalized to GAPDH. The 2−ΔΔCq method was used to
analyze mRNA expression (23). The
sequences of the primers were listed in Table I.
 | Table I.Oligonucleotide primers used reverse
transcription- quantitative polymerase chain reaction analysis. |
Table I.
Oligonucleotide primers used reverse
transcription- quantitative polymerase chain reaction analysis.
| Gene | Direction | Sequence (5′-3′) |
|---|
| miR-629 | F |
TGGGTTTACGTTGGGAGA |
|
| R |
GTGCAGGGTCCGAGGTATTC |
| U6 | F |
CTCGCTTCGGCAGCACA |
|
| R |
AACGCTTCACGAATTTGCGT |
| Bax | F |
CACCAGCTCTGAACAGATCATGA |
|
| R |
TCAGCCCATCTTCTTCCAGATGT |
| Bcl-2 | F |
CACCCCTGGCATCTTCTCCTT |
|
| R |
AGCGTCTTCAGAGACAGCCAG |
| Caspase-3 | F |
GATGTGGACGCAGCCAACCTCA |
|
| R |
TCCGGCAGTAGTCGCCTCTGAA |
| FOXO3 | F |
TCACGCACCAATTCTAACGC |
|
| R |
CACGGCTTGCTTACTGAAGG |
| GAPDH | F |
AGAAGGCTGGGGCTCATTTG |
|
| R |
GCAGGAGGCATTGCTGATGAT |
Western blotting
Western blot analysis was performed to assess
protein expression. Transfected SGC-7901 cells were lysed using
radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology, Haimen, China) and centrifuged at
10,000 × g for 15 min at 4°C. Protein concentration was then
determined using a BCA kit (Thermo Fisher Scientific, Inc.).
Subsequently, SDS-PAGE containing 50 mg protein/lane was performed
with 25% resolving gel. Proteins were transferred onto
polyvinylidene difluoride membranes, which were blocked with 15%
skimmed milk for 1 h at 37°C prior to experimentation. GAPDH was
utilized as an internal control. Then the membranes were incubated
with primary anti-FOXO3 (cat no. ab109629; 1:1,000), anti-Bcl-2
associated × (Bax; cat no. ab32503; 1:1,000), anti-B-cell lymphoma
2 (Bcl-2; cat no. ab32124; 1:1,000), anti-caspase-3 (cat no.
ab32351; 1:5,000) and anti-GAPDH (cat no. EPR16891; 1:10,000; all
Abcam, Cambridge, MA, USA) and horseradish peroxidase-conjugated
secondary antibodies (cat no. ab6721; 1:5,000; Abcam) overnight at
4°C and incubated at room temperature for 1 h. An ECL kit (cat. no.
K820500; Biovision Inc., Milpitas, CA, USA) was used to visualize
samples. The gray value was obtained using ImageJ analysis software
(National Institutes of Health, Bethesda, MD, USA).
MTT assay
At 48 h following plasmid transfection, an MTT assay
was performed to assess the viability of SGC-7901. Cells were
seeded into 96-well plate (5×103 cells/well). Purple
formazan was dissolved using DMSO and optical density was then
measured at 595 nm using a microplate reader at 0, 12, 24 and 48
h.
Flow cytometry assay
Following 48 h incubation, SGC-7901 cells were
digested with trypsin for 2 min at 37°C. The apoptosis rate of GC
cells was determined with an Annexin V-FITC Apoptosis Detection kit
(Abcam) according to the manufacturer's protocol. Cells were
stained with 5 µl Annexin V and propidium iodide (BD Bioscience,
Franklin Lakes, NJ, USA) in shade at 37°C for 15 min prior to
counting. The apoptosis rate of transfected SGC-7901 cells was then
counted using a flow cytometer (EPICS XL; Beckman Coulter, Inc.,
Fullerton, CA, USA) with FCS Express 3.0 software (DeNovo Software,
Glendale, CA, USA).
Bioinformatics analysis and the
dual-luciferase-reporter assay
miR-629 target gene prediction was determined using
TargetScan (http://www.targetscan.org/vert_71/). For the
luciferase reporter assay. The pMIR-REPORT™ Luciferase plasmid was
purchased from Thermo Fisher Scientific, Inc. SGC-7901 cells
(5×103) were seeded into 96-well plates and incubated
with RPMI-1640 medium at 37°C with 5% CO2 until a
confluence of 70% was reached. Subsequently, the miR-629 inhibitor
and negative control plasmid with 3′UTR-FOXO3-WT and
3′UTR-FOXO3-MUT were transfected into SGC-7901 cells using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) and
incubated at 37°C. After 48 h, the dual-luciferase reporter assay
kit (Promega Corporation, Madison, WI, USA) was utilized for
luciferase determination and luciferase activity was normalized to
that of Renilla.
Statistical analysis
All data were presented as the mean ± standard
deviation and were performed in triplicate. A Student's t-test was
performed for comparisons between two groups and one-way analysis
of variance followed by a Newman-Keuls post-hoc test was utilized
for the comparison of multiple groups. Analysis was performed using
GraphPad Prism version 5.01 software (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
FOXO3 is downregulated but miR-629 is
upregulated in GC tissues
The expression of miR-629 and FOXO3 mRNA was
determined using RT-qPCR and FOXO3 protein expression was assessed
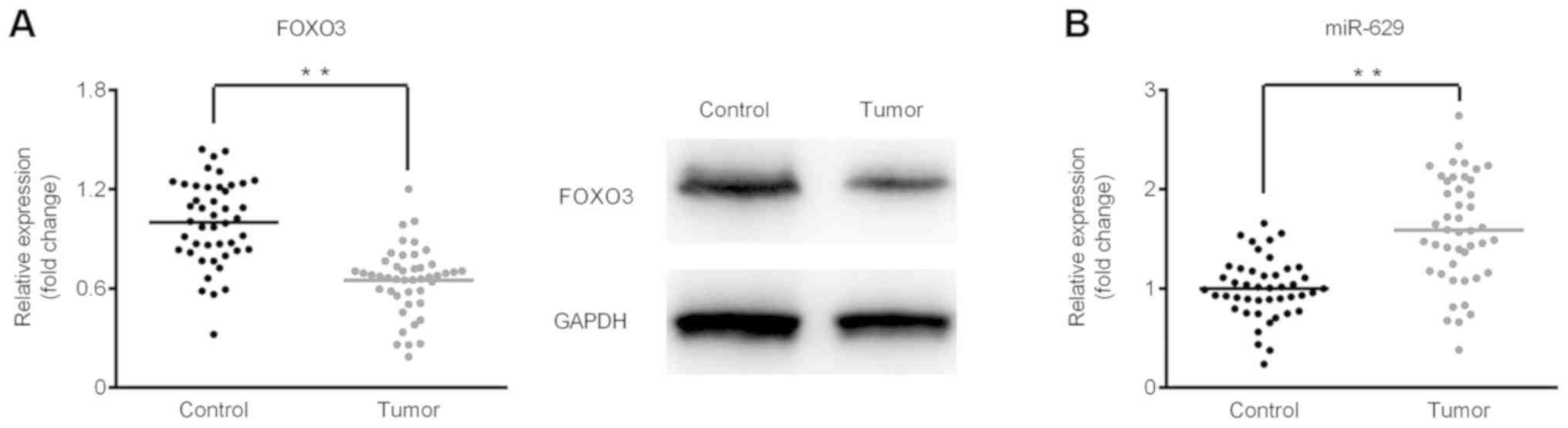
via western blot analysis. As shown in Fig. 1A, the results demonstrated that FOXO3
is significantly downregulated in GC tumor tissues at the mRNA and
protein level when compared with the control group. By contrast,
significant upregulation in miR-629 was observed in tumor tissues
compared with control tissues (Fig.
1B).
miR-629 is downregulated in SGC-7901
cells following inhibitor transfection
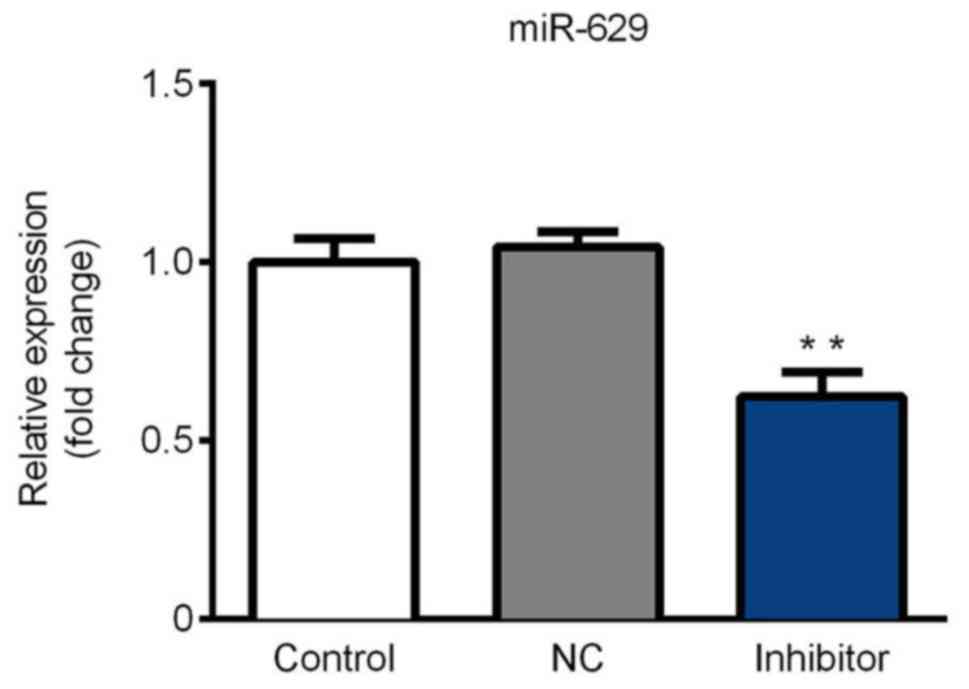
Cells were divided into three groups: The blank
control group, the miR-NC inhibitor group and the miR-629 inhibitor
group. As shown in Fig. 2, miR-629
expression was significantly decreased in the inhibitor group
compared with the control. These results indicate that the miR-629
inhibitor was successfully constructed and thus could be utilized
for further experimentation.
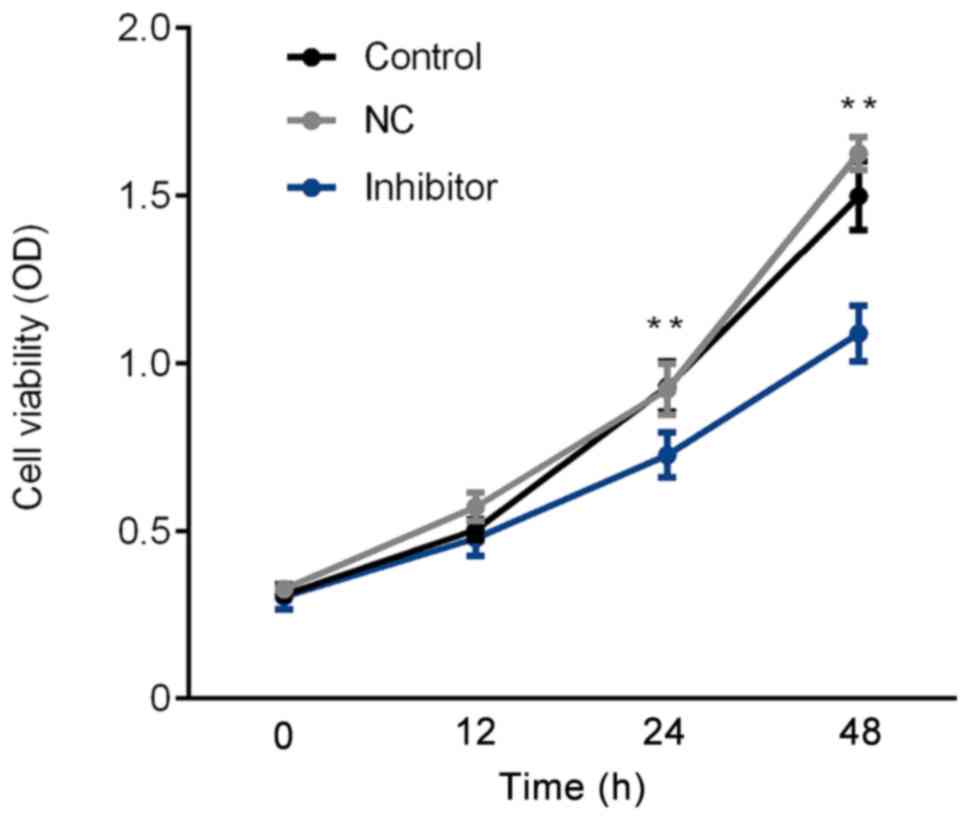
SGC-7901 cell proliferation rate is
inhibited by the miR-629 inhibitor
The proliferation rate of SGC-7901 cells was
assessed via an MTT assay. As presented in Fig. 3, compared with the control group, the
proliferation rate significantly decreased in the inhibitor group
following 24 and 48 h. Thus, the results demonstrated that the
miR-629 inhibitor induces a decrease in SGC-7901 cell
proliferation.
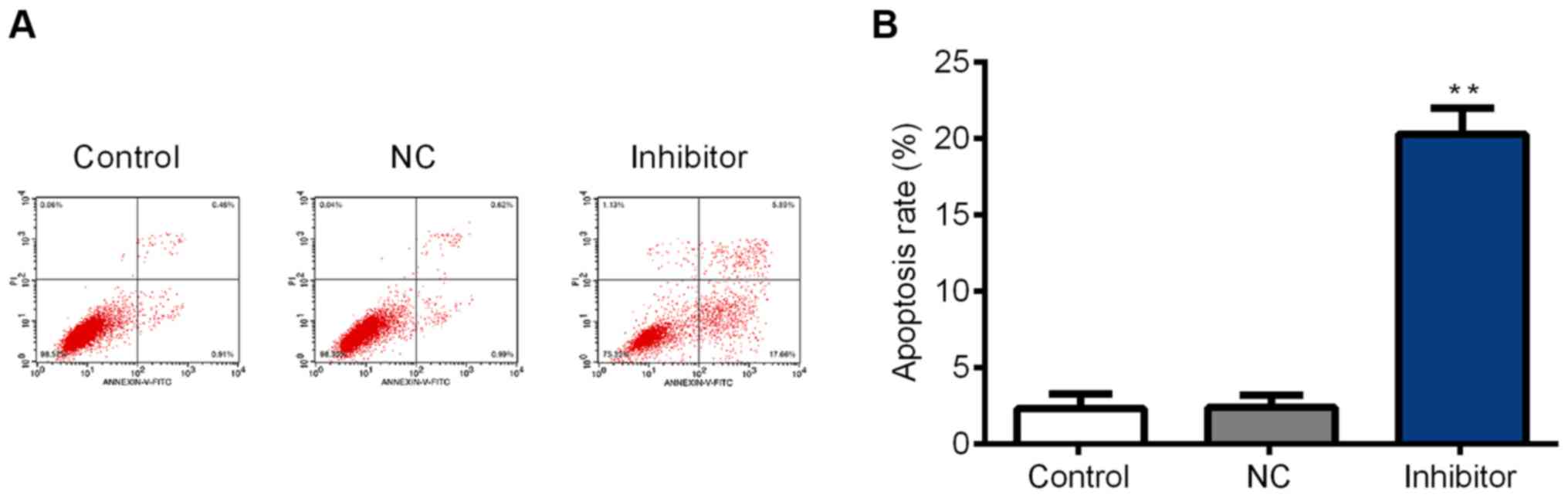
SGC-7901 cell apoptosis is promoted by
the miR-629 inhibitor
SGC-7901 cell apoptosis was assessed using flow
cytometry. The results indicated that the cell apoptosis rate was
significantly increased in the inhibitor group compared with the
control group (Fig. 4A and B). It
was therefore concluded that miR-629 inhibits SGC-7901 cell
apoptosis.
FOXO3, Bax and caspase-3 are
upregulated but Bcl-2 is downregulated in SGC-7901 cells following
inhibitor transfection
The expression of FOXO3, Bax, caspase-3 and Bcl-2 in
SGC-7901 cells was assessed following miR-629 inhibitor
transfection. The results demonstrated that the expression of
FOXO3, Bax and caspase-3 mRNA was significantly increased in the
inhibitor group compared with the control group. Additionally,
Bcl-2 expression was significantly decreased in the inhibitor group
compared with the control group (Fig.
5A-D). Furthermore, western blot analysis revealed that protein
expression was consistent with that of mRNA (Fig. 5E and F). These results indicate that
the miR-629 inhibitor downregulates Bcl-2 and upregulates the
expression of FOXO3, Bax and caspase-3.
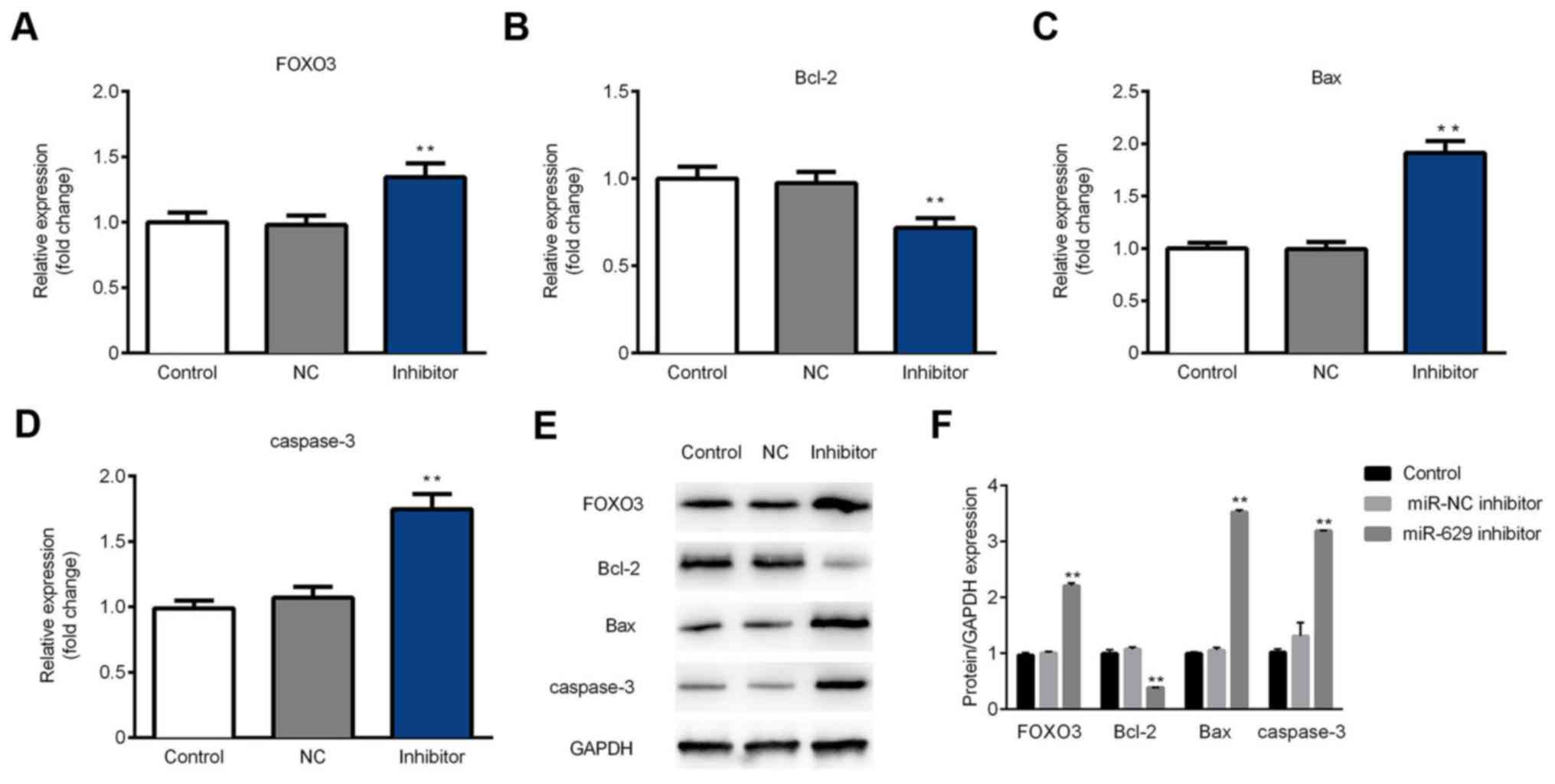 | Figure 5.miR-629 inhibitor transfection
upregulates FOXO3, Bax and caspase-3, and downregulates Bcl-2 in
SGC-7901 cells. The expression of (A) FOXO3, (B) Bax, (C) Bcl-2 and
(D) caspase-3 mRNA was determined using a reverse
transcription-quantitative polymerase chain reaction assay. (E) The
protein level of FOXO3, Bax, Bcl-2 and caspase-3 was determined
using western blotting. (F) Quantification of western blotting
results. **P<0.01 vs. the control group miR, microRNA; FOXO3,
forkhead box O3; Bax, Bcl-2 associated x; Bcl-2, B-cell lymphoma 2;
control, non-transfected group; NC, transfected with negative
control plasmids; inhibitor, transfected with miR-629 inhibitor
plasmids. |
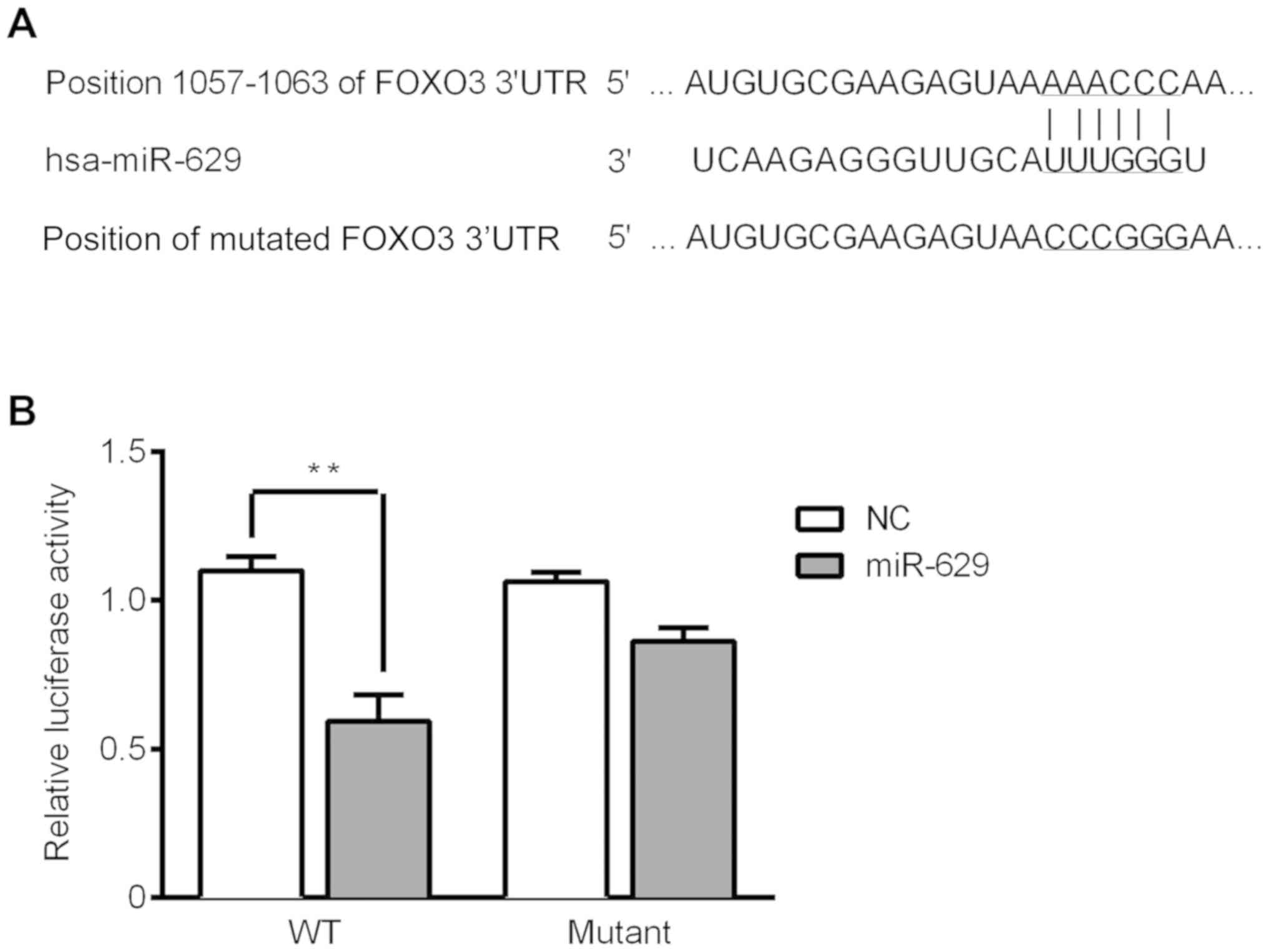
Luciferase reporter assay for target
verification
TargetScan was utilized to predict the target of
miR-629. The results revealed that FOXO3 was a target of miR-629
due to binding at the 3′UTR of FOXO3 (Fig. 6A). The luciferase activity
significantly reduced in the FOXO3 3′UTR-WT group compared with the
negative control group. Furthermore, there was no significant
difference between the negative control group and the FOXO3
3′UTR-MUT group (Fig. 6B). These
results indicate that FOXO3 is the direct target of miR-629.
Discussion
In congruence with a study by Shin et al
(7) the present study demonstrated
that miR-629 is overexpressed and FOXO3 is significantly
downregualted in GC. Furthermore, a study by Xiong et al
(24) revealed that sphingosine
kinase 1 induces the phosphorylation of FOXO3 and subsequent
downregulation of translational activity, resulting in
phosphoinositide 3-kinase/protein kinase B signaling. Further
studies should therefore assess the factors that affect the
expression of FOXO3 in GC. The results of the current study
revealed that miR-629 binds to FOXO3 and that the decreased
expression of the latter may be induced by GC. It has been
demonstrated that miR-629 promotes the progression of human
pancreatic cancer by targeting FOXO3, resulting in enhanced
pancreatic carcinoma cell proliferation and invasion (18). The present study also demonstrated
that downregulated miR-629 suppressed SGC-7901 cell
proliferation.
FOXO3 transcription factors are an evolutionarily
conserved subfamily of the forkhead transcription factors, which
are characterized by a forkhead DNA-binding domain (25). The FOXO subfamily comprises FOXO1,
FOXO3, FOXO4 and FOXO6 (26).
Furthermore, the mediation of these transcription factors is
determined by the availability of certain growth factors, such as
insulin and tumor necrosis factor α (26,27). In
addition, it has been revealed that FOXO transcription factors
exert regulatory effects on cell growth, the cell cycle, apoptosis
and defense against oxidative stress (28). The current study demonstrated that
the expression of FOXO3, Bax and caspase-3 was upregulated and that
Bcl-2 expression was suppressed, which was consistent with the
apoptosis results. These results indicate that suppressed miR-629
upregulated the expression of FOXO3, and promoted cell apoptosis by
reducing the expression of Bcl-2, and increasing BAX and Caspase 3
expression. A recent study has revealed that cyclin-dependent
kinase 6 protects against epithelial ovarian cancer by regulating
FOXO3 and promoting cell death (29).
The present study also revealed that miR-629 targets
FOXO3, resulting in the reduced expression of FOXO3 and the
progression of GC. The results further demonstrated that the
miR-629 inhibitor reduced cell proliferation and promoted cell
apoptosis. The expression of apoptosis-associated proteins was also
assessed and the results of western blotting indicated that cell
apoptosis activity had increased. Furthermore, the current study
revealed that miR-629 binds to the 3′UTR Of FOXO3, indicating that
it is a target of miR-629. Studies have demonstrated that various
miRNAs, including miR-223, miR-122 and miR-451 are associated with
the progression, migration and invasion of GC cell (30,31).
However, miR-223 also enhanced chemosensitivity and promoted the
apoptosis of GC cells by targeting FOXO3 (32). Additionally, TargetScan has revealed
that FOXO3 is the target of miR-629. Pancreatic β cells and
non-small cell lung cancer cells were also revealed to be
influenced by the sirtuin 1/FOXO3 and AMP-activated protein
kinase/FOXO3 signaling pathway (33,34).
Therefore, the impact of various additional factors, such as
transcription factors and epithelial-mesenchymal transition
factors, on the expression of FOXO3 and thus the progression of GC
should be assessed in the future.
In summary, the current study revealed that miR-629
was upregulated in GC and that the inhibition of miR-629 resulted
in an increase in FOXO3 expression. Furthermore, upregulated FOXO3
in SGC-7901 cells transfected with the miR-629 inhibitor may have
promoted the expression of Bax and caspase-3, and suppressed the
expression of Bcl-2, resulting in the repression of cell
proliferation and the promotion of cell apoptosis. In addition, the
luciferase reporter assay determined that FOXO3 is a direct target
of miR-629. Thus, miR-629 may serve essential roles in cell
proliferation and apoptosis in GC. Furthermore the inhibition of
miR-629 may be an effective therapy for patients with GC in the
future.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML drafted the manuscript. ML, YW and XL analyzed
the results and data, and collected materials. ZZ and LW conducted
the experiments. YL conceived of and designed the study, and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of The First Hospital of Shijiazhuang City (Shijiazhuang
City, China). All patients provided their written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cha Y, He Y, Ouyang K, Xiong H, Li J and
Yuan X: MicroRNA-140-5p suppresses cell proliferation and invasion
in gastric cancer by targeting WNT1 in the WNT/β-catenin signaling
pathway. Oncol Lett. 16:6369–6376. 2018.PubMed/NCBI
|
|
3
|
Hamashima C: Current issues and future
perspectives of gastric cancer screening. World J Gastroenterol.
20:13767–13774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He Z, Wang X, Huang C, Gao Y, Yang C, Zeng
P and Chen Z: The FENDRR/miR-214-3P/TET2 axis affects cell
malignant activity via RASSF1A methylation in gastric cancer. Am J
Transl Res. 10:3211–3223. 2018.PubMed/NCBI
|
|
5
|
Yang Y, Shen J, Yan D, Yuan B, Zhang S,
Wei J and Du T: Euchromatic histone lysine methyltransferase 1
regulates cancer development in human gastric cancer by regulating
E-cadherin. Oncol Lett. 15:9480–9486. 2018.PubMed/NCBI
|
|
6
|
Correa P: Gastric cancer: Overview.
Gastroenterol Clin North Am. 42:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shin VY, Ng EK, Chan VW, Kwong A and Chu
KM: A three-miRNA signature as promising non-invasive diagnostic
marker for gastric cancer. Mol Cancer. 14:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu HH, Lin WC and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:e12014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LK, Xie XN, Song XH, Su T, Chang XL,
Xu M, Liang B and Huang DY: Upregulation of miR-200b inhibits
hepatocellular carcinoma cell proliferation and migration by
targeting HMGB3 protein. Technol Cancer Res Treat.
17:15330338188064752018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lan H, Lu H, Wang X and Jin H: MicroRNAs
as potential biomarkers in cancer: Opportunities and challenges.
Biomed Res Int. 2015:1250942015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Schooneveld E, Wildiers H, Vergote I,
Vermeulen PB, Dirix LY and Van Laere SJ: Dysregulation of microRNAs
in breast cancer and their potential role as prognostic and
predictive biomarkers in patient management. Breast Cancer Res.
17:212015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Zhang S, Bao H, Mu S, Zhang B, Ma
H and Ma S: MicroRNA-365 promotes lung carcinogenesis by
downregulating the USP33/SLIT2/ROBO1 signaling pathway. Cancer Cell
Int. 18:642018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu HS and Xiao HS: MicroRNAs as potential
biomarkers for gastric cancer. World J Gastroenterol.
20:12007–12017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang M, Gu H, Qian H, Zhu W, Zhao C, Zhang
X, Tao Y, Zhang L and Xu W: miR-17-5p/20a are important markers for
gastric cancer and murine double minute 2 participates in their
functional regulation. Eur J Cancer. 49:2010–2021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun C, Yao X, Jiang Q and Sun X: miR-106b
targets DAB2 to promote hepatocellular carcinoma cell proliferation
and metastasis. Oncol Lett. 16:3063–3069. 2018.PubMed/NCBI
|
|
17
|
Liu LJ, Yu JJ and Xu XL: MicroRNA-93
inhibits apoptosis and promotes proliferation, invasion and
migration of renal cell carcinoma ACHN cells via the TGF-β/Smad
signaling pathway by targeting RUNX3. Am J Transl Res. 9:3499–3513.
2017.PubMed/NCBI
|
|
18
|
Yan H, Li Q, Wu J, Hu W, Jiang J, Shi L,
Yang X, Zhu D, Ji M and Wu C: MiR-629 promotes human pancreatic
cancer progression by targeting FOXO3. Cell Death Dis. 8:e31542017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Vincenzo S, Heijink IH, Noordhoek JA,
Cipollina C, Siena L, Bruno A, Ferraro M, Postma DS, Gjomarkaj M
and Pace E: SIRT1/FoxO3 axis alteration leads to aberrant immune
responses in bronchial epithelial cells. J Cell Mol Med.
22:2272–2282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SY, Kim HJ, Byeon HK, Kim DH and Kim
CH: FOXO3 induces ubiquitylation of AKT through MUL1 regulation.
Oncotarget. 8:110474–110489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu S, Yu Y, Zhang W, Yuan W, Zhao N, Li Q,
Cui Y, Wang Y, Li W, Sun Y and Liu T: FOXO3a promotes gastric
cancer cell migration and invasion through the induction of
cathepsin L. Oncotarget. 7:34773–34784. 2016.PubMed/NCBI
|
|
22
|
Park SH, Jang KY, Kim MJ, Yoon S, Jo Y,
Kwon SM, Kim KM, Kwon KS, Kim CY and Woo HG: Tumor suppressive
effect of PARP1 and FOXO3A in gastric cancers and its clinical
implications. Oncotarget. 6:44819–44831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong H, Wang J, Guan H, Wu J, Xu R, Wang
M, Rong X, Huang K, Huang J, Liao Q, et al: SphK1 confers
resistance to apoptosis in gastric cancer cells by downregulating
Bim via stimulating Akt/FoxO3a signaling. Oncol Rep. 32:1369–1373.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho KK, Myatt SS and Lam EW: Many forks in
the path: Cycling with FoxO. Oncogene. 27:2300–2311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hagenbuchner J, Kuznetsov A, Hermann M,
Hausott B, Obexer P and Ausserlechner MJ: FOXO3-induced reactive
oxygen species are regulated by BCL2L11 (Bim) and SESN3. J Cell
Sci. 125:1191–1203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Essers MA, Weijzen S, de Vries-Smits AM,
Saarloos I, de Ruiter ND, Bos JL and Burgering BM: FOXO
transcription factor activation by oxidative stress mediated by the
small GTPase Ral and JNK. EMBO J. 23:4802–4812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dall' Acqua A, Sonego M, Pellizzari I,
Pellarin I, Canzonieri V, D'Andrea S, Benevol S, Sorio R, Giorda G,
Califano D, et al: CDK6 protects epithelial ovarian cancer from
platinum-induced death via FOXO3 regulation. EMBO Mol Med.
9:1415–1433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang F, Xu Y, Liu C, Ma C, Zou S, Xu X,
Jia J and Liu Z: NF-κB/miR-223-3p/ARID1A axis is involved in
Helicobacter pylori CagA-induced gastric carcinogenesis and
progression. Cell Death Dis. 9:122018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu X, Gao F, Wang J, Tao L, Ye J, Ding L,
Ji W and Chen X: MiR-122-5p inhibits cell migration and invasion in
gastric cancer by down-regulating DUSP4. Cancer Biol Ther.
19:427–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng Q, He P and Wang Y: MicroRNA-223-3p
regulates cell chemo-sensitivity by targeting FOXO3 in prostatic
cancer. Gene. 658:152–158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang N, Zhang J, Qin M, Yi W, Yu S, Chen
Y, Guan J and Zhang R: Amelioration of streptozotocin-induced
pancreatic β cell damage by morin: Involvement of the
AMPK-FOXO3-catalase signaling pathway. Int J Mol Med. 41:1409–1418.
2018.PubMed/NCBI
|
|
34
|
Li J, Li P, Chen T, Gao G, Chen X, Du Y,
Zhang R, Yang R, Zhao W, Dun S, et al: Expression of microRNA-96
and its potential functions by targeting FOXO3 in non-small cell
lung cancer. Tumour Biol. 36:685–692. 2015. View Article : Google Scholar : PubMed/NCBI
|