Introduction
Coronary heart disease (CHD) is one of the leading
causes of mortality worldwide, accounting for one-third of all
mortalities worldwide each year (1).
As a severe type of CHD, acute myocardial infarction (AMI) is the
most common cause of mortality in China (2). The World Bank estimates that by 2030,
23 million Chinese patients will experience AMI annually (3). The most effective therapeutic measure
against myocardial ischemia is to restore heart perfusion. However,
the beneficial effects can be compromised by ischemia/reperfusion
injury (I/R) (4). Myocardial I/R can
induce further damage to the myocardium itself, leading to
metabolic and functional disorders (5). The underlying mechanism of I/R injury
may be associated with oxidative stress, the inflammatory response,
mitochondrial damage and cell apoptosis (6). In view of the dreaded complication of
reperfusion and the heavy social burden, it is urgently necessary
to further explore the mechanism of I/R pathogenesis and develop
effective intervention measures.
Remote ischemic post-conditioning (RIPostC), induced
by several episodes of transient I/R intervention on distant
tissues and organs away from the heart (such as the limbs, kidneys,
small intestine and skeletal muscle), is as an effective strategy
for myocardial protection against I/R injury (7). Due to its simple operation and
significant curative effects, RIPostC can be performed
noninvasively using a blood pressure cuff on the limb, and thus it
has gained the attention of numerous researchers (8–10).
RIPostC has been verified to have a beneficial effect in animal
studies and randomized clinical trials (11–14).
However, the underlying mechanism of RIPostC is still poorly
understood (15).
Rho-associated coiled-coil containing protein kinase
(ROCK), a serine/threonine kinase, has been identified as a
downstream effector of RhoA. The ROCK isoforms, ROCK1 and ROCK2,
were initially discovered as downstream targets of the small
guanosine triphosphate-binding protein RhoA (16). The RhoA/Rho-kinase signaling pathway
serves an important role in cardiovascular diseases including
myocardial infarction, heart failure, hypertension and
atherosclerosis (17). The
Rho-kinase-mediated signaling pathway induces enhanced myocardial
damage by mediating the phosphorylation of the downstream myosin
light chain (MLC) and the myosin phosphatase target subunit (MYPT1)
(18). Fasudil, a Rho-kinase
inhibitor, has a beneficial effect on myocardial I/R by reducing
myocardial infarct size, oxidative stress and cell apoptosis
(19,20). However, whether the cardioprotective
effect on RIPostC is associated with downregulated Rho-kinase
activity is unknown. Therefore, the present study was designed to
investigate the effect of Rho-kinase on RIPostC and try to
elucidate the underlying mechanism.
Materials and methods
Experimental animals
A total of 32 male Sprague-Dawley (SD) rats (weight,
250–300 g; age, 7–8 weeks), were purchased from the Laboratory
Animal Center of Bengbu Medical College. The rats had free access
to a normal diet and fresh water. All rats were kept at a constant
temperature (22–26°C) and humidity (50%) with a 12-h light/dark
cycle and were raised in individual cages. The animal research
study protocol was following ‘The Guide for the Care of Use of
Laboratory Animals’ published by the National Institute of Health
and were approved by the Animal Use and Care Committee of Bengbu
Medical College (21).
Materials and reagents
Fasudil was purchased from Tianjin Red Sun
Pharmaceutical Co., Ltd., (cat. no. 1604071). Creatine kinase (CK;
cat. no. 20170626), lactate dehydrogenase (LDH; cat. no. 20160606),
superoxide dismutase (SOD; cat. no. 20160608) and malondialdehyde
(MDA; cat. no. 20160620) assay kits were purchased from Nanjing
Jiancheng Institute of Biotechnology. The cardiac troponin I (cTnI)
ELISA kit was purchased from Calvin Biotechnology Co., Ltd., (cat.
no. CK-E30258R). TRIzol® reagent was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. (cat. no. 15596026).
RevertAid first strand cDNA synthesis kit was purchased from Thermo
Fisher Scientific, Inc. (cat. no. 00398085). PCR master mix was
purchased from Thermo Fisher Scientific, Inc. (cat. no. K0171).
QuickBlock Blocking solution was purchased from Beyotime Institute
of Biotechnology (cat. no. P0252). ROCK1, ROCK2, B-cell lymphoma 2
(Bcl-2), Bcl-2-associated X protein (Bax) and β-actin primers were
purchased from Sangon Biotech Co., Ltd. The following primary
antibodies were purchased from Cell Signaling Technology, Inc.:
Mouse anti-phosphorylated (p)-MLC (cat. no. 3675), rabbit anti-MLC
(cat. no. 3672), rabbit anti-p-MYPT1 (cat. no. 4563) and rabbit
anti-MYPT1 (cat. no. 2634). Rabbit GAPDH antibody was purchased
from Absin Bioscience Inc. (cat. no. JG26). Horseradish peroxidase
(HRP)-linked anti-rabbit immunoglobulin (IgG; cat. no. BST11J12B54)
and HRP-linked anti-mouse IgG (cat. no. BST11J12B50) were purchased
from Wuhan Boster Biological Technology Co., Ltd. Immobilon western
chemiluminescent HRP substrate (ECL) kit was acquired from EMD
Millipore (cat. no. WBKLS0100).
Establishment of the myocardial I/R
model
The rats were anesthetized by intraperitoneal
injection of chloral hydrate (400 mg/kg) prior to the operation.
Hemodynamic parameters and standard electrocardiograms (ECG) were
continuously measured by the MedLab biological signal collection
system to observe the changes of cardiac function in rats.
Following tracheal intubation, ventilation was applied via
respiratory equipment, with a respiratory rate of 70–80 times/min
and a tidal volume of 20–30 ml/kg (22). Then, the chest was gently opened
through the fourth and fifth ribs and the left anterior descending
coronary artery (LAD) was identified. The LAD was ligated using a
5–0 silk (2 mm) suture. Additionally, a medical latex tube (socket
inner diameter, 1.5 mm) was placed between the ligature and the
LAD. Myocardial ischemia was induced by tightening the ligature
around the latex tube. Successful surgically induced myocardial
ischemia was detected based on S-T segment elevation on an ECG
(23). After 45 min of ischemia, the
polyethylene tubes were loosened for 180 min to mimic reperfusion
(24).
Experimental groups
A total of 32 healthy male SD rats were randomly
divided into the following four groups (n=8 in each group): Sham
group (Sham), I/R group (I/R), RIPostC group (RIPostC) and the I/R
with fasudil group (I/R+Fas). In the Sham group, the LAD was
threaded but not ligated for 225 min in vivo. In the I/R
group, the LAD was ligated for 45 min (ischemia) followed by 180
min of the LAD open (reperfusion). In the RIPostC group, the same
operation as the I/R group was performed; then following 15 min of
ischemia, three cycles of right femoral artery clamping for 5 min
and declamping for 5 min were conducted prior to reperfusion, as
described in the authors previous study (24). In the I/R+Fas group, fasudil (10
mg/kg, a Rho-kinase inhibitor) was administered intravenously 5 min
prior to myocardial reperfusion in I/R-treated rats (25).
Measurement of plasma CK, LDH, MDA and
SOD
At the end of the reperfusion period, carotid artery
blood samples (~1.5 ml per rat) were collected with disposable
heparin blood vessels and centrifuged at 1,509 × g for 15 min at
4°C. The supernatant fluid was collected and stored at −80°C. The
MDA contents and LDH, CK and SOD activities were measured by
spectrophotometer at wavelengths of 532, 440, 660 and 550 nm,
respectively according to the manufacturer's protocol.
Measurement of myocardial cTnI
content
The cTnI content was measured using an ELISA kit at
a wavelength of 450 nm. The absorbances were measured and the
contents were calculated according the manufacturer's protocol.
Assessment of myocardial infarct
size
Infarct size was established by Evans blue and
triphenyltetrazolium chloride (TTC) staining as described
previously (21,24). After the rats were sacrificed, the
hearts were removed immediately and washed 2–3 times with PBS.
Using re-occlusion of LAD in isolated Langendorff-perfused
equipment, the hearts were perfused with 0.3 ml 1% Evans blue dye
to delineate the risk area. Following freezing at −80°C, the hearts
were cut into 2 mm thick sections along the left ventricular
transverse section and incubated with 1% TTC at 37°C for 20 min.
Subsequently, the sections were fixed in 4% paraformaldehyde
solution at 37°C for 30 min. The gray area indicated the percentage
infarct size (IS) and the red area represented the area at risk
(AAR) was; these were quantified by computerized planimetry using
ImageJ software (version 1.40; National Institutes of Health).
Infarct size was calculated as the percentage of IS to AAR.
RNA extraction and semi-quantitative
reverse transcription-polymerase chain reaction (RT-PCR) assay
detecting ROCK1, ROCK2, Bax and Bcl-2 mRNA expression
Total RNA was extracted from the ischemic myocardial
tissue using TRIzol® reagent. Total RNA (3 µg) was
reverse transcribed into cDNA at 42°C and 1.5 µl cDNA was utilized
for PCR amplification with the following temperature protocol:
Denaturation at 95°C for 3 min, followed by 40 cycles of
denaturation at 95°C for 30 sec, annealing (ROCK1, 58°C; ROCK2,
62.5°C; Bax, 62.5°C; Bcl-2, 62.5°C; and β-actin, 62.5°C) for 30
sec, and extension at 72°C for 30 sec, then 72°C for 10 min and
held at 4°C. The primer sequences are presented in Table I. The PCR products were analyzed on a
1.2% agarose gel. The densitometry results for the ROCK1, ROCK2,
Bcl-2 and Bax genes were compared with the corresponding β-actin
levels to analyze the relative expression, and the Bcl-2/Bax ratio
was calculated using a Tanon-3500 Gel imaging system (GIS
4.1.2).
 | Table I.Reverse transcription-PCR primers for
ROCK1, ROCK2, Bax, Bcl-2 and β-actin. |
Table I.
Reverse transcription-PCR primers for
ROCK1, ROCK2, Bax, Bcl-2 and β-actin.
| Gene | Primer | Sequence | Product (bp) |
|---|
| ROCK1 | Forward |
5′-GCAAATGCGGGAGTTACAAG-3′ | 314 |
|
| Reverse |
5′-CAAGCCGACTAACGGTATGATC-3′ |
|
| ROCK2 | Forward |
5′-AGAACCTGTCAAGCGTGGTAGTG-3′ | 339 |
|
| Reverse |
5′-GACAGCCATCCTTCTATTCGTGA-3′ |
|
| Bax | Forward |
5′-GGATCGAGCAGAGAGGATGG-3′ | 464 |
|
| Reverse |
5′-TGGTGAGTGAGGCAGTGAGG-3′ |
|
| Bcl-2 | Forward |
5′-CTGGTGGACAACATCGCTCTG-3′ | 228 |
|
| Reverse |
5′-GGTCTGCTGACCTCACTTGTG-3′ |
|
| β-actin | Forward |
5′-CTGTATGCCTCTGGTCGTAC-3′ | 214 |
|
| Reverse |
5′-TGATGTCACGCACGATTTCC-3′ |
|
Western blot analysis of the protein
expression of MLC, p-MLC, MYPT1 and p-MYPT1
Left anterior myocardium tissues from each group
were collected and homogenized in a lysis buffer, which contained
20 mmol/l Tris (pH 7.4), 150 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/l
EGTA, 1% Triton X-100, 0.1% SDS and 1% protease inhibitor cocktail.
The homogenates were sonicated by 3–5 cycles (30 sec sonication and
10 sec rest) at a frequency of 25 kHz and 60% amplitude before they
were centrifuged at 12,000 × g for 30 min at 4°C. The protein
concentration was determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology) (24). Total protein (45 µg) was separated by
SDS-PAGE using a 10% separation gel and a 5% concentrated gel, and
transferred to PVDF membranes. The membranes were blocked with
QuickBlock™ Blocking solution at 37°C for 2 h and then were
incubated with the primary antibodies at 4°C overnight. Immunoblots
were performed using the following antibodies: Rabbit GAPDH
antibody (1:4,000), rabbit MYPT antibody (1:500), rabbit p-MYPT1
antibody (1:2,000), rabbit MLC antibody (1:500) and mouse p-MLC
antibody (1:2,000). All membranes were incubated for 1 h with the
corresponding HRP-linked anti-rabbit IgG (1:8,000) or HRP-linked
anti-mouse IgG (1:4,000) secondary antibodies. The membranes were
analyzed by an ECL system. Gray values of the target bands were
analyzed using ImageJ software.
Statistical analysis
All values were expressed as the mean ± standard
deviation (n=8). Statistical comparisons were performed by one-way
analysis of variance and the Newman-Keuls test using SPSS 16.0
software (SPSS, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in plasma CK, LDH, MDA and SOD
levels in each group
Compared with the Sham group, the activities of CK
and LDH, and MDA contents were significantly increased in the I/R
group (P<0.01), while SOD activities were significantly
decreased (P<0.01). Compared with the I/R group, the activities
of CK and LDH, and MDA contents were significantly decreased
(P<0.01), and the SOD activities were significantly elevated in
the RIPostC and I/R+Fas groups (P<0.01). However, the levels of
CK, LDH, MDA and SOD were no significant differences between the
RIPostC and I/R+Fas groups (P>0.05; Fig. 1).
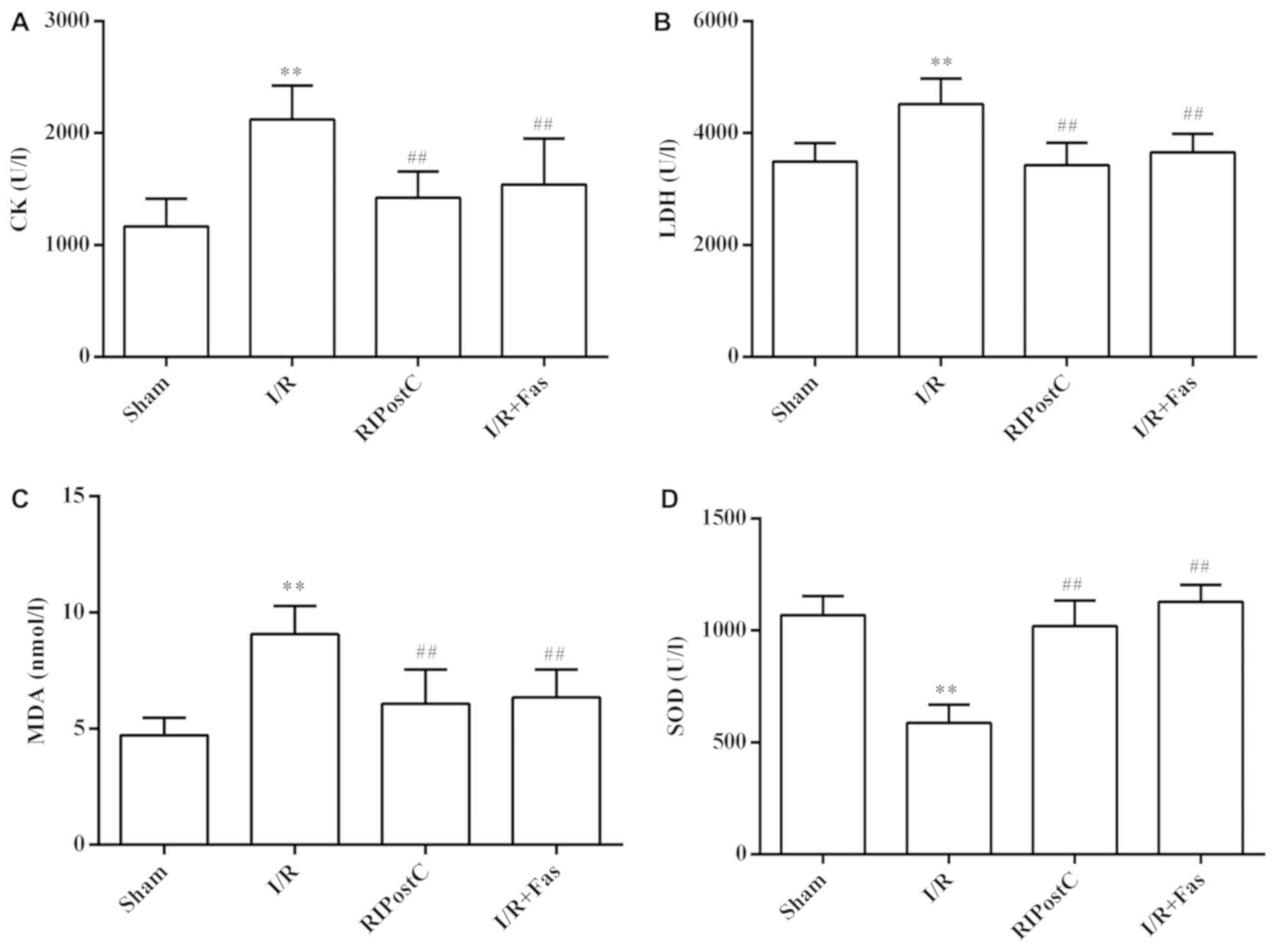 | Figure 1.Changes to CK, LDH, MDA and SOD
levels in the plasma. Effects of RIPostC and Rho-kinase on (A) CK
activity, (B) LDH activity, (C) MDA content and (D) SOD activity,
in the plasma of each groups. Values were expressed as the mean ±
standard deviation. **P<0.01 vs. Sham group;
##P<0.01 vs. I/R group. I/R, ischemia/reperfusion;
RIPostC, remote ischemic postconditioning; Fas, Fasudil; CK,
creatine kinase; LDH, lactate dehydrogenase; MDA, malondialdehyde;
SOD, superoxide dismutase. |
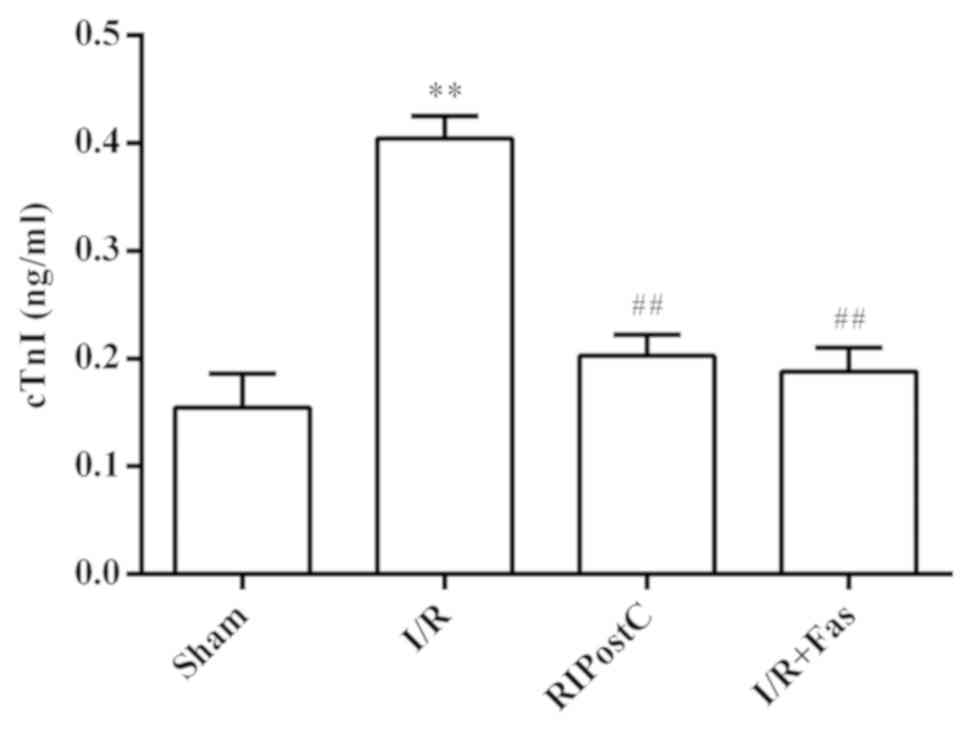
Changes in plasma cTnI levels in each
group
Compared with the Sham group, the contents of cTnI
were significantly increased in the I/R group (P<0.01). Compared
with the I/R group, the contents of cTnI were significantly
decreased in the RIPostC and I/R+Fas groups (P<0.01). However,
there were no significant differences in cTnI levels between the
RIPostC and I/R+Fas groups (P>0.05; Fig. 2).
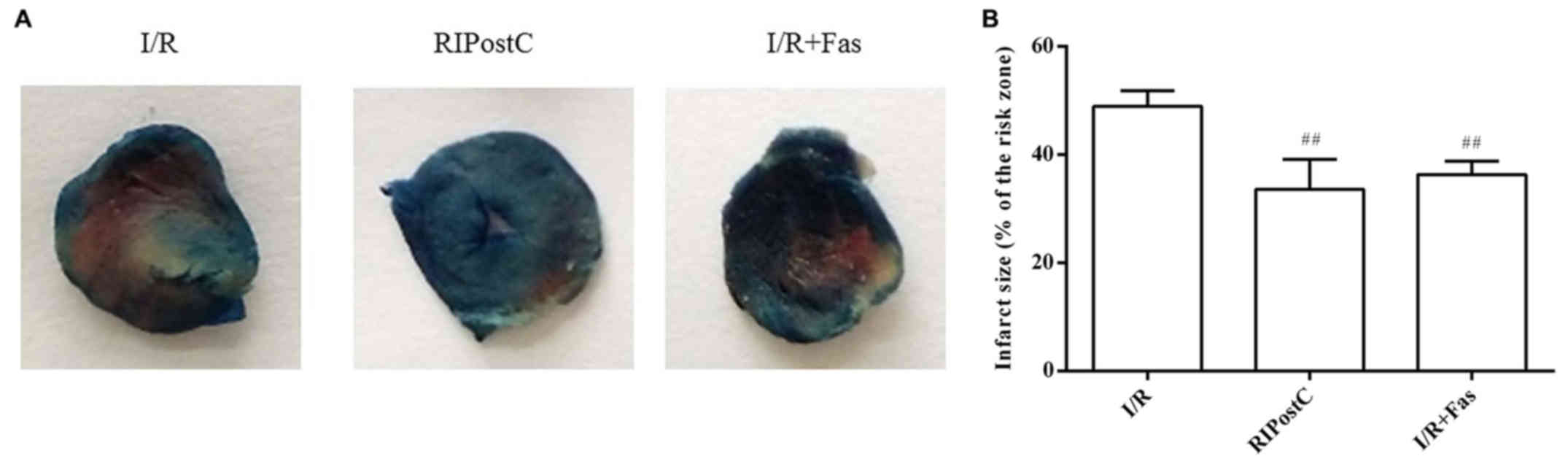
Changes in myocardial infarct size in
each group
Compared with the I/R group, the myocardial infarct
size was significantly decreased in the RIPostC and I/R+Fas groups
(P<0.01). There were no significant differences between the
RIPostC and I/R+Fas groups (P>0.05; Fig. 3).
Expression of ROCK1, ROCK2, Bax and
Bcl-2 mRNA in the myocardium
The RT-PCR results revealed that, compared with
those in the Sham group, the mRNA expression of ROCK1 and ROCK2 was
significantly increased (P<0.01), whereas the ratio of Bcl-2/Bax
was significantly decreased in the I/R group (P<0.01). However,
compared with the I/R group, the expression of ROCK1 and ROCK2 was
significantly decreased, and the ratio of Bcl-2/Bax was increased
in the RIPostC and I/R+Fas groups (P<0.01). In contrast to
RIPostC group, the expression of ROCK1 and ROCK2, and the ratio of
Bcl-2/Bax exhibited no statistical differences in the I/R+Fas group
(P>0.05; Fig. 4).
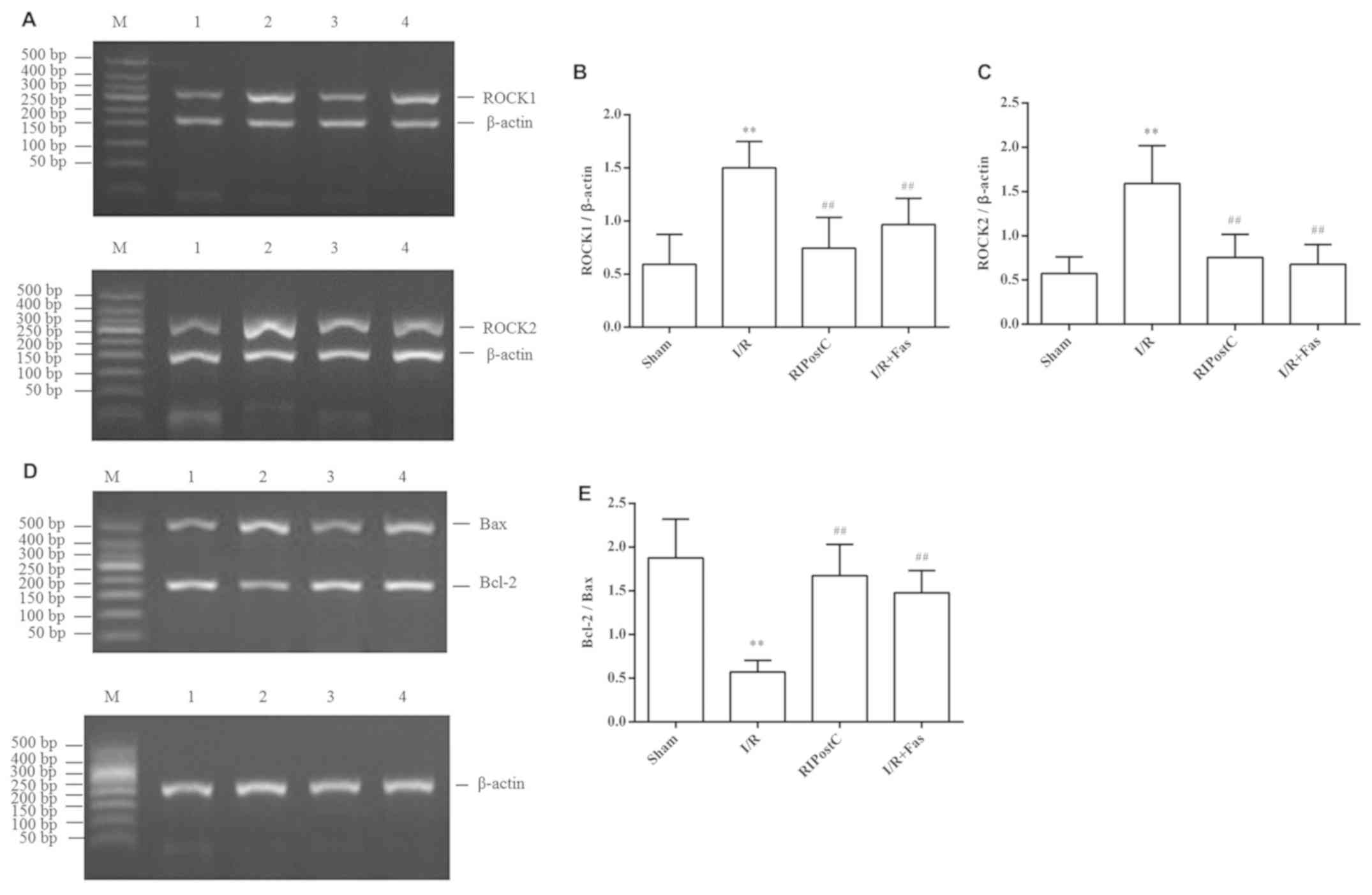 | Figure 4.Changes in the expression levels of
myocardial ROCK1, ROCK2, Bax and Bcl-2 at the mRNA level.
Expression levels of myocardial (A) ROCK1 and ROCK2 mRNA, (B)
quantification of the ROCK1/β-actin, (C) quantification of the
ROCK2/β-actin, (D) Bax and Bcl-2 mRNA, and (E) quantification of
the Bcl-2/Bax ratio for the different groups. Values were expressed
as the mean ± standard deviation. **P<0.01 vs. Sham group;
##P<0.01 vs. I/R group. M, marker; 1, Sham; 2, I/R;
3, RIPostC; 4, I/R+Fas; I/R, ischemia/reperfusion; RIPostC, remote
ischemic postconditioning; Fas, Fasudil; ROCK1, Rho-associated
coiled-coil containing protein kinase 1; ROCK2, Rho-associated
coiled-coil containing protein kinase 2; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein. |
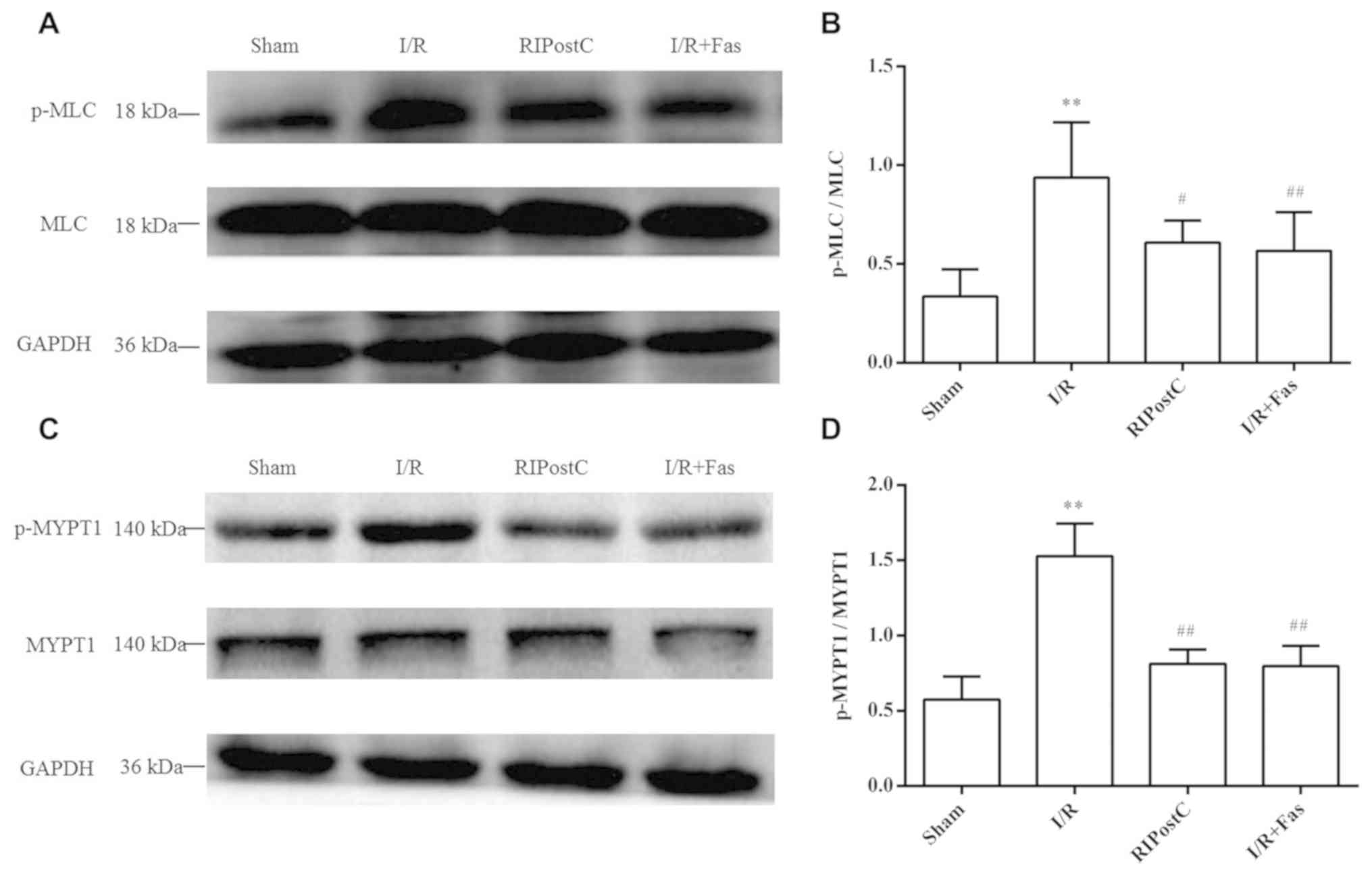
Protein expression of p-MLC, MLC,
p-MYPT1 and MYPT1
Compared with the Sham group, the protein expression
of p-MLC and p-MYPT1 was significantly increased in the I/R group
(P<0.01). By contrast, the protein expression of p-MLC and
p-MYPT1 in the RIPostC and I/R+Fas groups was decreased compared
with the I/R group (P<0.01; Fig.
5). In contrast to those of the RIPostC group, there were no
statistical differences in the I/R+Fas group (P>0.05; Fig. 4)
Discussion
In the present study, it was demonstrated that
RIPostC could provide protection against myocardial I/R injury.
Rho-kinase pathway served an important role in myocardial I/R
injury. The present study also demonstrated that the Rho-kinase
inhibitor fasudil produced attenuation of the myocardial infarction
and myocardial apoptosis associated with I/R. It was further
confirmed that RIPostC had a cardioprotective and anti-apoptotic
effect through inhibition of the Rho-kinase signaling pathway.
RIPostC, which is induced by several episodes of
brief I/R in distant organs from the heart, has been developed as
an effective strategy to protect against the harmful effects of I/R
injury (26–29). Although the protective role has been
widely recognized, the mechanism of the cardioprotective effect
induced by RIPostC remains to be fully elucidated (15). Myocardial I/R injury can cause the
generation of reactive oxygen species, calcium overload, the
inflammatory response, mitochondrial damage, lipid peroxidation and
further damage to the myocardial tissue (30). Myocardial infarction and the levels
of LDH, CK and cTnI are frequently used to quantify the amount of
myocardial damage (31,32). In addition, measuring MDA content and
SOD activity can reflect the degree of myocardial cell damage and
the production of oxygen free radicals (33). The present study showed that RIPostC
reduced the myocardial infarct size and inhibited the release of
LDH, CK, cTnI and MDA, while increasing the activity of SOD. These
results suggested that the RIPostC could effectively alleviate
myocardial I/R injury. It was also demonstrated that the Rho-kinase
inhibitor fasudil reduced infarct size, attenuated the increased
levels of LDH, cTnI, CK and MDA, and reduced the activity of SOD,
which had the cardioprotective effect similar to RIPostC. These
observations indicated that inhibition of Rho-kinase and RIPostC
could alleviate myocardial I/R injury by reducing the production of
free radicals.
Rho-kinase is a 160 kDa serine/threonine protein
kinase that acts as a downstream effector of the small G protein
RhoA. Rho-kinase has two highly homomeric isoforms, ROCK1 and
ROCK2, which regulate cell formation, migration, proliferation and
apoptosis (17). Both ROCK1 and
ROCK2 are expressed in vascular smooth muscle and the heart
(17,18). An increasing body of evidence has
demonstrated that the Rho-kinase pathway serves an important role
in myocardial I/R damage and inhibition of the Rho-kinase signaling
pathway has beneficial effects on cardiac functions and apoptosis
(20,34). Apoptosis is an essential contributor
to cardiac dysfunction (35). Bcl-2
family members serve important roles in regulating apoptotic
signaling. The balance in the expression levels of the
anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins play a major
role in the regulation of myocardial apoptotic cell death (24). Moreover, activation of Rho-kinase
enhances the contractions of vascular smooth muscle cells, leading
to coronary artery spasm and aggravating myocardial injury
(17,18). Certain studies have demonstrated that
MLC and MYPT1 acted as the principal downstream effector protein of
Rho-kinase, and their phosphorylation level could indirectly
reflect the activity of Rho-kinase (36). Rho-kinase can increase MLC
phosphorylation through direct effect on MLC or indirectly by
inactivating MLC phosphatase (MYPT1) (37,38).
Specifically, activation of Rho-kinase may increase calmodulin
formation, upregulate the concentration of intracellular
Ca2+ and induce the phosphorylation of MYPTl, which
inhibits the activity of MLC phosphatase (MYPT1), causing MLC
phosphorylation (37,39). In the present study, it was
demonstrated that the expression of ROCK1 and ROCK2 were
upregulated, the level of p-MLC and p-MYPT1 was increased, and the
ratio of Bcl-2/Bax was decreased in the I/R group. However, the
expression of ROCK1 and ROCK2 were downregulated, the level of
p-MLC and p-MYPT1 was decreased, and the ratio of Bcl-2/Bax was
increased in the RIPostC group compared with the I/R group.
Interestingly, the aforementioned index in the I/R with fasudil
group was similar to RIPostC group and no significant difference
was observed between RIPostC and I/R+Fas. The results revealed that
RIPostC could downregulate the activity of Rho-kinase as well as
inhibited the occurrence of myocardial apoptosis. These
observations suggested that RIPostC could attenuate the I/R-induced
injury in the myocardium by inhibiting the Rho-kinase signaling
pathway through its anti-apoptotic effect.
There were several limitations in this study that
are important to note. In the present study, it was only shown that
the inhibition of the Rho-kinase was associated with RIPostC, but
it was not investigated how RIPostC inhibits the Rho-kinase
signaling pathway. Furthermore, the present results confirmed that
RIPostC had a cardioprotective and anti-apoptotic effect through
inhibition of the Rho-kinase signaling pathway, however, the study
was not able to examine the Rho-kinase changes with pharmacological
intervention. In addition, further analysis of cardiac function
measurements and the direct evaluation of apoptosis are required to
verify the cardioprotective effect of RIPostC by inhibiting the
Rho-kinase pathway.
In conclusion, the present study demonstrated that
RIPostC could reduce heart infarct size and cardiomyocyte apoptosis
by reducing lipid peroxidation, suppressing oxidative stress and
inhibiting Rho-kinase activity. Thus, the Rho-kinase signaling
pathway may be an important mediator of RIPostC against myocardial
I/R injury in vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by research grants
from the Natural Science Foundation of China (grant no. 81770297),
the Natural Science Foundation of Anhui Province (grant no.
1508085QH150), the Natural Science of the Education Department of
Anhui Province (grant no. KJ2017A217), Outstanding Young Talents
Support Program in Colleges and Universities of Anhui Province
(grant nos. gxfxZD2016143 and gxfx2017062), and the National
College Students Innovation Project (grant no. 201610367011),
China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FM, QG and YY designed the study, performed the
experiments and drafted the manuscript. FN, ZYH, YLH and HJS
performed the experiments and collated the experimental data. XJJ
contributed to experiments, data interpretation and statistical
analysis. All authors revised the manuscript critically for
important intellectual content and gave the final approval of the
version to be published.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Institutional Animal Care and Use Committee of Bengbu Medical
College of China (Bengbu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson L, Oldridge N, Thompson DR,
Zwisler AD, Rees K, Martin N and Taylor RS: Exercise-based cardiac
rehabilitation for coronary heart disease: Cochrane systematic
review and meta-analysis. J Am Coll Cardiol. 67:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu G, Yang P, Zeng Y, Zhang S and Song J:
Danggui Buxue decoction promotes angiogenesis by up-regulation of
VEGFR1/2 expressions and down-regulation of sVEGFR1/2 expression in
myocardial infarction rat. J Chin Med Assoc. 81:37–46. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang H, Wang H, Liu T, Yang Z, Zhang R
and Han H: Co-cultured the MSCs and cardiomyocytes can promote the
growth of cardiomyocytes. Cytotechnology. 70:793–806. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumura K, Jeremy RW, Schaper J and
Becher LC: Progression of myocardial necrosis during reperfusion of
ischemic myocardium. Circulation. 97:795–804. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buja LM and Entman ML: Modes of myocardial
cell injury and cell death in ischemic heart disease. Circulation.
98:1355–1357. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Liu M, Sun R, Zeng Y, Chen S and
Zhang P: Protective approaches against myocardial ischemia
reperfusion injury. Exp Ther Med. 12:3823–3829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kerendi F, Kin H, Halkos ME, Jiang R,
Zatta AJ, Zhao ZQ, Guyton RA and Vinten-Johansen J: Remote
postconditioning. Brief renal ischemia and reperfusion applied
before coronary artery reperfusion reduces myocardial infarct size
via endogenous activation of adenosine receptors. Basic Res
Cardiol. 100:404–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hausenloy DJ and Yellon DM: Ischaemic
conditioning and reperfusion injury. Nat Rev Cardiol. 13:193–209.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang J, Xu D, Guo Q, Ou B, Ling Q, Li J,
Yang Z and Tang W: Remote ischemic postconditioning improves
myocardial dysfunction via the risk and safe pathways in a rat
model of severe hemorrhagic shock. Shock. 49:460–465. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li CM, Zhang XH, Ma XJ and Luo M: Limb
ischemic postconditioning protects myocardium from
ischemia-reperfusion injury. Scand Cardiovasc J. 40:312–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andreka G, Vertesaljai M, Szantho G, Font
G, Piroth Z, Fontos G, Juhasz ED, Szekely L, Szelid Z, Turner MS,
et al: Remote ischaemic postconditioning protects the heart during
acute myocardial infarction in pigs. Heart. 93:749–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deftereos S, Giannopoulos G, Tzalamouras
V, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V,
Karageorgiou S, Avramides D, Toutouzas K, et al: Renoprotective
effect of remote ischemic post-Conditioning by intermittent balloon
inflations in patients undergoing percutaneous coronary
intervention. J Am Coll Cardiol. 61:1949–1955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dezfulian C, Garrett M and Gonzalez NR:
Clinical application of preconditioning and postconditioning to
achieve neuroprotection. Transl Stroke Res. 4:19–24. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu X, Lv T, Yang SF, Zhang XH and Miao YF:
Limb remote ischemic post-conditioning reduces injury and improves
long-term behavioral recovery in rats following subarachnoid
hemorrhage: Possible involvement of the autophagic process. Mol Med
Rep. 17:21–30. 2018.PubMed/NCBI
|
|
15
|
Aimo A, Borrelli C, Giannoni A,
Pastormerlo LE, Barison A, Mirizzi G, Emdin M and Passino C:
Cardioprotection by remote ischemic conditioning: Mechanisms and
clinical evidences. World J Cardiol. 7:621–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liao JK, Seto M and Noma K: Rho kinase
(ROCK) inhibitors. J Cardiovasc Pharmacol. 50:17–24. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Satoh K, Fukumoto Y and Shimokawa H:
Rho-kinase: Important new therapeutic target in cardiovascular
diseases. Am J Physiol Heart Circ Physiol. 301:H287–H296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimokawa H, Sunamura S and Satoh K:
RhoA/Rho-kinase in the cardiovascular system. Circ Res.
118:352–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye HW, Fang TT, Gu XY, Wang Y, Zhu GY, Yu
Y and Gao Q: Role of autophagy in fasudil-induced Rho kinase
inhibition for protection against myocardial ischemia-reperfusion
injury in rats. Nan Fang Yi Ke Da Xue Xue Bao. 36:1706–1711.
2016.(In Chinese). PubMed/NCBI
|
|
20
|
Zhang J, Liu XB, Cheng C, Xu DL, Lu QH and
Ji XP: Rho-kinase inhibition is involved in the activation of
PI3-kinase/Akt during ischemic-preconditioning-ind-uced
cardiomyocyte apoptosis. Int J Clin Exp Med. 7:4107–4114.
2014.PubMed/NCBI
|
|
21
|
Zhang WP, Zong QF, Gao Q, Yu Y, Gu XY,
Wang Y, Li ZH and Ge M: Effects of endomorphin-1 postconditioning
on myocardial ischemia/reperfusion injury and myocardial cell
apoptosis in a rat model. Mol Med Rep. 14:3992–3998. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng XY, Gu XY, Gao Q, Zong QF, Li XH and
Zhang Y: Effects of dexmedetomidine postconditioning on myocardial
ischemia and the role of the PI3K/Akt-dependent signaling pathway
in reperfusion injury. Mol Med Rep. 14:797–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Fan Z, Yang J, Ding J, Yang C and
Chen L: microRNA-22 attenuates myocardial ischemia-reperfusion
injury via an anti-inflammatory mechanism in rats. Exp Ther Med.
12:3249–3255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Jia XJ, Zong QF, Zhang GJ, Ye HW, Hu
J, Gao Q and Guan SD: Remote ischemic postconditioning protects the
heart by upregulating ALDH2 expression levels through the PI3K/Akt
signaling pathway. Mol Med Rep. 10:536–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Zhu W, Tao J, Xin P, Liu M, Li J and
Wei M: Fasudil protects the heart against ischemia-reperfusion
injury by attenuating endoplasmic reticulum stress and modulating
SERCA activity: The differential role for PI3K/Akt and JAK2/STAT3
signaling pathways. PLos One. 7:e481152012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmidt MR, Sloth AD, Johnsen J and Bøtker
HE: Remote ischemic conditioning: The cardiologist's perspective. J
Cardiovasc Med (Hagerstown). 13:667–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liem DA, Verdouw PD and Duncker DJ:
Transient limb ischemia induces remote ischemic preconditioning in
vivo. Circulation. 107:e218–e219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roubille F, Franck-Miclo A, Covinhes A,
Lafont C, Cransac F, Combes S, Vincent A, Fontanaud P,
Sportouch-Dukhan C, Redt-Clouet C, et al: Delayed postconditioning
in the mouse heart in vivo. Circulation. 124:1330–1336. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xin P, Zhu W, Li J, Ma S, Wang L, Liu M,
Li J, Wei M and Redington AN: Combined local ischemic
postconditioning and remote perconditioning recapitulate
cardioprotective effects of local ischemic preconditioning. Am J
Physiol Heart Circ Physiol. 298:H1819–H1831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalogeris T, Bao Y and Korthuis RJ:
Mitochondrial reactive oxygen species: A double edged sword in
ischemia/reperfusion vs preconditioning. Redox Boil. 2:702–714.
2014. View Article : Google Scholar
|
|
31
|
Mythili S and Malathi N: Diagnostic
markers of acute myocardial infarction. Biomed Rep. 3:743–748.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
O'Brien PJ: Cardiac troponin is the most
effective translational safety biomarker for myocardial injury in
cardiotoxicity. Toxicology. 245:206–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao YH, Chen L, Ma YL and He QY: Chronic
intermittent hypoxia aggravates cardiomyocyte apoptosis in rat
ovariectomized model. Chin Med J (Engl). 125:3087–3092.
2012.PubMed/NCBI
|
|
34
|
Bian H, Zhou Y, Yu B, Shang D, Liu F, Li B
and Qi J: Rho-kinase signaling pathway promotes the expression of
PARP to accelerate cardiomyocyte apoptosis in ischemia/reperfusion.
Mol Med Rep. 16:2002–2008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng Y, Tan J, Li H, Kong X, Liu Y, Guo
R, Li G, Yang B and Pei M: Cardioprotective effects of total
flavonoids from Jinhe Yangxin prescription by activating the
PI3K/Akt signaling pathway in myocardial ischemia injury. Biomed
Pharmacother. 98:308–317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiao Y, Fan YF, Wang YL, Zhang JY, Chen S
and Chen ZW: Protective effect and mechanism of total flavones from
rhododendron simsii planch flower on cultured rat cardiomyocytes
with anoxia and reoxygenation. Evid Based Complement Alternat Med.
2015:8635312015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi J and Wei L: Rho kinase in the
regulation of cell death and survival. Arch Immunol Ther Exp
(Warsz). 55:61–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amano M, Ito M, Kimura K, Fukata Y,
Chihara K, Nakano T, Matsuura Y and Kaibuchi K: Phosphorylation and
activation of myosin by Rho-associated kinase (Rho-kinase). J Biol
Chem. 271:20246–20249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Wang X, Liu W and Zhang L: Role of
the Rho/ROCK signaling pathway in the protective effects of fasudil
against acute lung injury in septic rats. Mol Med Rep.
18:4486–4498. 2018.PubMed/NCBI
|