Introduction
Diabetic nephropathy (DN) represents one of the
major chronic complications of diabetes, causing a progressive
decline in glomerular filtration rate (1). In the process of DN initiation and
progression, abnormal activation of the renin angiotensin system
(RAS), both locally and systemically, generates functional
abnormalities in the vasculature. This leads to endothelial
dysfunction and extracellular matrix deposition, contributing to
sclerosis, fibrosis and progressive DN (2,3).
Angiotensin II is known to cause endothelial damage and
dysfunction, which then promotes reactive oxygen species (ROS)
production and NF-κB activation, potentiating hypertension-induced
injury to the glomerular filtration barrier (4,5). Various
studies have demonstrated that angiotensin II is a proinflammatory,
pro-oxidant peptide that contributes substantially to kidney tissue
injury beyond the hemodynamic influences on vascular tone and
intraglomerular pressure (6,7). In turn, angiotensin-converting enzyme
inhibitors (ACEIs) can inhibit the generation of angiotensin II,
thereby effectively blocking ROS, whilst also reducing blood
pressure and urinary protein excretion, delaying the deterioration
of renal function. These effects have been confirmed by a large
number of experiments, and ACEIs have accordingly been used as
basic drugs to treat DN in the clinic (8,9). In
particular, fosinopril (FP) has been revealed to have significant
benefits in the early stages of DN in clinical trials (10). However, the mechanism of action of
ACEIs or FP in mediating renal protection is not yet fully
understood.
Previous studies have demonstrated that the
pathogenesis of DN is very complex (1,11,12).
Notably, one of the best animal models available to study DN is the
Otsuka Long-Evans Tokushima Fatty (OLETF) rat (13). OLETF rats can spontaneously develop
late-onset hyperphagia, mild obesity, late-onset hyperglycemia,
hypertension, dyslipidemia and advanced DN. At 12–20 weeks of age,
OLETF rats exhibit mild obesity and hyperinsulinemia. Late-onset
hyperglycemia is noted by 18 weeks of age. At 22 weeks of age,
OLETF rats develop overt albuminuria and at the age of 54 weeks,
advanced renal changes such as macroalbuminuria, nodular lesions,
diffuse glomerulosclerosis and tubulointerstitial fibrosis are
present, which is comparable to the symptoms of human DN (14). Long-Evans Tokushima Otsuka (LETO)
rats are available as a normal control for OLETF rats, as they
carry a similar genetic background but do not spontaneously develop
diabetes (15).
Therefore, to investigate the mechanisms of action
of ACEIs in the treatment of DN, the present study examined the
effects of the ACEI FP on protein expression in the renal cortex of
OLETF rat using mass spectrometry-based profiling. By incorporating
a research model with a consistent genetic background, the
experimental design reduced the difficulty of protein analysis
inherent in a natural population with obvious heterogeneity. In
addition, kidney cortex proteins were separated into soluble and
insoluble protein fractions to further overcome the complexity of
the OLETF rat kidney cortex proteome. The results of the current
study may elucidate novel concepts regarding the underlying
mechanism of FP in DN.
Materials and methods
Animals and experimental design
Four-week-old male LETO rats (n=18; weight, 164±6 g)
and age-matched OLETF rats (n=24; weight, 207±19 g) that were
provided by the Tokushima Research Institute (Otsuka
Pharmaceutical). All rats were housed at 22±3°C and 50±10% humidity
using a 12-h light/dark cycle. All animals were given free access
to standard rat chow and water.
Three groups of rats at 12 weeks of age were
prepared as follows: i) Non-diabetic LETO rats provided with
distilled water (LETO control; n=18); ii) diabetic OLETF rats
provided with distilled water (OLETF control; n=12); and iii) OLETF
rats administered FP (Monopril; Bristol-Myers Squibb) at 0.833
mg/kg body weight/day (OLETF + FP; n=12). FP was dissolved in
distilled water and administered once daily by intragastric
administration. At 36 weeks of age, 10 rats from the LETO control
group and 7 rats from the other two groups were euthanized with an
intraperitoneal injection of pentobarbital (200 mg/kg). The
remaining rats in each group (8 LETO controls and 5 from each of
the other groups) were sacrificed at 56 weeks of age. After the
rats were sacrificed, the kidneys were removed and separated into
two pieces for histopathological examination and proteomic
analysis. The present study was approved by the Ethics Committee of
the China-Japan Friendship Institute of Clinical Medicine (no.
ZR05016) and performed in accordance with the Guiding Principles
for the Care and Use of Laboratory Animals (16).
MTT assay
An MTT assay was performed to analyze whether FP
damaged renal tubular NRK-52E cells (Cell Resource Center of
Shanghai Institutes for Biological Science) under normal and high
glucose conditions. Cells were seeded into 96-well plates
(2×103 cells/well) in 200 µl culture medium (DMEM
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. 31600-034)
supplemented with 5% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.; 16000-044)) treated with different FP
concentrations (0, 0.05, 0.10, 0.20, 0.41, 0.83, 1.66, 3.33, 6.66,
13.32 and 26.65 mg/ml) under normal (5 mM) and high (30 mM) glucose
media for 48 h at 37°C. Following treatment, cells were washed
twice with PBS and 20 µl MTT (2 mg/ml) was subsequently added into
each well. Cells were further incubated for 4 h at 37°C, after
which DMSO was added into each well to dissolve formazan crystals.
Absorption was determined at a wavelength 490 nm using a Bio-Rad
680 microplate reader (Bio-Rad Laboratories, Inc.).
Determination of body weight, blood
glucose and urinary protein
The body weights of the rats were measured at 4-week
intervals. Blood samples from the tail vein were taken at 4-week
intervals and blood glucose levels were measured using a One Touch
Ultrablood glucose monitoring system (LifeScan, Inc.). Rats were
housed individually in metabolic cages (Suzhou Fengshi Laboratory
Animal Equipment Co., Ltd.) for urinary collection for 24 h at
4-week intervals.
Histological examination of the
kidney
Sections of kidney tissue were removed and
immediately fixed in 10% phosphate buffered formalin solution at
room temperature for 24 h and embedded in paraffin for further
histological and immunohistochemical analysis. Sections (3 µm) from
each sample were cut and stained with periodic acid Schiff's stain
for evaluation of the degree of glomerulosclerosis, defined as a
thickening of the basement membrane and mesangial expansion from 40
glomeruli in each section at ×400 magnification (17). Tubulointerstitial and vascular damage
were assessed on periodic acid Schiff-stained paraffin sections at
×100 magnification using a similar scoring system (0–4) as
previously described (18). The
observer was blinded to all tissue samples.
Immunohistochemistry
Kidney injury molecule-1 (KIM-1) immunohistochemical
staining was performed to analyze whether FP caused OLETF renal
damage. All procedures were performed in accordance with the
Elivision™ Plus Two-step System PV-6000 kit protocol (Zymed; Thermo
Fisher Scientific, Inc.; cat. no. PV6000). Paraffin sections (3 µm
thick) as aforementioned were deparaffinized in xylene and
rehydrated in descending alcohol series, after which endogenous
enzyme activity was blocked via incubation in 3% hydrogen peroxide
for 10 min at room temperature. Antigen retrieval was performed by
incubating samples with 0.01 mol/l sodium citrate buffer (pH 6.0)
at 115°C for 1.5 min. Tissue sections were subsequently incubated
with primary polyclonal antibodies against KIM-1 (Boster Biological
Technology; cat. no. BA35317; 1:100) overnight at 4°C. After
washing with PBS, sections were incubated with secondary antibodies
(included in the Dako Elivision™ Plus Two-step System PV-6000 kit;
Zymed; Thermo Fisher Scientific, Inc,) for 25 min at 37°C. The
signal was subsequently visualized using the Diaminobenzidine.
Following counterstaining with hematoxylin for 15 min at room
temperature, positive immunostaining was scored by two blinded
experienced pathologists. Immunohistochemistry results were
evaluated using an Olympus BX53 upright light microscope and scored
by examining 10 random representative fields at a 400×
magnification. The staining intensity of each visual field was
graded from 0 to 3+, as descried by Zhang (19).
Extraction of renal cortical soluble
and insoluble protein fractions
The method of extraction of soluble and insoluble
protein was based on a previously described protocol with some
modifications (20). Briefly, the
renal cortical tissue samples from the LETO, OLETF and OLETF
treated with FP groups were ground into powder in liquid nitrogen
individually. Soluble lysis buffer [40 mM Tris, pH 7.5; 1%
dithiothreitol; 1% IPG buffer, pH 3–10; and protease inhibitor
cocktail (Roche Diagnostics)] were added to dissolve the powder,
and the mixture was then centrifuged at 40,000 × g at 4°C for 30
min to separate the soluble and insoluble fractions. The soluble
fraction was further centrifuged at 40,000 × g at 4°C for 30 min to
separate out any remaining insoluble content. The insoluble
fraction was washed twice with soluble lysis buffer (details as
aforementioned) to wash out any soluble proteins. The insoluble
pellet was suspended in insoluble lysis buffer (40 mM Tris, pH 7.5;
7 M urea; 2 M thiourea; 4% CHAPS; 1% dithiothreitol; 1% IPG buffer,
pH 3–10; and complete protease inhibitor cocktail) and then
subjected to ultrasound homogenization at 5 sec pulsed intervals at
20 kHz for 1 min on ice. The homogenized pellet was then put on ice
for 30 min and the solution was spun at 40,000 × g at 14°C for 30
min to separate out any insoluble content. Protein concentration
was determined using the Bradford protein assay on a GeneQuant 1300
spectrometer (GE Healthcare Life Sciences). The protein extracts
were stored at −80°C for subsequent two-dimensional electrophoresis
(2-DE) analysis.
2-DE and matrix assisted laser
desorption/ionization time-of-flight mass spectrometry (MALDI-TOF
MS)
Each prepared soluble and insoluble protein was
separated by 2-DE individually and the differentially expressed
proteins were identified by MALDI-TOF MS using a method described
previously (21). In brief, samples
of 1.3 mg soluble or insoluble protein were loaded on an
immobilized pH gradient gel strip (pH 3–10 NL, 24 cm; GE
Healthcare) using the in-gel rehydration mode. Proteins were
separated by first dimensional separation (Ettan IPGphor 3
Isoelectric Focusing Unit; GE Healthcare), and 13% total monomer
concentration and 3% weight percentage of crosslinker second
dimensional separation (Ettan DALT II system; GE Healthcare). The
separated protein spots were visualized using colloidal Coomassie
blue staining according to the manufacturer's protocol (GE
Healthcare), and the resultant images were analyzed using
ImageMaster 2D Platinum 6.0 software (GE Healthcare), according to
manufacturer's protocol. Statistical analysis was carried out using
the t-test and false discovery rate as described by Biron et
al (22). The differentially
expressed protein spots were cut manually from the gels with a
stainless-steel scalpel. The in-gel digestion and MALDI-TOF MS of
each excised protein spot were performed by the National Center of
Biomedical Analysis (Beijing, China).
Database search
The obtained MS data were identified by using the
Mascot search engine (www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH=PMF)
according to the specific peptide mass fingerprints (PMF).
Identified proteins were further checked for reliability following
the criteria by Biron et al (22) as follows: i) molecular weight search
Score >60 (calculated as −10*Log (P); P<0.05, default
threshold); ii) proportion of the theoretical sequence of the
protein covered by MS data ≥20%; and iii) compared with theoretical
values, a molecular mass variation <30% and isoelectric point
variation <2.0. Each identified candidate protein entry was
traced to its corresponding official gene name using the Protein
Information Resource (Georgetown University Medical Center).
Functional classification of
differentially expressed proteins
To obtain an overview of the differentially
expressed protein functions of the OLETF rat kidney cortex, the
online tool DAVID Bioinformatics Resources 6.7 (https://david-d.ncifcrf.gov/) was used to perform
enrichment analysis using gene ontology (GO) terms and KEGG
pathways (23). The threshold was
set as P<0.05 to explore the overrepresentation of biological
terms and signaling pathways.
Western blot analysis
As not all identified proteins had commercial
antibodies and due to economic limitations, heat shock protein
family A member 9 (Hspa9) and glutathione peroxidase 3 (Gpx3) were
selected as representative proteins and examined by western blot
analysis to confirm their expression alterations. Protein samples
from 2-DE analysis (15 µg) were separated via SDS-PAGE using a 12%
polyacrylamide gel and electro-transferred to a nitrocellulose
membrane in a Trans-Blot® Semi-dry Electrophoretic
Transfer Cell (Bio-Rad Laboratories, Inc.). Nonspecific bands were
blocked in TBS-T (25 mM Tris, 150 mM NaCl and 0.05% Tween-20; pH
7.5) containing 5% skimmed milk at room temperature for 1 h.
Membranes were subsequently incubated with a primary antibody
against Hspa9 (Santa Cruz Biotechnology, Inc.; 1:1,000; cat. no.
SC-133137), Gpx3 (Abcam; 1:1,000; cat. no. ab256470) and β-actin
(Tianjin Sungene Biotech Co.; 1:5,000; cat. no. KM9001) overnight
at 4°C followed by incubation with an anti-mouse (Jackson
ImmunoResearch Laboratories, Inc.; 1:3,000; cat. no. 115-035-003)
or anti-rabbit IgG (Jackson, 1:3,000; cat. no. 111-035-003)
horseradish peroxidase-conjugated secondary antibodies at room
temperature for 1 h. The immunocomplexes were visualized by
enhanced chemiluminescence using the Amersham ECL Western Blotting
Detection kit (GE Healthcare). The signals were acquired by the
chemiDoc™XR+ molecular imager (Bio-Rad Laboratories, Inc.) and then
quantified using Quantity-One 4.31 software (Bio-Rad Laboratories,
Inc.).
Statistical analysis
SigmaPlot 12.5 (Systat Software, Inc.) was used to
analyze data and determine statistical differences. Each experiment
was at independently replicated 3 times and data were presented as
the mean ± standard deviation. Data that met the criteria for
parametric tests were analyzed by Student's t-test (two groups) or
by a one-way ANOVA and a subsequent Bonferroni post-hoc test (more
than two groups). Groups of data that failed tests for normality
and equal variance were analyzed by the nonparametric
Kruskal-Wallis test followed by Dunn's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
FP treatment lowers 24-h urinary
protein levels in OLETF rats
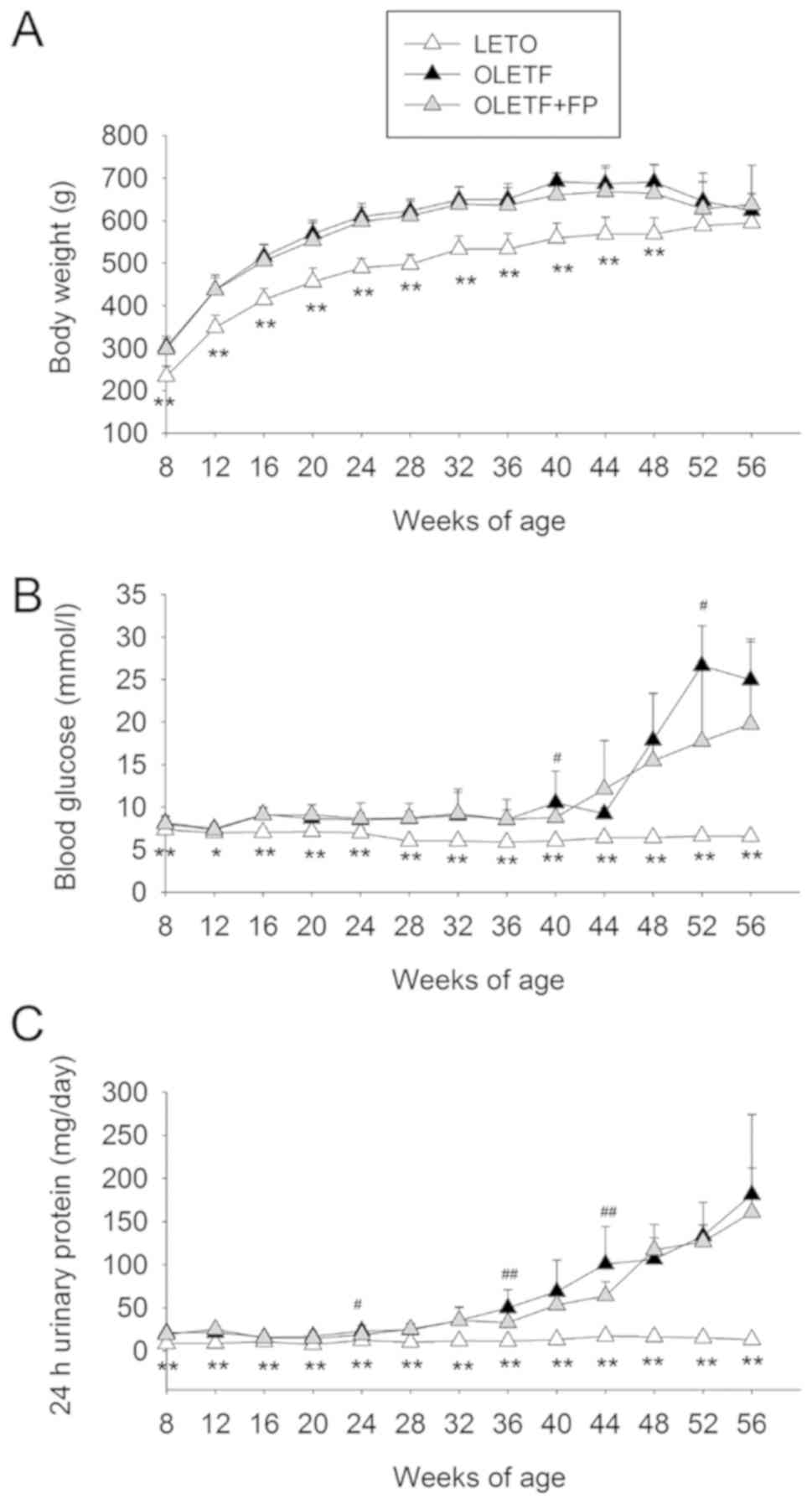
The body weights of OLETF rats were greater than
those of LETO rats between 12 and 48 weeks; however, no significant
differences were observed when compared with OLETF rats treated
with FP. In addition, at weeks 52 and 56, body weight did not
differ significantly among the groups (Fig. 1A). Blood glucose levels for the LETO
control rats did not change throughout the study period. Over the
same period of time, age-matched OLETF rat blood glucose levels
were statistically higher. The blood glucose levels of OLETF rats
treated with FP also increased during the study period, with levels
significantly different from those of the OLETF control group at
weeks 40 and 52 (Fig. 1B). Urinary
protein levels in all OLETF rats gradually and continually
increased during the entire study period compared with the levels
in the LETO rats, which remained unchanged. OLETF rats treated with
FP exhibited significantly lower 24-h urinary protein levels than
those exhibited by the OLETF control group at weeks 24, 36 and 44
(Fig. 1C).
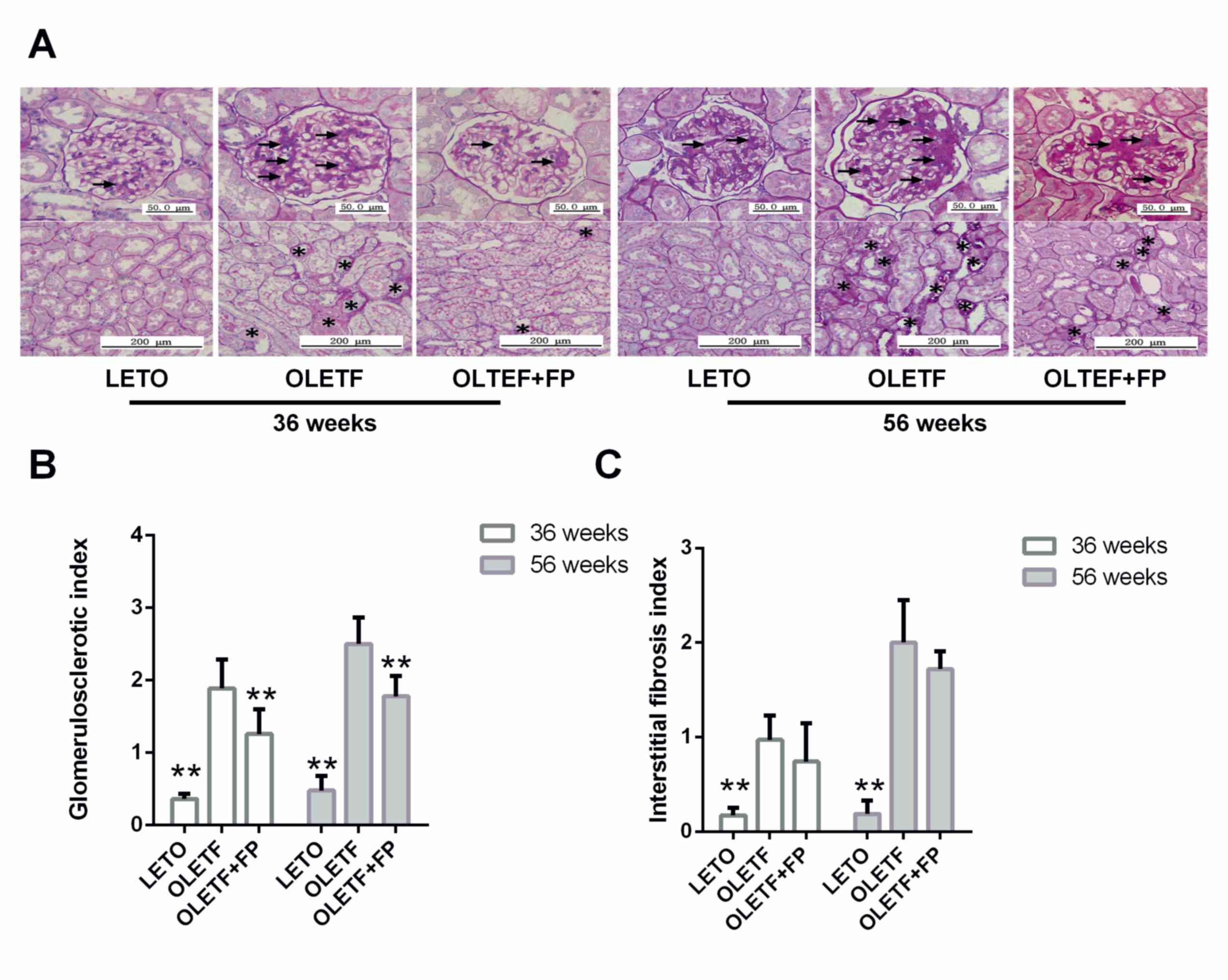
FP treatment ameliorates histological
kidney changes in OLETF rats
Glomerular lesions in OLETF rats were characterized
by hyalinosis, thickening of the basement membrane, mesangial
expansion and sclerosis (Fig. 2A).
These pathological changes were more severe at 56 weeks of age than
at 36 weeks. Interstitial fibrosis in OLETF rats was focal and mild
at 36 weeks of age, whereas at 56 weeks of age severe changes
occurred that included protein cast, tubular dilation or atrophy
and infiltration of inflammatory cells (Fig. 2A). Treatment with FP significantly
ameliorated glomerulosclerosis in OLETF rats at both 36 and 56
weeks of age (Fig. 2B), but was
unable to attenuate the increase in interstitial fibrosis (Fig. 2C).
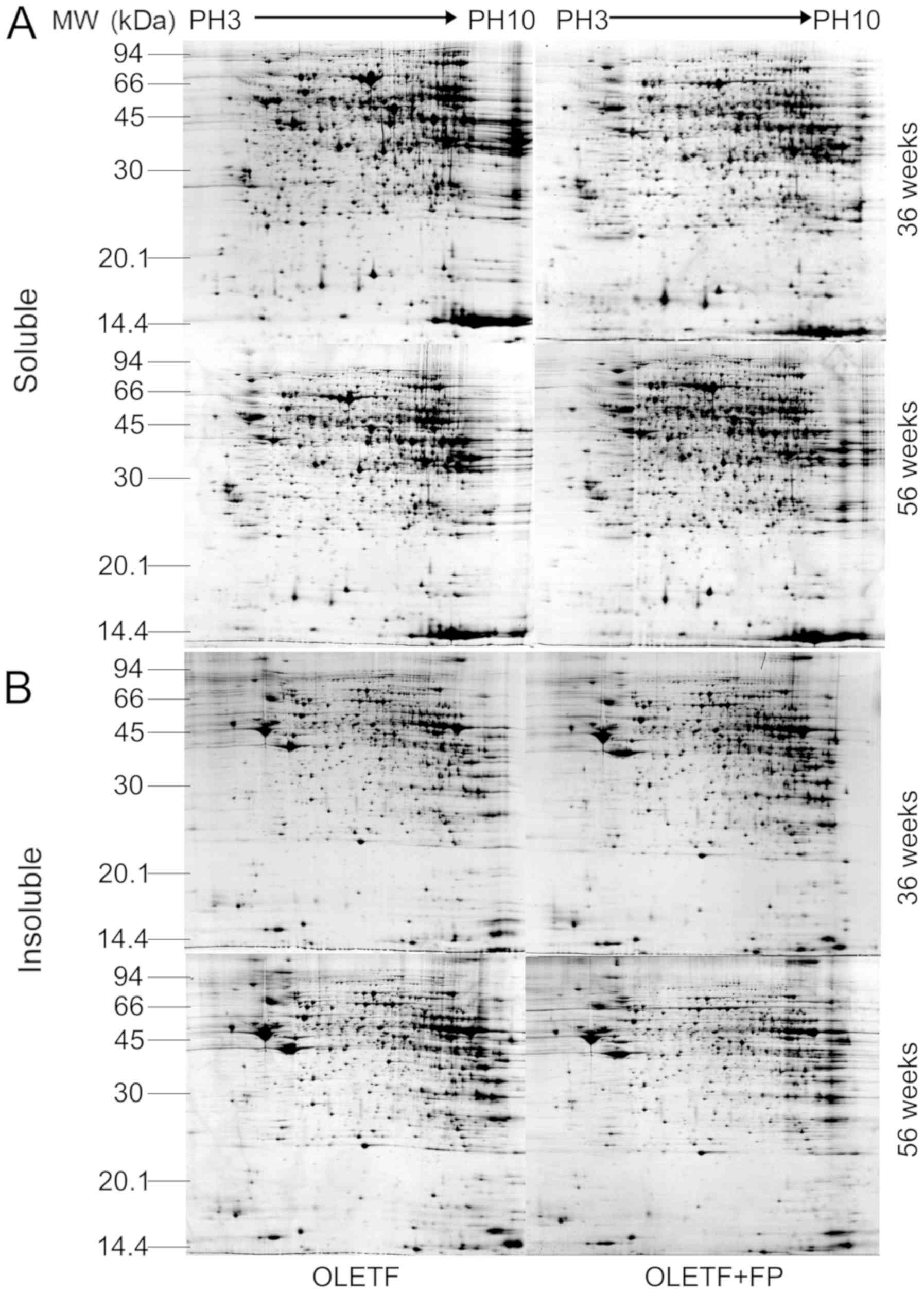
Comparative proteomic analysis of
renal cortices from untreated and FP-treated OLETF rats
Fig. 3 displays
representative 2-DE patterns of soluble and insoluble renal cortex
proteins from untreated and FP-treated OLETF rats. Comparison of
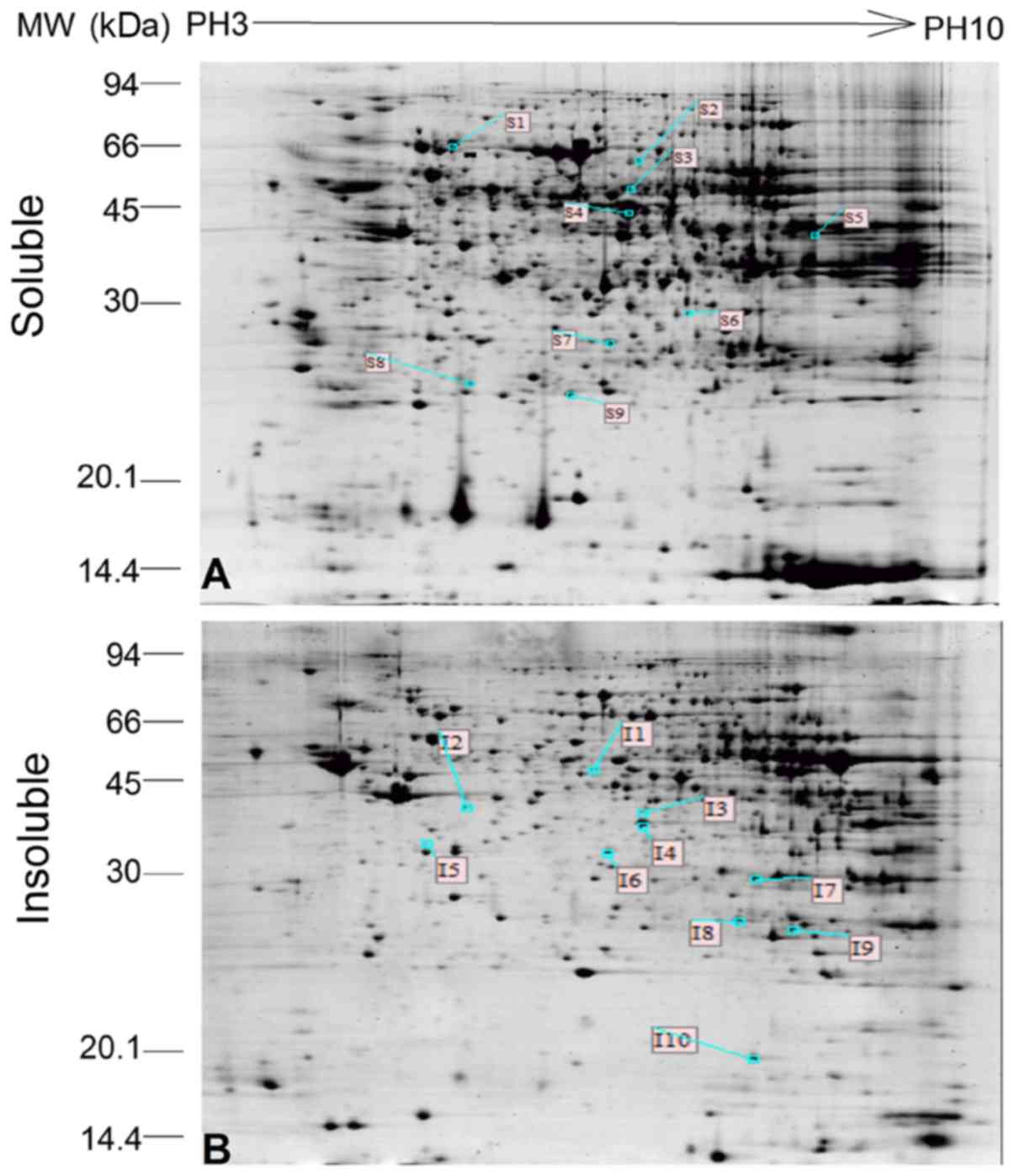
the protein profiles from the two groups identified that 9 protein
spots (S1-S9) were differentially expressed in the soluble
subproteome and 10 protein spots (I1-I10) were differentially
expressed in the insoluble subproteome (Fig. 4). These protein spots were submitted
for MALDI-TOF MS analysis. All protein spots were successfully
isolated and their PMF, protein name and related data were obtained
in the corresponding database and are listed in Table I. Certain different protein spots
were identified as the same protein, likely because of different
translational or post-translational modifications leading to
changes in physicochemical characteristics during protein
expression, resulting in their separation into different protein
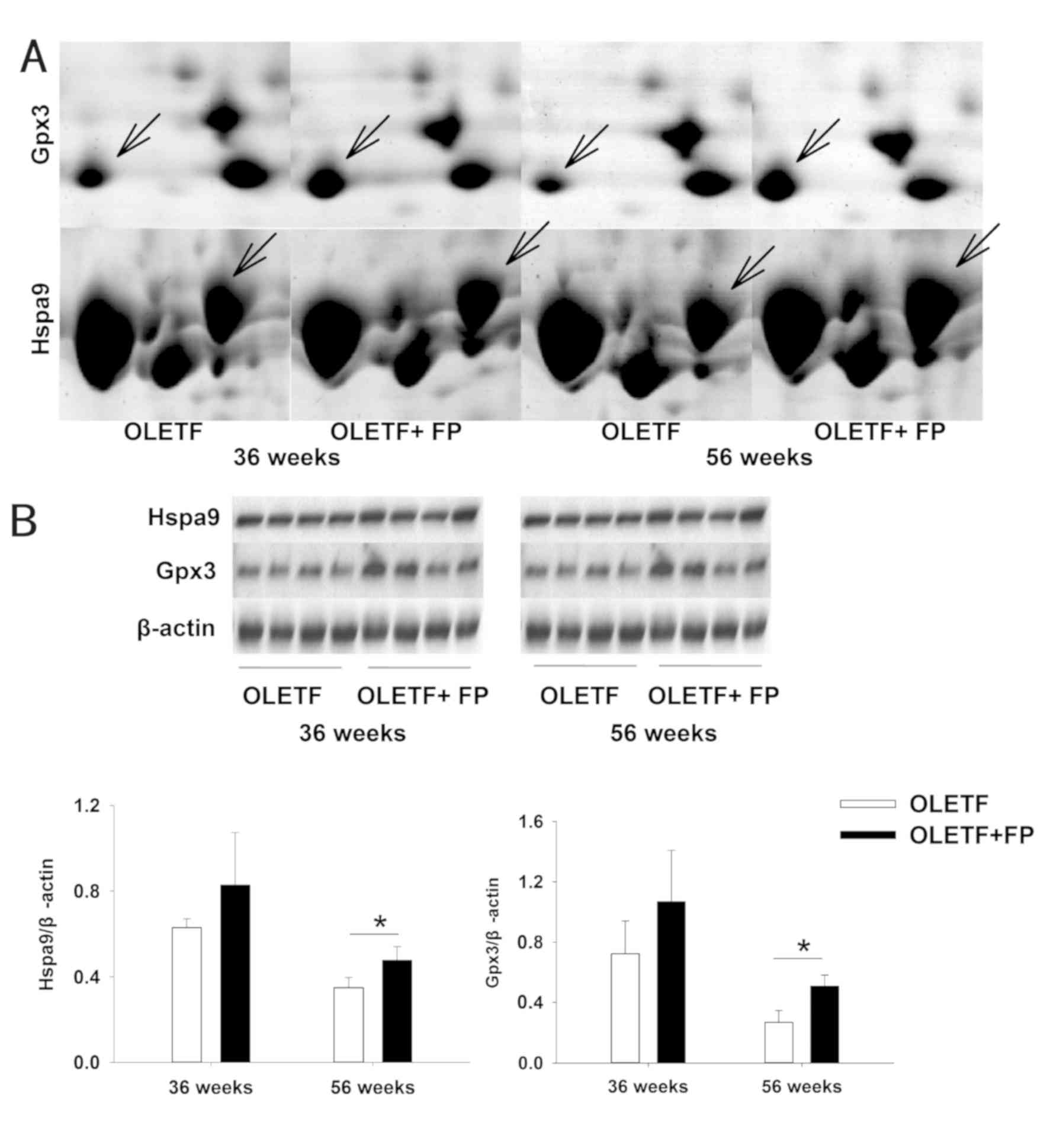
spots in 2-DE. The differentially expression of Hspa9 and Gpx3 were
further validated by western blot analysis. As shown in Fig. 5, the expression levels of Hspa9 and
Gpx3 at 56 weeks were similar to those observed in the 2-DE gels.
The abundances of the proteins increased significantly in the renal
cortex of OLETF rats treated with FP at 56 weeks.
 | Table I.Effect of fosinopril on the renal
cortex protein expression profile of OLETF rats. |
Table I.
Effect of fosinopril on the renal
cortex protein expression profile of OLETF rats.
| Spot no. | GI no. | Protein name | Gene name | Mass | Sequence coverage
(%) | MOWSE score | Age, weeks | Protein
abundance |
|---|
| S1 | gi|1000439 | grp75 | Hspa9 | 32/48 | 46 | 239 | 36 | N |
|
|
|
|
|
|
|
| 56 | ↑ |
| S2 | gi|40254595 |
Dihydropyrimidinase- | Dpysl2 | 19/57 | 43 | 108 | 36 | ↓ |
|
|
| related protein
2 |
|
|
|
| 56 | ↓ |
| S3 | gi|149030730 | Selenium
binding | Selenbp1 | 11/35 | 35 | 88 | 36 | ↓ |
|
|
| protein 2 |
|
|
|
| 56 | ↓ |
| S4 | gi|158186649 | α-enolase | Eno1 | 26/46 | 66 | 222 | 36 | ↓ |
|
|
|
|
|
|
|
| 56 | N |
| S5 | gi|40254752 |
Phosphoglycerate | Pgk1 | 21/33 | 56 | 204 | 36 | N |
|
|
| kinase 1 |
|
|
|
| 56 | ↓ |
| S6 | gi|157823267 |
S-formylglutathione | Esd | 9/39 | 33 | 62 | 36 | N |
|
|
| hydrolase isoform
a |
|
|
|
| 56 | ↑ |
| S7 | gi|51491893 | Phenazine
biosynthesis- | Pbld1 | 10/21 | 32 | 126 | 36 | ↓ |
|
|
| like
domain-containing protein |
|
|
|
| 56 | ↓ |
| S8 | gi|6978515 | Apolipoprotein
A-I | Apoa1 | 14/59 | 48 | 83 | 36 | N |
|
|
| preproprotein |
|
|
|
| 56 | ↑ |
| S9 | gi|6723180 | Plasma
glutathione | Gpx3 | 15/21 | 42 | 136 | 36 | ↑ |
|
|
| peroxidase
precursor |
|
|
|
| 56 | ↑ |
| I1 | gi|158186649 | α-enolase | Eno1 | 8/16 | 27 | 80 | 36 | N |
|
|
|
|
|
|
|
| 56 | ↑ |
| I2 | gi|51036635 | Fructose-1, | Fbp1 | 13/19 | 33 | 144 | 36 | N |
|
|
| 6-bisphosphatase
1 |
|
|
|
| 56 | ↑ |
| I3 | gi|170295834 | NADH
dehydrogenase | Ndufa10 | 15/26 | 31 | 175 | 36 | ↑ |
|
|
| [ubiquinone] 1
alpha subcomplex subunit 10, mitochondrial precursor |
|
|
|
| 56 | ↑ |
| I4 | gi|170295834 | NADH
dehydrogenase | Ndufa10 | 17/24 | 56 | 241 | 36 | ↓ |
|
|
| [ubiquinone] 1
alpha subcomplex subunit 10, mitochondrial precursor |
|
|
|
| 56 | N |
| I5 | gi|56090293 | Pyruvate
Dehydrogenase | Pdhb | 16/33 | 34 | 108 | 36 | ↑ |
|
|
| E1 component
subunit beta, mitochondrial precursor |
|
|
|
| 56 | N |
| I6 | gi|15100179 | Malate
dehydrogenase, | Mdh1 | 10/16 | 30 | 128 | 36 | N |
|
|
| cytoplasmic isoform
Mdh1 |
|
|
|
| 56 | ↑ |
| I7 | gi|57527204 | Electron
transfer | Etfa | 20/54 | 58 | 168 | 36 | N |
|
|
| flavoprotein
subunit alpha, mitochondrial precursor |
|
|
|
| 56 | ↓ |
| I8 | gi|24159081 | Chain A, crystal
structure | Echs1 | 21/40 | 56 | 158 | 36 | N |
|
|
| analysis of rat
enoyl-Coa hydratase in complex with hexadienoyl-Coa |
|
|
|
| 56 | ↓ |
| I9 | gi|68163417 | Acylpyruvase
FAHD1, | Fahd1 | 10/17 | 32 | 90 | 36 | ↓ |
|
|
| mitochondrial |
|
|
|
| 56 | ↓ |
| I10 | gi|55926145 | Nucleoside
diphosphate | Nme2 | 8/12 | 55 | 102 | 36 | N |
|
|
| kinase B |
|
|
|
| 56 | ↓ |
DAVID analysis revealed over-representation of
pathways and terms (Table II) in
the differentially expressed proteins from this tissue.
Specifically, ‘glycolysis/gluconeogenesis’ was over-represented
among the identified KEGG pathways. ‘Generation of precursor
metabolites’, ‘energy, glucose metabolic process’ and ‘oxidation
reduction’ were over-represented in biological processes.
Furthermore, differentially expressed proteins were
over-represented in the mitochondrion and soluble fraction. Within
these, there was over-representation of molecular functions
including ‘magnesium ion binding’, ‘selenium binding’ and
‘hydro-lyase activity’.
 | Table II.DAVID analysis of differentially
expressed proteins in the kidney cortices of untreated and
fosinopril-treated OLETF rats. |
Table II.
DAVID analysis of differentially
expressed proteins in the kidney cortices of untreated and
fosinopril-treated OLETF rats.
| Category | Term | Genes | Count | Percentage | P-value |
|---|
| KEGG pathway |
Glycolysis/gluconeogenesis | Fbp1, Pgk1, Pdhb,
Eno1 | 4 | 25 | 0.0005 |
| Biological process
metabolites and energy | Generation of
precursor | Ndufa10, Pgk1,
Pdhb, Eno1, Etfa, Mdh1 | 6 | 37.5 | <0.0001 |
| Biological
process | Glycolysis | Pgk1, Pdhb, Eno1,
Mdh1 | 4 | 25 | <0.0001 |
| Biological
process | Glucose metabolic
process | Fbp1, Pgk1, Pdhb,
Eno1, Mdh1 | 5 | 31.25 | <0.0001 |
| Biological
process | Monosaccharide
catabolic process | Pgk1, Pdhb, Eno1,
Mdh1 | 4 | 25 | <0.0001 |
| Biological
process | Hexose metabolic
process | Fbp1, Pgk1, Pdhb,
Eno1, Mdh1 | 5 | 31.25 | <0.0001 |
| Biological
process | Alcohol catabolic
process | Pgk1, Pdhb, Eno1,
Mdh1 | 4 | 25 | 0.0001 |
| Biological
process | Cellular
carbohydrate catabolic process | Pgk1, Pdhb, Eno1,
Mdh1 | 4 | 25 | 0.0001 |
| Biological
process | Monosaccharide
metabolic process | Fbp1, Pgk1, Pdhb,
Eno1, Mdh1 | 5 | 31.25 | 0.0001 |
| Biological
process | Carbohydrate
catabolic process | Pgk1, Pdhb, Eno1,
Mdh1 | 4 | 25 | 0.0001 |
| Biological
process | Pyruvate metabolic
process | Fbp1, Pgk1,
Pdhb | 3 | 18.75 | 0.0010 |
| Biological
process | Oxidation
reduction | Gpx3, Ndufa10,
Pdhb, Etfa, Mdh1 | 5 | 31.25 | 0.0032 |
| Biological
process | Coenzyme metabolic
process | Gpx3, Pdhb,
Mdh1 | 3 | 18.75 | 0.0123 |
| Biological
process | Cofactor metabolic
process | Gpx3, Pdhb,
Mdh1 | 3 | 18.75 | 0.0192 |
| Biological
process |
Gluconeogenesis | Fbp1, Pgk1 | 2 | 12.5 | 0.0245 |
| Biological
process | Hexose biosynthetic
process | Fbp1, Pgk1 | 2 | 12.5 | 0.0287 |
| Biological
process | Monosaccharide
biosynthetic process | Fbp1, Pgk1 | 2 | 12.5 | 0.0370 |
| Biological
process | Acetyl-CoA
metabolic process | Pdhb, Mdh1 | 2 | 12.5 | 0.0401 |
| Biological
process | Alcohol
biosynthetic process | Fbp1, Pgk1 | 2 | 12.5 | 0.0463 |
| Biological
process | Response to
drug | Apoa1, Gpx3,
Ndufa10 | 3 | 18.75 | 0.0467 |
| Subcellular
localization | Mitochondrial
matrix | Echs1, Ndufa10,
Pdhb, Etfa, Hspa9 | 5 | 31.25 | 0.0001 |
| Subcellular
localization | Mitochondrial
lumen | Echs1, Ndufa10,
Pdhb, Etfa, Hspa9 | 5 | 31.25 | 0.0001 |
| Subcellular
localization | Mitochondrion | Fahd1, Nme2, Echs1,
Dpysl2, Ndufa10, Pdhb, Etfa, Mdh1, Hspa9 | 9 | 56.25 | 0.0001 |
| Subcellular
localization | Soluble
fraction | Gpx3, Fbp1, Pgk1,
Eno1, Mdh1 | 5 | 31.25 | 0.0006 |
| Subcellular
localization | Intracellular
organelle lumen | Apoa1, Echs1,
Ndufa10, Pdhb, Etfa, Hspa9 | 6 | 37.5 | 0.0182 |
| Subcellular
localization | Organelle
lumen | Apoa1, Echs1,
Ndufa10, Pdhb, Etfa, Hspa9 | 6 | 37.5 | 0.0210 |
| Subcellular
localization | Membrane-enclosed
lumen | Apoa1, Echs1,
Ndufa10, Pdhb, Etfa, Hspa9 | 6 | 37.5 | 0.0235 |
| Molecular
function | Magnesium ion
binding | Fahd1, Nme2, Fbp1,
Eno1 | 4 | 25 | 0.0044 |
| Molecular
function | Selenium
binding | Gpx3, Selenbp1 | 2 | 12.5 | 0.0209 |
| Molecular
function | Hydro-lyase
activity | Echs1, Eno1 | 2 | 12.5 | 0.0436 |
Discussion
DN is a main cause of end-stage renal disease that
currently has no effective treatment to block or reverse its
pathological process. A number of clinical and basic studies have
investigated ACEIs/angiotensin II receptor blockers (24–26) for
the treatment of DN to delay the progression of the disease, and
these drugs have been recommended by the Kidney Disease Outcomes
Quality Initiative as first-line clinical treatments (27). However, studies have identified that
ACEIs demonstrate some nephrotoxicity since ACEIs, including FP,
may cause an exaggerated or progressive decline in renal function
in conditions such as renal-artery stenosis (28,29) and
polycystic kidney disease (30), due
to their preferential vasodilation of the renal efferent
arteriole.
The present study determined that the ACEI drug FP
reduced the pathological damage to renal tissue in OLETF rats and
reduced urinary protein excretion. FP significantly inhibited the
expression of KIM-1, an early marker of renal injury in OLETF rats
(Fig. S2), indicating that FP
exerts good renoprotective effects. In vitro experiments
also confirmed that FP had a significant effect on the
proliferation of NRK-52E cells at 13.32 µg/ml, which was ~119 times
the maximum blood concentration in human (112±8 ng/ml; Fig. S1) (31), suggesting that FP improves diabetic
kidney injury with no obvious nephrotoxic effects.
In the development of DN, proteinuria not only
serves as an important marker for evaluating pathological
progression but also participates in the development of renal
fibrosis (32). Albumin and other
proteins that appear in urine due to abnormal filtration, can
interact with the glomerular mesangium and proximal tubule
(33), which activates a wide array
of intracellular signaling pathways, such as the ERK, NF-κB and
protein kinase C pathways (34).
This induces fibrotic substances, such as transforming growth
factor-β, collagens, C-C motif chemokine 2 and metalloproteinase
inhibitor 1 (33,35,36), and
ultimately leads to fibrosis and loss of renal function (37). The present results demonstrated that
FP alleviated proteinuria in OLETF rats, suggesting that this may
have a role in improving diabetic glomerular sclerosis and
tubulointerstitial fibrosis. Subsequent experiments investigating
the renal cortex proteomics profile in OLETF rats determined that
FP treatment resulted in significant changes in the abundance of 17
proteins, including Hspa9 and Gpx3. Bioinformatics analysis
indicated that the function of these differentially expressed
proteins likely participated in biological processes such as
oxidative stress and glucose metabolism. Therefore, FP may have a
renoprotective role by increasing the expression of Hspa9 to
inhibit oxidative stress, consequently reducing tissue and cell
damage.
In the present study, compared with the OLETF
control, treatment with FP caused a significant increase in the
abundance of the plasma glutathione peroxidase precursor Gpx3. Gpx
comprises an important part of the antioxidant mechanism in body
(38,39) and is actively expressed in human
kidney proximal tubule cells (40).
Previous research determined that hyperglycemia induces the
overproduction of superoxide by the mitochondrial electron
transport chain, leading to an increase in ROS greater than the
antioxidant capacity of the body, resulting in cell and tissue
damage (12). In addition, high
glucose can stimulate 12-lipoxygenasein podocytes, which also leads
to oxidative stress through the generation of superoxide (41). Based on such observations, Brownlee
(12) proposed that the
overproduction of superoxide was likely to be a common mechanism
for the pathogenesis of diabetic complications. Consistent with
this, angiotensin II has been shown to induce ROS production and
the activation of NF-κB and mitogen activated protein kinase in
diabetic glomeruli and mesangial cells (34). As angiotensin II is an important
medium of oxidative stress, studies have accordingly begun to
explore the use of ACEIs for reducing oxidative stress levels in
patients with DN. Notably, these trials have demonstrated that
ACEIs and angiotensin II receptor blockers are able to reduce
oxidative stress and inflammation in patients with DN and that this
effect is independent of the antihypertensive effect (42,43). It
has been suggested that the specific mechanism may involve a
reduction of oxidative stress-associated damage by blocking the
NADPH oxidase pathway (44) by
decreasing the elevation in ROS generation induced by
platelet-derived growth factor (45). Another potential mechanism might
involve attenuating apoptosis and intracellular ROS formation
induced by angiotensin II through modulation of the toll like
receptor 4/MYD88 innate immune signal transduction adaptor pathway
(46). Furthermore, previous studies
determined that Gpx3 interacts with podocin, which is used as a
marker of podocytes (47,48) and is associated with microalbuminuria
in DN (49). Taken together, Gpx3
may serve an important role in the development of DN. In the
present study, it was determined that FP treatment increased the
Gpx3 precursor concentration in the renal cortex of OLETF rats.
These results suggested that FP could inhibit the development of DN
by stimulating increased Gpx3 expression.
The results of the present study also indicated that
FP affected the expression of Hspa9, which has begun to attract
attention regarding its role in the pathogenesis of DN (50). Hspa9 also known as glucose regulated
protein 75, mortalin, mitochondria heat shock protein 70 and
peptide binding protein 74, is mainly localized in the
mitochondria, where it functions as a molecular chaperone, serving
a central role in the elaborate translocation system for the
efficient import and export of proteins and maintaining the
function of the mitochondria (51).
Mutations in Hspa9 cause the degeneration of nerve cells and have a
role in the pathogenesis of Parkinson's disease (52). Hspa9 binds to p53 protein in the
cytoplasm and inhibits its function acting as an anti-apoptotic
factor. Consistent with this, Hspa9 is found to be upregulated in
many human cancer types, suggesting its association with the
occurrence and metastasis of malignant tumors (53). In addition, high levels of Hspa9
expression can protect the cell from a variety of stress-induced
damage types, reducing the effects of various physical and chemical
factors. For example, it has been shown that Hspa9 overexpression
can reduce the damage to cells mediated by glucose deprivation and
that the protective effect may be achieved by inhibiting the
accumulation of ROS (54). The
antioxidant function has also been confirmed by many other studies
(55–57), including the normalization of
high-glucose-induced enhanced ROS production through the endogenous
overexpression of translocase of inner mitochondrial membrane
(Tim)44 combined with facilitated import of antioxidative enzymes
such as superoxide dismutase and GPx, as Tim44 forms a complex with
Hspa9 and Tim23 to affect mitochondrial transmembrane transport
(55). Other research has
demonstrated that at the mRNA level, α-lipoic acid, a type of
antioxidant, can increase the expression of Hspa9 in both
streptozotocin-induced diabetic and non-diabetic rats (58). In addition, Hspa9 can integrate with
podoplanin, which is mainly expressed in podocytes, indicating an
important role of Hspa9 in development of DN (59). Furthermore, ACEIs can affect the
expression of Hspa9, as has been demonstrated by a study where
treatment with the ACEI captopril delayed the apoptotic tube
collapse of bovine retinal endothelial cells and upregulated the
level of Hspa9 expression in these cells (60). Taken together, it can be further
hypothesized that the oxidative stress-inhibitory or renoprotective
effects of ACEIs correlate with the upregulation of Hspa9
expression.
In conclusion, the present study determined that 17
proteins were significantly changed in the FP-treated kidney
cortex. The increased expression levels of Hspa9 and Gpx3 following
FP treatment were confirmed by western blot analysis These
identified differentially expressed proteins may not only improve
understanding of the mechanism of ACEIs in OLETF rats, but may also
provide potential drug targets for the treatment of DN.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from
National Natural Science Foundation of China (grant nos. 81873140,
81620108031, 81773958 and 81100518) and Universities Technology
Research Project of Hebei province (grant no. QN2014013).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, YL, HZ and PL designed the present study and
wrote the manuscript. ZL and HZ performed the majority of
experiments. ZP and XW performed western blotting.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of China-Japan Friendship Hospital (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Umanath K and Lewis JB: Update on diabetic
nephropathy: Core curriculum 2018. Am J Kidney Dis. 71:884–895.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yerram P, Karuparthi PR, Hesemann L, Horst
J and Whaley-Connell A: Chronic kidney disease and cardiovascular
risk. J Am Soc Hypertens. 1:178–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Botdorf J, Chaudhary K and Whaley-Connell
A: Hypertension in cardiovascular and kidney disease. Cardiorenal
Med. 1:183–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayden MR, Sowers KM, Pulakat L,
Joginpally T, Krueger B, Whaley-Connell A and Sowers JR: Possible
mechanisms of local tissue renin-angiotensin system activation in
the cardiorenal metabolic syndrome and type 2 diabetes mellitus.
Cardiorenal Med. 1:193–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Huang XR, Chen HY, Penninger JM and
Lan HY: Loss of angiotensin-converting enzyme 2 enhances
TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal
inflammation in a mouse model of obstructive nephropathy. Lab
Invest. 92:650–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nistala R, Whaley-Connell A and Sowers JR:
Redox control of renal function and hypertension. Antioxid Redox
Signal. 10:2047–2089. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobori H, Nangaku M, Navar LG and
Nishiyama A: The intrarenal renin-angiotensin system: From
physiology to the pathobiology of hypertension and kidney disease.
Pharmacol Rev. 59:251–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karalliedde J and Viberti G: Evidence for
renoprotection by blockade of the renin-angiotensin-aldosterone
system in hypertension and diabetes. J Hum Hypertens. 20:239–253.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunz R, Friedrich C, Wolbers M and Mann
JF: Meta-analysis: Effect of monotherapy and combination therapy
with inhibitors of the renin angiotensin system on proteinuria in
renal disease. Ann Intern Med. 148:30–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balakumar P, Arora MK, Ganti SS, Reddy J
and Singh M: Recent advances in pharmacotherapy for diabetic
nephropathy: Current perspectives and future directions. Pharmacol
Res. 60:24–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magee C, Grieve DJ, Watson CJ and Brazil
DP: Diabetic nephropathy: A Tangled Web to Unweave. Cardiovasc
Drugs Ther. 31:579–592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brownlee M: The pathobiology of diabetic
complications: A unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong LL, Wu H, Cui WP, Zhou WH, Luo P, Sun
J, Yuan H and Miao LN: Advances in murine models of diabetic
nephropathy. J Diabetes Res. 2013:7975482013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Li P, Burczynski FJ, Gong Y, Choy
P, Sha H and Li J: Attenuation of diabetic nephropathy in Otsuka
Long-Evans Tokushima fatty (OLETF) rats with a combination of
Chinese herbs (Tangshen Formula). Evid Based Complement Alternat
Med. 2011:6137372011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawano K, Hirashima T, Mori S, Saitoh Y,
Kurosumi M and Natori T: Spontaneous long-term hyperglycemic rat
with diabetic complications. Otsuka Long-Evans Tokushima Fatty
(OLETF) strain. Diabetes. 41:1422–1428. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Council NR: Guide for the care and use of
laboratory animals: Eighth editionThe National Academies Press;
Washington, DC: 2011
|
|
17
|
Forbes JM, Thallas V, Thomas MC, Founds
HW, Burns WC, Jerums G and Cooper ME: The breakdown of preexisting
advanced glycation end products is associated with reduced renal
fibrosis in experimental diabetes. FASEB J. 17:1762–1764. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Veniant M, Heudes D, Clozel JP, Bruneval P
and Menard J: Calcium blockade versus ACE inhibition in clipped and
unclipped kidneys of 2K-1C rats. Kidney Int. 46:421–429. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang PL, Rothblum LI, Han WK, Blasick TM,
Potdar S and Bonventre JV: Kidney injury molecule-1 expression in
transplant biopsies is a sensitive measure of cell injury. Kidney
Int. 73:608–614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramachandra Rao SP, Wassell R, Shaw MA and
Sharma K: Profiling of human mesangial cell subproteomes reveals a
role for calmodulin in glucose uptake. Am J Physiol Renal Physiol.
292:F1182–F1189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Zhang H, Dong X, Burczynski FJ, Choy
P, Yang F, Liu H, Li P and Gong Y: Proteomic profile of primary
isolated rat mesangial cells in high-glucose culture condition and
decreased expression of PSMA6 in renal cortex of diabetic rats.
Biochem Cell Biol. 88:635–648. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Biron DG, Brun C, Lefevre T, Lebarbenchon
C, Loxdale HD, Chevenet F, Brizard JP and Thomas F: The pitfalls of
proteomics experiments without the correct use of bioinformatics
tools. Proteomics. 6:5577–5596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson SA and Spurney RF: Twenty years
after ACEIs and ARBs: Emerging treatment strategies for diabetic
nephropathy. Am J Physiol Renal Physiol. 309:F807–F820. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu FY, Lin FJ, Ou HT, Huang SH and Wang
CC: Renoprotective effect of angiotensin-converting enzyme
inhibitors and angiotensin II receptor blockers in diabetic
patients with proteinuria. Kidney Blood Press Res. 42:358–368.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shani M, Vinker S and Feldman L: End-stage
renal disease and adherence to angiotensin-converting enzyme
inhibitors and angiotensin receptor blockers among patients with
diabetes. J Clin Hypertens (Greenwich). 19:627–631. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rossing K, Jacobsen P, Pietraszek L and
Parving HH: Renoprotective effects of adding angiotensin II
receptor blocker to maximal recommended doses of ACE inhibitor in
diabetic nephropathy: A randomized double-blind crossover trial.
Diabetes Care. 26:2268–2274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bakris GL and Weir MR:
Angiotensin-converting enzyme inhibitor-associated elevations in
serum creatinine: Is this a cause for concern? Arch Intern Med.
160:685–693. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van de Ven PJ, Beutler JJ, Kaatee R, Beek
FJ, Mali WP and Koomans HA: Angiotensin converting enzyme
inhibitor-induced renal dysfunction in atherosclerotic renovascular
disease. Kidney Int. 53:986–993. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chapman AB, Gabow PA and Schrier RW:
Reversible renal failure associated with angiotensin-converting
enzyme inhibitors in polycystic kidney disease. Ann Intern Med.
115:769–773. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singhvi SM, Duchin KL, Morrison RA,
Willard DA, Everett DW and Frantz M: Disposition of fosinopril
sodium in healthy subjects. Br J Clin Pharmacol. 25:9–15. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lambers Heerspink HJ and Gansevoort RT:
Albuminuria is an appropriate therapeutic target in patients with
CKD: The pro view. Clin J Am Soc Nephrol. 10:1079–1088. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Diwakar R, Pearson AL, Colville-Nash P,
Brunskill NJ and Dockrell ME: The role played by endocytosis in
albumin-induced secretion of TGF-beta1 by proximal tubular
epithelial cells. Am J Physiol Renal Physiol. 292:F1464–F1470.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moon JY, Tanimoto M, Gohda T, Hagiwara S,
Yamazaki T, Ohara I, Murakoshi M, Aoki T, Ishikawa Y, Lee SH, et
al: Attenuating effect of angiotensin-(1–7) on angiotensin
II-mediated NAD(P)H oxidase activation in type 2 diabetic
nephropathy of KK-A(y)/Ta mice. Am J Physiol Renal Physiol.
300:F1271–F1282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stephan JP, Mao W, Filvaroff E, Cai L,
Rabkin R and Pan G: Albumin stimulates the accumulation of
extracellular matrix in renal tubular epithelial cells. Am J
Nephrol. 24:14–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wolf G, Schroeder R, Ziyadeh FN and Stahl
RA: Albumin up-regulates the type II transforming growth
factor-beta receptor in cultured proximal tubular cells. Kidney
Int. 66:1849–1858. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burton C and Harris KP: The role of
proteinuria in the progression of chronic renal failure. Am J
Kidney Dis. 27:765–775. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Behne D and Kyriakopoulos A: Mammalian
selenium-containing proteins. Annu Rev Nutr. 21:453–473. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haung W, Koralewska-Makar A, Bauer B and
Akesson B: Extracellular glutathione peroxidase and ascorbic acid
in aqueous humor and serum of patients operated on for cataract.
Clin Chim Acta. 261:117–130. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Whitin JC, Bhamre S, Tham DM and Cohen HJ:
Extracellular glutathione peroxidase is secreted basolaterally by
human renal proximal tubule cells. Am J Physiol Renal Physiol.
283:F20–F28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang SW, Natarajan R, Shahed A, Nast CC,
LaPage J, Mundel P, Kashtan C and Adler SG: Role of 12-lipoxygenase
in the stimulation of p38 mitogen-activated protein kinase and
collagen alpha5(IV) in experimental diabetic nephropathy and in
glucose-stimulated podocytes. J Am Soc Nephrol. 14:3178–3187. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
de Cavanagh EM, Inserra F, Toblli J,
Stella I, Fraga CG and Ferder L: Enalapril attenuates oxidative
stress in diabetic rats. Hypertension. 38:1130–1136. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ogawa S, Mori T, Nako K, Kato T, Takeuchi
K and Ito S: Angiotensin II type 1 receptor blockers reduce urinary
oxidative stress markers in hypertensive diabetic nephropathy.
Hypertension. 47:699–705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sonta T, Inoguchi T, Matsumoto S, Yasukawa
K, Inuo M, Tsubouchi H, Sonoda N, Kobayashi K, Utsumi H and Nawata
H: In vivo imaging of oxidative stress in the kidney of diabetic
mice and its normalization by angiotensin II type 1 receptor
blocker. Biochem Biophys Res Commun. 330:415–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ishizawa K, Izawa-Ishizawa Y, Dorjsuren N,
Miki E, Kihira Y, Ikeda Y, Hamano S, Kawazoe K, Minakuchi K, Tomita
S, et al: Angiotensin II receptor blocker attenuates PDGF-induced
mesangial cell migration in a receptor-independent manner. Nephrol
Dial Transplant. 25:364–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lv J, Jia R, Yang D, Zhu J and Ding G:
Candesartan attenuates Angiotensin II-induced mesangial cell
apoptosis via TLR4/MyD88 pathway. Biochem Biophys Res Commun.
380:81–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park SJ, Lee BH and Kim DJ: Identification
of proteins that interact with podocin using the yeast 2-hybrid
system. Yonsei Med J. 50:273–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Morito N, Yoh K, Ojima M, Okamura M,
Nakamura M, Hamada M, Shimohata H, Moriguchi T, Yamagata K and
Takahashi S: Overexpression of Mafb in podocytes protects against
diabetic nephropathy. J Am Soc Nephrol. 25:2546–2557. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chiu YW, Kuo MC, Kuo HT, Chang JM, Guh JY,
Lai YH and Chen HC: Alterations of glomerular and extracellular
levels of glutathione peroxidase in patients and experimental rats
with diabetic nephropathy. J Lab Clin Med. 145:181–186. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fan QL, Yang G, Liu XD, Ma JF, Feng JM,
Jiang Y and Wang LN: Effect of losartan on the glomerular protein
expression profile of type 2 diabetic KKAy mice. J Nephrol.
26:517–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kaul SC, Deocaris CC and Wadhwa R: Three
faces of mortalin: A housekeeper, guardian and killer. Exp
Gerontol. 42:263–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wadhwa R, Ryu J, Ahn HM, Saxena N,
Chaudhary A, Yun CO and Kaul SC: Functional significance of point
mutations in stress chaperone mortalin and their relevance to
Parkinson disease. J Biol Chem. 290:8447–8456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Deocaris CC, Widodo N, Shrestha BG, Kaur
K, Ohtaka M, Yamasaki K, Kaul SC and Wadhwa R: Mortalin sensitizes
human cancer cells to MKT-077-induced senescence. Cancer Lett.
252:259–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu Y, Liu W, Song XD and Zuo J: Effect of
GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP
level, mitochondrial membrane potential and ROS accumulation
following glucose deprivation in PC12 cells. Mol Cell Biochem.
268:45–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Matsuoka T, Wada J, Hashimoto I, Zhang Y,
Eguchi J, Ogawa N, Shikata K, Kanwar YS and Makino H: Gene delivery
of Tim44 reduces mitochondrial superoxide production and
ameliorates neointimal proliferation of injured carotid artery in
diabetic rats. Diabetes. 54:2882–2890. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lv Y, Li Y, Zhang D, Zhang A, Guo W and
Zhu S: HMGB1-induced asthmatic airway inflammation through
GRP75-mediated enhancement of ER-mitochondrial Ca2+
transfer and ROS increased. J Cell Biochem. 119:4205–4215. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Honrath B, Metz I, Bendridi N, Rieusset J,
Culmsee C and Dolga AM: Glucose-regulated protein 75 determines
ER-mitochondrial coupling and sensitivity to oxidative stress in
neuronal cells. Cell Death Discov. 3:170762017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Oksala NK, Lappalainen J, Laaksonen DE,
Khanna S, Kaarniranta K, Sen CK and Atalay M: Alpha-lipoic Acid
modulates heat shock factor-1 expression in streptozotocin-induced
diabetic rat kidney. Antioxid Redox Signal. 9:497–506. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tsuneki M, Maruyama S, Yamazaki M, Xu B,
Essa A, Abé T, Babkair H, Cheng J, Yamamoto T and Saku T:
Extracellular heat shock protein A9 is a novel interaction partner
of podoplanin in oral squamous cell carcinoma cells. Biochem
Biophys Res Commun. 434:124–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hamdi HK and Castellon R: ACE inhibition
actively promotes cell survival by altering gene expression.
Biochem Biophys Res Commun. 310:1227–1235. 2003. View Article : Google Scholar : PubMed/NCBI
|