Introduction
Benign esophageal strictures are common
digestive-tract diseases and may be caused by several factors,
including corrosive damage, radioactive damage and injuries
following endoscopic or surgical operations (1–3). Due to
recent advances in the field of endoscopic technology, the risk of
esophageal stricture caused by extensive resection of esophageal
mucosa following endoscopic mucosal resection or endoscopic
submucosal dissection has increased to >80% (4,5).
Therefore, the prevention and treatment of benign esophageal
strictures has become a matter of increasing urgency. Dilatation by
balloon or Bougie dilators may not maintain long-lasting effects,
resulting in the recurrence of the strictures. It has been
indicated that repeated injection of steroids, a suggested
treatment of strictures, may lead to steroid-associated side
effects (6,7). Treatments associated with cell and
tissue engineering, which are still at a preliminary stage, are
currently not available and require further development suggesting
that their complete mechanisms of action remain to be fully
elucidated (8).
At present, temporary stent placement is one of the
most commonly used methods for the prevention and treatment of
esophageal strictures. Luminal patency may be shaped through
supporting force from the stent. However, this radial force causes
tissue proliferation and re-stenosis in addition to lumen
dilatation (9). Furthermore,
secondary injuries to esophageal mucosa, including inflammatory
reaction and fibrosis, may occur while the stent is removed from
the esophagus.
At present, secondary damage during stent removal is
inevitable due to the radial force of the stents. Previous studies
performed by our group reported on the design of novel detachable
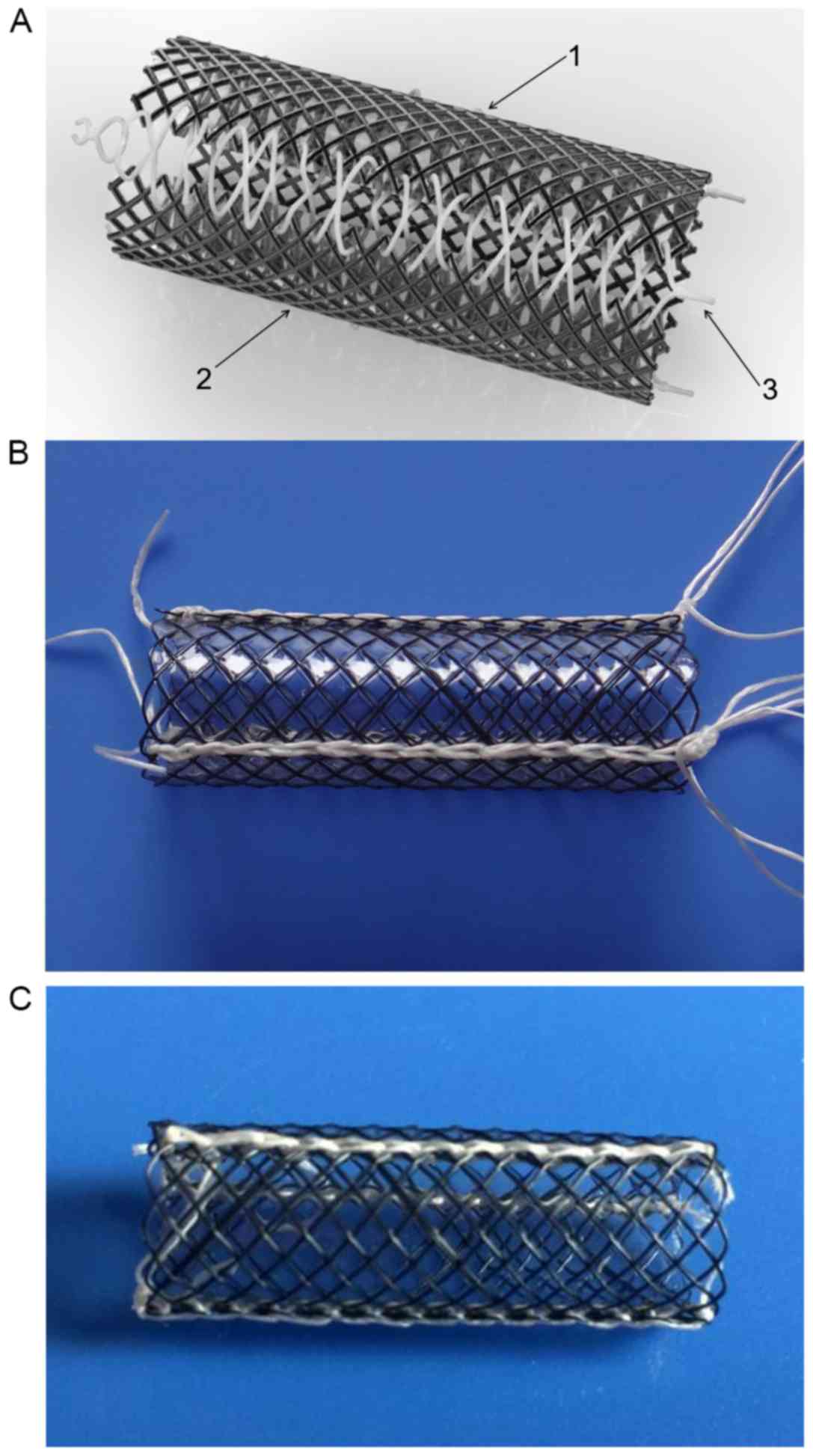
stents (10,11). The novel stents exhibit the following
features: i) The stents are composed of 3 fully covered metallic
meshes; ii) the metallic meshes are connected with lines and the
stents may be disassembled into 3 pieces when the connecting lines
are removed; iii) he design that allows for extraction of the
stents without radial force is unique. In the present study, 2
subtypes of the novel detachable stent were employed. The first
subtype was the detachable stent (DS) and the second was the
biodegradable stent (BS; Fig. 1). In
the present study, in vitro and in vivo experiments
were performed to test the supporting effects and the extent of
local mucosal damage when the debris of the stents was
retracted.
Materials and methods
Manufacture of stents
In the present study, the mold used for each animal
(10 mm in diameter and 70 mm in length; Shandong Medical
Instrumental Institute) was manufactured as an aluminous cylinder
with small bulges on its surface. The wire of the nickel-titanium
memory alloy (0.13 mm in diameter; Grinm Advanced Materials Co.,
Ltd) was braided into metallic mesh pieces on the mold and placed
into a high-temperature cabinet-type electric furnace (SXL-2;
Jinghong Experimental Equipment Co., Ltd) for heat-setting. The
temperature range was 510–550°C and the heating time ranged from 15
to 35 min. Subsequently, the metallic mesh pieces were dried with
anhydrous alcohol and immersed in silicon solution for coating. The
fully covered pieces were removed and dried in an oven at 120°C for
1 h.
The connecting line was composed of a surgical
suture line in the DS and of a poly(lactic-co-glycolic acid) (PLGA)
thread (0.4–1.2 mm in diameter; Shandong Medical Instrumental
Institute,) in the BS. The suture line was braided with a monoline
chain structure pattern. The tension was adjusted by a 100 g
balancing weight every 3 stitches. At the proximal end of the
stent, a loop knot was made for stent retrieval.
Assessment of stent-supporting
properties. Measurement of expansion point and softening point
The stent was immersed in a beaker containing normal
saline at 0°C for 2 min. External pressure was applied to deform
it. When the length of the short axis in the stent was ~0, 50 ml
normal saline was slowly added to the beaker under constant
stirring. The water temperature was recorded with an accuracy of
0.1°C when the sample was restored to 90% of its original shape.
This critical temperature was defined as the expansion point. The
softening point was defined as the temperature, which was equal to
the expansion point minus 25°C.
Measurement of stent flexibility
When the stent was bent by 30°, its diameter was
measured by a vernier caliper with an accuracy of 0.01 mm. The
stent flexibility was calculated using the following formula:
(d/D)×100%, where d was the original diameter of the stent and D
was the minimum diameter at the bending place.
Measurement of radial compression
ratio
The stent was compressed to the maximum extent at a
temperature range of 25–35°C and packed into the ‘gauge’ of a hard
pipe ranging from 4.0–7.0 mm in diameter. The radial compression
ratio was calculated as r/R (r was the original diameter when the
stent expanded and R was the minimum inner diameter of the ‘gauge’
where the stent was loaded).
Measurement of radial force
The radial force of the stent was measured with a
pressure-testing machine (AGS-H; Shimadzu Corp.). The radial force
was considered as the force along the stent when the short axis
length of the stent was equal to half of its original diameter.
Esophageal stricture model and animal
grouping
A total of 20 New Zealand white rabbits were used
for the animal experiment of stricture modeling. The present study
was approved by the Ethics Committee of Shandong Provincial
Hospital affiliated to Shandong University (Jinan, China; no.
2017-002). The details of the procedures were as previously
described (10–12). Following fasting of the rabbits for
24 h, they were anesthetized with 3% sodium pentobarbital (30
mg/kg) and placed in the left lateral decubitus position. A total
of 1 ml 4% sodium hydroxide solution was introduced orally into
each animal to produce corrosive stricture injury in the middle of
the esophagus.
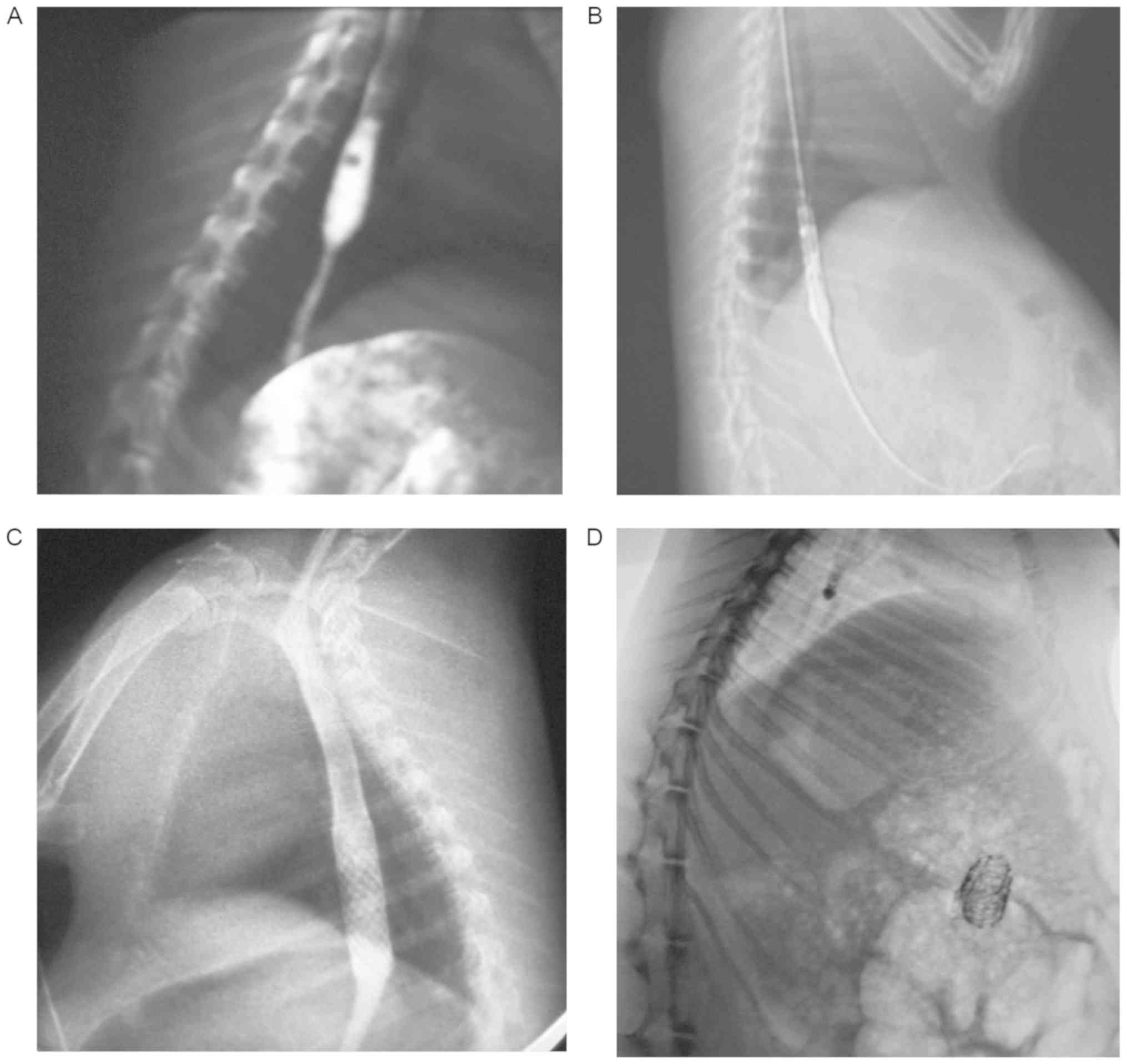
After 2 weeks, all of the rabbits were evaluated
using a fluoroscope (Prestige; General Electric Co.). The criterion
to confirm modeling success was the diameter of stricture injuries
being <1/2 of the maximal diameter of the enlarged esophagus
above the site of injury (Figs. 2A
and 3). The success rate was 90%.
The rabbits with corrosive stricture were randomly assigned into 3
groups as follows: DS, BS and control groups. A total of 6 rabbits
were included in each group. The rabbits of the control group did
not undergo any treatment.
All animals were observed by endoscopy and
fluoroscopy weekly. The animals in the BS group were observed by
fluoroscopy at 1-day intervals from the end of the 4th week. The
general condition and body weight of the rabbits was evaluated
weekly. Complications, including stent migration, aspiration,
bleeding, perforation, fistula and animal death were recorded.
Rabbits that exhibited strictures of >80% of the lumen were
euthanized. Observation of the esophageal stricture was performed
macroscopically (via endoscopic and fluoroscopic observation) and
histologically at the end of the 8th week.
Stent placement and removal
Following anesthetization, a guidewire was inserted
into the rabbit's stomach. The stent was sheathed in the delivery
system and introduced using a guidewire. Subsequently, the stents
were placed and expanded across the stricture injuries using
fluoroscopy (Fig. 2B and C).
The connecting PLGA of the BS group was degraded and
the metallic mesh pieces moved to the stomach cavity (Fig. 2D). The debris of the stents was
extracted via endoscopy using biopsy forceps (FB-19K-1; Olympus
Optical Co. Ltd.).
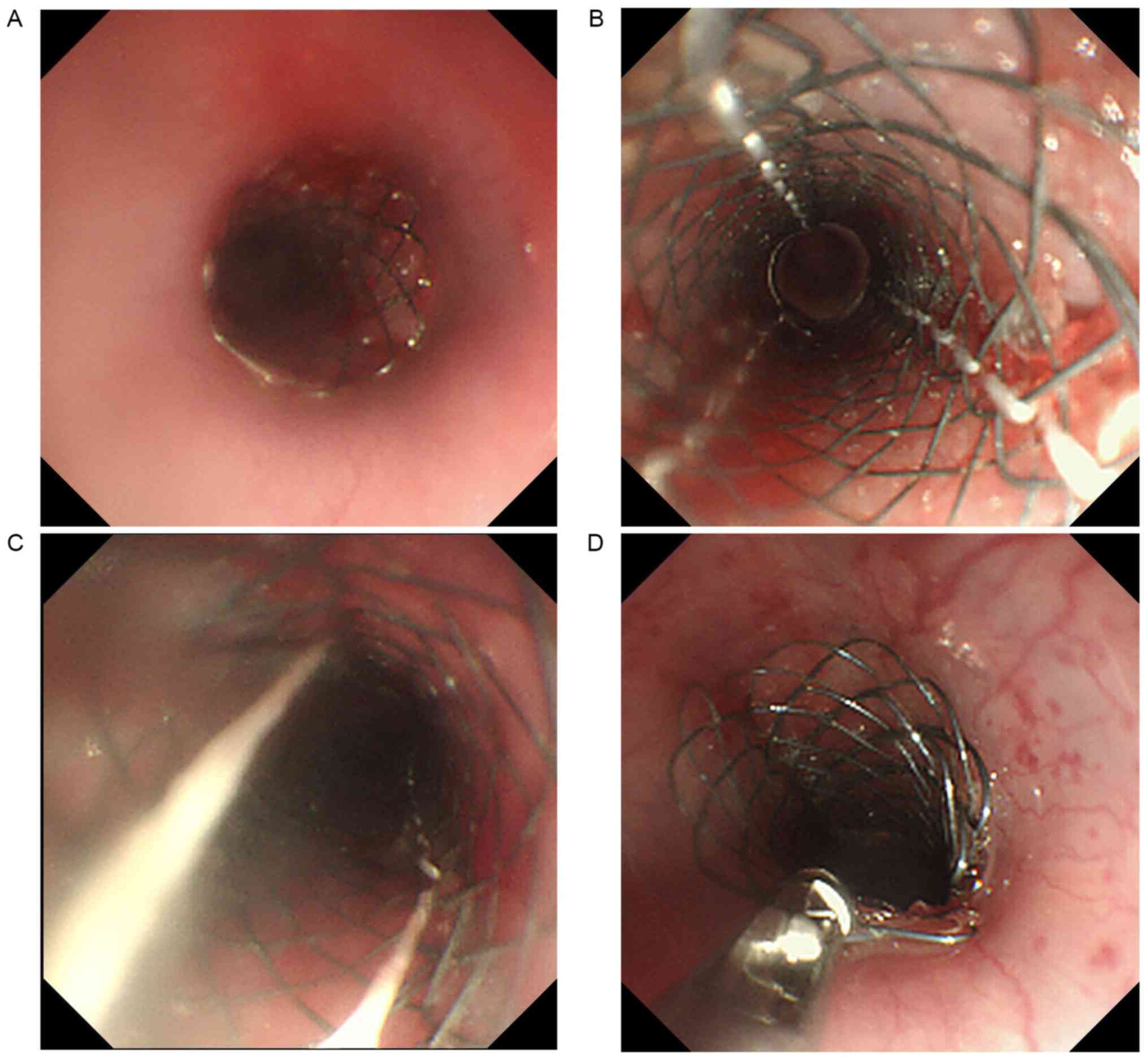
The removal operations were performed at the 8th
week with an ultraslim endoscope and biopsy forceps. The details of
the procedure were as follows (Fig.
4): The string of the slipknot was removed by biopsy forceps
and the connecting lines were subsequently untied. The metallic
mesh pieces were disassembled. As a result, the radial force of the
stent was absent. Finally, the debris of the pieces was easily
removed by biopsy forceps.
Hematoxylin and eosin staining
(H&E)
Resection of the esophagus was conducted after stent
removal in order to obtain histological samples. The resected
esophagi were fixed in 10% formalin overnight at room temperature.
The specimens were embedded in paraffin and sliced into 5 µm thick
sections. H&E staining was performed at room temperature
according to routine protocols. After deparaffinization and
rehydration, the slides were immersed in hematoxylin solution and
then dipped in 1% acid ethanol (1% HCl in 70% ethanol) 5 times.
Then the slides were rinsed with distilled water and stained with
eosin solution for 3 min. After dehydration with graded alcohol
solutions and clearing with xylene, the slides were sealed by
neutral balata. The slides were examined using an Olympus BX41
fluorescence microscope (Olympus Corporation). A total of 3
sections were collected from the stricture of each rabbit and at
least 2 fields of view from each were imaged.
Statistical analysis
The data were analyzed using SPSS 22.0 software (IBM
Corp.). The numerical variables were compared by one-way analysis
of variance. Subsequently, the least-significant differences test
was applied as a post hoc test. Fisher's exact test was used to
compare the categorical variables. P<0.05 was considered to
indicate a significant difference.
Results
Stent assessment
The stent diameter and length were 10±0.5 and 30±5
mm, respectively. The properties of the 2 types of stents were
identical. The softening point was 0±5°C and the expansion point
was 25±3°C. These results indicated that the stent was able to be
compressed into the stent delivery system at ~0°C and that it was
able to expand well in vivo. The stent flexibility was
estimated to be 94%. The radial compression ratio was >2.5 and
the radial force was 6.1 N. The physical properties indicated that
the flexibility, radial compression ratio and radial force of the
stent met the design requirements (Table
I).
 | Table I.Properties of the two types of
detachable stent. |
Table I.
Properties of the two types of
detachable stent.
| Property | Biodegradable
stent | Detachable stent |
|---|
| Softening point | 0±5°C | 0±5°C |
| Expansion point | 25±3°C | 25±3°C |
| Stent
flexibility | 94% | 94% |
| Radial compression
ratio | >2.5 | >2.5 |
| Radial force | 6.1 N | 6.1 N |
General condition and endoscopic
examination
A total of 18 out of 20 rabbits met the criteria of
the successfully established stricture model and were randomly
assigned into 3 groups. Their average weight exhibited no apparent
difference (Table II). The stent
exhibited good expansion in the esophagus and covered the narrow
part of the organ. All rabbits in the DS group survived during the
observation period and no complications were observed. One animal
of the BS group did not survive due to perforation. In addition,
the animals in the control group did not survive due to severe
stricture formation within 4 weeks.
 | Table II.Characteristics of the animals
used. |
Table II.
Characteristics of the animals
used.
| Item | Control group
(n) | BS group (n) | DS group (n) | P-value (Control vs.
BS) | P-value (Control vs.
DS) | P-value (BS vs.
DS) |
|---|
| Initial number of
rabbits | 6 | 6 | 6 | – | – |
|
| Average weight
(kg)a | 2.54±0.13 | 2.55±0.17 | 2.56±0.17 | 0.844 | 0.816 | 0.971 |
| Stent
implantation | 0 | 6 | 6 | – | – | – |
| Survival | 0 | 5 | 6 | 0.015 | 0.02 | 1 |
| Degraded stent | – | 5 | – | – | – | – |
| Complications |
|
|
|
|
|
|
|
Proliferation | – | 1 | 0 | – | – | 0.455 |
| Tissue
rupture | – | 0 | 2 | – | – | 0.455 |
| Minor
bleeding | – | 1 | 2 | – | – | 1 |
| Stent removal | – | 5 | 6 |
|
| 1 |
| Stricture rate
(%)b | 61.5±10.0 | 15.0±4.1 | 15.9±7.7 | <0.001 | <0.001 | 0.836 |
The stents of the BS group were degraded and dropped
into the stomach between weeks 6 and 7 (2 in 6 weeks and 3 in 7
weeks). The debris of the stent had moved to the stomach and was
easily extracted using endoscopy and biopsy forceps. One rabbit did
not survive due to perforation at the 4th week. Minor bleeding
occurred in only one animal. Stent removal was not associated with
any severe complications. In the DS group, the removal procedures
were easy and smooth. A total of 2 cases exhibited minor
hemorrhage. The minor bleeding did not need special treatment by
endoscope and was resolved spontaneously. The stricture rate,
incidence of complications, survival and stent removal exhibited no
significant differences between the BS and DS groups.
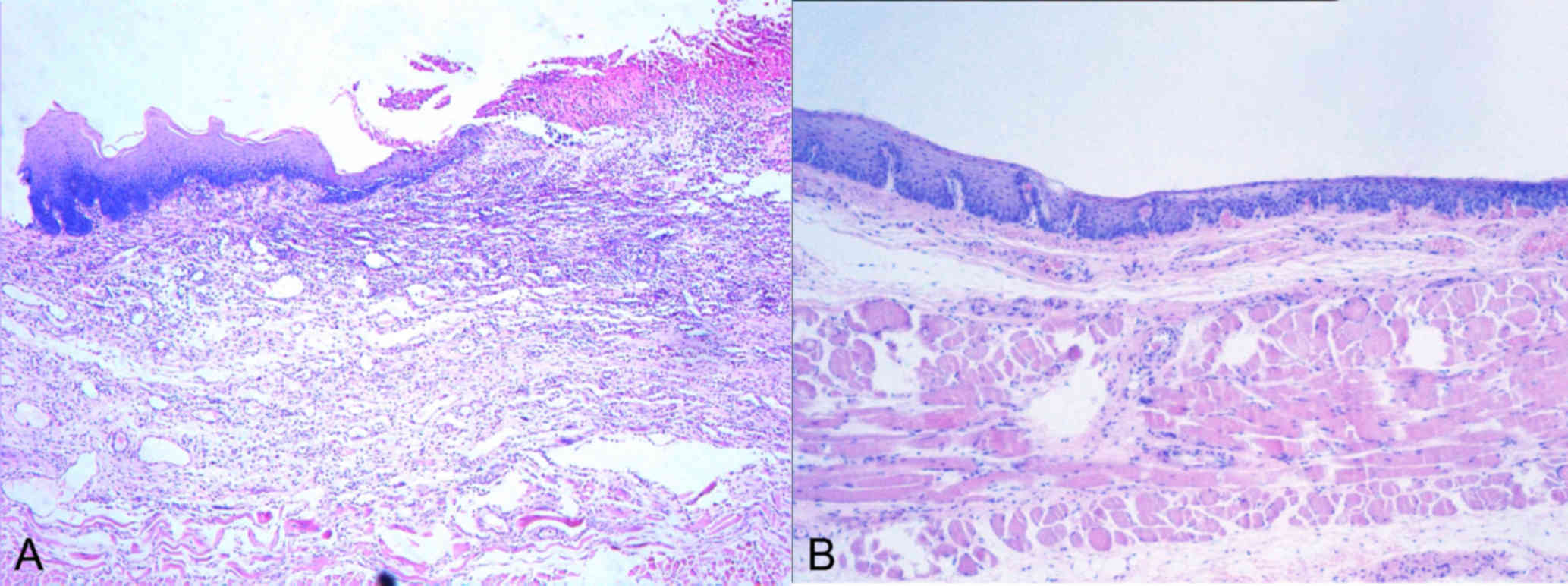
Rate of stricture and histological
evaluation
The rates of esophageal stricture at the time the
stent was removed from the esophagus in the BS and DS groups were
significantly lower than those in the control group (Table II). With this regard, no significant
differences were noted between the BS and DS groups. A histological
sample was taken after the removal procedure. Inflammation and the
formation of granulated tissue was observed using microscopy.
Tissue proliferation and development of ulcers were detected in the
incised esophageal specimens. The sample of a rabbit with minor
bleeding in the DS group was used for histological observation
(Fig. 5). These observations
indicated the presence of newly formed tissue in this sample. This
finding suggested that the novel stents may prevent secondary
damage during stent removal.
Discussion
Temporary stent implantation is considered as a
viable management for the prevention and treatment of benign
esophageal strictures, due to its long-lasting dilatation effect.
At present, commercially available esophageal stents may be
classified as self-expanding metallic stents (SEMSs),
self-expanding plastic stents (SEPSs) and BS. Although uncovered
SEMSs have been successfully used in palliative therapy of
malignant esophageal strictures, hyperproliferation and tissue
embedding on the contact surface of the meshes impede their
application in benign strictures (13,14).
Covered SEMSs exert a strong radial force and may alleviate
hyperproliferation and tissue embedding. These stents have become
the most commonly used type of stent in benign esophageal
stricture. Kim et al (15)
reported that the most common complication of SEMS placement was
tissue in-and over-growth (31%). The migratory rate of fully
covered SEMSs ranged from 17.5 to 39% (16–18).
Hyperproliferation and tissue embedding stimulated by SEPSs were
less common than those stimulated by SEMSs (19). However, the migratory rate of SEPSs
was generally higher than that of SEMSs. Studies have estimated
that the rate of SEPS migration ranged from 7 to 85% (19–23).
Removal of the migrated stents may also cause secondary damage of
the mucosa due to the radial force of the stents. BS were
introduced to avoid the secondary damage associated with stent
removal. Satio et al (24)
reported on a biodegradable esophageal stent, which was knitted
from poly-l-lactic acid monofilaments in the same manner as the
Ultraflex stent(Boston Scientific, MA, USA). The Ultraflex stent,
which is a partially covered nitinol alloy stent knitted into a
specific geometric figure is a commonly used commercially available
stent in the field of esophageal stricture. The stricture was
dilated prior to stent insertion and the rate of stent migration
was 77%. Recent studies focused on the application of Ella BD stent
(Ella-SX, s.r.o., Hradec Králové, Czech Republic), which is
composed of polydioxanone monofilaments (25). Tissue in-and over-growth was a common
feature of the studies into the effectiveness of the Ella BD stent
(Ella-CS, s.r.o., Hradec Králové, Czech Republic) and occurred due
the uncovered surface of the biodegradable material (26–28). The
production of a fully-covered BS has been proposed to solve this
problem, although this type of material has not been previously
tested (29).
Temporary stent implantation may reduce the
inflammatory reaction and fibrosis during stent placement. This may
be applied to avoid secondary injuries to the esophageal lumen when
the stent is removed. In order to address this application, a new
series of stents was designed and developed. In the present study,
the softening point of the novel DS/BS was 0±5°C and the expansion
point was 25±3°C. These values indicated that the stent was able to
be compressed into the stent delivery system at ~0°C. Furthermore,
the results demonstrated that the stent expanded well when it was
inserted into the esophageal lumen. Tanaka et al (30) reported that the radial forces of
commercially available esophageal stents ranged from 3.6 to 11.5 N.
The radial force of the DS was estimated to be 6.2 N. This novel
stent was comparable with the commercially available stents in
providing sufficient support. In the present study, the rate of
esophageal stricture in the BS and DS groups was significantly
lower than that in the control group. No significant differences
were noted in esophageal stricture between the BS and the DS
groups. The results indicated that the novel stents may provide
long-lasting dilatation effects to maintain luminal patency.
Although the radial force of the stent provides a
long-lasting dilation effect, this always prevented the extraction
of the stent. Stent retrieval usually caused secondary injuries of
the esophageal mucosa and induced inflammatory reaction and
restenosis. Eloubeidi and Lopes (31) reported that in 20 and 80% of the
cases, regular and double-channel therapeutic endoscopy was
adopted, respectively (18% of the cases required assistance of one
rat-tooth forceps, one rat-tooth forceps and a snare were used in
27% of cases, 2 rat-tooth forceps were used in 50% of cases and
other modalities were applied in 5% of cases). Hirdes et al
(32) demonstrated the feasibility
and removability of the ‘stent-in-stent’ technique. The authors
used a fully-covered stent of the same or slightly larger size. The
stent was inserted into the initially placed embedded stent and the
2 stents were removed simultaneously when the pressure resulted in
necrosis of the overgrowth tissue at 10–14 days following
placement. In the present study, a single-channel ultraslim
endoscope and biopsy forceps were used for stent removal.
In the DS group, the process of stent removal was
smooth and easy. The radial force of the DS was eliminated by
removing the string of the thread. The connecting threads were
pulled out of the stent, resulting in its collapse. The metallic
pieces were removed successively. A total of 2 rabbits were
selected for further study following stent retrieval as these two
rabbits exhibited minor bleeding during stent removal. The animals
exhibited severe inflammation of the esophagus and ulcer formation
with the presence of minor bleeding. Inflammation may cause
bleeding during stent retrieval. Proliferation or granulation of
the tissue and ulceration are common in tissues after stent
implantation. As a result, when the stent is pulled free from the
esophagus, the contact surface of the stent and blunt traction can
severely damage the esophageal tissue where new tissue or a
pressure ulcer has formed. In the 2 cases, the minor bleeding did
not need special treatment by endoscope and resolved spontaneously.
This illustrated that the novel DS may be used to avoid severe
secondary injury during stent removal.
In the BS group, the stents were degraded and
transferred to the stomach between weeks 6 and 7. Only one rabbit
did not survive. The debris of the stent was transferred to the
stomach and was easily extracted using biopsy forceps and
endoscopy. Minor bleeding occurred in one case, though in the
absence of severe complications associated with stent removal. At
present, this novel BS is the only fully-covered stent, which may
in part be biodegraded. Euthanasia and separation of esophagus were
conducted to obtain histological specimens of rabbit tissue. This
tissue showed clear proliferation or granulation following
ulceration when compared with normal tissue. Severe inflammation
may cause tissue vulnerability. When the pieces of the stent were
removed from the esophagus, secondary damage, such as bleeding and
tissue rupture, inevitably occurred due to friction between the
surface of the stent pieces and the inflamed esophageal mucosa. In
the absence of the radial force of the stent, the bleeding
associated with stent removal was minor. This result suggested that
the newly designed detachable stents may avoid secondary damage
during stent removal procedure.
The limitations of the present study were the lack
of additional in vitro and clinical data to support the
in vivo results. Large patient studies are required to
collect additional data regarding the efficacy of DS/BS used for
the treatment of benign esophageal strictures. Further studies
should focus on different shapes of stent, which may be used to
treat different types of disease in the digestive tract.
In conclusion, the present study indicated that the
novel DS/BS for the treatment of esophageal stricture was safe and
easily placed. This novel type of stent may offer long-lasting
supporting effects and avoid secondary injuries to the esophageal
lumen during stent removal.
Acknowledgements
The authors would like to thank Mr. Dong Wang and
Mr. Junqi Li from the Institute of Shandong Provincial Medical
Instruments (Jinan, China) for their technical support.
Funding
The present study was supported by a grant from the
Natural Science Foundation of Shandong Province, China (grant no.
ZR201702230130).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS performed the study, analyzed and interpreted the
data and drafted the manuscript. QSP participated in the animal
experiments and in the endoscopic operation. DX assisted with the
endoscopic operation and analyzed the data. JYL provided technical
support and participated in the endoscopic operation. JL
participated in the experimental design and supervised the study.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Provincial Hospital affiliated to Shandong
University (Jinan, China; no. 2017-002).
Patient consent for publication
Not applicable
Competing interests
JYL and JL own the patent for the detachable stents
(patent no. ZL2011-10323099.5, 2011). The remaining authors have no
competing interests.
References
|
1
|
Hasan M and Maple JT: Traversing difficult
esophageal strictures from the retrograde apprpach. Tech
Gastrointest Endosc. 10:149–154. 2008. View Article : Google Scholar
|
|
2
|
Shah JN: Benign refractory esophageal
strictures: Widening the endoscopist's role. Gastrointest Endosc.
63:164–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luchtefeld MA, Milsom JW, Senagore A,
Surrell JA and Mazier WP: Colorectal anastomotic stenosis: Results
of a survey of the ASCRS membership. Dis Colon Rectum. 32:733–736.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mori H, Rafiq K, Kobara H, Fujihara S,
Nishiyama N, Oryuu M, Suzuki Y and Masaki T: Steroid permeation
into the artificial ulcer by combined steroid gel application and
balloon dilatation: Prevention of esophageal stricture. J
Gastroenterol Hepatol. 28:999–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isomoto H, Yamaguchi N, Nakayama T,
Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S
and Nakao K: Management of esophageal stricture after complete
circular endoscopic submucosal dissection for superficial
esophageal squamous cell carcinoma. BMC Gastroenterol. 11:462011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi S, Kanai N, Ohki S, Takagi R,
Yamaguchi N, Isomoto H, Kasai Y, Hosoi T, Nakao K, Eguchi S, et al:
Prevention of esophageal strictures after endoscopic submucosal
dissection. World J Gastroenterol. 20:15098–15109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamashina T, Uedo N, Fujii M, Ishihara R,
Mikamori M, Motoori M, Yano M and Iishi H: Delayed perforation
after intralesional triamcinolone injection for esophageal
stricture following endoscopic submucosal dissection. Endoscopy. 45
(Suppl 2) UCTN:E922013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang J, Ye S, Ji X, Liu F and Li Z:
Deployment of carboxymethyl cellulose sheets to prevent esophageal
stricture after full circumferential endoscopic submucosal
dissection: A porcine model. Dig Endosc. 30:608–615. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JH, Song HY, Park JY, Kim JH, Kim YH,
Kim JH and Kim SB: Temporary stent placement with concurrent
chemoradiation therapy in patients with unresectable oesophageal
carcinoma: Is there an optimal time for stent removal? Eur Radiol.
23:1940–1945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Shang L, Liu JY and Qin CY: Newly
designed ‘pieced’ stent in a rabbit model of benign esophageal
stricture. World J Gastroenterol. 21:8629–8635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Shang L, Liu J and Qin C: A novel
biodegradable esophageal stent: Results from mechanical and animal
experiments. Am J Transl Res. 8:1108–1114. 2016.PubMed/NCBI
|
|
12
|
Thompson JN: Corrosive esophageal
injuries. II. An investigation of treatment methods and
histochemical analysis of esophageal strictures in a new animal
model. Laryngoscope. 97:1191–1202. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kozarek RA: Expandable endoprostheses for
gastrointestinal stenoses. Gastrointest Endosc Clin North Am.
4:279–295. 1994. View Article : Google Scholar
|
|
14
|
Tan BS, Kennedy C, Morgan R, Owen W and
Adam A: Using uncovered metallic endoprostheses to treat recurrent
benign esophageal strictures. AJR Am J Roentgenol. 169:1281–1284.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JH, Song HY, Choi EK, Kim KR, Shin JH
and Lim JO: Temporary metallic stent placement in the treatment of
refractory benign esophageal strictures: Results and factors
associated with outcome in 55 patients. Eur Radiol. 19:384–390.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Senousy BE, Gupte AR, Draganov PV,
Forsmark CE and Wagh MS: Fully covered Alimaxx esophageal metal
stents in the endoscopic treatment of benign esophageal diseases.
Dig Dis Sci. 55:3399–3403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
EI Hajj II, Imperiale TF, Rex DK, Ballard
D, Kesler KA, Birdas TJ, Fatima H, Kessler WR and DeWitt JM:
Treatment of esophageal leaks, fistulae, and perforations with
temporary stents: Evaluation of efficacy, adverse events, and
factors associated with successful outcomes. Gastrointest Endosc.
79:589–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilson JL, Louie BE, Farivar AS, Vallières
E and Aye RW: Fully covered self-expanding metal stents are
effective for benign esophagogatric disruptions and strictures. J
Gastrointest Surg. 17:2045–2050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dua KS, Vleggaar FP, Santharam R and
Siersema PD: Removable self-expanding plastic esophageal stent as a
continuous, non-permanent dilator in treating refractory benign
esophageal strictures: A prospective two-center study. Am J
Gastroenterol. 103:2988–2994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh YS, Kochman ML, Ahmad NA and Ginsberg
GG: Clinical outcomes after self-expanding plastic stent placement
for refractory benign esophageal strictures. Dig Dis Sci.
55:1344–1348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Repici A, Conio M, De Angelis C, Battaglia
E, Musso A, Pellicano R, Goss M, Venezia G, Rizzetto M and Saracco
G: Temporary placement of an expandable polyester silicone-covered
stent for treatmet of refractory benign esophageal stricture.
Gastrointest Endosc. 60:513–519. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Triester SL, Fleischer DE and Sharma VK:
Failure of self-expanding plastic stents in treatment of refractory
benign esopohageal strictures. Endoscopy. 38:533–537. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barthel JS, Kelley ST and Klapman JB:
Management of persistent gastroesophageal anastomotic strictures
with removalbe self-expandable polyester silicon-covered(Pollyflex)
stents: An alternative to serial dilation. Gastrointest Endosc.
67:546–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saito Y, Tanaka T, Andoh A, Minematsu H,
Hata K, Tsujikawa T, Nitta N, Murata K and Fujiyama Y: Novel
biodegradable stents for benign esophageal strictures following
endoscopic submucosal dissection. Dig Dis Sci. 53:330–333. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Boeckel PG, Vleggaar FP and Siersema
PD: Biodegradable stent placement in the esophagus. Expert Rev Med
Devices. 10:37–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dumoulin FL and Plassmann D: Tissue
hyperplasia following placement of biodegradable stent for a
refractory esophageal stricture: Treatment with argon plasm
coagulation. Endoscopy. 44 (Suppl 2) UCTN:E356–E357. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hair CS and Devonshire DA: Severe
hyperplastic tissue stenosis of a novel biodegradable esophageal
stent and subsequent successful management with high-pressure
balloon dilation. Endoscopy. 42 (Suppl 2):E132–E133. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Hooft JE, van Berge Henegouwen MI,
Rauws EA, Bergman JJ, Busch OR and Fockens P: Endoscopic treatment
of benign anastomotic esophagogastric strictures with a
biodegradable stent. Gastrointest Endosc. 73:1043–1047. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Boeckel PG, Vleggaar FP and Siersema
PD: A comparison of temporary self-expanding plastic and
biodegradable stents for refractory benign esophageal strictures.
Clin Gastroenterol Heppatol. 9:653–659. 2011. View Article : Google Scholar
|
|
30
|
Tanaka T, Takahashi M, Nitta N, Furukawa
A, Andoh A, Saito Y, Fujiyama Y and Murata K: Newly developed
biodegradable stents for benign gastrointestinal tract stenoses: A
preliminary clinical trial. Digestion. 74:199–205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eloubeidi MA and Lopes TL: Novel removable
internally fully covered self-expanding metal esophageal stent:
Feasibility, technique of removal, and tissue response in humans.
Am J Gastroenterol. 104:1374–1381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirdes MM, Siersema PH, Houben MH, Weusten
BL and Vleggaar FP: Stent-in-stent technique for removal of
embedded esophageal self-expanding metal stents. Am J
Gastroenterol. 106:286–293. 2011. View Article : Google Scholar : PubMed/NCBI
|