Introduction
Migraine is a neurological condition with common,
chronic and multifactorial effects, characterized by severe attacks
of headache and dysfunction in the autonomic nervous system. It is
listed as one of the most debilitating neurological diseases and
the seventh-highest cause of disability worldwide (1). Migraine affects 11% of adults, and is
mostly associated with the 25–30 years age group (2). During migraine attacks, severe headache
may last from hours to days and severely impairs the lives of
affected individuals. In addition, migraine increases the risk of
suffering from stroke or myocardial infarction (3).
The prevailing theory on the pathogenesis of
migraine is that it arises from the trigeminovascular system (TMVS)
(4). When stimulating the trigeminal
nerve of a patient with migraine with either smell or pain, the
blood oxygen level-dependent (BOLD) response increases in those
patients. The BOLD response is an indirect method that measures
neuronal activity by recording associated changes in cerebral
hemodynamics (5). This functional
response is associated with an increased patient pain rating on
trigeminal stimulation. During a migraine headache, the TMVS
becomes sensitized and excessively active for an extended period of
time. The TMVS then releases excitatory neurotransmitters and
pro-inflammatory mediators, including calcitonin gene-related
peptide (CGRP), substance P (SP), 5-hydroxytryptamine (5-HT) and
glutamic acid (Glu) (6). SP is a
neurotransmitter that transmits pain, which produces potent
vasodilatation via the direct (endothelium-independent) relaxation
of vascular smooth muscle and has a potent role in relaxing blood
vessels (7). These pro-inflammatory
mediators may directly activate the nociceptors, generate action
potentials or facilitate the firing of the nociceptors, resulting
in peripheral sensitization and hyperalgesia (8,9). They
are also able to evoke the release of additional pro-inflammatory
mediators, causing aseptic neuroinflammation (10). This may initiate the c-fos early gene
expression and c-fos protein expression in the medulla oblongata,
located in the brainstem (11).
Changes in these neurotransmitters lead to strengthening of injury
signals and eventually to migraine (12). Nitroglycerin is able to rapidly
expand cerebral vessels and activate the trigeminal nucleus. The
trigeminal nucleus then releases endogenous nitric oxide, keeping
the TMVS sensitive (10). Therefore,
the model generated using nitroglycerin to induce migraine in
experimental animals is commonly used to study the effectiveness of
novel drugs for treating migraine and the associated mechanisms
(13).
According to previous studies, there are certain
issues with drugs used to treat migraine, including drug resistance
and dependence (14). Among triptan
prescription medications, the most common side effects are racing
heartbeat, warm sensations and chest pressure. Among other
prescription medications, tiredness (69%) and difficulty thinking
clearly (35%) were the most common side effects reported (15).
Traditional Chinese Medicine has the scope and
long-reaching experience for the treatment of migraine (16,17),
with acupuncture being a good example. Scutellaria
baicalensis, Du Liang soft capsule, and other Chinese patent
medicines have also proved effective for migraine (18–21).
Fructus Viticis is the dried fruit of Vitex trifolia
L. var. simplicifolia cham. or Vitex trifolia L. The major
active components of Fructus Viticis are flavonoids,
diterpenes, steroids and iridoids (22). For quality control of Fructus
Viticis, three flavonoids, 3,6,7-trimethylquercetagetin,
casticin and luteolin, are used as reference substances (23). Fructus Viticis is widely used
as a Traditional Chinese Medicine for treating migraine and its
efficacy has been proven (24).
However, it is currently unknown whether Fructus Viticis
provides analgesia or inhibits neuroinflammation induced in the
TMVS during migraine, and the potential roles of Fructus
Viticis to inhibit pain signal transmission have remained
elusive. Therefore, the present study was performed to investigate
the mechanisms of action of Fructus Viticis to relieve
migraine, and for this purpose, a migraine treatment experiment was
designed and two classic analgesic experiments were used. The
underlying mechanisms of the effects of Fructus Viticis
methanolic extract (VME) in rats with nitroglycerin-induced
migraine were explored by determining the plasma levels of Glu,
aspartic acid (Asp), γ-aminobutyric acid (GABA), norepinephrine
(NE), 5-HT, SP and CGRP, as well as c-fos-expressing cells in the
brain stem.
Materials and methods
Chemical materials
Rizatriptan benzoate tablets were purchased from
OuLi Pharmaceutical Co., Ltd. Fructus Viticis samples, which
is the dry fruit of Vitex trifolia L, were obtained from the
Hehuachi tradition herbal medicine market (Chengdu, China) and
authenticated by Professor Yuntong Ma, Chengdu university of
Traditional Chinese Medicine (Chengdu, China). The sample of the
plant material was deposited into a Specimen bank of the Institute
of Materia Medica Integration and Transformation for Brain
Disorders (No. 510106180514WWen001). VME and rizatriptan benzoate
tablets were respectively suspended in a 0.5% carboxymethyl
cellulose (CMC)-Na solution for gastrointestinal administration.
Nitroglycerin (5 mg/ml) was obtained from YiMin Pharmaceutical Co.,
Ltd. 5-HT, GABA, NE, Asp and Glu standards were purchased from
Sigma-Aldrich (Merck KGaA). Rabbit polyclonal c-fos antibody (cat.
no. ab209794) was provided by Abcam. Horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat. no. sc-2004) was
obtained from Santa Cruz Biotechnology, Inc. Aspirin enteric-coated
tablets were purchased from Bayer Healthcare Co., Ltd. Morphine
hydrochloride injection was obtained from Shenyang First
Pharmaceutical Co., Ltd. Normal goat serum was purchased from
Zhejiang Tianhang Biotechnology Co., LTD. Acetic acid was purchased
from Chengdu Kelong Chemical Reagent Factory. Chengdu Alfa
Biotechnology Co., Ltd. provided casticin, luteolin and
3,6,7-trimethylquercetagetin. A SP ELISA kit (cat. no. ERC112-96)
was purchased from ExCell Bio Biotechnology, Inc. A CGRP ELISA kit
(cat. no. E-EL-R0135c) was obtained from Elabscience Biotechnology,
Inc.
Preparation and analysis of VME
extract
The whole Fructus Viticis samples were
shredded and soaked for 30 min at room temperature each in volumes
of methanol that were five times the weight of Fructus
Viticis and underwent reflux extraction at 65°C for 30 min. The
extract was then collected, following which the aforementioned
reflux extraction process was repeated again. For administration of
the extract to the animals, the methanol was removed from the
VME extract in advance in a vacuum using rotary evaporator
where the residue was dissolved in 0.5% CMC-Na (used as the vehicle
in the control and administration groups) at a concentration of 20
g/ml. The Agilent 1260 Infinity High-Performance Liquid
Chromatography instrument (Agilent Technologies, Inc.) was used to
analyze the extract solution and chromatographic separation was
performed using an Ultimate AQ-C18 Column (4.6×250 mm, 5 µm;
Agilent Technologies, Inc.) with a column temperature of 25°C. A
gradient-elution was provided with water as solvent A and
acetonitrile as solvent B, using a flow rate of 1.0 ml/min, the
elution started at 70:30 A:B (v/v) with the gradient gradually
increased to 50:50 (A:B, v/v) for 15 min, and maintained 15 min,
where 20 µl sample was injected and the effluent absorbance was
measured at 254 nm. The concentration of luteolin,
3,6,7-trimethylquercetagetin and casticin was determined, which was
performed using luteolin, 3,6,7-trimethylquercetagetin and casticin
as reference compounds to verify the authenticity and quality of
Fructus Viticis.
Animal experiments
All animal care and experimental procedures
(including the analgesic experiments and migraine experiment) were
approved by the Ethics Committee for Animal Experiments of the
Institute of Materia Medica Integration and Transformation for
Brain Disorders (Chengdu, China). A total of 60 male Sprague Dawley
rats (weight, 250–300 g; age, 8 weeks) were purchased from the
Experimental Animal Center of Sichuan Academy of Medical Sciences
(certificate no. SCXK2013-15). Furthermore, 18 male Sprague Dawley
rats (weight, 180–220 g; age, 6 weeks) were purchased from
Chongqing Enswell Biotechnology Co., Ltd. (certificate no.
SCXK20170003). A total of 48 female and 16 male Kunming mice
(weight, 16–22 g; age, 5 weeks) were obtained from Dashuo
Biological Technology Co., Ltd. (certificate no. SCXK2013-24). All
of the animals were housed at a suitable temperature (23±2°C) and
humidity (40–70%) with ad libitum access to water and food
and a standard 12-h light/dark cycle, where the male animals were
kept separately from the females. They were acclimatized to their
housing environment for seven days prior to experimentation. All
animal experiments complied with the Animal Research: Reporting
in vivo Experiments guidelines and were performed in
accordance with the National Institutes of Health (NIH) Guide for
the Care and Use of Laboratory Animals (NIH Publication no. 8023,
revised 1978).
Analgesic experiments
General
Two classic analgesic experiments were used to
verify the analgesic effect of Fructus Viticis. One was the
hot plate test and the other was the acetic acid-induced writhing
test. Subsequently, a rat model was used to test the effect of
Fructus Viticis on migraine. All products that were injected
into the animals had been sterilized.
Hot plate test
The procedures performed in the present study was
performed according to a previously published protocol (25). First, all female mice were placed on
a hot plate at a temperature of 55±0.5°C to test the pain
threshold; the pain reaction of the mice was paw licking (the time
from placing on the hot plate to the occurrence of paw licking was
recorded as the paw licking time). The mice were tested twice, with
no less than 5 min between each test. Onset of paw licking was
considered as the basic pain threshold. Mice with paw licking times
of >30 sec and <5 sec were removed and the remaining female
mice (with a paw licking time of 5–30 sec) were randomly divided
into four groups. These included a control group, a morphine group,
a VME high-dose group and a VME low-dose group (n=8 for each
group). The VME high-dose, VME low-dose, control and morphine
groups were gastrointestinally administered with corresponding
concentrations of VME or 0.5% CMC-Na. The oral gavage
administration was given once a day for five days. At 30 min prior
to the hot plate test, the morphine group received an
intraperitoneal injection of 0.1% morphine hydrochloride (0.01
ml/g). The pain threshold of all groups was determined at 30, 60,
90 and 120 min after administration. If the paw licking time was
>60 sec, the pain threshold was recorded as 60 sec.
Acetic acid-induced writhing test
The procedures performed for this test were adopted
from that reported previously (26).
A total of 16 male and 16 female mice were randomly divided into
four groups: A control group, aspirin group, VME high-dose group
and VME extract low-dose group. The VME high-dose group, the VME
low-dose group and the control group received gastrointestinal
administration of corresponding concentrations of VME or 0.5%
CMC-Na. The aspirin group was administered with 0.6 g/kg
enteric-coated aspirin by oral gavage. The administration continued
for 5 days, given once a day (27).
Each mouse received 0.7% acetic acid solution in normal saline by
intraperitoneal injection at a dosage of 0.2 ml each hour after the
last drug administration. The pain latency and number of times of
writhing were observed and recorded within 15 min.
Migraine experiment
The method used in the present study was according
to a previously published protocol (21). A total of 60 rats were randomly
divided into five groups: Control, model, rizatriptan, VME
high-dose (7 g/kg) and low-dose (1.75 g/kg) groups (n=12). The
dosage of Fructus Viticis for clinical use in human patients
is 30 g/day, which may be converted to dose for rats of 3.5
g/kg/day based on a weight change algorithm (28). The high-dose group received twice the
clinical dose whereas the low-dose group received half the clinical
dose (29). The clinical dose of
rizatriptan for human patients is 10 mg/d, which may be converted
to 1 mg/kg for rats. The control group, model group, VME high-dose
group and VME low-dose group received the corresponding
concentrations of VME or 0.5% CMC-Na only by oral gavage
administration. The administration continued for 15 days, given
once a day.
At 30 min after the last administration, rats in the
model, rizatriptan, VME high-dose and VME low-dose groups were
injected subcutaneously with nitroglycerin at a dose of 10 mg/kg.
The migraine animal model used in the present study was based on
that established in previous studies (30). The rats in the control group were
injected subcutaneously with physiological saline at a dose of 2
ml/kg. A few minutes later, the rats in the model group appeared
restless, with characteristic symptoms of red ears, head
scratching, cage climbing and body shaking. These symptoms lasted
for ~2 h. Red ears, head scratching and cage climbing signs were
more typical than other symptom characteristics. These three
symptoms were therefore used to evaluate whether the migraine model
was successfully established. For each rat, the latency of ear
redness was recorded. If latency was >60 min, then the latency
was recorded as 60 min. The numbers of times of head scratching and
cage climbing within 150 min were recorded.
Quantitative analysis of 5-HTe, NE,
Glu, Asp and GABA
The method used was described previously (31–33). At
150 min after modeling, six randomly selected rats in each group
were euthanized. First, 3% pentobarbital sodium was
intraperitoneally injected at a dosage of 60 mg/kg and when the
righting reflex disappeared, rats were decapitated. The brain was
taken out and rapidly frozen at −80°C.
For testing for NE and 5-HT, a standard curve was
recorded and analysis was performed using an Acquity
Ultra-Performance Liquid Chromatography/Synapt G2 Quadrupole
Time-of-Flight mass spectrometer (Waters China, Ltd.). The entire
brain was collected for amino acid detection. Analysis of Asp, GABA
and Glu was performed using the Agilent 1260 Infinity system
(Agilent Technologies, Inc.) coupled with a Nova-Pak®
C18 (5 µm) column (Waters China, Ltd). The chromatographic analysis
method used was that described in the AccQ-Tag amino acid chemical
reagent package (Waters China, Ltd.). The Waters AccQ-Tag amino
acid chemical reagent package contains Amino acid derivatization
reagent, Nova-Pak® C18 (5 µm) column, amino acid
standards and an instruction of operation and analysis.
ELISA for CGRP and SP
At 150 min after modeling, six rats from each group
were anesthetized with 1.5% isoflurane. Blood samples were
collected from the abdominal aorta. Subsequently, CGRP and SP were
detected using the ELISA kits according to the manufacturer's
protocols.
Immunohistochemical analysis
Immunohistochemical staining of the brain stem was
performed using a previously described method (34–36). The
brain stem was fixed with fresh 4% paraformaldehyde solution.
Paraffin-embedded sections with a thickness of 4 µm were then
prepared, where the slices were deparaffinized and rehydrated using
a decreasing ethanol gradient before being blocked with 10% normal
goat serum at room temperature for 1 h. The tissue sections were
incubated with rabbit anti-c-fos primary antibody (1:50) at 4°C for
48 h before incubation with goat anti-rabbit secondary antibody
(1:200) at 25°C for 1 h. Then nuclei were counterstained with
hematoxylin at room temperature for 2 min. The number of
c-fos-immunoreactive cells (c-fos IR cells) was determined.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Each group of data was analyzed using SPSS 25 software
(IBM Corp.). Kolmogorov-Smirnov was used to verify whether the data
conformed to the normal distribution. Data with a normal
distribution were compared using one-way analysis of variance
followed by Tukey's post-hoc test, P<0.05 or P<0.01 were
considered to indicate statistically significant differences.
Statistical analyses of the results were performed out in a
double-blinded fashion.
Results
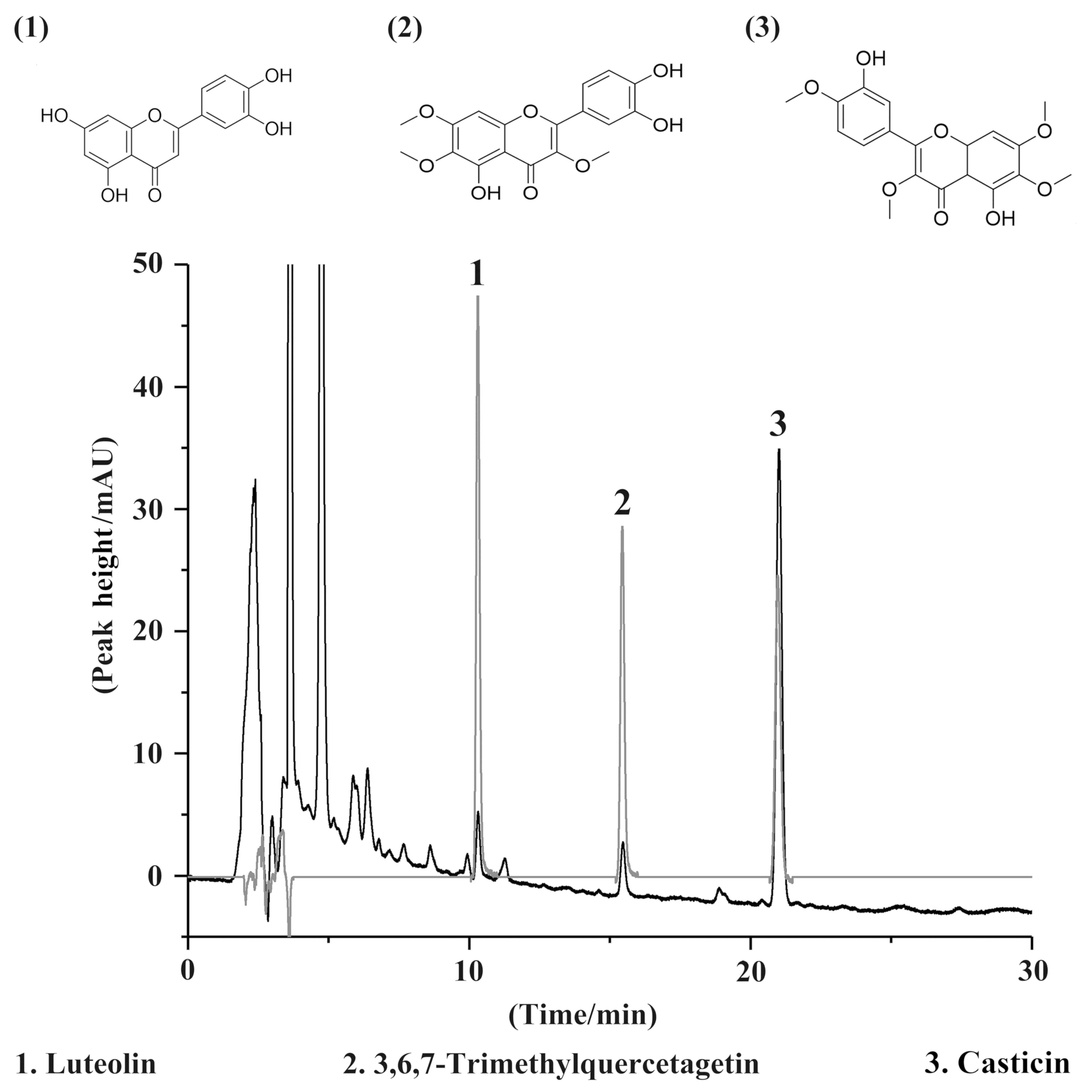
Quality control of VME
Luteolin, 3,6,7-trimethylquercetagetin and casticin
are characteristic components of Fructus Viticis and were
detected by HPLC. A chromatogram of the extracts and reference
compounds is provided in Fig. 1. All
three compounds were detected in the VME and the content of
casticin met the Chinese Pharmacopoeia standard of casticin
>0.03% (37).
Analgesic effect of VME
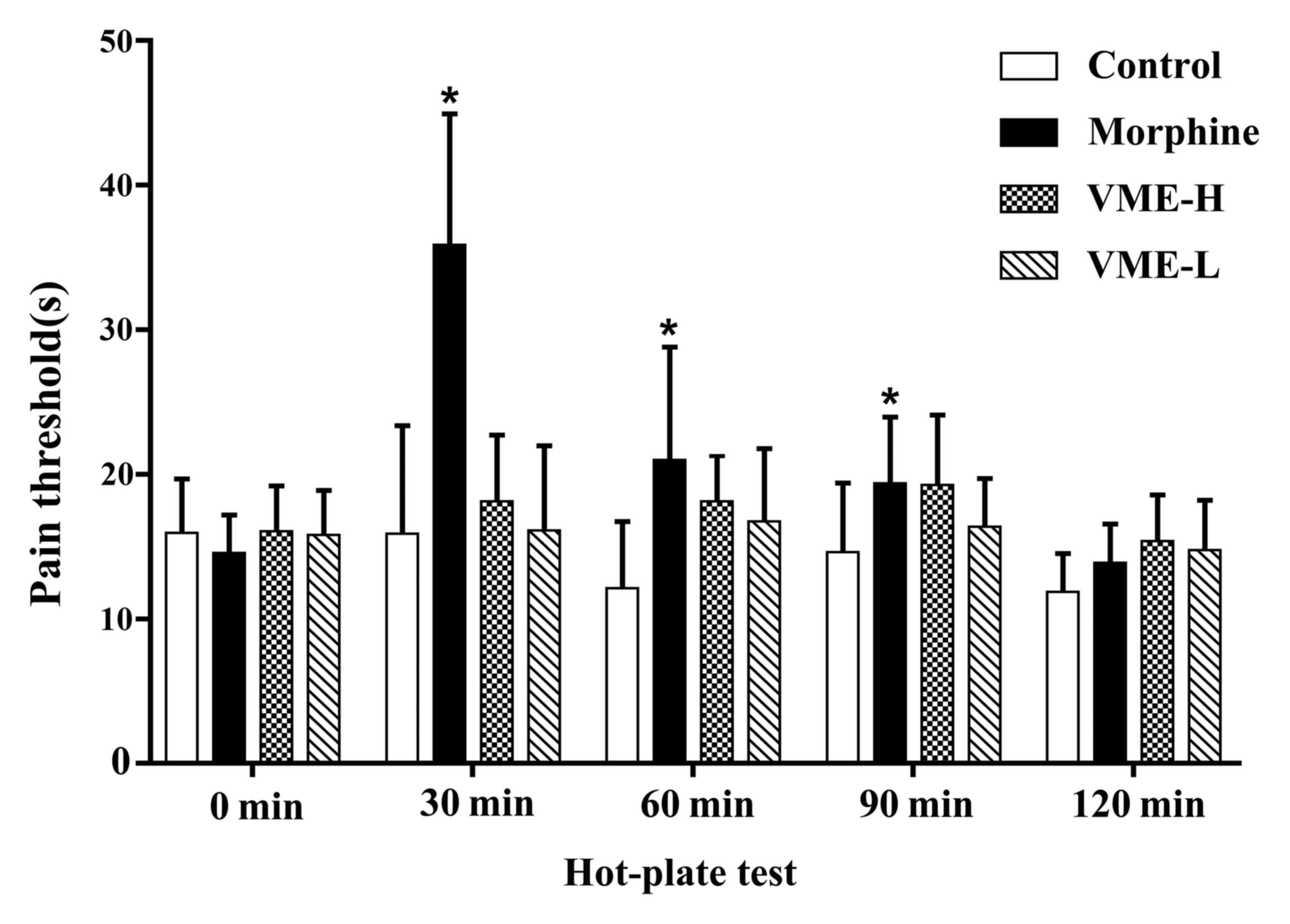
The results of the hot plate test are provided in
Fig. 2, indicating that the morphine
significantly decreased the pain threshold at 30, 60 and 90 min
(P<0.05), and the high dose of VME decreased the pain threshold
slightly at the same time points, but no significance was observed
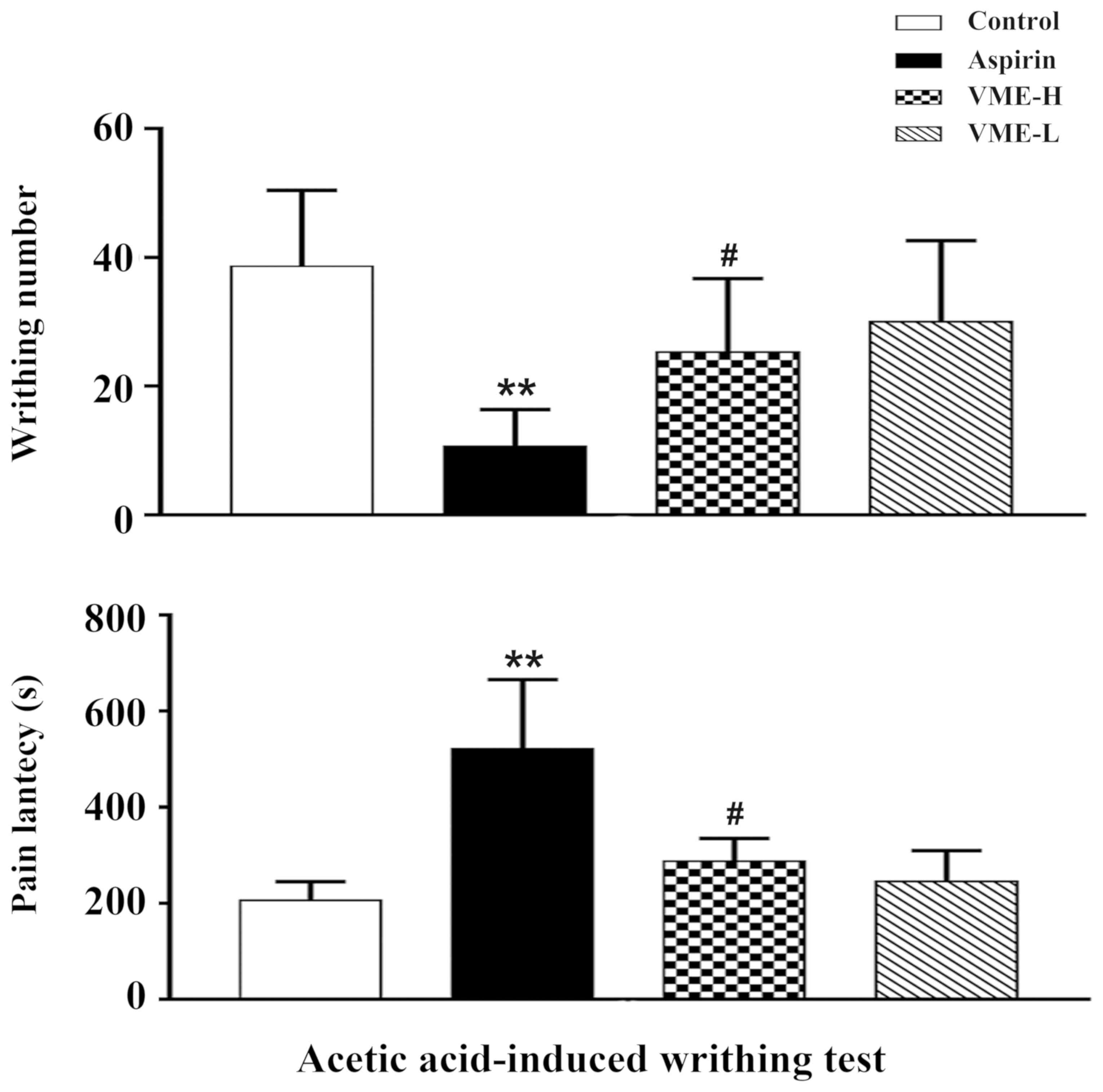
(P>0.05). As depicted in Fig. 3,
the acetic acid-induced writhing test indicated that the aspirin
obviously reduced the pain latency and the number of times of
writhing (P<0.01 for each). In addition, the high dose VME
reduced the pain latency (P<0.05) and the number of times of
writhing (P<0.05).
Effect of VME on migraine-like
behaviour
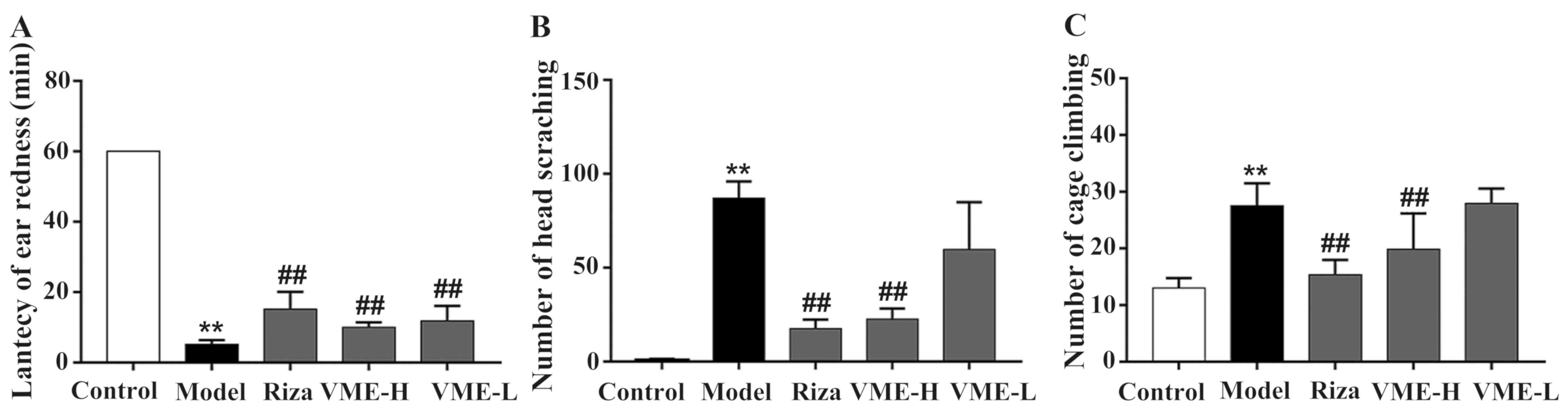
The rats of the model group had migraine-like
responses after 5 min of nitroglycerin injection, including the use
of fore- or hind paws to scratch the head and development of ear
redness. There was no ear redness in the control group after
subcutaneous injection of normal saline and within 120 min, only
one or two episodes of head scratching and a few incidents of cage
climbing were observed (Fig. 4),
which may have been at random. Compared with the model group, there
were significant differences regarding these parameters (P<0.01
for each). The rats in the model group exhibited shorter latencies
of ear redness, as well as frequent head scratching and cage
climbing, with an increased amount of discomfort-associated
behaviour and a modeling success rate of 100% (P<0.01). The
latency of ear redness in the rizatriptan and VME high-dose group
was significantly higher compared with that in the model group
(P<0.01; Fig. 4A). The amount of
head scratching and cage climbing was also significantly lower than
that of the model group (P<0.01; Fig.
4B and C). The effect in the VME low-dose group compared with
the trend in the model group was not statistically significant.
Effect of VME on neurotransmitters and
amino acids in the rat model of migraine
As presented in Fig.
5, the effect of the test drugs on various neurotransmitters in
the brain of rats with migraine was assessed. The 5-HT levels in
the model group were lower than those in the control group
(P<0.05), while pre-treatment with rizatriptan and VME at the
high dose inhibited this phenomenon (P<0.01; P<0.05, Fig. 5A). Furthermore, subcutaneous
injection of nitroglycerin reduced the level of NE, while this was
prevented by pre-treatment with high-dose rizatriptan and VME
(P<0.01, for each; Fig. 5B). The
low dose of VME had no significant effect on rats with migraine
(P>0.05).
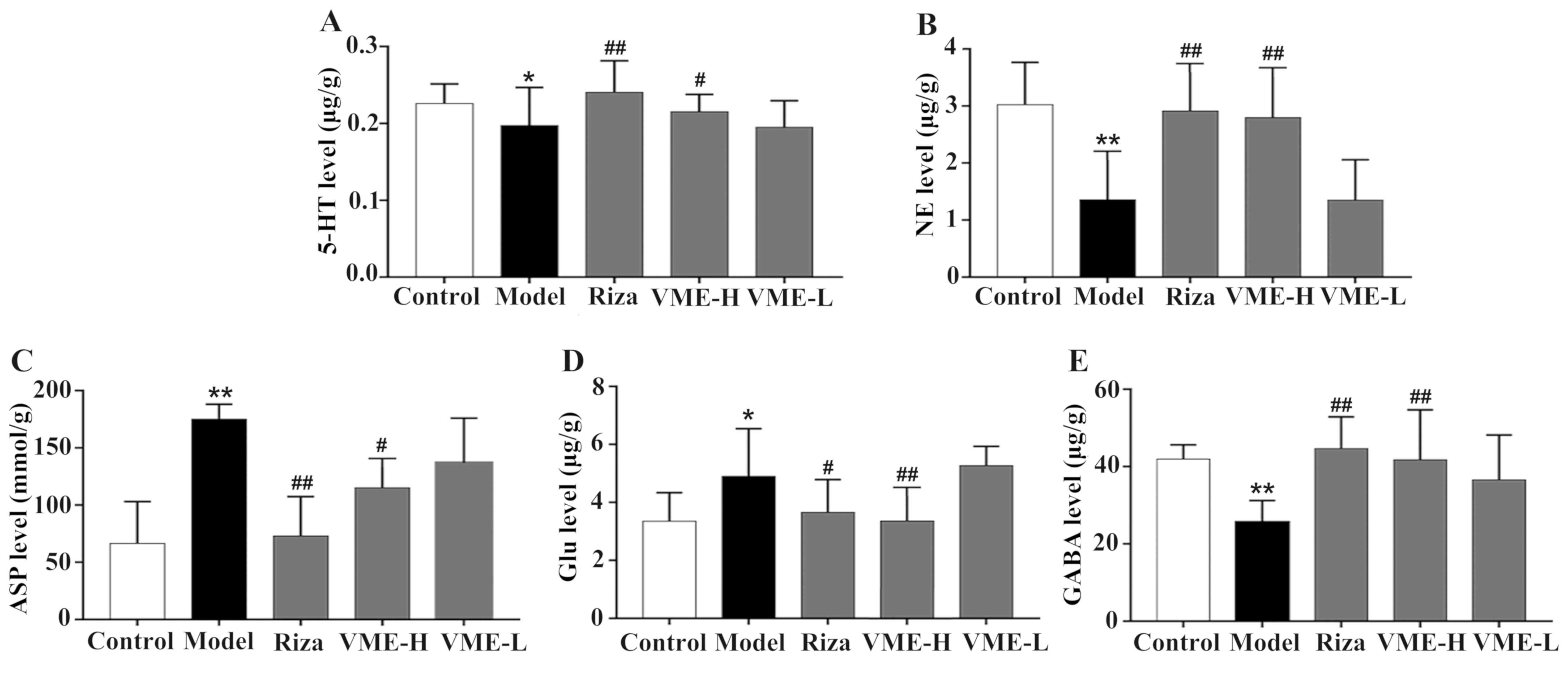 | Figure 5.Effect of VME on the levels of (A)
5-hydroxytryptamine, (B) NE, (C) ASP, (D) Glu and (E) GABA induced
by subcutaneous injection of nitroglycerin. Values are expressed as
the mean ± standard deviation (n=6). *P<0.05, **P<0.01 vs.
control group; #P<0.05, ##P<0.01 vs.
model group. Riza, rizatriptan benzoate; VME-H/L, high/low
concentration of VME; NE, norepinephrine; ASP, aspartic acid; Glu,
glutamic acid; GABA, γ-aminobutyric acid. |
The amino acid contents are provided in Fig. 5C-E. The Asp and Glu levels were
increased following subcutaneous injection of nitroglycerin
(P<0.01), which was significantly inhibited by pre-treatment
with high-dose VME (P<0.05 and P<0.01, respectively), which
was similar to the effect of rizatriptan (P<0.01 and P<0.05,
respectively). The levels of GABA were decreased in the model group
(P<0.05), and in comparison, they were significantly increased
in the rizatriptan and high-dose VME groups (P<0.01).
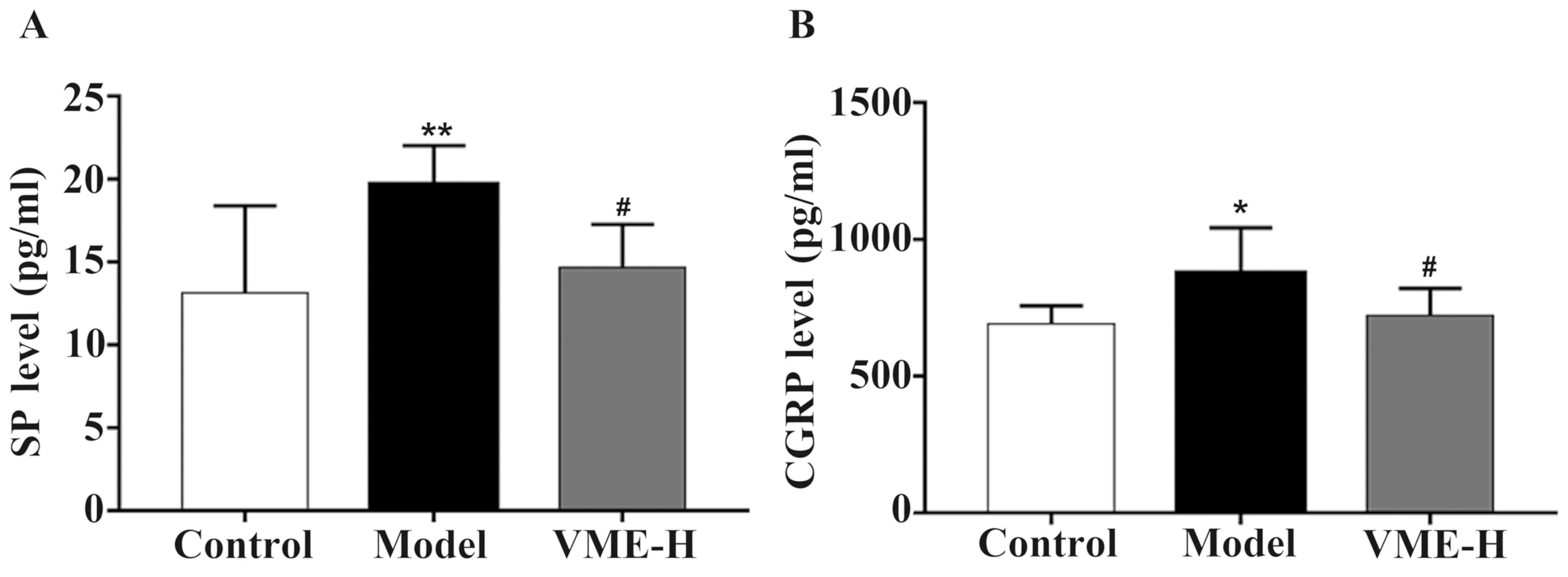
Effect of VME on SP and CGRP in the
rat model of migraine
The levels of SP and CGRP are provided in Fig. 6A and B, respectively. The levels of
SP and CGRP were increased following subcutaneous injection of
nitroglycerin (P<0.01 and P<0.05, respectively), which was
significantly inhibited by pre-treatment high-dose VME (P<0.05).
There was no significant difference in the SP and CGRP content
between the high-dose VME and control groups (P>0.05).
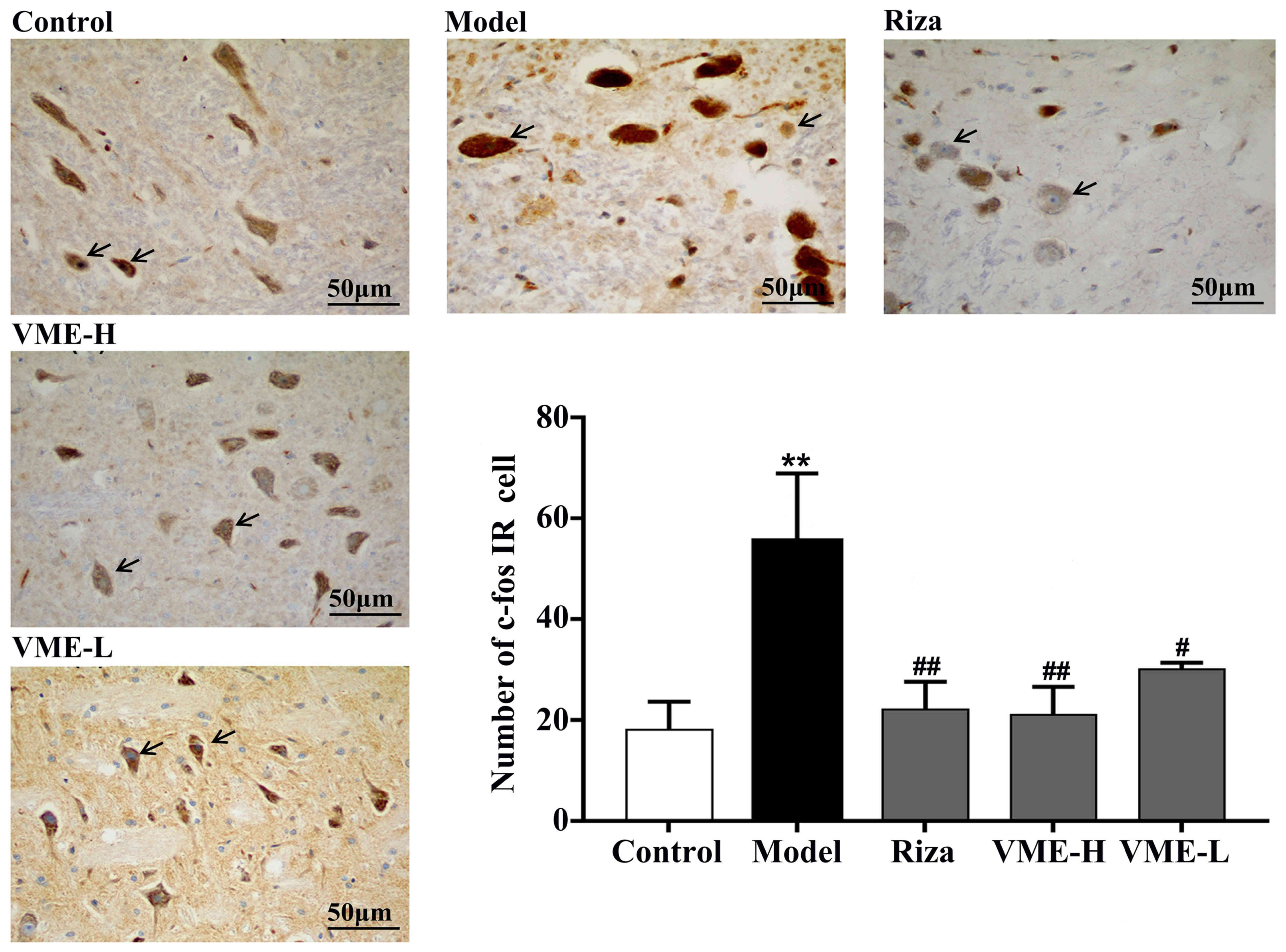
Effect of VME on c-fos expression in
brain stem neurons of the rat model of migraine
C-fos-positive staining in the brain stem neurons
was assessed as a central pain sensory marker. The number of c-fos
IR cells in the rats of different groups was determined (Fig. 7). In the migraine model group, the
number of c-fos IR cells was significantly higher than that of the
control group (P<0.01). The number of c-fos IR cells in the
brain stem of the VME high-dose group, the rizatriptan and VME
low-dose groups were significantly lower than that in the model
group (P<0.01 or P<0.05), indicating that VME reduced the
migraine-associated expression of c-fos in the brain stem.
Discussion
Migraine attacks seriously affect the lives of
patients. During the onset process of migraine, patients experience
irritability (38). Several studies
indicated that nitroglycerin administration in rodents may be used
to generate a model to resemble migraine in humans (13,39).
After intraperitoneal injection of nitroglycerin, the level of
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptor and the phosphorylation of glutamate A1 at Ser831 in the
posterior spinal trigeminal nerve were reported to be increased and
central sensitization was enhanced (40). Nitroglycerin, as a nitric oxide
promoter (41), activates the TMVS
and induces hyperalgesia in rats. Model rats exhibit similar pain
responses to those of patients with migraine, and associated
signs/behaviours in those rats include ear redness, head scratching
and cage climbing. Therefore, behavioural testing may be used to
assess the level of migraine pain in rats. In the present study,
the behavioural tests indicated that rats in the model group
exhibited characteristics of restlessness, with an increased
latency of ear redness, head scratching and cage climbing, whereas
pre-treatment with Fructus Viticis alleviated the
migraine-like headache response. Thus, the rat model of migraine
was successfully established. According to different requirements,
a variety of migraine models have been previously established, and
for each model, it should be determined whether it was successfully
confirmed based on the corresponding behavioural criteria. In
addition to the migraine model induced by subcutaneous injection of
nitroglycerin, further examples include the dural electrical
stimulation model and the electrophysiology chemical provocation
model (42–44).
NE and 5-HT are neurotransmitters, the
downregulation of which are associated with migraine; consequently,
they are widely applied as an auxiliary parameter in the diagnosis
of migraine (43). Subcutaneous
injection of nitroglycerin as an external stimulus may stimulate
excitatory signaling in the TMVS. NE and 5-HT are critical
regulatory factors and may inhibit transcription of the CGRP gene
and prevent CGRP release to decrease the level of CGRP. Drugs that
reduce CGRP have been used in the clinic to alleviate migraine
(45). Simultaneously, the two
neurotransmitters are able to increase the binding of GABA to
glycine neurons and α2 5-HT receptors to reduce the damaging effect
of external stimuli to the TMVS (46). In the present study, when compared to
the model group, the 5-HT and NE levels of rats in the high-dose
VME groups were increased.
The trigeminal nucleus is where the cell bodies of
the trigeminal nerve are aggregated in the brain stem. It is the
largest cranial nucleus, spanning the entire brain stem and
extending from the midbrain to the medulla. SP and CGRP are
vasoactive neuropeptides released by activated peripheral
nociceptive sensory nerve terminals (47). It is generally recognized that
migraine headaches result from activation and sensitization of
trigeminal sensory afferent fibers, leading to the release of
neuropeptides including CGRP and SP. This triggers an inflammatory
cascade that causes neurogenic inflammation (48). In response to prolonged TMVS, SP and
CGRP are released from the trigeminal sensory nerves in the area of
the dural blood vessels, leading to endothelium-dependent
vasodilation, increased microvascular permeability, as well as
subsequent plasma and protein extravasation, causing
neuroinflammation. SP is the prime mediator, via neurokinin (NK)-1
receptors, of plasma leakage at the site of inflammation (49,50),
whereas CGRP and SP induce vasodilatation. The involvement of SP in
plasma leakage and neurogenic inflammation has been well
established by the ability of NK-1 receptor antagonists and SP
immunoneutralization to attenuate neurogenic exudative responses to
a variety of stimuli (51). A
significant increase in plasma SP and CGRP levels has been
demonstrated during the headache phase of migraine.
In the present study, VME was indicated to have
definite analgesic effects, making it apt for migraine treatment.
The mechanism for this is likely linked to the regulation of
abnormal contents of amino acids and neurotransmitters and the
reduction of SP, CGRP and positive expression of c-fos in the brain
stem. There is an accumulation of evidence demonstrating that
numerous traditional Chinese medicines, which are widely used for
treating various neurological headaches, confer beneficial and
reliable therapeutic effects (52–54).
Fructus Viticis is a common prescription in Traditional
Chinese Medicine. It has been reported that 25% percent of
Fructus Viticis use is for clinical purposes (24). In addition, Fructus Viticis is
an ideal drug for migraine prevention for three reasons: First,
casticin, the major active component of Fructus Viticis, is
anti-inflammatory and inhibits nitrous oxide production in cells
(55). Luteolin is another flavone
of Fructus Viticis and is able to ameliorate hyperalgesia in
the central nervous system (56). A
previous study demonstrated that luteolin has anti-nociceptive
effects on acute thermal pain and persistent inflammatory pain in
rodents (57). Previous research has
also suggested that luteolin displays anxiolytic and sedative
effects through interaction with benzodiazepine binding sites
(58). Finally, Fructus
Viticis is a traditional medicine that is widely used in Asian
countries, including China, Korea and Japan, to treat migraine,
headache and inflammation (57,59).
Data from the present study suggest that the
methanolic extract of Fructus Viticis may be used to treat
disturbances of amino acid metabolism. During the migraine aura and
headache phase, amino acid levels are abnormal. When the harmful
signal is generated, extreme depolarization of glial and neuronal
cell membranes results in disruption of ionic gradients, a rise in
extracellular potassium concentrations and release of glutamate
(12). Furthermore, the release of
neurotransmitter GABA may inhibit transmission of nerve signaling,
modulate pain thresholds and produce analgesic effects (60–62).
These two different types of amino acid have opposite functions.
The present results indicated that high-dose and low-dose VME
significantly increased GABA levels in rats. Furthermore, SP, Glu
and Asp have been proven to mediate the transmission of excitatory
signals to the synapse (63,64). In clinical studies on migraine
patients, elevated levels of Glu and Asp were detected in the
cerebrospinal fluid (65). The
balance between the two types of neurotransmitter provides a
framework in which other factors regulate functions of the human
brain (66). The experimental
results of the present study indicated that the content of Asp and
Glu was high in the model group and decreased in the drug treatment
groups.
The present results indicated that methanolic
extract of Fructus Viticis alleviated pain-like behavior in
a rat models of migraine, reduced pain sensitization of the
trigeminal nerve and decreased the expression of c-fos. c-fos
protein, encoded by the c-fos gene, is often used to evaluate
nervous activity (67). Subcutaneous
injection of nitroglycerin in rats may lead to elevation of CGRP
(9), which in turn is able to induce
the expression of the c-fos gene in the trigeminal nucleus caudalis
(68). As indicated in a previous
study, the amount of c-fos-expressing neurons is increased in the
brain stem nuclei of rats with migraine (11). C-fos is commonly accepted as a neural
marker of pain in the central nervous system. Upon apparent
hyperalgesic sensitization of the central nervous system, c-fos
protein is expressed. Therefore, the amount of c-fos IR neurons
may, to a certain degree, determine the extent of migraine. Due to
its immediacy, it may be assumed that c-fos is one of the earliest
indicators of successful migraine modeling. However, this is
nonspecific, as c-fos is also expressed during tumorigenesis as a
subcomponent of the macromolecular activator protein 1 complex
(69), which is associated with
neoplastic transformation and progression. For instance, it was
reported that c-fos has a role in endometrial, cervical and
colorectal cancer (70,71).
In conclusion, the present study demonstrated that
VME has an analgesic effect in mice and is able to mitigate the
migraine-like pain response to nitroglycerin in model rats. CGRP
and SP, as vasoactive neuropeptides released by activated
peripheral nociceptive sensory nerve terminals during migraine,
were reduced in the group treated with high-dose VME. The
therapeutic efficacy is likely a result of the inhibitory effect on
the transmission of trigeminal nerve injury signals. The present
study provides a further scientific basis for the anti-migraine
effects of Fructus Viticis.
Acknowledgements
The authors gratefully acknowledge the provided the
hot plate and other experimental facilities of the Institute of
Material Medica Integration and Transformation for Brain Disorders
(Chengdu, China).
Funding
This study was supported by the National Nature
Science Foundation of China (grant no. 81303082) and Major R&D
(Major Projects) Projects in Sichuan Province (grant no.
19ZDYF0600).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from teh corresponding author on reasonable
request.
Authors' contributions
SX and KF conceived the project, WW, TP and LQ
performed the experiments. HC performed data analysis. JW
supervised the project. WW drafted and revised the manuscript.
Ethics approval and consent to
participate
The protocols were approved by the animal
experimental ethics committee of Ethics Committee for Animal
Experiments of the Institute of Materia Medica Integration and
Transformation for Brain Disorders (grant no. 20131017; Chengdu,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2015 Neurological Disorders
Collaborator Group, : Global, regional, and national burden of
neurological disorders during 1990–2015: A systematic analysis for
the Global Burden of Disease Study 2015. Lancet Neurol. 16:877–897.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patton GC, Olsson CA, Skirbekk V, Saffery
R, Wlodek ME, Azzopardi PS, Stonawski M, Rasmussen B, Spry E,
Francis K, et al: Adolescence and the next generation. Nature.
554:458–466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mawet J, Kurth T and Ayata C: Migraine and
stroke: In search of shared mechanisms. Cephalalgia. 35:165–181.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bolay H, Reuter U, Dunn AK, Huang Z, Boas
DA and Moskowitz MA: Intrinsic brain activity triggers trigeminal
meningeal afferents in a migraine model. Nat Med. 8:136–142. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asghar MS, Hansen AE, Larsson HB, Olesen J
and Ashina M: Effect of CGRP and sumatriptan on the BOLD response
in visual cortex. J Headache Pain. 13:159–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iyengar S, Ossipov MH and Johnson KW: The
role of calcitonin gene-related peptide in peripheral and central
pain mechanisms including migraine. Pain. 158:543–559. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyoshi H and Nakaya Y: Calcitonin
gene-related peptide activates the K+ channels of vascular smooth
muscle cells via adenylate cyclase. Basic Res Cardiol. 90:332–336.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basbaum AI, Bautista DM, Scherrer G and
Julius D: Cellular and molecular mechanisms of pain. Cell.
139:267–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Demartini C, Tassorelli C, Zanaboni AM,
Tonsi G, Francesconi O, Nativi C and Greco R: The role of the
transient receptor potential ankyrin type-1 (TRPA1) channel in
migraine pain: Evaluation in an animal model. J Headache Pain.
18:942017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borkum JM: Migraine triggers and oxidative
stress: A narrative review and synthesis. Headache. 56:12–35. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bohár Z, Fejes-Szabó A, Tar L, Varga H,
Tajti J, Párdutz Á and Vécsei L: Evaluation of c-Fos
immunoreactivity in the rat brainstem nuclei relevant in migraine
pathogenesis after electrical stimulation of the trigeminal
ganglion. Neurol Sci. 34:1597–1604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dodick DW: Migraine. Lancet.
391:1315–1330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moye LS and Pradhan AAA: Animal model of
chronic migraine-associated pain. Curr Protoc Neurosci.
80:9.60.1–9.60.9. 2017. View
Article : Google Scholar
|
|
14
|
Rains JC, Penzien DB, McCrory DC and Gray
RN: Behavioral headache treatment: History, review of the empirical
literature, and methodological critique. Headache. 45 (45
Suppl):S92–S109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gallagher RM and Kunkel R: Migraine
medication attributes important for patient compliance: Concerns
about side effects may delay treatment. Headache. 43:36–43. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen DQ, Cao G, Chen H, Argyopoulos CP, Yu
H, Su W, Chen L, Samuels DC, Zhuang S, Bayliss GP, et al:
Identification of serum metabolites associating with chronic kidney
disease progression and anti-fibrotic effect of
5-methoxytryptophan. Nat Commun. 10:14762019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen DQ, Feng YL, Cao G and Zhao YY:
Natural products as a source for antifibrosis therapy. Trends
Pharmacol Sci. 39:937–952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Chen J, Li Y, Sun X, Chang X,
Zheng H, Gong B, Huang Y, Yang M, Wu X, et al: The Long-term effect
of acupuncture for migraine prophylaxis: A randomized clinical
trial. JAMA Intern Med. 177:508–515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun YY, Zhang WJ, Dong CL, Zhang XF, Ji J,
Wang X, Wang L, Hu WL, Du WJ, Cui CL, et al: Baicalin alleviates
Nitroglycerin-induced migraine in rats via the trigeminovascular
system. Phytother Res. 31:899–905. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Pan X, Xu Y, Lu X, He S, He R and
Gong M: Optimization of combinations of ginsenoside-Rg1,
ginsenoside-Rb1, evodiamine and rutaecarpine for effective therapy
of mouse migraine. J Nat Med. 70:207–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou M, Tang Q, Xue Q, Zhang X, Liu Y, Yang
S, Chen L and Xu X: Pharmacodynamic action and mechanism of Du
Liang soft capsule, a traditional Chinese medicine capsule, on
treating nitroglycerin-induced migraine. J Ethnopharmacol.
195:231–237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Xin HL, Zhang QY, Zheng HC, Rahman K
and Qin LP: Anti-nociceptive and anti-hyperprolactinemia activities
of Fructus Viticis and its effective fractions and chemical
constituents. Phytomedicine. 14:668–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan R, Wang D, Yu Z, Wang X and Lan T:
Preparative isolation and purification of the active components
from Viticis Fructus by high-speed counter-current
chromatography. Se Pu. 28:1043–1047. 2010.(In Chinese). PubMed/NCBI
|
|
24
|
Yan YY, Dai JZ, Yang CX and Zhang GM:
Analysis of traditional Chinese medicine syndromes in 159 German
migraine patients. Zhongguo Zhong Yao Za Zhi. 42:2599–2605.
2017.(In Chinese). PubMed/NCBI
|
|
25
|
Liu P, Xing B, Chu Z, Liu F, Lei G, Zhu L,
Gao Y, Chen T and Dang YH: Dopamine D3 receptor knockout mice
exhibitabnormal nociception in a sex-different manner. J Neurosci
Res. 95:1438–1445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang G, Hu Z, Song X, Cui Q, Fu Q, Jia R,
Zou Y, Li L and Yin Z: Analgesic and Anti-inflammatory activities
of resveratrol through classic models in mice and rats. Evid Based
Complement Alternat Med. 2017:51975672017.PubMed/NCBI
|
|
27
|
Zakaria A, Jais MR and Ishak R: Analgesic
properties of nigella sativa and eucheuma cottonii extracts. J Nat
Sci Biol Med. 9:23–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nair AB and Jacob S: A simple practice
guide for dose conversion between animals and human. J Basic Clin
Pharm. 7:27–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu W, Dai Y, Ma T, Wei J, Chen H and Xu S:
Tongluo Xingnao effervescent tablet reverses memory deficit and
reduces plaque load in APPswe/PS1dE9 mice. Exp Ther Med.
15:4005–4013. 2018.PubMed/NCBI
|
|
30
|
Burstein R, Noseda R and Borsook D:
Migraine: Multiple processes, complex pathophysiology. J Neurosci.
35:6619–6629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pradhan AA, Smith ML, McGuire B, Tarash I,
Evans CJ and Charles A: Characterization of a novel model of
chronic migraine. Pain. 155:269–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Y, Liu S, Shu H, Xing Y and Tao F:
AMPA receptor GluA1 Ser831 phosphorylation is critical for
nitroglycerin-induced migraine-like pain. Neuropharmacology.
133:462–469. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ben Aissa M, Tipton AF, Bertels Z, Gandhi
R, Moye LS, Novack M, Bennett BM, Wang Y, Litosh V, Lee SH, et al:
Soluble guanylyl cyclase is a critical regulator of
migraine-associated pain. Cephalalgia. 38:1471–1484. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Becerra L, Bishop J, Barmettler G, Kainz
V, Burstein R and Borsook D: Brain network alterations in the
inflammatory soup animal model of migraine. Brain Res. 1660:36–46.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Munro G, Jansen-Olesen I and Olesen J:
Animal models of pain and migraine in drug discovery. Drug Discov
Today. 22:1103–1111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
D'Andrea G and Leon A: Pathogenesis of
migraine: From neurotransmitters to neuromodulators and beyond.
Neurol Sci. 31 (Suppl 1):S1–S7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
State Pharmacopoeia Committee, .
Pharmacopoeia of the People's Republic of China. Part I. Beijing:
China Medical Science and Technology Press. 2015.
|
|
38
|
Silberstein SD, Dodick DW, Bigal ME, Yeung
PP, Goadsby PJ, Blankenbiller T, Grozinski-Wolff M, Yang R, Ma Y
and Aycardi E: Fremanezumab for the preventive treatment of chronic
migraine. N Engl J Med. 377:2113–2122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akerman S, Holland PR, Lasalandra MP and
Goadsby PJ: Endocannabinoids in the brainstem modulate dural
trigeminovascular nociceptive traffic via CB1 and ‘triptan’
receptors: Implications in migraine. J Neurosci. 33:14869–14877.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Malhotra R: Understanding migraine:
Potential role of neurogenic inflammation. Ann Indian Acad Neurol.
19:175–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martins LB, Teixeira AL and Domingues RB:
Neurotrophins and migraine. Vitam Horm. 104:459–473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rosa AC and Fantozzi R: The role of
histamine in neurogenic inflammation. Br J Pharmacol. 170:38–45.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Williamson DJ, Hargreaves RJ, Hill RG and
Shepheard SL: Intravital microscope studies on the effects of
neurokinin agonists and calcitonin gene-related peptide on dural
vessel diameter in the anaesthetized rat. Cephalalgia. 17:518–524.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alvaro G and Di Fabio R: Neurokinin 1
receptor antagonists-current prospects. Curr Opin Drug Discov
Devel. 10:613–621. 2007.PubMed/NCBI
|
|
45
|
Zhou L, Pinyi C, Ling L, Zhang Y, Liu X,
Wu Y, Jiang L, Cheng D, Huang W, Pettigrew JC and Yi D: Systematic
review and meta-analysis of traditional Chinese medicine in the
treatment of migraines. Am J Chin Med. 41:1011–1025. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu HQ, Zhou YH and Wang XL: Clinical study
on effect of Xiaoyao Nose Drops in stopping episode of Migraine.
Chin J Integr Med. 12:112–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li JC, Shen XF, Meng XL, Zhang Y and Lai
XR: Analgesic effect and mechanism of the three TCM-herbal
drug-combination Tou Feng Yu pill on treatment of migraine.
Phytomedicine. 18:788–794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song HM, Park GH, Park SB, Kim HS, Son HJ,
Um Y and Jeong JB: Vitex rotundifolia Fruit Suppresses the
proliferation of human colorectal cancer cells through
Down-regulation of Cyclin D1 and CDK4 via proteasomal-dependent
degradation and transcriptional inhibition. Am J Chin Med.
46:191–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hara K, Haranishi Y, Terada T, Takahashi
Y, Nakamura M and Sata T: Effects of intrathecal and
intracerebroventricular administration of luteolin in a rat
neuropathic pain model. Pharmacol Biochem Behav. 125:78–84. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Backhouse N, Delporte C, Apablaza C,
Farías M, Goïty L, Arrau S, Negrete R, Castro C and Miranda H:
Antinociceptive activity of Buddleja globosa (matico) in several
models of pain. J Ethnopharmacol. 119:160–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ishisaka M, Kakefuda K, Yamauchi M,
Tsuruma K, Shimazawa M, Tsuruta A and Hara H: Luteolin shows an
antidepressant-like effect via suppressing endoplasmic reticulum
stress. Biol Pharm Bull. 34:1481–1486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee C, Lee JW, Jin Q, Lee HJ, Lee SJ, Lee
D, Lee MK, Lee CK, Hong JT, Lee MK and Hwang BY: Anti-inflammatory
constituents from the fruits of Vitex rotundifolia. Bioorg Med Chem
Lett. 23:6010–6014. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ambrosini A, Di Lorenzo C, Coppola G and
Pierelli F: Use of Vitex agnus-castus in migrainous women with
premenstrual syndrome: An open-label clinical observation. Acta
Neurol Belg. 113:25–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Noseda R, Borsook D and Burstein R:
Neuropeptides and neurotransmitters that modulate thalamo-cortical
pathways relevant to migraine headache. Headache. 57 (Suppl
2):S97–S111. 2017. View Article : Google Scholar
|
|
55
|
Andreou AP, Shields KG and Goadsby PJ:
GABA and valproate modulate trigeminovascular nociceptive
transmission in the thalamus. Neurobiol Dis. 37:314–323. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Janssen SP, Truin M, Van Kleef M and
Joosten EA: Differential GABAergic disinhibition during the
development of painful peripheral neuropathy. Neuroscience.
184:183–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu X, Han Y, Xiong W, Liu W, Lu S, Li J,
Wang H and Fan Z: Effects of heating coagulation of middle
meningeal artery on plasma CGRP level and c-fos expression in
migraine rat triggered by nitroglycerin. Neurol Sci. 32:589–594.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
van IJzendoorn DGP, Forghany Z, Liebelt F,
Vertegaal AC, Jochemsen AG, Bovée JVMG, Szuhai K and Baker DA:
Functional analyses of a human vascular tumor FOS variant identify
a novel degradation mechanism and a link to tumorigenesis. J Biol
Chem. 292:21282–21290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang YH, Liang S, Xu DS, Lin X, He CY,
Feng Y and Hong YL: Effect and mechanism of senkyunolide I as an
anti-migraine compound from Ligusticum chuanxiong. J Pharm
Pharmacol. 63:261–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tassorelli C and Joseph SA: Systemic
nitroglycerin induces Fos immunoreactivity in brainstem and
forebrain structures of the rat. Brain Res. 682:167–181. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pan X, Wang M, Wu Y, Lu X, Shang Y, Xu Y,
Zhai Y, Li J, Li Z and Gong M: Identification of active ingredients
in Wuzhuyu decoction improving migraine in mice by spectral
efficiency association. Mol Med Rep. 12:1524–1534. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang ZH, Vaziri ND, Wei F, Cheng XL, Bai
X and Zhao YY: An integrated lipidomics and metabolomics reveal
nephroprotective effect and biochemical mechanism of Rheum
officinale in chronic renal failure. Sci Rep. 6:221512016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Q, Zhao Y, Guo Y, Cao DY, Tian YL,
Yao FR and Wang HS: Electrophysiological evidence for the
interaction of substance P and glutamate on Adelta and C afferent
fibre activity in rat hairy skin. Clin Exp Pharmacol Physiol.
33:1128–1133. 2007. View Article : Google Scholar
|
|
64
|
Gundersen V, Chaudhry FA, Bjaalie JG,
Fonnum F, Ottersen OP and Storm-Mathisen J: Synaptic vesicular
localization and exocytosis of L-aspartate in excitatory nerve
terminals: A quantitative immunogold analysis in rat hippocampus. J
Neurosci. 18:6059–6070. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vikelis M and Mitsikostas DD: The role of
glutamate and its receptors in migraine. CNS Neurol Disord Drug
Targets. 6:251–257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gao R and Penzes P: Common mechanisms of
excitatory and inhibitory imbalance in schizophrenia and autism
spectrum disorders. Curr Mol Med. 15:146–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Young D, Ma J, Cherkerzian S, Froimowitz
MP, Ennulat DJ, Cohen BM, Evans ML and Lange N: Automated
identification of Fos expression. Biostatistics. 3:351–364. 2001.
View Article : Google Scholar
|
|
68
|
Chen DQ, Chen H, Chen L, Vaziri ND, Wang
M, Li XR and Zhao YY: The link between phenotype and fatty acid
metabolism in advanced chronic kidney disease. Nephrol Dial
Transplant. 32:1154–1166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dou F, Miao H, Wang JW, Chen L, Wang M,
Chen H, Wen AD and Zhao YY: An integrated lipidomics and phenotype
study reveals protective effect and biochemical mechanism of
traditionally used Alisma orientale Juzepzuk in chronic
renal disease. Front Pharmacol. 9:532018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pandey MK, Liu G, Cooper TK and Mulder KM:
Knockdown of c-Fos suppresses the growth of human colon carcinoma
cells in athymic mice. Int J Cancer. 130:213–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lu C, Shen Q, DuPré E, Kim H, Hilsenbeck S
and Brown PH: cFos is critical for MCF-7 breast cancer cell growth.
Oncogene. 24:6516–6524. 2005. View Article : Google Scholar : PubMed/NCBI
|