Introduction
Percutaneous CT-guided lung biopsy has increasingly
been adopted as a validated technology in clinical practice. The
most common complications of percutaneous CT-guided lung biopsy are
pneumothorax and hemoptysis. In general, treatment is not required,
except in a small number of serious cases. Systemic air embolism is
one of the rare complications of percutaneous CT-guided lung biopsy
that may have serious consequences (1–4). If an
air embolism occurs in the coronary or cerebral artery, it may lead
to acute myocardial or cerebral infarction, or even death (5–8).
However, if the complications are identified early, so that timely
and effective treatment measures are taken, the rare and serious
complications are usually non-fatal. Hyperbaric oxygen therapy is
currently recognized as an effective treatment.
As the complications of systemic circulation air
embolism frequently occur in the process of puncture or within a
short time after puncture (3,5,7), the operator should be well aware of the
symptoms that may accompany the complications. Where air embolism
is suspected, head and chest CT should be reviewed immediately
after the operation, so as to take treatment measures as soon as
possible.
In recent years, as understanding of the
complications of percutaneous CT-guided lung biopsy has improved,
an increasing number of studies have examined air embolism, but few
have performed comprehensive analyses of multiple domestic cases
(3,7,8). In the
present study, risk factors associated with air embolism following
lung biopsy were assessed, based on data collected between 2014 and
2018, and the complications and outcomes of 19 patients who
developed air embolism were summarized. In addition, the associated
risk factors and treatments were discussed to provide insight into
and a reference for the prevention, early detection and treatment
of systemic air embolism.
Materials and methods
Patients
A total of 2,026 patients (1,155 males and 871
females) with an average age of 58.7±10.6 years (range, 7–86 years)
subjected to percutaneous CT-guided lung biopsy between January
2014 and June 2018 at the Affiliated Hospital of Qingdao University
(Qingdao, China) were retrospectively enrolled. Each patient
provided written informed consent to undergo percutaneous CT-guided
lung biopsy. For patients under 18 years of age, their parents or
guardians provided written informed consent. All patients underwent
routine CT scanning and certain patients underwent enhanced CT
scanning.
Equipment and methods
All patients underwent a routine blood test and
coagulation test prior to the percutaneous lung biopsy. Patients
with frequent cough were administered cough suppressants and
received breath-hold training prior to the test. The puncture sites
and positions were selected on the basis of previous CT images of
the patients.
CT scanning was performed using a 16-slice CT
scanner (Brilliance CT, Philips Healthcare). The scanning range was
3–4 cm above and below the lesion center, the scanning slice
thickness was 3–5 mm and the interval between slices was also 3–5
mm. A catheter grid surface tag combined with the CT scanner
positioning cursor were used to determine the puncture location,
depth and direction. Routine disinfection was performed and the
syringe was maintained at a site along the direction of puncture
after local anesthesia, and an additional CT scan was performed
over the puncture area to confirm the puncture path.
The coaxial cannula needle was quickly advanced
through the pleura in a predetermined direction under a breath
hold, and CT scanning was performed to observe the tip after the
needle reached the preset depth. Subsequently, the puncture angle
and depth of the needle were adjusted to ensure that all procedures
were performed within the pleura. When the needle was inserted in
the lesion or at the edge of the lesion, the needle core was
withdrawn, and an 18-G or a 20-G biopsy needle (Bard Biopsy; BD
Biosciences) was inserted to complete the sampling procedure. The
decision to take a second sample was based on the quality of the
first sample obtained and the tolerance of the patient.
All patients underwent a chest CT scan after
percutaneous lung biopsy, but only those patients with symptoms of
a suspected cerebrovascular air embolism or those with an air
embolism in the systemic circulation after the chest CT examination
underwent a CT scan of the brain. Chest and brain CT scanning were
performed after the puncture to determine whether a systemic air
embolism had occurred in the cerebrovascular artery, left atrium,
left ventricle, coronary artery, aorta or its three branches. A
systemic air embolism was confirmed when CT values <-200
Hounsfield units were observed in two sequential images (9). All patients received careful
observation and monitoring of vital signs within 2 h after the
operation. In addition, all patients who were confirmed to have
systemic air embolism underwent brain MRI examination and color
Doppler echocardiography one week later to determine whether they
had delayed cerebral infarction or myocardial infarction.
Statistical analysis
The factors that may lead to air embolism were
divided into three categories: Patient characteristics (e.g. age
and sex), puncture process (e.g. location and depth) and the nature
of the lesion (e.g. sampling frequency and time). Statistical
analyses were performed using SPSS (version 19.0; IBM Corp.).
Independent-sample t-tests were used to evaluate the differences
between numerical variables. Pearson χ2 was employed to
evaluate the differences between categorical variables. P<0.05
was considered to indicate statistical significance.
Results
The incidence and location of systemic
air embolism
A total of 19 cases of systemic air embolism were
identified among the 2,026 patients who underwent percutaneous
CT-guided lung biopsy, with an incidence rate of ~0.9%. Details of
the 19 patients with air embolisms are provided in Table I. Among the 19 patients, 3 (15.8%)
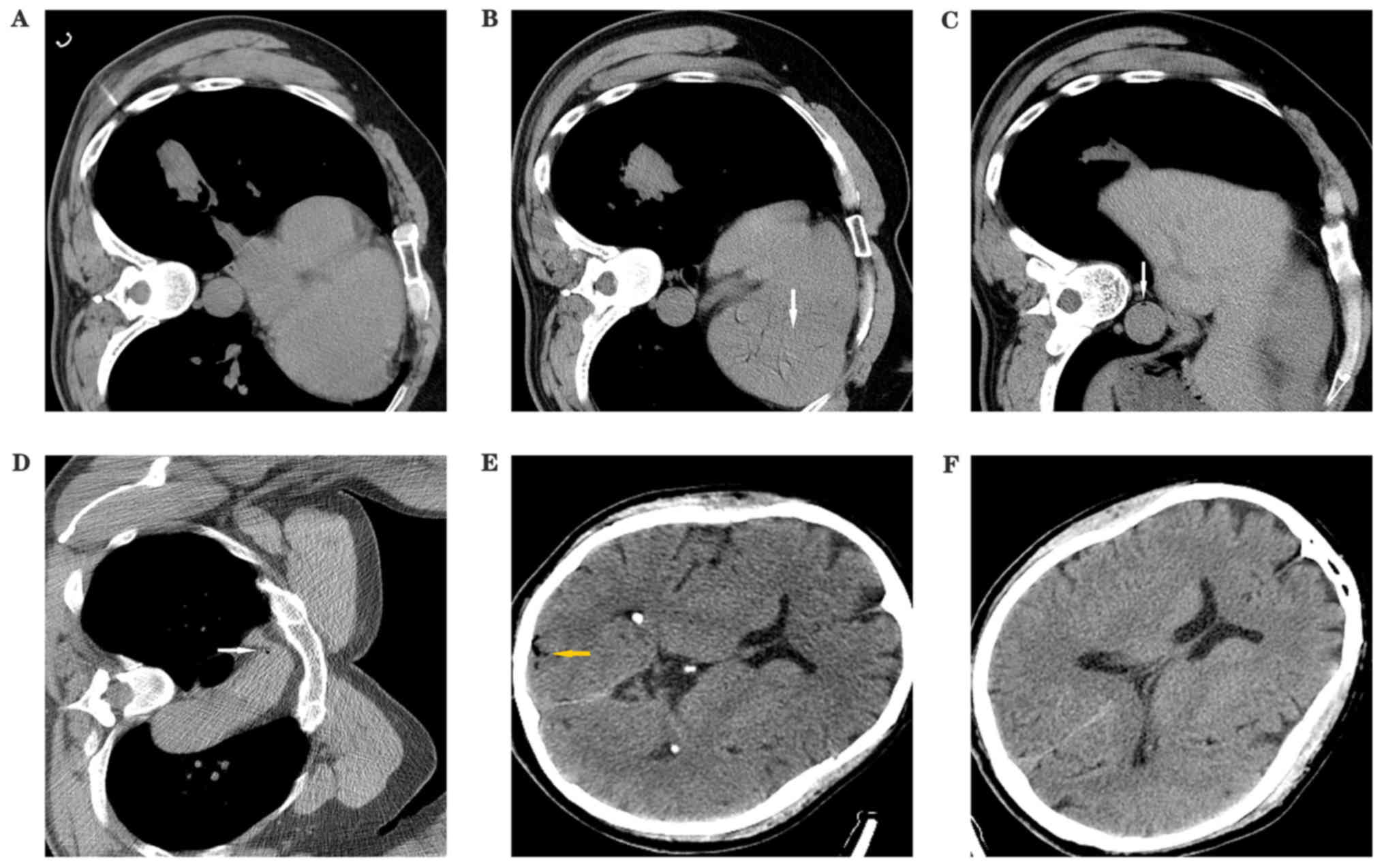
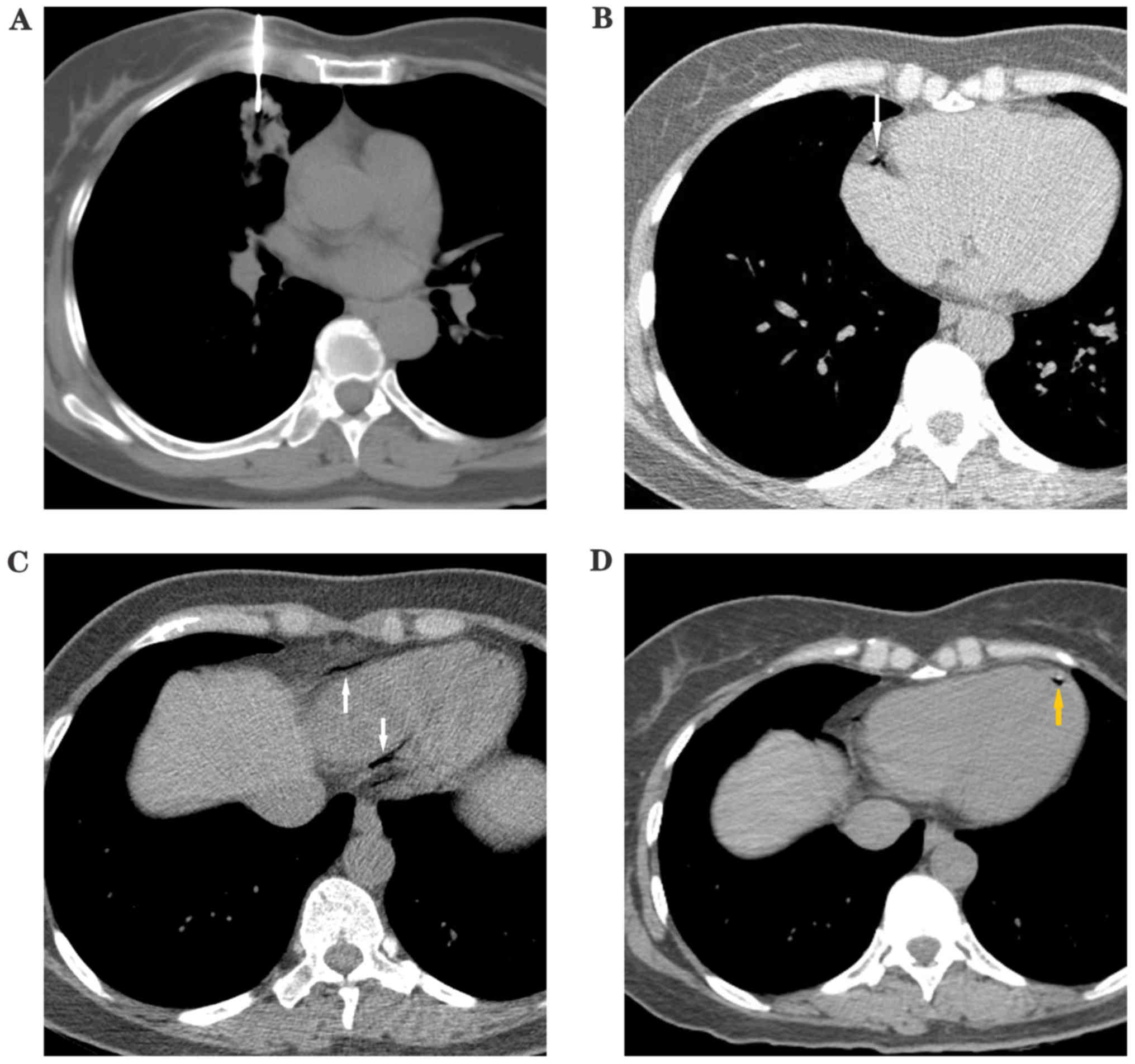
had obvious symptoms, Fig. 1 shows
the CT of a patient with a small quantity of air in the left
ventricle, aorta and in the cerebral blood vessels. Fig. 2 shows a patient with a right coronary
artery embolism. Three patients with obvious symptoms were treated
with high flow inhalation of 100% oxygen, hypertensive drugs and
fluid supplementation. The remaining 16 patients without obvious
symptoms were treated with inhalation of pure oxygen only. All 19
patients underwent CT 30 min later, which showed that their air
embolism had dissipated. The most common site of the air embolism
was the left ventricle (17/19, 89.5%), with other sites including
the aorta, left atrium, brachiocephalic trunk, cerebrovascular
artery, internal carotid and coronary artery. After one week, the
19 patients with systemic air embolism were examined by brain MRI
and color Doppler echocardiography, but no delayed myocardial
infarction or cerebral infarction was identified.
 | Table I.Conditions of the 19 patients with air
embolism. |
Table I.
Conditions of the 19 patients with air
embolism.
| Patient no. | Location | Symptoms | Treatment | Outcome |
|---|
| 1 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 2 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 3 | Left ventricle,
cerebrovascular artery | Low blood pressure,
convulsions | 100% oxygen,
hyperbaric oxygen | Survival without
sequelae |
| 4 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 5 | Left ventricle,
cerebrovascular artery | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 6 | Left ventricle,
brachiocephalic artery | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 7 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 8 | Left ventricle,
descending aorta | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 9 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 10 | Left ventricle, right
coronary artery | Pain and tightness in
the chest, hypotension, shock | 100% oxygen, shock
correctiona | Survival without
sequelae |
| 11 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 12 | Left atrium | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 13 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 14 | Left ventricle,
descending aorta | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 15 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 16 | Aortic arch, left
internal carotid artery | Unconsciousness, low
blood pressure | 100% oxygen, shock
correctiona, hyperbaric
oxygen | Survival without
sequelae |
| 17 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 18 | Left ventricle,
descending aorta | Asymptomatic | 100% oxygen | Survival without
sequelae |
| 19 | Left ventricle | Asymptomatic | 100% oxygen | Survival without
sequelae |
Risk factors
The factors predicted to influence systemic air
embolism occurrence during percutaneous CT-guided lung biopsy are
provided in Table II. The relative
location of the lesion above the level of the left atrium during
puncture and coughing during the puncture procedure significantly
influenced the occurrence of air embolism (P<0.05). No
statistically significant differences were identified between any
of the other factors.
 | Table II.Factors with the potential to
influence the occurrence of air embolism. |
Table II.
Factors with the potential to
influence the occurrence of air embolism.
| Characteristic | Air embolism
group | Control group | P-value |
|---|
| Age (years) | 56.5±9.1 | 59.6±11.3 | 0.233 |
| Sex [N (NT%)] |
| Male | 9 (47%) | 1,146 (57%) | 0.394 |
|
Female | 10 (53%) | 861 (43%) |
|
| Emphysema diagnosis
[N (NT%)] |
|
Positive | 7 (37%) | 675 (34%) | 0.768 |
|
Negative | 12 (63%) | 1,332 (66%) |
|
| Embolism size
(mm) | 32.1±18.6 | 42.3±23.2 | 0.056 |
| Region of lung for
biopsya [N (NT%)] |
| Upper
and middle | 10 (53%) | 1,178 (59%) | 0.593 |
|
Below | 9 (47%) | 829 (41%) |
|
| Biopsy depth
(mm) | 26.4±15.7 | 20.5±26.3 |
>0.05b |
| Lesion
statusc [N (NT%)] |
|
Solid | 14 (74%) | 1,632 (81%) | 0.396 |
|
Non-solid | 5 (27%) | 375 (19%) |
|
| Patient position [N
(NT%)] |
|
Supine | 8 (42%) | 1,219 (61%) | 0.098 |
| Prone
and lateral | 11 (58%) | 788 (39%) |
|
| Biopsy needle size
[N (NT%)] |
|
18G | 18 (95%) | 1,959 (98%) | 0.417 |
|
20G | 1 (5%) | 48 (2%) |
|
| Location of lesion
relative to left atrium [N (NT%)] |
|
Above | 17 (89%) | 1,091 (54%) | 0.002 |
|
Below | 2 (11%) | 916 (46%) |
|
| Presence of trocar
[N (NT%)] |
|
Trocar | 17 (89%) | 1,560 (78%) | 0.220 |
| No
trocar | 2 (11%) | 447 (22%) |
|
| Patient cough [N
(NT%)] |
|
Cough | 14 (74%) | 912 (45%) | 0.014 |
| No
cough | 5 (26%) | 1,095 (55%) |
|
| Operation duration
(min) | 25.8±7.0 | 23.6±9.9 | 0.334 |
| Number of biopsies
taken | 2.2±1.2 | 2.5±1.6 | 0.415 |
| Pneumothorax status
[N (NT%)] |
|
Positive | 7 (37%) | 468 (23%) | 0.166 |
|
Negative | 12 (63%) | 1,539 (77%) |
|
| Pulmonary
hemorrhage at the puncture site [N (NT%)] |
|
Positive | 15 (79%) | 1,188 (59%) | 0.081 |
|
Negative | 4 (21%) | 819 (41%) |
|
Discussion
Systemic air embolism is a rare complication of
percutaneous CT-guided lung biopsy. A 2006 study described the
incidence rate of air embolism after percutaneous CT-guided lung
biopsy to be 0.061% (2). However, an
increased interest and understanding of this condition led to a
study in 2012 to report an incidence of 3.8% (9). One potential reason for this difference
in incidence rate is that early studies may only have counted cases
with severe symptoms, while most asymptomatic patients were ignored
(2,9). According to one study, only 26.1% of
air embolism cases were detected during or immediately after biopsy
(9). In the present study, a general
lung and brain CT examination was performed in all 2,026 patients
who underwent percutaneous CT-guided lung biopsy and the incidence
of air embolism was 0.9%. A small amount of air had entered the
circulation in all 19 patients with systemic air embolism, but 16
of them did not have obvious clinical symptoms. The reason for this
may be that these patients have less air entering the systemic
circulation and had received high-flow 100% oxygen inhalation
therapy after air embolism was discovered. Oxygen inhalation
therapy with 100% oxygen dissipates the air within embolized
bubbles due to accelerated nitrogen resorption and improves
oxygenation of ischemic tissue. The most common site of the air
embolism was the left ventricle.
When a substantial amount of air enters the systemic
or cerebrovascular circulation or the coronary artery, clinical
symptoms, including aphasia, hemiplegia, fainting, arrhythmia,
cardiac arrest, a decrease in blood pressure and shock, may arise.
In such cases, another chest and brain CT should be performed to
confirm the air embolism, and once confirmed, the patient should be
rapidly placed in a Trendelenburg position or in the right lateral
decubitus position and administered 100% high-flow oxygen.
Administration of oxygen is an effective treatment for hypoxia and
eliminates air bubbles by establishing a diffusion gradient that
favors the egress of air from the bubbles (10). The right lateral decubitus position
prevents air from entering the systemic circulation, as the left
ventricular outflow tract lies inferiorly, and the air within the
left ventricle remains in the non-dependent superior aspect and is
positioned away from the aorta (11). However, it may be hypothesized that
the probability of air entering the left coronary artery is
increased in that position, although no better heart and lung
recovery rescue position is available. Therefore, timely
intervention and the Trendelenburg position may be a better option.
Hyperbaric oxygen therapy is currently recognized as an effective
treatment for systemic air embolism, as it eliminates air within
embolized bubbles by accelerating nitrogen resorption and improves
the oxygenation of ischemic tissues (11–13).
However, hyperbaric oxygen therapy is contraindicated for patients
with severe emphysema, pulmonary bulla or post-operative
pneumothorax.
In the present study, air embolisms occurred in the
cerebral circulation and the left internal carotid in 3 of 19
affected patients: One patient was asymptomatic and the others
presented with symptoms, including hypotension, convulsions and/or
unconsciousness. No clinical sequelae were observed after oxygen
therapy. In addition, 1 patient had an air embolism in the right
coronary artery and exhibited chest pain, tightness and
hypotension-associated shock, but no sequelae remained after timely
intervention.
Although the prognosis in this group of 19 patients
was good, systemic air embolism may potentially have severe
consequences. The prognosis is poor, particularly when the embolism
is located in the coronary and cerebral arteries, with a mortality
rate of as high as 26.3% (4). Thus,
patients with an air embolism should receive focused care to
improve their prognoses.
In the present study, coughing during the procedure
and the relative location of the lesion above the level of the left
atrium during percutaneous CT-guided lung biopsy significantly
increased the occurrence of air embolism complications. Usually,
the pressure in the pulmonary vein is ~10 cm H2O, but
the pressure in the lung may sharply increase to ≥180 cm
H2O when the glottis is opened during coughing (14). During the biopsy process, coughing
may be provoked by bleeding around the vascular injury site caused
by the needle. When the pulmonary pressure increases substantially,
alveolar air may easily enter the left atrium through a
bronchovenous fistula or other air-containing spaces and pulmonary
veins, and then travel to the left ventricle to result in a
systemic air embolism. If coughing persists after the extraction of
specimens, the procedure should be promptly terminated and a chest
CT should be performed as soon as possible to evaluate the risk of
air embolism; in addition, patients with frequent cough should be
administered medicine to relieve their cough. Numerous studies
reporting air embolism caused by coughing have been published in
the past decade (15–17).
If the location of the lesion is above the level of
the left atrium, the venous pressure around the lesion is
relatively low. A higher position results in lower pressure and air
more easily enters the systemic circulation through the vulnerable
blood vessels. A prone position is one of the risk factors for air
embolism, according to previous studies (9,18).
However, in the present study, there was no significant difference
in the incidence of air embolism between two groups in different
positions. The reason for adopting a prone position may be that the
patient's lesions are located in the dorsal and basal segments of
the lower lobe of the lung (9,19,20). The
results of the present study suggest that when the prone position
is selected for lesions close to the dorsal pleura the location of
the lesion is usually higher than the level of the left atrium,
which increases the probability of developing an air embolism.
Therefore, the puncture position may not be an independent risk
factor, whereas the relative location of the lesion above the level
of the left atrium during puncture is a risk factor. In addition, a
supine position should be selected if possible during the puncture
of the basal segment or the lower lobe of the lung to avoid the
complication of an air embolism (9).
Of note, the use of a trocar did not affect the
occurrence of air embolisms. The amount of air in a 25-cm trocar is
only ~0.017 cm3. This small amount of air does not
affect the body, even if it enters the circulation, as according to
previous experimental studies on dogs, at least 2–3 ml of air must
be injected into the pulmonary veins to be fatal (15). In a previous study (17), lesions located near the bottom of the
lungs were intractable due to the large movements associated with
breathing, resulting in difficulty establishing a successful
puncture. Theoretically, a deeper puncture site is more likely to
damage the surrounding blood vessels and cause a systemic air
embolism. However, according to the statistical analyses performed
in the present study, the average depth in the air embolism group
was greater than that in the group without air embolism, though the
difference was not statistically significant. In previous studies,
additional factors, including the coaxial technique, needle size,
bleeding, lesion size of <1 cm and frequency of sampling, were
also reported as risk factors, although they remain controversial
(3,14,17,19,20). In
the present study, no significant differences in the other risk
factors assessed were observed between the two groups.
The present study has several limitations. As this
was a single-center retrospective study there was inevitably bias
in the selection of research subjects. Furthermore, the interaction
between different factors was not assessed. In addition, the
potential risk factors mentioned above, including the application
of coaxial technique, needle size, lesion size and frequency of
sampling, are not certain to be independent risk predictors. To
confirm this will require future multi-factor logistic regression
analysis of further large patient cohorts.
In conclusion, in the present study, the incidence
of air embolism complications was ~0.9%. Cough and the relative
location of the lesion above the left atrium during puncture may be
risk factors for systemic air embolism following percutaneous lung
biopsy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
requesr.
Authors' contributions
SHL and QF performed the experiments, analyzed the
data and wrote the manuscript. HLY and YBH provided technical
support during experimentation and were involved in drafting the
manuscript. QY, ZXZ and BPZ aquired, analyzed and interpreted the
data. CYZ designed the experiments and gave final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics Review
board of Qingdao University. Written informed consent was obtained
from all patients or gaurdains.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richardson CM, Pointon KS, Manhire AR and
Macfarlane JT: Percutaneous lung biopsies: A survey of UK practice
based on 5444 biopsies. Br J Radiol. 75:731–735. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomiyama N, Yasuhara Y, Nakajima Y, Adachi
S, Arai Y, Kusumoto M, Eguchi K, Kuriyama K, Sakai F, Noguchi M, et
al: CT-guided needle biopsy of lung lesions: A survey of severe
complication based on 9783 biopsies in Japan. Eur J Radiol.
59:60–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Um SJ, Lee SK, Yang DK, Son C, Kim KN, Lee
KN and Kim YS: Four cases of a cerebral air embolism complicating a
percutaneous transthoracic needle biopsy. Korean J Radiol.
10:81–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ibukuro K, Tanaka R, Takeguchi T, Fukuda
H, Abe S and Tobe K: Air embolism and needle track implantation
complicating CT-guided percutaneous thoracic biopsy:
Single-institution experience. AJR Am J Roentgenol. 193:430–436.
2009. View Article : Google Scholar
|
|
5
|
Cheng HM, Chiang KH, Chang PY, Chou YF,
Huang HW, Chou AS and Yen PS: Coronary artery air embolism: A
potentially fatal complication of CT-guided percutaneous lung
biopsy. Br J Radiol. 83:83–85. 2010. View Article : Google Scholar
|
|
6
|
Kodama F, Ogawa T, Hashimoto M, Tanabe Y,
Suto Y and Kato T: Fatal air embolism as a complication of
CT-guided needle biopsy of the lung. J Comput Assist Tomogr.
23:949–951. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghafoori M and Varedi P: Systemic air
embolism after percutaneous transthoracic needle biopsy of the
lung. Emerg Radiol. 15:353–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mokhlesi B, Ansaarie I, Bader M, Tareen M
and Boatman J: Coronary artery air embolism complicating a
CT-guided transthoracic needle biopsy of the lung. Chest.
121:993–996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freund MC, Petersen J, Goder KC, Bunse T,
Wiedermann F and Glodny B: Systemic air embolism during
percutaneous core needle biopsy of the lung: Frequency and risk
factors. BMC Pulm Med. 12:22012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Liew HD, Conkin J and Burkard ME: The
oxygen window and decompression bubbles: Estimates and
significance. Aviat Space Environ Med. 64:859–865. 1993.PubMed/NCBI
|
|
11
|
Hare SS, Gupta A, Goncalves AT, Souza CA,
Matzinger F and Seely JM: Systemic arterial air embolism after
percutaneous lung biopsy. Clin Radiol. 66:589–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashizawa K, Watanabe H, Morooka H and
Hayashi K: Hyperbaric oxygen therapy for air embolism complicating
CT-guided needle biopsy of the lung. AJR Am J Roentgenol.
182:1606–1607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohashi S, Endoh H, Honda T, Komura N and
Satoh K: Cerebral air embolism complicating percutaneous
thin-needle biopsy of the lung: Complete neurological recovery
after hyperbaric oxygen therapy. J Anesth. 15:233–236. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agostoni E and Rahn H: Abdominal and
thoracic pressures at different lung volumes. J Appl Physiol.
15:1087–1092. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishikawa Y, Matsuguma H, Nakahara R, Ui A,
Suzuki H and Yokoi K: Arterial air embolism: A rare but
life-threatening complication of percutaneous needle biopsy of the
lung. Ann Thorac Surg. 87:16222009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kau T, Rabitsch E, Celedin S, Habernig SM,
Weber JR and Hausegger KA: When coughing can cause stroke-a
case-based update on cerebral air embolism complicating biopsy of
the lung. Cardiovasc Intervent Radiol. 31:848–853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishii H, Hiraki T, Gobara H, Fujiwara H,
Mimura H, Yasui K, Doke T, Mukai T, Kurokawa H, Ando Y, et al: Risk
factors for systemic air embolism as a complication of percutaneous
CT-guided lung biopsy: Multicenter case-control study. Cardiovasc
Intervent Radiol. 37:1312–1320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muth CM and Shank ES: Gas embolism. N Engl
J Med. 342:476–482. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhatia S: Systemic air embolism following
CT-guided lung biopsy. J Vasc Interv Radiol. 20:709–711. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arnold BW and Zwiebel WJ: Percutaneous
transthoracic needle biopsy complicated by air embolism. AJR Am J
Roentgenol. 178:1400–1402. 2002. View Article : Google Scholar : PubMed/NCBI
|