Introduction
Brachial plexus root avulsion (BPRA), as a primary
injury, is one of the most serious axonal injuries that may lead to
multiple pathophysiological processes in the spinal cord (1,2). These
secondary processes often involve an altered expression of specific
genes and proteins associated with inflammation, apoptosis,
mitochondrial oxidative phosphorylation and oxidative stress,
which, in turn, contribute to the apoptotic death of the affected
motor neurons with subsequent permanent paralysis of the
ipsilateral upper limb muscles (2).
The spinal cord ventral horn is where the somatic
motor neuron subtypes, namely γ, β and α motor neurons, are
located. These subtypes are responsible for functional movements
that are lost following a brachial plexus/spinal cord injury
(3). β motor neurons are the
smallest and less abundant of the somatic motor neuron subtypes.
The molecular and electrical properties of β motor neurons are
poorly characterized and largely remain unidentified (3,4). γ motor
neurons contribute ~30% to the motor neuron pool and have smaller
cell bodies compared with the largest motor neurons, α motor
neurons (1–4). Functionally, γ motor neurons innervate
the intrafusal muscle fibers, which modulate the sensitivity of
muscle spindles to stretch (3–5). α motor
neurons are the predominant cell type in the motor neuron pool.
They innervate force-generating extrafusal muscle fibers at
neuromuscular junctions. Notably, in mice, the muscle fiber targets
of γ and α motor neurons can be distinguished by estrogen-related
receptor (ERR) transcription factor expression (5). It is unknown if the same is true for
rats, which represent an important experimental animal model for
brachial plexus injuries.
ERRs are a small group of orphan nuclear receptor
transcription factors with 3 isoforms, namely ERRα (NR3B1), ERRβ
(NR3B2) and ERRγ (NR3B3) (6–9). The 3 mammalian ERR genes have been
implicated in diverse physiological processes, ranging from
placental development to bone density maintenance (10,11). The
ERRα and ERRγ isoforms have been demonstrated to perform core
functions in the regulation of metabolic genes and cellular energy
metabolism in skeletal muscle and heart (9–12). By
contrast, the expression of ERRβ is only observed in restricted
embryonic tissues (13), and its
disruption causes fetal lethality resulting from impaired
placentation (14). In addition, the
post-natal expression of the ERRβ gene is highly restricted
(15), therefore, less is known
regarding its role in adult physiology (13). ERRα interacts with peroxisome
proliferator-activated receptor γ coactivator-1α to stimulate
vascular endothelial growth factor expression and angiogenesis in a
hypoxia-inducible factor-1α-independent pathway following traumatic
spinal cord injury (16). Genomic
studies have demonstrated that ERRα and ERRγ target a common set of
gene promoters associated with fatty acid oxidation, oxidative
phosphorylation and muscle contraction. Furthermore, due to the
potential of overlapping target genes of ERRα and ERRγ (17), the in vivo physiological
importance of ERRs, particularly in neurons, remains to be
determined.
Notably, in mice, experiments have revealed that
ERRγ deficiency accelerates the progression of pathologic processes
and implicates the ERRs as etiological factors in diseases
(18–20). In the central nervous system of mice,
ERRγ was highly expressed during neuronal differentiation (15). This transcription factor is also
typically expressed at high levels in mature γ but not α motor
neurons of mice, forming a basis for distinguishing these 2 cell
types (5,20,21). Pei
et al (20) also indicated
that ERRγ orchestrates the expression of a distinct neural gene
network that promotes mitochondrial oxidative metabolism, thereby
revealing the extraordinary neuronal dependence on glucose. In
addition, ERRγ defects in neuronal metabolism, particularly in
mitochondrial oxidative phosphorylation, have been associated with
ageing and diverse human neurological diseases (22). Results from gain- and
loss-of-function models developed to characterize ERR function, and
the use of small synthetic molecules to modulate their activity,
have demonstrated the role of ERR in the control of skeletal
muscle, heart and musculoskeletal physiology (9). Taken together, these data presented
ERRγ as a potential therapeutic target and a subject for further
study, due to its co-localization with transcription factors
involved in post-avulsion reactions. To the best of our knowledge,
the pattern of expression of ERRγ in the rat spinal cord,
especially following BPRA, is unknown. Rats have often been
selected as candidates for BPRA and spinal cord injury experiments,
not only because they are readily available, but also due to their
post-injury morphological, biochemical and functional changes that
are similar to those observed in human patients (23). The present study aimed to explore the
post-brachial plexus injury expression profile of the transcription
factor ERRγ and determine whether it may be used to define
functionally distinct motor neuron sub-populations in the rat
spinal cord.
Materials and methods
Animal model
A total of 35 adult female Sprague Dawley rats
(weight, 180–250 g; age, 8–10 weeks) were purchased from the
Laboratory Animal Centre of Sun Yat-sen University. The rats were
housed under a 12-hour light/dark cycle, with ad libitum
access to rat chow and water. All surgical procedures were
conducted aseptically, in accordance with the Chinese National
Health and Medical Research Council animal ethics guidelines. The
experiments were approved by the Sun Yat-sen University Animal
Experimentation Ethics Committee.
BPRA surgery
BPRA was performed as previously described (24,25) In
brief, the rats were anesthetized with a mixture of ketamine (80
mg/kg) and xylazine (8 mg/kg) administered intramuscularly (IM).
While in the supine position, the right brachial plexus was exposed
and identified, and its roots (C5-T1) were isolated under a
dissecting microscope (magnification ×10). Extra-vertebral avulsion
of the ventral and dorsal roots was then performed. The ventral and
dorsal roots, in addition to the dorsal root ganglia, were cut off
at the distal ends of the avulsed spinal nerves and examined under
the microscope to confirm the success of the surgery.
Retrograde labelling of the injured
spinal motor neurons with fluorogold (FG)
A total of 3 days prior to BPRA surgery, FG
retrograde labelling of the avulsion-injured motor neurons was
performed on 5 adult SD rats; procedures were performed as
previously described (26,27). Briefly, the rats were anesthetized
with a mixture of ketamine (80 mg/kg) and xylazine (8 mg/kg)
(Fujian Gutian Pharmaceutical Co., Ltd.) administered IM, and laid
in supine position under the dissecting microscope for surgery.
Following the identification of the right brachial plexus, the C7
and C8 spinal nerve roots were injected with 2% FG (2% w/v;
Fluorochrome, LLC). A micropipette with the FG solution (2.0 µl for
60 sec) was slowly injected under the epineurium into the proximal
stumps of the C7 and C8 nerve roots. The injection site was then
clamped with micro forceps for an additional 10 sec to ensure all
of the axons had been cut. Finally, the muscle, fascia and skin
were sutured successively in layers. Then, 3 days later, all rats
in this group were anesthetized again. In prone position under the
dissecting microscope, the rats underwent laminectomy of the C6 to
C7 vertebrae, cutting of the dura mater and opening of the
subarachnoid space to expose the dorsal and ventral roots of the C7
and C8 spinal nerves. Following positive identification, all of the
dorsal and ventral rootlets of C7 and C8 were pulled out using a
micropipette hook. Muscles, fascia and skin were then sutured
successively in layers and the rats were allowed to survive for 3
days until sacrifice. Tissues were processed for FG and cyclic
AMP-dependent transcription factor 3 (ATF-3) immunofluorescence as
previously described (27).
Western blot preparation and
analysis
To analyze the ERRγ protein levels in the spinal
cord segment that underwent root avulsion, 10 rats were sacrificed
with a lethal dose of a mixture of ketamine (320 mg/kg) and
xylazine (32 mg/kg) at 2 (n=5) and 4 weeks (n=5) following BPRA.
Following confirmation of the absence of corneal and pain reflexes,
the C7/C8 spinal segments were rapidly exposed, dissected and
divided into left and right halves. The avulsed side was the right
side, with the left side used as a control. Western blot analysis
was performed as previously described (28). Samples (pooled left sides, right
sides, and left and right of C7/C8 spinal segments of normal rats)
were sonicated on ice in lysis buffer with 0.1% protease inhibitor
and 0.5% PMSF to extract the total protein using the Total Protein
Extraction Sample kit according to the manufacturer's protocol
(Nanjing KeyGen Biotech Co., Ltd.). Protein concentration in each
sample was determined using BCA protein assay kit, according to the
manufacturer's protocol. The samples were diluted in an equal
volume of 5X SDS loading buffer. The proteins in the samples (40
µg) were then separated using a 10% TGX™ FastCast™ Acrylamide kit
(Bio-Rad Laboratories, Inc.), transferred onto PVDF membranes and
then blocked with 5% milk in TBST solution for 2 h at room
temperature. Next, the PVDF membranes were probed with ERR3
(1:2,000; cat. no., sc66883, Santa Cruz Biotechnology, Inc) and
GAPDH (1:2,000; cat. no., SAB1405848; Sigma-Aldrich: Merck KGaA)
primary antibodies diluted in Western Blot Immune Booster solution
1 (Santa Cruz Biotechnology, Inc.) overnight at 4°C. These
membranes were then washed and probed with horseradish
peroxidase-conjugated goat anti-mouse IgG (1:5,000; cat. no.,
AP127P; Merck KGaA) and anti-rabbit IgG (1:5,000; cat. no., AP107P;
Merck KGaA) secondary antibodies for 2 h at room temperature,
developed using enhanced chemiluminescence substrate and then
exposed on BioMax film (Kodak). Exposed films were scanned, and
protein bands quantified using Image-Pro Plus software version 6.0
(Media Cybernetics, Inc.).
Immunohistochemistry (IHC) and
immunofluorescence (IF)
The IHC and IF procedures were performed as
previously described (27). Briefly,
rats were deeply anesthetized with an intramuscular injection of a
mixture of ketamine (80 mg/kg) and xylazine (8 mg/kg) at 2 (5
experimental rats and 5 control) and 4 weeks (5 experimental rats
and 5 control) following BPRA surgery, and perfused transcardially
with normal saline, followed by 4% paraformaldehyde in 0.1 M PBS
(pH 7.4). Following perfusion, the C7 and C8 spinal segments of
each rat were carefully removed, immersed in fixative (4%
paraformaldehyde for 4 h at 4°C) and then stored in 30% (v/v)
sucrose solution in PBS overnight. For IHC staining, frozen
transverse sections (35 µm) were washed 3 times with 0.01 M PBS for
10 min and incubated in 0.3% peroxide in methanol (100%) at room
temperature for 15 min to eliminate endogenous peroxidase activity.
Following washing in PBS, the sections were incubated in 3% BSA
(NeoFroxx GmbH) and 0.3% Triton X-100 in 0.01 M PBS at room
temperature for 30 min and then for 72 h at 4°C with the following
primary antibodies: Anti-ERRγ (1:500; cat. no., sc66883; Santa Cruz
Biotechnology, Inc.). Following washing in PBS, sections were
incubated with the anti-rabbit IgG secondary antibody (1:5,000;
cat. no., 31470; Invitrogen; Thermo Fisher Scientific, Inc.) at
room temperature for 2 h. The sections were then rinsed and
incubated with ABC reagents (1:500; Wuhan Boster Biological
Technology, Ltd.) at room temperature for 45 min. The sections were
then washed thoroughly and incubated in 0.05 DAB (Nanjing KeyGen
Biotech Co., Ltd.) and 0.01% H2O2 for 3–5 min
until a brown reaction product was observed.
For IF double-labelling, sections from 5 rats were
incubated in 3% BSA and 0.3% Triton X-100 in 0.01 M PBS at room
temperature for 30 min and incubated with the following primary
antibodies for 72 h at 4°C: Anti- Neuronal Nuclei (1:500; cat. no.,
ab104224; Abcam); anti-ERRγ (1:500; cat. no., sc66883; Santa Cruz
Biotechnology, Inc.); and anti-ATF-3 (1:500; cat. no., sc81189;
Santa Cruz Biotech Inc.). Following washing in PBS, the sections
were incubated with fluorescein isothiocyanate-conjugated
anti-mouse IgG (1:1,000; cat. no., F-2761; Invitrogen; Thermo
Fisher Scientific, Inc.), TRITC-conjugated anti-Rabbit IgG
(1:1,000; cat. no., A18750; Invitrogen; Thermo Fisher Scientific,
Inc.) at room temperature for 2 h in the dark. The sections were
washed again in PBS, mounted on glass slides and examined under a
fluorescence microscope at ×10 and ×20 magnification (Zeiss AG).
The staining specificity was verified by the omission of primary
antibodies.
The mean of a total number of ERRγ-positive motor
neurons in 10 serial IHC sections of the ventral horns of C7 and C8
spinal segment was calculated in each rat. The positive motor
neurons, the ones exhibiting visibly stained nuclei of the spinal
cord, were counted under a microscope at a magnification, ×20 (Carl
Zeiss AG) as described previously (29,30).
Enumeration of motor neurons, pooling of means and data analysis
were performed by two independent persons blinded to the
treatment/sidedness of the groups.
Statistical analysis
Enumeration of motor neurons, pooling of means and
data analysis were performed by two independent persons blinded to
the treatment sidedness of the groups. The data are presented as
the mean ± standard error of the mean and were analyzed using SPSS
v.16.0 software (SPSS, Inc.). A one-way analysis of variance was
used to analyze the differences among groups, followed by a
post-hoc Bonferroni test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Avulsion-induced ATF-3 is a marker of
injured motor neurons
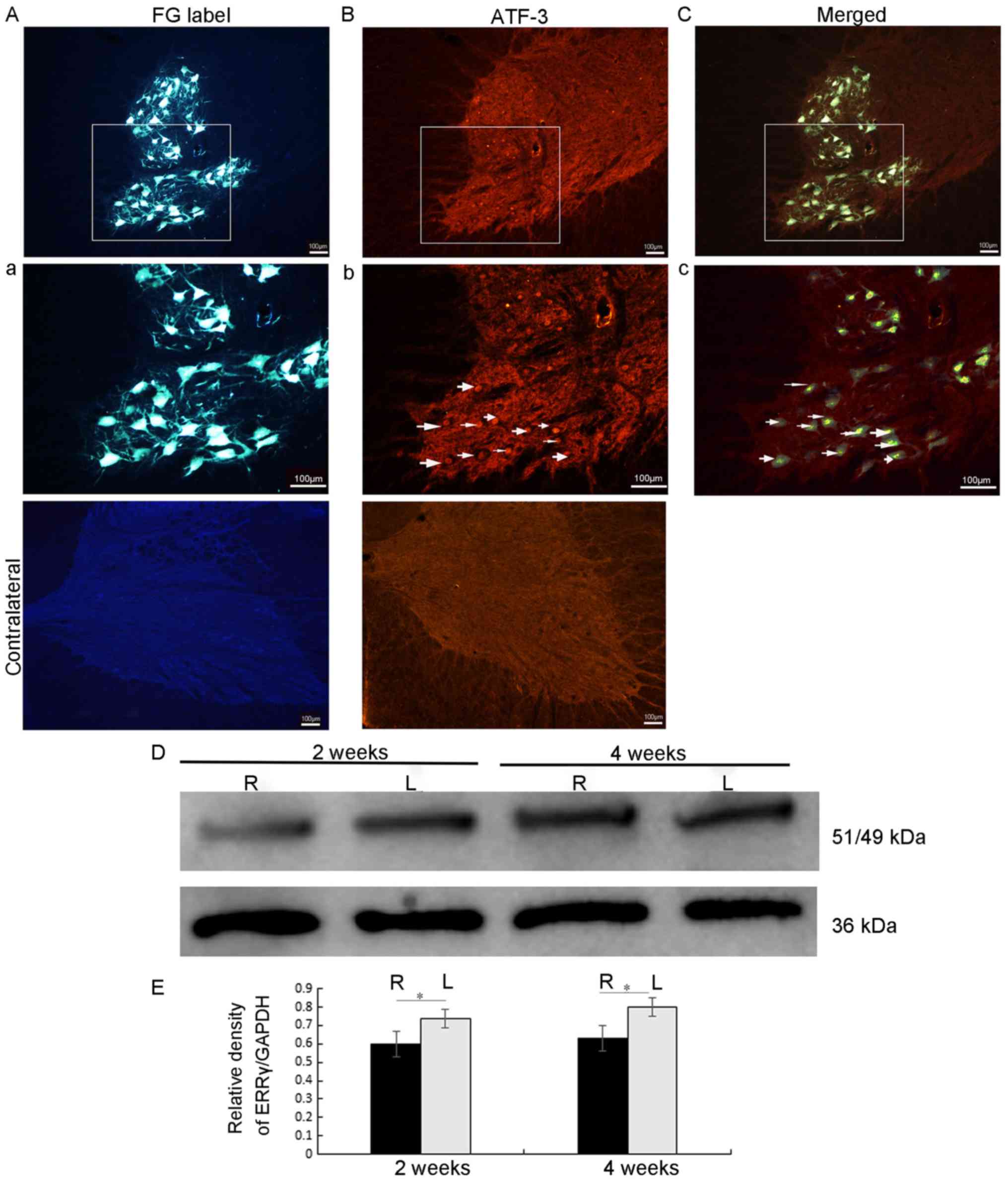
At the end of the 6 days after the FG retrograde
labelling, the C7 and C8 spinal cord sections from 5 rats were
examined under a fluorescence microscope. The results indicated
that motor neurons in the ipsilateral ventral horn were labelled by
FG (Fig. 1). In addition, almost all
motor neurons, including their cell bodies and their dendritic
processes, were labelled by the FG in the ipsilateral C7 and C8
ventral horns (Fig. 1Aa). In the
FG-labelled motor neurons, the FG dye was taken up by the cut end
of the axons in the middle and lower trunks of the brachial plexus
and then transported to the cytoplasm of the motor neuron cell
bodies in the C7 and 8 ventral horn segments via retrograde
transport (Fig. 1). Therefore,
FG-labelled motor neurons represented the injured motor neurons,
whose axons had been severed. Fig.
1Bb also indicated that, on day 3 following avulsion, ATF-3 was
expressed in the nuclei of motor neurons whose axons had been
severed by root avulsion. It was, therefore, only expressed in the
ipsilateral ventral horn of the spinal cord corresponding to the
level of the root avulsion. Fig. 1Cc
additionally demonstrates the co-localization of FG with ATF-3,
thereby providing evidence that the ATF-3 is a viable marker of
injured motor neurons.
Avulsion decreases ERRγ protein
expression in the ipsilateral half of injured spinal cords
The expression of the ERRγ protein in the spinal
cord following root avulsion was assessed by western blot analysis
using an anti-ERRγ antibody. At each time point [2 weeks (n=5) and
4 weeks (n=5)], the results of the representative western blot
analysis suggested that the ERRγ protein level in the ipsilateral
half, the avulsed half of the spinal cord, was significantly
decreased compared with that of the contralateral half (all
P<0.05; Fig. 1D and E).
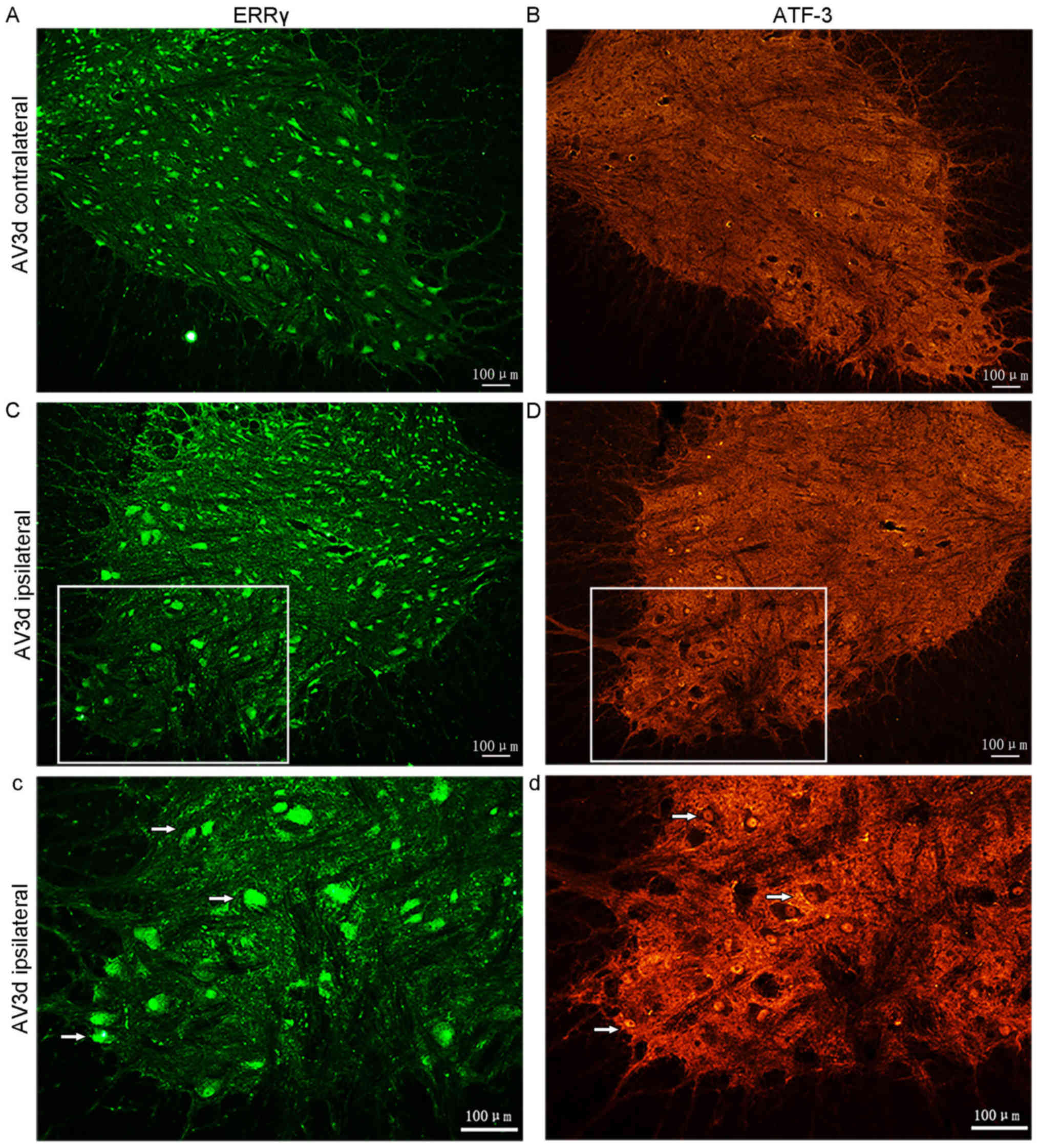
Avulsion-induced death of the ERRγ and
ATF-3 positive motor neurons
In the ERRγ-stained sections, the numbers of
positive motor neurons were notably decreased in the ipsilateral
side compared with those in the contralateral side (Fig. 2A-Cc). The data also demonstrated that
the positive signal for ERRγ was present in both small and large
motor neurons. These results, therefore, demonstrated that there
was constitutive expression of ERRγ and that BPRA induced ERRγ
downregulation in motor neurons in the injured segment of the
spinal cord.
In addition, the expression of ATF-3 in the spinal
cord following root avulsion was also assessed using IF. Adjacent
sections were either stained with an antibody against ERRγ or
ATF-3. At 3 days following avulsion, no ATF-3 positive neurons were
identified on the contralateral ventral horns of the C7 and C8
(n=5). However, ATF-3 was detected on the ipsilateral side of
corresponding segments (Fig. 2B-Dd)
(n=5).
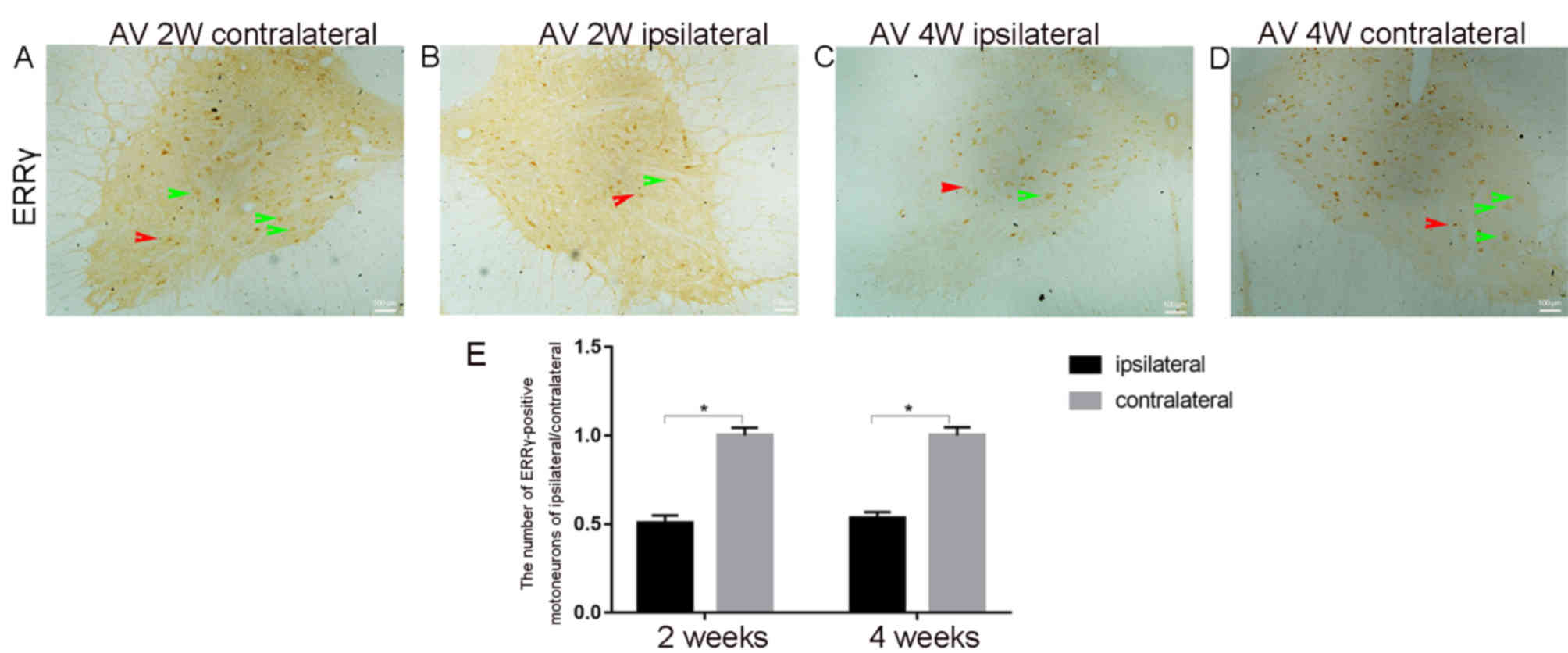
In Fig. 3, the
representative IHC images 2 weeks post-avulsion indicate fewer
ERRγ-positive motor neurons in the ipsilateral injured side
(Fig. 3B) compared with in the
contralateral side (Fig. 3A). This
trend was also observed 4 weeks post-avulsion (Fig. 3C and D). The ERRγ-positive signal was
present in both smaller γ (red arrow) and larger α (green arrow)
motor neurons. The results of immunoreaction cell counting in the
ventral horns demonstrated that the average number of the
ERRγ-positive neurons in the ipsilateral ventral horn on day 14 was
9±1 (n=5) and on day 28 was 11±0.5 (n=5) (Fig. 3E). On the contralateral ventral horn,
the number of ERRγ-positive neurons on day 14 was 18±1, while on
day 28 it was 21±1.5 (Fig. 3E).
Further observations and statistical analysis indicated that
avulsion significantly decreased the number of ERRγ-positive motor
neurons in the ipsilateral ventral horn, both on days 14 and 28
compared with the number in the contralateral ventral horn (all
P<0.05; Fig. 3E).
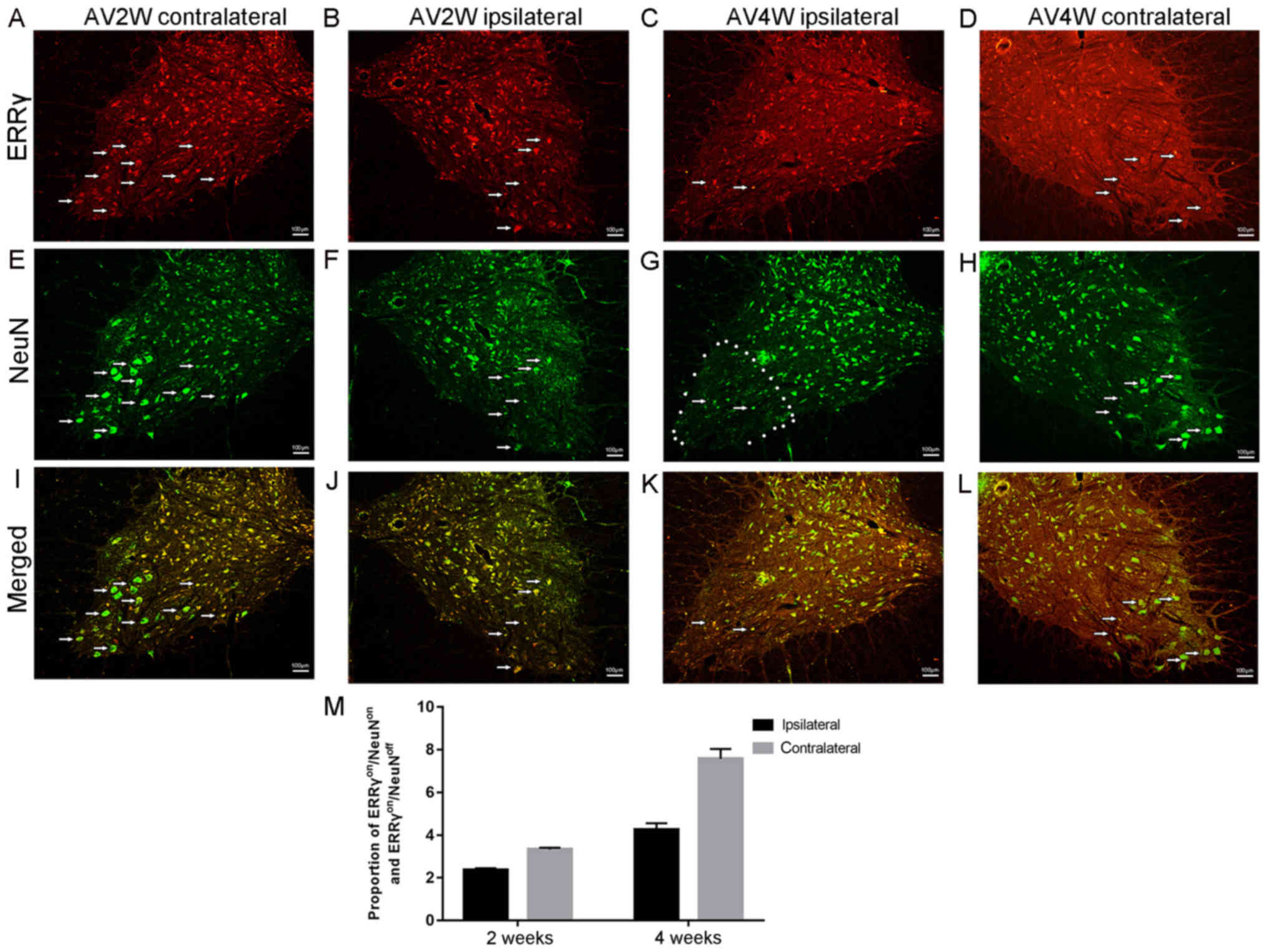
ERRγ nonspecific expression in γ and α
motor neurons
In mice, γ and α motor neurons were distinguished
based on the expression of ERRγ (5).
In the present study involving a rat model, the IF double-labelling
reaction results indicated that the positive immunoreactions of the
NeuN antibody ERRγ (red, Fig. 4A-D)
and (green, Fig. 4E-H) were
concentrated in the cytoplasm and nuclei of the spinal neurons in
the contralateral ventral horns of the injured spinal segments,
respectively. The area that was used for cell counting is marked in
Fig. 4G. Almost all ERRγ-positive
motor neurons were also NeuN-positive (Fig. 4I-L). However, a few motor neurons
were ERRγon/NeuNoff. These results
demonstrated that the ERRγon/NeuNoff status
of motor neurons can be identified as γ motor neurons (Fig. 4M). In addition, the presence of
ERRγon/NeuNoff implies that ERRγ was not
specific to a single cell subtype, the γ motor neuron.
Discussion
The aim of the present study was to describe the
post-brachial plexus avulsion expression profile of ERRγ and
determine whether ERRγ and NeuN have complementary expression
profiles in γ and α motor neurons.
In a previous study, ATF-3-positive immunoreaction
was demonstrated to be a marker of neuronal survival and
regenerative competency following experimental avulsion injury and,
as such, the authors suggested the use of ATF-3 as a regenerative
marker of the affected motor neurons (31). In the present study, ATF-3 was not
expressed on the intact half of the spinal cord (the contralateral
side), but was markedly induced in the avulsion side of the spinal
motor neurons of the corresponding cord segments. The FG-labelled
motor neurons represented the injured motor neurons, as these
FG-labelled motor neuron somata received the FG tracer that had
been taken up and retrogradely transported by the remaining stumps
of the axons. These results were in concordance with our previous
study (27). ATF-3 expression also
occurred only in the nuclei of the FG-positive neurons, as
previously demonstrated (27). In
previous studies, ATF-3 was revealed to be induced in the liver
upon intoxication or hepatectomy, in the heart upon myocardial
ischemia or ischemia-reperfusion, and in the kidney upon renal
ischemia-reperfusion (32,33). In each of these cases, including
those in our studies, ATF-3 was notably and consistently induced in
the corresponding tissues that are exposed to the stress signal.
Therefore, ATF-3 may serve an important role in the general early
response to stress. The present data suggested that ATF-3 may be a
specific phenotypic marker of BPRA-injured motor neurons, as BPRA
resulted in an early and sustained expression of ATF-3 in the
injured spinal motor neurons. In the ipsilateral spinal cord horn,
avulsion induced a sustained expression of ATF-3 in the nuclei.
In the present study, it was identified that there
was a consistent decrease in the levels of ERRγ protein expression
on the brachial plexus cord avulsion-injured side of the spinal
cord. These results were consistent with the other results of the
present study, indicating that the number of ERRγ-positive motor
neurons was also decreased in the same injured side over the same
observation period. In combination, these results indicated that
the effects of brachial plexus injury may have led to the death of
ERRγ-positive motor neurons. This result was not in agreement with
earlier data that γ motor neurons are largely spared following
secondary spinal cord injury (34,35). In
a previous study (34), the authors
postulated that the surviving γ motor neurons may have indirectly
exacerbated the death of motor neurons through a regimen of
excitotoxic proprioceptive afferent (IA) feedback on α motor
neurons. Although the model in the present study was not able to
molecularly distinguish γ from α motor neurons, and there may also
be confounding caused by a decrease in the size of the dying α
motor neurons; future studies should focus on the identification of
these subtypes to determine whether this phenomenon may be involved
in present neuronal cell loss. In addition, well-established causes
of motor neuron loss following avulsion injuries, such as nitric
oxide (25,28,36) or
JNK-mediated (phosphorylated c-Jun) apoptosis (37) could not be ruled out. It could be
suggested that, in this case, all axons of the motor neuron pool
under study were severed, therefore, the interaction whereby γ
causes α motor neuron degeneration could only happen at the level
of the spinal cord ventral horn. This is an additional avenue for
further study.
ERRs, the first orphan nuclear receptors, share
sequence homology with members of the nuclear receptor superfamily
(38,39). Studies have identified that the ERRs
control vast metabolic gene networks and are key regulators of
energy metabolism, particularly in response to various
environmental challenges or biological stresses (13). However, results from multiple
genome-wide binding site location analyses have suggested that ERRs
may also be major orchestrators of other biosynthetic pathways and
biological processes; furthermore, this regulation is likely to be
cell- and tissue-specific. ERRγ was the first orphan receptor to be
identified due to its interaction with transcriptional
coactivators. In a number of respects, ERRγ functions like a
classical nuclear receptor. Although it has been demonstrated that
ERRγ is a functional transcriptional activator, its true
physiological role remains to be determined. The highest expression
of ERRγ occurs around days 11–15 of mouse embryonic development, a
period of very active organogenesis (15). ERRγ was also expressed in selected
adult tissues such as the heart, kidney and muscle (40). ERRγ has roughly similar temporal
patterns of expression in mouse embryos, and somewhat similar-
although not identical- distributions in adult tissues (15,27,41). The
transcription factor ERRγ is expressed at high levels in γ but not
α motor neurons, whereas the neuronal DNA binding protein, NeuN,
marks α but not γ motor neurons in mice (5). The present study aimed to determine
whether γ and α motor neurons in the spinal cord of the rats are
distinguishable on the basis of their profile of expression of
transcription factors and other molecular markers, as previously
reported in mice (5). In this
previous study, both the distribution and frequency of small, ERR
positive/RNA binding protein fox-1 homolog 3 (NeuN) negative
(ERRγon/NeuNoff) motor neurons in this previous study matched the
profile expected for γ motor neurons (5). Results from the present study
demonstrated that ERRγon/NeuNoff neurons
could be assumed to be γ motor neurons; however, not all
ERRγon (positive) neurons were γ motor neurons. Even the
largest neurons in the ventral horn (α motor neurons) also
exhibited the ERRγ signal in the present study. In light of the
above results, further studies are required to establish those
transcription factors that may be used to mark and positively
distinguish between motor neuron subtypes within the motor neuron
pool. In the present study, it was apparent that ERRγ was
promiscuous, and therefore did not qualify as a molecular marker,
as it did in mice (5).
The data from the present study proposed the
relevance of ERRγ in mediating motor neuron response to
avulsion-associated stress. It was apparent that the ERRγ
expression level was decreased on the injured side of the spinal
cord, indicating that it may participate in certain
response-to-injury signaling pathways or have a constitutive
expression role in normal spinal cord cells. To the best of our
knowledge, the present results provided the first insights into the
role of ERRγ in the spinal cord, as a novel approach toward
understanding specific motor neuron response to BPRA stress. We
hypothesized that a decrease in the ERRγ expression, specifically
in the injured motor neurons that eventually died, may imply a
neuroprotective role should it have been present. However, further
studies are required to verify the exact role of ERRγ. It is well
established that the neuroendocrine pathways that regulate
gonadotropin release in rodents are sexually dimorphic and
profoundly affected by neonatal estrogens (42). It would be important and interesting
for future studies to explore the differences in ERR expression
following BPRAs and elucidate their co-localization a well as roles
in neuroprotection and neurodegeneration, if any.
In conclusion, the data of the present study on ERRγ
expression demonstrated that γ and α motor neurons cannot be
distinguished molecularly, and, as a result, there are no
complementary profiles of DNA binding protein and ERRγ expression.
Therefore, contrary to previous data from mice (5), the principle that spinal motor neurons
may be fractionated into functionally distinct subtypes on the
basis of their profile of transcription factors (ERRγ and NeuN)
does not appear to extend to neuronal subtypes within single motor
neuron pools in Sprague Dawley rats. Instead, in rats, there is at
least a tendency that ERRγ and NeuN are expressed in the same
subpopulations of motor neurons in the rat spinal cord, due to the
observed expression promiscuity. However, the downregulation of
ERRγ in the injured side of the spinal cord is an intriguing result
that should be investigated further.
Acknowledgements
The authors would like to thank Professor Fu Rao of
the Anatomy Department of Sun Yat-sen University, China for general
scientific review of the manuscript.
Funding
The present study was supported by a research grant
from the National Science Foundation Council of China (grant no.
31471030).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LHZ conceptualised and designed the study. GY, PLMZ
and LL performed the experiments. KZ, YT and ZL assisted with some
of the experiments, and performed data analysis and cell counting.
GY and PLMZ wrote the manuscript draft, all authors revised it and
then PLMZ made final edits to the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Sun Yat-sen
University Animal Experimentation Ethics Committee prior to the
execution of the present study. This study was conducted in
accordance with the Chinese National Health and Medical Research
Council (NHMRC) animal ethics guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu Y, Liu H, Hua X, Xu WD, Xu JG and Gu
YD: Supplementary motor cortical changes explored by resting-state
functional connectivity in brachial plexus injury. World Neurosurg.
88:300–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noristani HN, Sabourin JC, Boukhaddaoui H,
Chan-Seng E, Gerber YN and Perrin FE: Spinal cord injury induces
astroglial conversion towards neuronal lineage. Mol Neurodeger.
11:68–81. 2016. View Article : Google Scholar
|
|
3
|
Stifani N: Motor neurons and the
generation of spinal motor neuron diversity. Front Cell Neurosci.
8:293–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manuel M and Zytnicki D: Alpha, beta and
gamma motoneurons: Functional diversity in the motor system's final
pathway. J Integr Neurosci. 10:243–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friese A, Kaltschmidt JA, Ladle DR,
Sigrist M, Jessell TM and Arber S: Gamma and alpha motor neurons
distinguished by expression of transcription factor ERR3. Proc Natl
Acad Sci USA. 106:13588–13593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tremblay AM and Giguère V: The NR3B
subgroup: An overview. Nucl Recept Signal. 5:e0092009.
|
|
7
|
Giguère V: Transcriptional control of
energy homeostasis by the estrogen related receptors. Endocr Rev.
29:677–696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deblois G and Giguère V: Functional and
physiological genomics of estrogenrelated receptors (ERRs) in
health and disease. Biochim Biophys Acta. 1812:1032–1040. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huss JM, Garbacz WG and Xie W:
Constitutive activities of estrogen-related receptors:
Transcriptional regulation of metabolism by the ERR pathways in
health and disease. Biochim Biophys Acta. 1852:1912–1927. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He H, Xi G and Lu X: Molecular cloning,
characterization, and expression analysis of an estrogen
receptor-related receptor homologue in the cricket, Teleogryllus
emma. J Insect Sci. 10:188–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshihara E, Wei Z, Lin CS, Fang S,
Ahmadian M, Kida Y, Tseng T, Dai Y, Yu RT, Liddle C, et al: ERRγ is
required for the metabolic maturation of therapeutically functional
glucose-responsive β cells. Cell Metab. 23:622–634. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deblois G and Giguère V: Oestrogen-related
receptors in breast cancer: Control of cellular metabolism and
beyond. Nat Rev Cancer. 13:27–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Audet-Walsh É and Giguère V: The multiple
universes of estrogen-related receptor alpha and gamma in metabolic
control and related diseases. Acta Pharmacol Sin. 36:51–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo J, Sladek R, Bader JA, Matthyssen A,
Rossant J and Giguère V: Placental abnormalities in mouse embryos
lacking the orphan nuclear receptor ERR-β. Nature. 388:778–782.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonnelye E, Vanacker JM, Spruyt N, Alric
S, Fournier B, Desbiens X and Laudet V: Expression of the
estrogen-related receptor 1 (ERR-1) orphan receptor during mouse
development. Mech Dev. 65:71–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu JZ, Long H, Wu TD, Zhou Y and Lu HB:
The effect of estrogen-related receptor α on the regulation of
angiogenesis after spinal cord injury. Neuroscience. 290:570–580.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dufour CR, Wilson BJ, Huss JM, Kelly DP,
Alaynick WA, Downes M, Evans RM, Blanchette M and Giguere V:
Genome-wide orchestration of cardiac functions by the orphan
nuclear receptors ERRα and γ. Cell Metab. 5:345–356. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon DH, Eom GH, Kee HJ, Nam YS, Cho YK,
Kim DK, Koo JY, Kim HS, Nam KI, Kim KK, et al: Estrogen-related
receptor gamma induces cardiac hypertrophy by activating GATA4. J
Mol Cell Cardiol. 65:88–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murray J, Auwerx J and Huss JM: Impaired
myogenesis in estrogen-related receptor γ (ERRγ)-deficient skeletal
myocytes due to oxidative stress. FASEB J. 27:135–150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pei L, Mu Y, Leblanc M, Alaynick W, Barish
GD, Pankratz M, Tseng TW, Kaufman S, Liddle C, Yu RT, et al:
Dependence of hippocampal function on ERRγ-regulated mitochondrial
metabolism. Cell Metab. 21:628–636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kida YS, Kawamura T, Wei Z, Sogo T,
Jacinto S, Shigeno A, Kushige H, Yoshihara E, Liddle C, Ecker JR,
et al: ERRs mediate a metabolic switch required for somatic cell
reprogramming to pluripotency. Cell Stem Cell. 16:547–555. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mattson MP, Gleichmann M and Cheng A:
Mitochondria in neuroplasticity and neurological disorders. Neuron.
60:748–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fleming JC, Norenberg MD, Ramsay DA,
Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD
and Weaver LC: The cellular inflammatory response in human spinal
cords after injury. Brain. 129:3249–3269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou LH, Han S, Xie YY, Wang LL and Yao
ZB: Differences in c-jun and nNOS expression levels in motoneurons
following different kinds of axonal injury in adult rats. Brain
Cell Biol. 36:213–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu W, Liuzzi FJ, Schinco FP, Depto AS, Li
Y, Mong JA, Dawson TM and Snyder SH: Neuronal nitric oxide synthase
is induced in spinal neurons by traumatic injury. Neurosci.
61:719–726. 1994. View Article : Google Scholar
|
|
26
|
Fu R, Tang Y, Ling ZM, Li YQ, Cheng X,
Song FH, Zhou LH and Wu W: Lithium enhances survival and regrowth
of spinal motoneurons after ventral root avulsion. BMC Neurosci.
15:84–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Y, Ling ZM, Fu R, Li YQ, Cheng X,
Song FH, Luo HX and Zhou LH: Time-specific microRNA changes during
spinal motoneuron degeneration in adult rats following unilateral
BPRA: Ipsilateral vs. contralateral changes. BMC Neurosci.
15:92–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Yan L, Zhao X, Wu W and Zhou LH:
The diversity of nNOS gene expression in avulsion-injured spinal
motoneurons among laboratory rodents. Nitric Oxide. 22:37–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L and Wu W: Antisense oligos to
neuronal nitric oxide synthase aggravate motoneuron death induced
by spinal root avulsion in the adult rat. Exp Neurol. 197:84–92.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li YQ, Tang Y, Fu R, Meng QH, Zhou X, Ling
ZM, Cheng X, Tian SW, Wang GJ, Liu XG and Zhou LH: Efficient
labeling in vitro with non-ionic gadolinium magnetic
resonance imaging contrast agent and fluorescent transfection agent
in bone marrow stromal cells of neonatal rats. Mol Med Rep.
12:913–920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Linda H, Skold MK and Ochsmann T:
Activating transcription factor 3, a useful marker for a
regenerative response after nerve root injury. Front Neurol.
2:30–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen BP, Wolfgang CD and Hai T: Analysis
of ATF3, a transcription factor induced by physiological stresses
and modulated by gadd153/Chop10. Mol Cell Biol. 16:1157–1168. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin T, Sandhu G, Wolfgang CD, Burrier A,
Webb RL, Rigel DF, Hai T and Whelan J: Tissue-specific pattern of
stress kinase activation in ischemic/reperfused heart and kidney. J
Biol Chem. 272:19943–19950. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Powis RA and Gillingwater TH: Selective
loss of alpha motor neurons with sparing of gamma motor neurons and
spinal cord cholinergic neurons in a mouse model of spinal muscular
atrophy. J Anat. 228:443–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lalancette-Hebert M, Sharma A, Lyashchenko
AK and Shneider NA: Gamma motor neurons survive and exacerbate
alpha motor neuron degeneration in ALS. Proc Natl Acad Sci USA.
113:E8316–E8325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martín MC, Balfagón G, Minoves N and
Blanco J: Androgen deprivation increases neuronal nitric oxide
metabolism and its vasodilator effect in rat mesenteric arteries.
Nitric Oxide. 12:163–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Raivich G, Bohatschek M, Da Costa C, Iwata
O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L,
Wolfer DP, et al: The AP-1 transcription factor c-jun is required
for efficient axonal regeneration. Neuron. 43:57–67. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giguère V, Yang N, Segui P and Evans RM:
Identification of a new class of steroid hormone receptors. Nature.
331:91–94. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hong H, Yang L and Stallcup: MR
Hormone-independent transcriptional activation and coactivator
binding by novel orphan nuclear receptor ERR3. J Biol Chem.
274:22618–22626. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Z and Teng CT: Interplay between
estrogen-related receptor alpha (ERRalpha) and gamma (ERRgamma) on
the regulation of ERRalpha gene expression. Mol Cell Endocrinol.
264:128–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sladek R, Bader JA and Giguère V: The
orphan nuclear receptor estrogenrelated receptor alpha is a
transcriptional regulator of the human medium-chain acyl coenzyme A
dehydrogenase gene. Mol Cell Biol. 17:5400–5409. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao J and Patisaul HB: Sexually dimorphic
expression of hypothalamic estrogen receptors α and β and Kiss1 in
neonatal male and female rats. J Comp Neurol. 519:2954–2977. 2011.
View Article : Google Scholar : PubMed/NCBI
|