Introduction
Hypertension as a clinically common disease is
divided into primary and secondary hypertension, of which the
former is more common (1).
Hypertension endangers the health of adults and children, and
causes multi-organ diseases, especially diseases in the
cardiovascular system. The disease worsens with time if the
patients are not treated properly (2). Hypertension usually leads to heart
failure, left ventricular hypertrophy and other complications,
which cause significant heart injury, affecting left ventricular
diastolic function and result in cardiac dysfunction (3). The progression of hypertensive heart
disease includes myocardial fibrosis and changes in left
ventricular geometry, without signs and symptoms of heart failure
or left ventricular systolic dysfunction before functional changes
(4,5). Half of the patients with hypertension
have diastolic dysfunction, which has proved closely related to the
increasing incidence and morbidity of cardiovascular diseases and
the progression of heart failure (6). Patients with diastolic dysfunction have
increased year by year with the high incidence of hypertension and
its complications (7).
Hypertension is usually treated with angiotensin
converting enzyme inhibitor (ACEI), angiotensin II receptor
antagonist (ARB), calcium antagonist (CCB) and β receptor blockers.
These drugs can relieve hypertensive patients' left ventricular
diastolic function, clinical signs and symptoms (5,8,9). Galectin-3 (Gal-3), which is a member of
the galectin family and a β-galactoside-binding protein of 30-kDa,
plays an important role in immune regulation (10). As a pathogenic factor of heart
failure, Gal-3 not only causes myocardial inflammation and fibrosis
and affects left ventricular systolic function, but also promotes
cardiac remodeling, myocardial fibrosis and heart failure (11).
According to a previous study, Gal-3 is closely
related to the development and progression of cardiovascular
diseases, expected to be a biomarker for assisting the diagnosis
and prognosis of cardiac diseases (12). However, the role of Gal-3 in the
development, progression and treatment of hypertension complicated
with diastolic dysfunction remains unclear. Therefore, GAL-3
expression in patients with the disease was detected in this study
to explore the role of Gal-3 in the development, progression and
treatment of the disease.
Patients and methods
General information
One hundred patients with hypertension complicated
with diastolic dysfunction admitted to The First Affiliated
Hospital of Jiamusi University (Jiamusi, China) from June 2017 to
December 2018 were enrolled as group A. The patients consisted of
53 males and 47 females, aged 46–75 years with an average age of
63.57±10.38 years. According to the New York Heart Association
(NYHA) Functional Classification (13), there were 20 patients with grade I,
26 patients with grade II, 30 patients with grade III and 24
patients with grade IV in group A. Inclusion criteria for group A:
Patients who met the diagnostic criteria for hypertension in the
World Health Organization/International Society of Hypertension
(WHO/ISH) (14); patients with an
E/A value of cardiac color ultrasound <1; patients with a
diastolic pressure ≥90 mmHg or a systolic pressure ≥140 mmHg.
Exclusion criteria: Those with secondary hypertension, coronary
heart disease, valvular heart disease, congenital heart disease,
cerebrovascular diseases, cardiomyopathy, diabetes, acute pulmonary
embolism, connective tissue diseases, nervous system diseases,
immune diseases, endocrine and metabolic diseases, malignant tumors
or severe hepatorenal damage; those with mental illness and a
family history of psychosis; those who had taken diuretic drugs to
reduce blood pressure within the past 3 months; those who were not
treated according to the doctor's advice. Further 80 individuals
undergoing physical examination during the same period served as
group B. The subjects in group B consisted of 46 males and 34
females, aged 41–69 years with an average age of 60.69±10.67 years.
The inclusion criteria were applied to group A, and the subjects in
group B were healthy controls.
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Jiamusi University. The subjects
and their families were informed and signed a fully informed
consent form.
Therapeutic methods and efficacy
evaluation
Patients in group A were routinely treated, which
included smoking cessation, temperance, reduction of sodium intake,
weight and fat intake, and increasing of aerobic exercise. Their
myocardial function and blood pressure were monitored in real-time.
According to conditions, the patients were treated with ACEI, ARB,
CCB and β receptor blockers for 3 months, with diuresis, potassium
excretion and correction of acid-base equilibrium disorders given
at the same time. Adverse reactions during the treatment were
observed. Grading: Markedly effective: Symptoms and signs
disappeared completely, and improvement of cardiac function was
grade II. Effective: Symptoms and signs were improved, and
improvement of cardiac function was grade I. Invalid: Symptoms,
signs and cardiac function had no significant change and even
worsened.
Echocardiography
GE Vivid 7 color ultrasonic device (General Electric
Company) and M3S probe with 2.5 MHz were used for analysis and
measurement by specialist physicians in ultrasonic medicine.
Subjects were in supine position breathing calmly, and their ECG
was synchronously recorded to obtain the standard two-dimensional
images of sections of the heart, and the frequency spectrums of
valve orifices and pulmonary vein. Left ventricular diastolic
function was determined with respect to mitral valve early peak
flow velocity (EPFV), atrial peak flow velocity (APFV), maximum
flow velocity ratio of early to atrial diastole (EPFV/APFV) and
peak mitral annulus velocity (E/E′).
Detection methods
Five milliliters of fasting peripheral blood was
extracted, placed in a vacuum tube free of anticoagulant, and
centrifuged at 670.8 × g, at 20–25°C for 10 min to separate serum.
An Elx-800 microplate reader (BioTek) was used to detect serum
Gal-3 concentration, with steps carried out in accordance with the
instructions of human Gal-3 enzyme-linked immunosorbent assay
(ELISA) kit (Renjie Biotechnology Co., Ltd.). The kit and the
sample to be tested were taken out 20 min in advance to balance
with room temperature. Sample wells, standard wells and blank wells
were set up. The standard wells were added with 50 µl of standard
substances with different concentrations, the sample wells were
added with 50 µl of the sample to be tested, nothing was added to
the blank wells. The wells were added with 100 µl of horseradish
peroxidase (HRP)-labeled detection secondary goat anti-human IgG
monoclonal antibody (1:250; cat. no. A0201; Beyotime Institute of
Biotechnology) (https://www.beyotime.com/product/A0201.htm), covered
with tectorial membranes, and incubated at 37°C for 1 h. After
that, they were added with 350 µl of cleaning solution and allowed
to stand for 1 min. The liquid in wells was discarded and the wells
were dried with absorbent paper. The wells were washed 5 times.
Next, the wells were added with 50 µl each of substrates A and B,
and incubated at 37°C for 15 min in the dark. Finally, they were
added with 50 µl of stop solution. The Elx-800 microplate reader
was used to detect OD values of wells at 450 nm within 15 min, in
order to calculate Gal-3 concentration.
Statistical analysis
SPSS 20.0 (IBM Corporation) was used to
statistically analyze the data, GraphPad Prism 7 (GraphPad
Software, Inc.) to plot figures. Enumeration data were expressed as
[n (%)], and Chi-square test was used for comparison of enumeration
data between groups. Measurement data were expressed as mean ±
standard deviation (mean ± SD), and independent samples t-test was
used for comparison of measurement data between groups, paired
t-test for comparison before and after treatment within groups.
Spearman's test was used for correlation analysis. The receiver
operating characteristic (ROC) curve was used to plot the
diagnostic value of Gal-3 concentration before treatment for
efficacy. P<0.05 indicated a statistically significant
difference.
Results
Comparison of general information
There was no significant difference between group A
and group B in terms of sex, age, history of smoking, history of
drinking, place of residence, educational level, fasting blood
glucose (FBG), hemoglobin (Hb), red blood cell (RBC) count,
platelet (PLT) count, aspartate aminotransferase (AST), alanine
aminotransferase (ALT), uric acid or serum creatinine (P>0.05)
(Table I).
 | Table I.Comparison of general information [n
(%)] (mean ± SD). |
Table I.
Comparison of general information [n
(%)] (mean ± SD).
| Category | Group A (n=100) | Group B (n=80) | t/χ2 | P-value |
|---|
| Sex |
|
| 0.364 | 0.547 |
| Male | 53 (53.00) | 46 (57.50) |
|
|
|
Female | 47 (47.00) | 34 (42.50) |
|
|
| Age (years) | 63.57±10.38 | 60.69±10.67 | 1.827 | 0.069 |
| History of
smoking |
|
| 0.190 | 0.663 |
| Yes | 57 (57.00) | 43 (53.75) |
|
|
| No | 43 (43.00) | 37 (46.25) |
|
|
| History of
drinking |
|
| 0.251 | 0.616 |
| Yes | 55 (55.00) | 41 (51.25) |
|
|
| No | 45 (45.00) | 39 (48.75) |
|
|
| Place of
residence |
|
| 0.641 | 0.423 |
| City | 67 (67.00) | 49 (61.25) |
|
|
|
Countryside | 33 (33.00) | 31 (38.75) |
|
|
| Educational
level |
|
| 0.818 | 0.366 |
|
≥University | 42 (42.00) | 39 (48.75) |
|
|
|
<University | 58 (58.00) | 41 (51.25) |
|
|
| FBG (mmol/l) | 5.53±1.37 | 5.74±2.26 | 0.769 | 0.443 |
| Hb (g/l) | 116.58±10.85 | 119.57±13.53 | 1.646 | 0.102 |
| RBC
(×1012/l) | 4.63±0.35 | 4.71±0.45 | 1.342 | 0.181 |
| PLT
(×109/l) | 147.48±15.28 | 151.52±16.37 | 1.708 | 0.090 |
| AST (U/l) | 21.73±9.65 | 20.45±8.65 | 0.926 | 0.356 |
| ALT (U/l) | 19.42±8.26 | 17.35±9.41 | 1.570 | 0.118 |
| Uric acid
(µmol/l) | 298.16±65.37 | 281.52±45.38 | 1.934 | 0.055 |
| Serum creatinine
(µmol/l) | 79.13±14.16 | 75.61±12.59 | 1.740 | 0.084 |
| Cardiac function
grading |
| Grade
I | 20 (20.00) | – |
|
|
| Grade
II | 26 (26.00) | – |
|
|
| Grade
III | 30 (30.00) | – |
|
|
| Grade
IV | 24 (24.00) | – |
|
|
Comparison of Gal-3 concentration
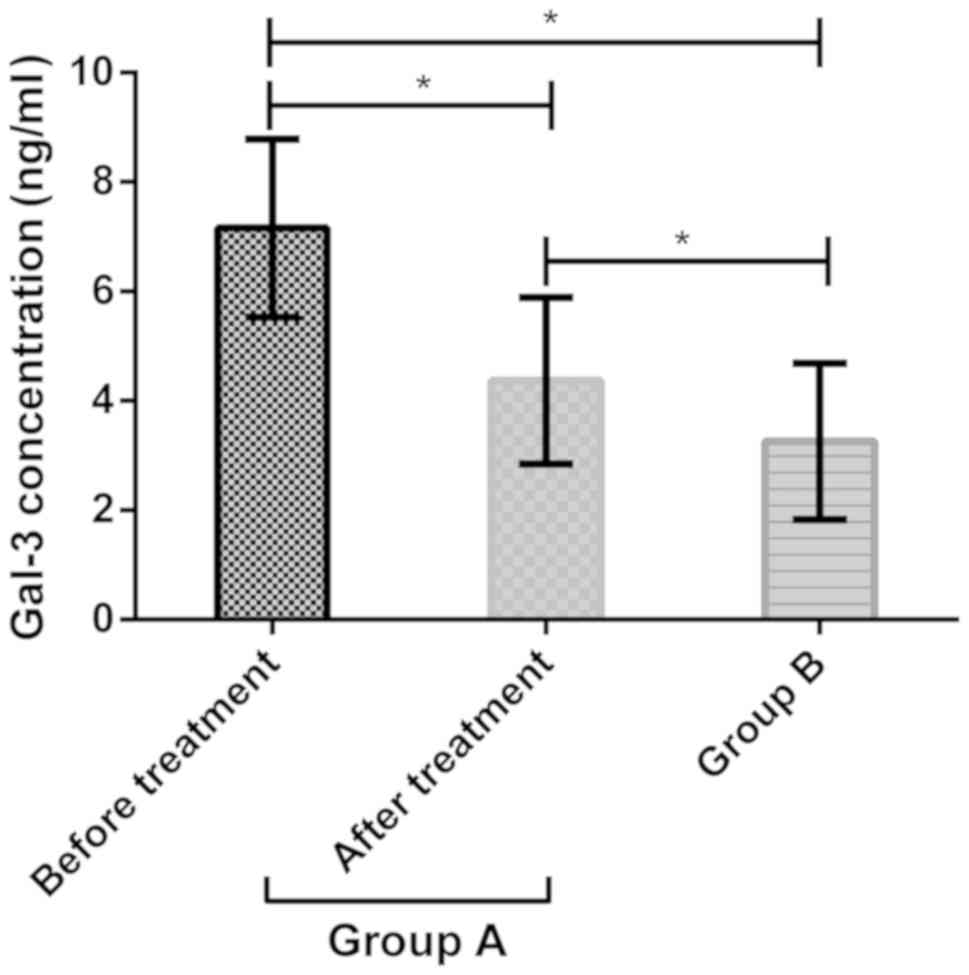
In group A, Gal-3 concentration was 7.16±1.63 ng/ml
before treatment and 4.37±1.52 ng/ml after treatment. The
concentration was 3.26±1.43 ng/ml in group B. Gal-3 concentration
in group A was significantly higher than that in group B
(P<0.05). After treatment, the concentration in group A was
significantly lower than that before treatment (P<0.05), but
significantly higher than that in group B (P<0.05) (Fig. 1).
Correlation of Gal-3 concentration
with cardiac function grading in group A
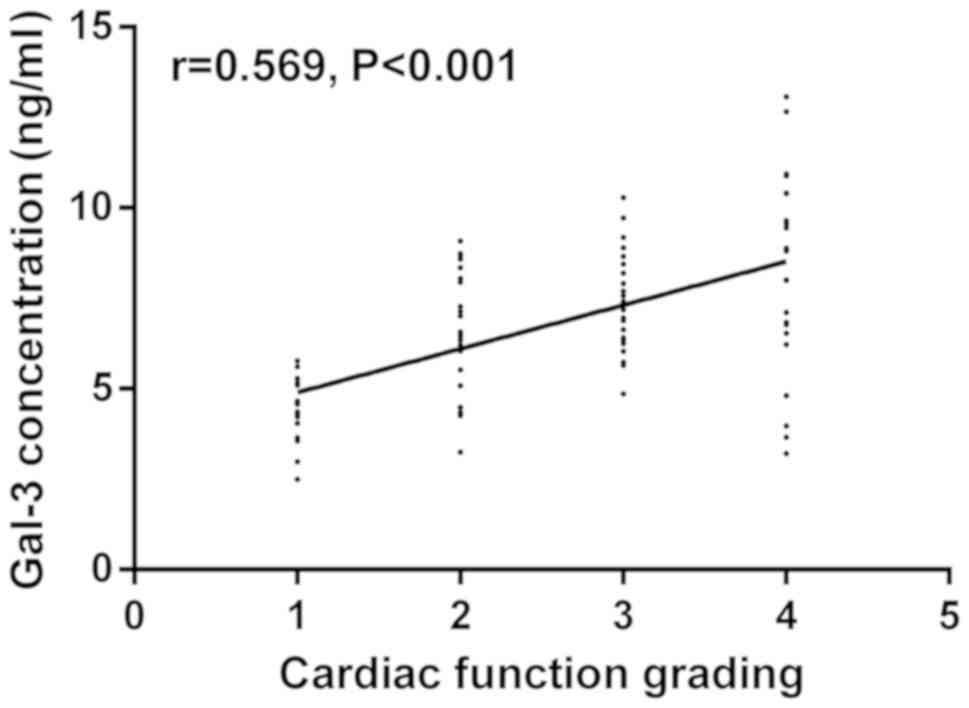
Gal-3 concentration was significantly higher in
patients with cardiac function grades II, III and IV than that in
patients with grade I (P<0.05). The concentration increased with
the rise of cardiac function grading. Cardiac function grade I was
set as 1, grade II as 2, grade III as 3 and grade IV as 4.
According to Spearman's correlation, Gal-3 concentration was
positively correlated with cardiac function grading (r=0.569,
P<0.001) (Table II and Fig. 2).
 | Table II.Correlation of Gal-3 concentration
with cardiac function grading in group A (mean ± SD). |
Table II.
Correlation of Gal-3 concentration
with cardiac function grading in group A (mean ± SD).
| Cardiac function
grading | n | Gal-3 (ng/ml) |
|---|
| Grade I | 20 | 4.63±0.89 |
| Grade II | 26 |
6.74±1.43a |
| Grade III | 30 |
7.89±1.76a,b |
| Grade IV | 24 |
8.37±2.26a–c |
| F-value | – | 21.370 |
| P-value | – | <0.001 |
Comparison of diastolic function
indexes
Compared with before treatment in group A, patients
after treatment had significantly higher EPFV and EPFV/APFV
(P<0.05), but significantly lower APFV and E/E′ (P<0.05).
Compared with group B, patients in group A after treatment had
significantly lower EPFV and EPFV/APFV (P<0.05), but
significantly higher APFV and E/E′ (P<0.05) (Table III).
 | Table III.Comparison of diastolic function
indexes before and after treatment in group A, and between group A
and group B (mean ± SD). |
Table III.
Comparison of diastolic function
indexes before and after treatment in group A, and between group A
and group B (mean ± SD).
|
| Group A
(n=100) |
|
|---|
|
|
|
|
|---|
| Indexes | Before
treatment | After
treatment | Group B (n=80) |
|---|
| EPFV (m/sec) | 0.67±0.13 |
0.76±0.17a |
0.81±0.14a,b |
| APFV (m/sec) | 0.81±0.15 |
0.72±0.14a |
0.63±0.12a,b |
| EPFV/APFV | 0.84±0.13 |
1.03±0.16a |
1.22±0.16a,b |
| E/E′ | 17.24±4.57 |
13.42±3.18a |
7.86±2.54a,b |
Gal-3 concentration before treatment
in the effective and invalid groups and its predictive value for
efficacy
During treatment, group A had 2 patients with nausea
and 1 patient with dizziness, none of whom were treated. After
treatment, group A had 39 markedly effective patients, 32 effective
patients and 29 invalid patients. According to efficacy after
treatment, the patients in group A were divided into the effective
group (71 patients) and the invalid group (29 patients). Before
treatment, Gal-3 concentration in the effective group was 6.41±1.73
ng/ml, significantly lower than 9.16±2.37 ng/ml in the invalid
group (P<0.05). The ROC curve of Gal-3 concentration before
treatment was plotted for diagnosing the efficacy for hypertension
complicated with diastolic dysfunction. The AUC of Gal-3
concentration was 0.792 (95% CI, 0.691–0.894), the sensitivity was
73.24% and the specificity was 79.31%, with an optimal cut-off
value of 7.68 ng/ml (Table IV and
Fig. 3).
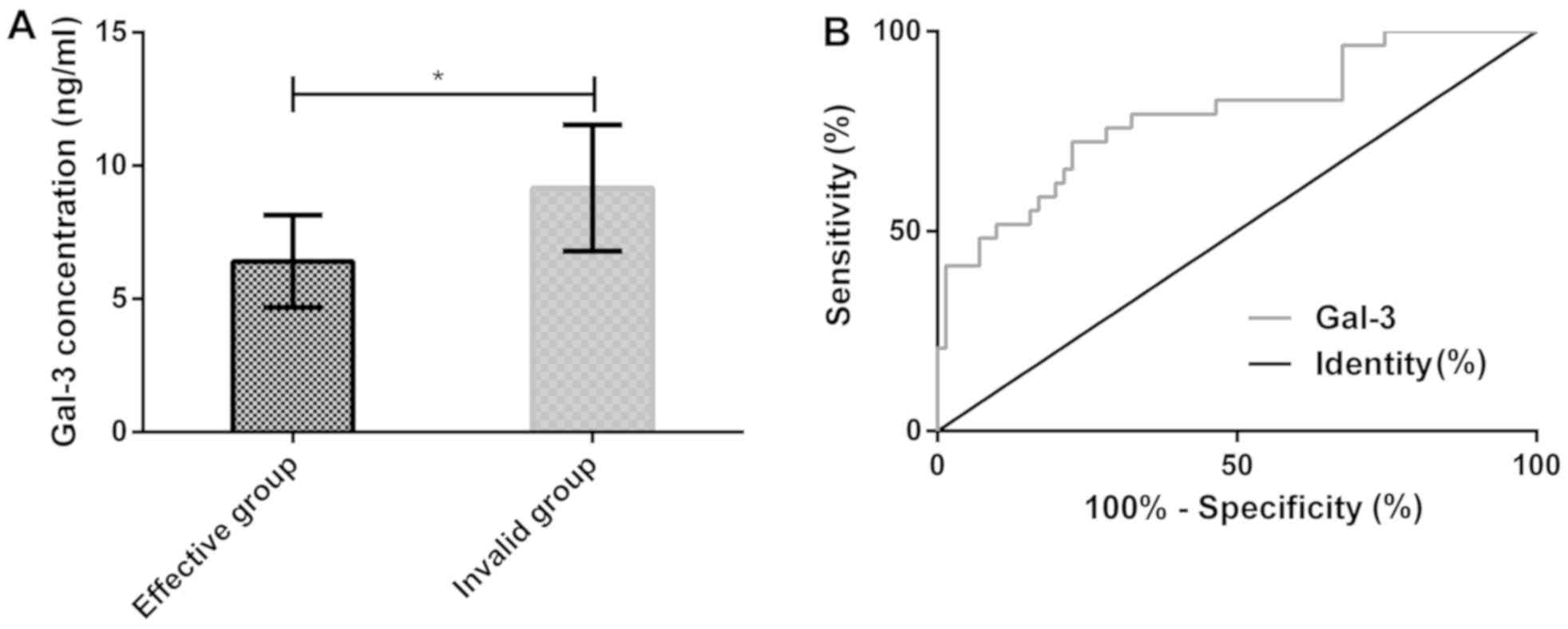 | Figure 3.Serum Gal-3 concentration before
treatment in the effective and invalid groups and its predictive
value for efficacy. (A) According to ELISA, before treatment, serum
Gal-3 concentration in the effective group was significantly lower
than that in the invalid group (P<0.05). (B) According to the
ROC curve, the AUC of serum Gal-3 concentration for evaluating
efficacy was 0.792 (95% CI, 0.691–0.894), the sensitivity was
73.24% and the specificity was 79.31%, with an optimal cut-off
value of 7.68 ng/ml. *P<0.05. Gal-3, galectin-3; ELISA,
enzyme-linked immunosorbent assay; AUC, area under curve; ROC,
receiver operating characteristic. |
 | Table IV.ROC curve analysis of serum Gal-3
concentration predicting the therapeutic effect of hypertension
combined with diastolic dysfunction. |
Table IV.
ROC curve analysis of serum Gal-3
concentration predicting the therapeutic effect of hypertension
combined with diastolic dysfunction.
| Diagnostic
indicator | AUC | 95% CI | Sth. Error | Cut-off | Sensitivity
(%) | Specificity
(%) |
|---|
| Gal-3 (ng/ml) | 0.792 | 0.691–0.894 | 0.052 | 7.68 | 73.24 | 79.31 |
Discussion
With an increasing incidence in recent years,
hypertension is an important risk factor for many cardiovascular
diseases (15). Major cardiac
changes in patients with hypertension are left ventricular
enlargement and hypertrophy, which are leading causes of cardiac
diastolic dysfunction (16). In the
early stage of cardiac diastolic dysfunction, the symptoms and
signs of heart failure are unapparent. However, as the disease
progresses, most patients with cardiac diastolic dysfunction suffer
from secondary systolic dysfunction, aggravated signs and symptoms
of heart failure (17). Therefore,
it is particularly important to find biomarkers related to the
development, progression and treatment of hypertension complicated
with diastolic dysfunction.
As a β-galactoside-binding lectin and a member of
the soluble protein family, Gal-3 present in the nucleus promotes
vascular injury and cardiovascular fibrosis. In patients with heart
failure, plasma Gal-3 level has been proven to be the best
predictor of short-term prognosis (18). There are many studies on Gal-3 in
hypertension and heart failure. According to Calvier et al
(19), Gal-3 concentration in plasma
of patients with pulmonary hypertension significantly increases, so
Gal-3 plays an important role in the pathophysiological process of
the disease, and it can be used as a biomarker for reflecting the
functional state and disease progression of the disease. According
to Singh et al (20), Gal-3 is involved in the pathogenesis
of cardiac fibrosis which is a leading cause of diastolic
dysfunction, so it can be used as a predictor of diastolic
dysfunction in patients with atrial fibrillation and heart failure.
The results of this study showed that Gal-3 concentration in group
A was significantly higher than that in group B, and positively
correlated with cardiac function grading; that is, Gal-3
concentration significantly increased with the rise of cardiac
function grading. These findings indicate that Gal-3 may be
involved in the development and progression of hypertension
complicated with diastolic dysfunction, and it increases with the
aggravation of the disease. This is similar to the findings of
previous studies. The pathological process of hypertensive disease
progression is myocardial fibrosis (21). Therefore, Gal-3 as a marker for
myocardial fibrosis (22) may
gradually increase with the deterioration of cardiac function and
the aggravation of myocardial fibrosis.
Clinically, patients with hypertension are commonly
treated with ACEI, ARB, CCB and β-receptor blockers, which can
control blood pressure, improve cardiac structure and function, and
inhibit myocardial fibrosis and the release of inflammatory
cytokines. They can also reduce the left ventricle and its cardiac
chamber, and improve cardiac diastolic function (23–26). In
the present study, after treatment, Gal-3 concentration in group A
was significantly lower than that before treatment, but
significantly higher than that in group B. Although the patients'
Gal-3 concentration after treatment did not return to normal, their
cardiac diastolic function was improved. Therefore, the inhibition
of Gal-3 concentration may be one of the therapeutic mechanisms. In
a study by Calvier et al (27), the increasing expression of Gal-3 in
experimental hyperaldosteronism is related to cardiac and renal
fibrosis and dysfunction. Inhibition or blocking of the expression
through drugs reduces cardiac and renal fibrosis, so Gal-3 can be
used as a new target for pharmacological intervention. According to
a study by Vergaro et al (28), the inhibition of Gal-3 expression and
protein content prevents isoproterenol-induced left ventricular
dysfunction and fibrosis in mice. Therefore, Gal-3 concentration
may affect the treatment of the disease. In this study, before
treatment, Gal-3 concentration in the effective group was
significantly lower than that in the invalid group. According to
the ROC curve, Gal-3 concentration before treatment had a high
diagnostic value for the clinical efficacy. These findings indicate
that Gal-3 concentration before treatment has a predictive value
for efficacy.
This study confirmed the role of Gal-3 concentration
in the development, progression and treatment of patients with
hypertension complicated with diastolic dysfunction, but it still
has deficiencies in design. Drugs were given based on the patients'
signs and symptoms, so their specific effects on Gal-3
concentration remain unclear and risk factors affecting the onset
and treatment of the disease were not explored. These deficiencies
require further research to support the results of this study.
In conclusion, Gal-3 may be involved in the
development and progression of hypertension complicated with
diastolic dysfunction. Its concentration increases with the rise of
cardiac function grading but significantly decreases after
treatment. Therefore, Gal-3 concentration before treatment can be
used as a potential predictor of efficacy.
Acknowledgements
Not applicable.
Funding
This study was supported by Heilongjiang Provincial
Health and Family Planning Commission Research Project
2017-381.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TD and HL conceived and designed the study. TD, HL,
SW and WC were responsible for the collection and analysis of the
experimental data. HL and WC interpreted the data and drafted the
manuscript. TD revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Jiamusi University (Jiamusi,
China). Signed informed consents were obtained from the patients or
the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leung AA, Nerenberg K, Daskalopoulou SS,
McBrien K, Zarnke KB, Dasgupta K, Cloutier L, Gelfer M,
Lamarre-Cliche M, Milot A, et al CHEP Guidelines Task Force, :
Hypertension Canada's 2016 Canadian hypertension education program
guidelines for blood pressure measurement, diagnosis, assessment of
risk, prevention, and treatment of hypertension. Can J Cardiol.
32:569–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oluboka OJ, Katzman MA, Habert J, McIntosh
D, MacQueen GM, Milev RV, McIntyre RS and Blier P: Functional
recovery in major depressive disorder: Providing early optimal
treatment for the individual patient. Int J Neuropsychopharmacol.
21:128–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Soullier C, Niamkey JT, Ricci JE,
Messner-Pellenc P, Brunet X and Schuster I: Hypertensive patients
with left ventricular hypertrophy have global left atrial
dysfunction and impaired atrio-ventricular coupling. J Hypertens.
34:1615–1620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ravassa S, López B, Querejeta R, Echegaray
K, San José G, Moreno MU, Beaumont FJ, González A and Díez J:
Phenotyping of myocardial fibrosis in hypertensive patients with
heart failure. Influence on clinical outcome. J Hypertens.
35:853–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nazário Leão R and Marques da Silva P:
Diastolic dysfunction in hypertension. Hipertens Riesgo Vasc.
34:128–139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ginelli P and Bella JN: Treatment of
diastolic dysfunction in hypertension. Nutr Metab Cardiovasc Dis.
22:613–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nadruz W, Shah AM and Solomon SD:
Diastolic dysfunction and hypertension. Med Clin North Am.
101:7–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Sun N, Jiang X and Xi Y:
Comparative efficacy of β-blockers on mortality and cardiovascular
outcomes in patients with hypertension: A systematic review and
network meta-analysis. J Am Soc Hypertens. 11:394–401. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wallentin F, Wettermark B and Kahan T:
Drug treatment of hypertension in Sweden in relation to sex, age,
and comorbidity. J Clin Hypertens (Greenwich). 20:106–114. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anyfanti P, Gkaliagkousi E, Gavriilaki E,
Triantafyllou A, Dolgyras P, Galanopoulou V, Aslanidis S and Douma
S: Association of galectin-3 with markers of myocardial function,
atherosclerosis, and vascular fibrosis in patients with rheumatoid
arthritis. Clin Cardiol. 42:62–68. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
González GE, Rhaleb NE, D'Ambrosio MA,
Nakagawa P, Liao TD, Peterson EL, Leung P, Dai X, Janic B, Liu YH,
et al: Cardiac-deleterious role of galectin-3 in chronic
angiotensin II-induced hypertension. Am J Physiol Heart Circ
Physiol. 311:H1287–H1296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jagodzinski A, Havulinna AS, Appelbaum S,
Zeller T, Jousilahti P, Skytte-Johanssen S, Hughes MF, Blankenberg
S and Salomaa V: Predictive value of galectin-3 for incident
cardiovascular disease and heart failure in the population-based
FINRISK 1997 cohort. Int J Cardiol. 192:33–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meijers WC, van der Velde AR and de Boer
RA: ST2 and galectin-3: Ready for prime time? EJIFCC. 27:238–252.
2016.PubMed/NCBI
|
|
14
|
Ghorpade AG, Shrivastava SR, Kar SS,
Sarkar S, Majgi SM and Roy G: Estimation of the cardiovascular risk
using World Health Organization/International Society of
Hypertension (WHO/ISH) risk prediction charts in a rural population
of South India. Int J Health Policy Manag. 4:531–536. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Chen Z, Zhang L, Wang X, Hao G,
Zhang Z, Shao L, Tian Y, Dong Y, Zheng C, et al China hypertension
survey investigators, : Status of hypertension in China: Results
from the China hypertension survey, 2012–2015. Circulation.
137:2344–2356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bansal M, Kasliwal RR and Trehan N:
Relationship between different cardiovascular risk scores and
measures of subclinical atherosclerosis in an Indian population.
Indian Heart J. 67:332–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deckx S, Heggermont W, Carai P, Rienks M,
Dresselaers T, Himmelreich U, van Leeuwen R, Lommen W, van der
Velden J, Gonzalez A, et al: Osteoglycin prevents the development
of age-related diastolic dysfunction during pressure overload by
reducing cardiac fibrosis and inflammation. Matrix Biol.
66:110–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maiolino G, Rossitto G, Pedon L, Cesari M,
Frigo AC, Azzolini M, Plebani M and Rossi GP: Galectin-3 predicts
long-term cardiovascular death in high-risk patients with coronary
artery disease. Arterioscler Thromb Vasc Biol. 35:725–732. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calvier L, Legchenko E, Grimm L, Sallmon
H, Hatch A, Plouffe BD, Schroeder C, Bauersachs J, Murthy SK and
Hansmann G: Galectin-3 and aldosterone as potential tandem
biomarkers in pulmonary arterial hypertension. Heart. 102:390–396.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh T, Pilania M, Jat GS and Kumar R:
Ambiguity about selection of cardiovascular risk stratification
tools: Evidence from a North Indian rural population. Indian J
Community Med. 43:170–174. 2018.PubMed/NCBI
|
|
21
|
Wu H, Chen L, Xie J, Li R, Li GN, Chen QH,
Zhang XL, Kang LN and Xu B: Periostin expression induced by
oxidative stress contributes to myocardial fibrosis in a rat model
of high salt-induced hypertension. Mol Med Rep. 14:776–782. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hogas S, Bilha SC, Branisteanu D, Hogas M,
Gaipov A, Kanbay M and Covic A: Potential novel biomarkers of
cardiovascular dysfunction and disease: Cardiotrophin-1, adipokines
and galectin-3. Arch Med Sci. 13:897–913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gorostidi M and de la Sierra A:
Combination therapy in hypertension. Adv Ther. 30:320–336. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shah SJ and Stafford RS: Current trends of
hypertension treatment in the United States. Am J Hypertens.
30:1008–1014. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taddei S: Combination therapy in
hypertension: What are the best options according to clinical
pharmacology principles and controlled clinical trial evidence? Am
J Cardiovasc Drugs. 15:185–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gradman AH: Strategies for combination
therapy in hypertension. Curr Opin Nephrol Hypertens. 21:486–491.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calvier L, Martinez-Martinez E, Miana M,
Cachofeiro V, Rousseau E, Sádaba JR, Zannad F, Rossignol P and
López-Andrés N: The impact of galectin-3 inhibition on
aldosterone-induced cardiac and renal injuries. JACC Heart Fail.
3:59–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vergaro G, Prud'homme M, Fazal L, Merval
R, Passino C, Emdin M, Samuel JL, Cohen Solal A and Delcayre C:
Inhibition of galectin-3 pathway prevents isoproterenol-induced
left ventricular dysfunction and fibrosis in mice. Hypertension.
67:606–612. 2016. View Article : Google Scholar : PubMed/NCBI
|