Introduction
Gestational diabetes mellitus (GDM) (1,2) is a
disease that occurs during pregnancy, and its incidence rate has
gradually increased. Lack of pregnancy knowledge, along with
unreasonable diet, glycemic control and pregnancy weight gain lead
to GDM in pregnant women (3). These
are important factors in becoming a high-risk pregnant woman. If
not controlled in time, the disease may have a severe impact on
mothers and infants.
Most pregnant women with GDM cannot control their
blood glucose effectively through diet control and exercise therapy
(4). Consequently, they are often
dependent on drug therapy to control their blood glucose level.
Insulin is often selected for the treatment of diabetes as it can
reduce blood glucose levels (5).
However, it has no obvious effect on the treatment of insulin
resistance in patients with GDM; thus, there are still some
deficiencies in its efficacy. Metformin is an insulin sensitizer
that adjusts glucose metabolism by adjusting insulin sensitivity
(6). This constitutes an important
reason for improving insulin sensitivity in vivo.
Increasing insulin resistance in GDM patients is the
result of increased secretion of progesterone, estrogen and other
substances in the middle and late stages of gestation, resulting in
decreased insulin sensitivity, unstable blood glucose level, and
abnormal glucose metabolism and various complications (7). Diabetic nephropathy is easy to occur
during the treatment of GDM (8). Due
to the inconspicuous early symptoms, diabetic nephropathy is not
easy to detect, and develops to the point of severe damage when
identified at late stage. While cystatin C (Cys C) is a sensitive
indicator for early diagnosis of damage to glomerular filtration
function, it can reflect the damage degree of patients with GDM
complicated with renal disease (9).
In addition, when the body's blood glucose level is at a high
concentration, patients may lose excessive amounts of fluid
(10,11). The level of homocysteine (Hcy) also
increases, accelerating the pathological changes of small blood
vessels and aggravating the course of GDM (10,11).
Therefore, the monitoring of the two serum factors is conducive to
the prevention and timely detection of some related
complications.
Previous findings have shown that insulin combined
with metformin have an effect of declining the levels of blood
sugar (12). However, few studies on
the joint treatment of the two concerning the detection of serum
Cys C and Hcy levels in patients with GDM and their effects on
mothers and infants are currently available. In the present study,
we explored the effect of the combined therapy of insulin and
metformin on serum Cys C and Hcy levels, and the maternal and
neonatal outcomes in GDM patients.
Patients and methods
General data
A total of 80 cases of GDM patients admitted to the
department of obstetrics and gynecology of Liaocheng Third People's
Hospital (Liaocheng, China) from July 2015 to July 2017 were
selected, 42 cases of patients treated with insulin combined with
metformin were included in the study group, and 38 cases of
patients treated with insulin alone were included in the control
group. There were no significant differences in age, BMI and
gestational weeks (P>0.05). Patients who met one of the
diagnostic criteria for GDM were included in the study: fasting
blood glucose (FPG) ≥5.1 mmol/l, blood glucose after 1 h (1hPG)
≥10.0 mmol/l, and postprandial blood glucose after 2 h (2hPG) ≥8.5
mmol/l (13). Exclusion criteria
were: patients who had gestational diabetes during previous
pregnancy; patients with liver and kidney dysfunction; patients
with communication and cognitive dysfunction; patients who did not
cooperate with the study requirements.
All the patients and their families agreed to
participate in the experiment and signed an informed consent. The
present study was approved by the Ethics Committee of the Liaocheng
Third People's Hospital.
Therapeutic schedule
Regular exercise and diet management were adopted in
both groups. Patients in the control group received insulin
treatment [import drug registration certificate no. X19990279[96],
medicine authorized number: J-70[2]; Novo Nordisk (China)
Pharmaceuticals Co., Ltd.], at the dose of 0.2–0.25 U/(kg·day).
Patients in the study group received treatment of insulin combined
with metformin. The insulin dose was 0.2–0.25 U/(kg·day), and the
metformin was given orally at 0.5 g/time twice a day. The dosage
could be increased or decreased according to the patient's blood
glucose level, and the highest dose could not exceed 3 g/day. The
treatment continued until the birth of the newborn.
Observational indexes
Blood glucose levels of GDM patients in both groups
were observed before and after treatment, and FBG and 2hPG levels
were measured and recorded in all patients before and after
treatment.
Venous blood (5 ml) of the pregnant women was
extracted before and after treatment and centrifuged at 2,600 × g
for 10 min at 4°C. Then, the serum was placed in the refrigerator
(AU5800 automatic biochemical analyzer; Beckmen Coulter). ELISA was
used to detect the level of Hcy in serum (EY-elisa2279; Shanghai
Yiyan Biological Technology Co., Ltd.). Immunoturbidimetry was used
to detect the serum Cys C level (59400368211; Hangzhou Deangel
Biological Engineering Co., Ltd.).
Maternal and neonatal outcomes between the two
groups were compared, including maternal delivery methods: Cesarean
section, preterm delivery and labor induction. The number of
newborns with hypoglycemia, macrosomia, neonatal jaundice, and
fetal malformation was recorded.
UmAlb levels of pregnant women in the two groups
were compared, special protein immunoturbidimetry was used in the
detection. The detection instrument was Beckman DXC600 automatic
biochemical detector (Beckmen Coulter).
The incidence of adverse reactions was compared
between the two groups, including nausea, vomiting, hypertension
and diarrhea.
Statistical analysis
In this study, SPSS 20.0 [Boyi zhixun (Beijing)
Information Technology Co., Ltd. [was used for statistical analysis
of experimental data. Chi-square test was used for enumeration
data. Mean ± standard deviation was used for measurement data. The
t-test was used for comparison between the two groups, and paired
t-test was used for comparison before and after treatment of the
two groups. GraphPad Prism 6 software was used to draw the
experimental illustrations. P<0.05 was considered a
statistically significant difference.
Results
Comparison of general data of the two
groups
There were no significant differences in general
data such as age, BMI and gestational weeks (P>0.05) (Table I).
 | Table I.Comparison of general data of the two
groups. |
Table I.
Comparison of general data of the two
groups.
| Factors | Research group
(n=42) | Control group
(n=38) | t/χ2
value | P-value |
|---|
| Age (years) |
|
| 0.179 | 0.673 |
| ≤26 | 28 (66.67) | 27 (71.05) |
|
|
|
>26 | 14 (33.33) | 11 (28.94) |
|
|
| Family history |
|
| 0.038 | 0.846 |
| With | 5 (11.90) | 4 (10.52) |
|
|
|
Without | 37 (88.10) | 34 (89.47) |
|
|
| Embryo number |
|
| 0.011 | 0.918 |
| Single
birth | 40 (95.24) | 36 (94.74) |
|
|
|
Twins | 2 (4.76) | 3 (7.89) |
|
|
| History of
abortion |
|
| 0.506 | 0.613 |
| With | 8 (19.05) | 9 (23.68) |
|
|
|
Without | 34 (80.95) | 29 (76.32) |
|
|
| BMI before pregnancy
(kg/m2) | 21.45±0.38 | 21.83±0.26 | 0.111 | 0.739 |
| Body growth during
gestation (kg) | 16.24±0.27 | 16.34±0.34 | 0.128 | 0.617 |
| Gravidity | 1.67±0.38 | 1.58±0.25 | 0.220 | 1.237 |
| Parity | 1.35±0.26 | 1.46±0.37 | 0.125 | 1.550 |
| Gestational
weeks | 27.47±2.27 | 27.42±1.82 | 0.108 | 0.914 |
Comparison of FBG and 2hPG levels
before and after treatment in the two groups
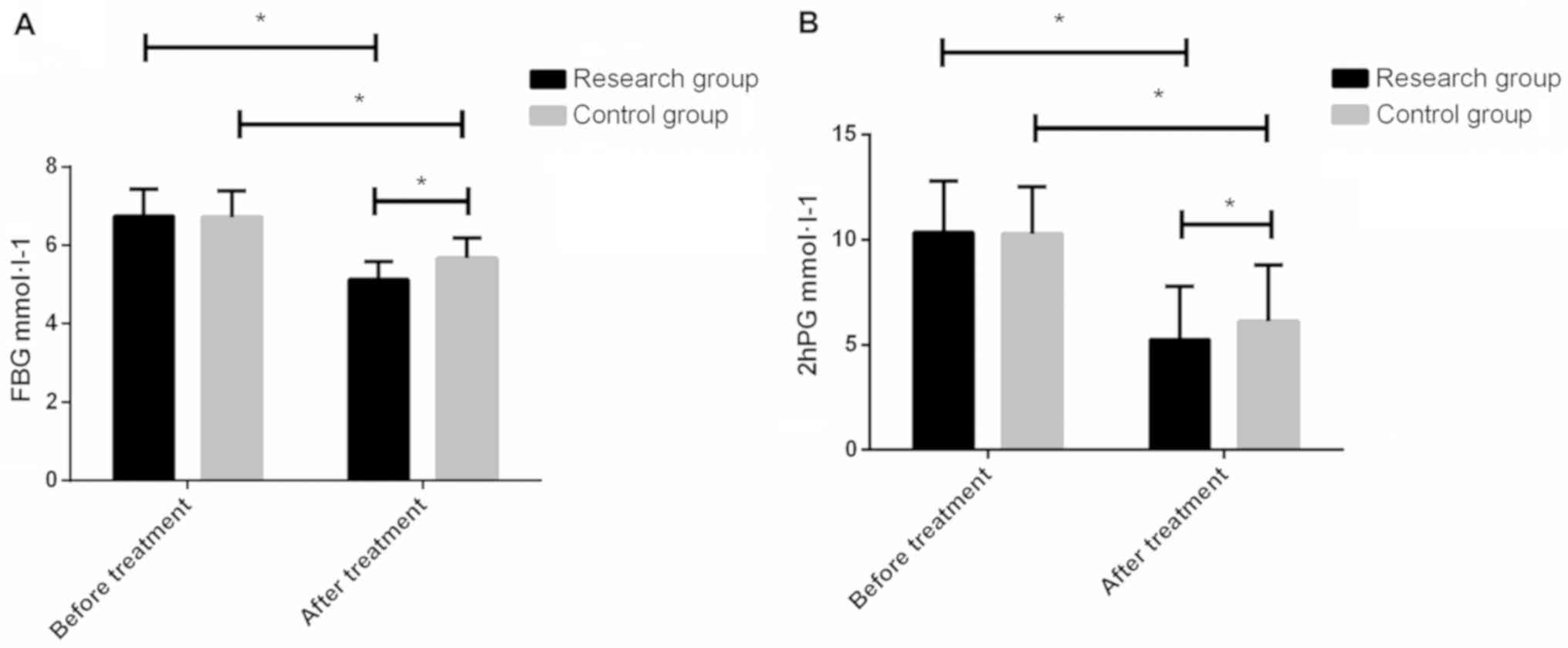
The FBG content in the study group and the control
group before treatment was 6.75±0.68 and 6.72±0.67
mmol·l−1, and the 2hPG content was 10.34±2.45 and
10.27±2.23 mmol·l−1, respectively. After treatment, the
content of FBG was 5.13±0.46 and 5.67±0.52 mmol·l−1,
respectively. The 2hPG content was 5.24±2.56 and 6.12±2.67
mmol·l−1, respectively. There were no significant
differences between the study and control groups before treatment
(P>0.05), and the levels of FBG and 2hPG in the study group
after treatment were lower than those in the control group
(P<0.05) (Fig. 1).
Content of serum Cys C and Hcy of
patients in the two groups before and after treatment
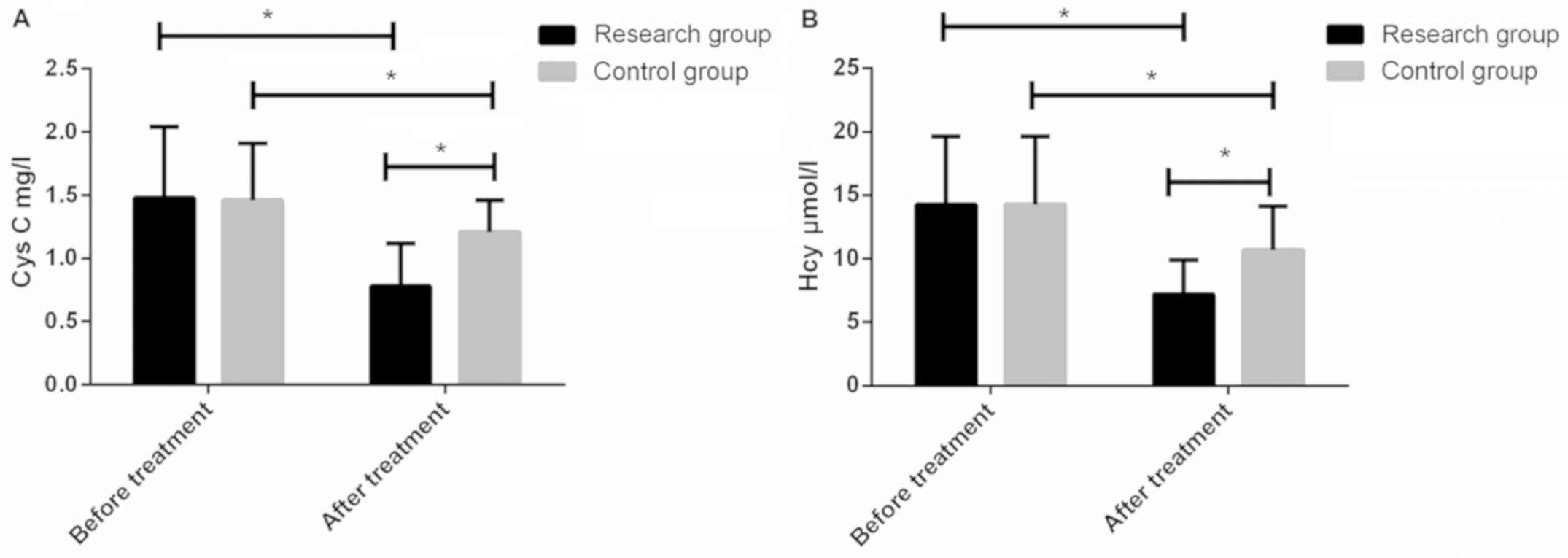
Serum Cys C contents in the study group and control
group before treatment were 1.480.56 and 1.460.45 mg/l,
respectively. The contents of serum Hcy were 14.25 and 14.285.34
µmol/l, respectively. There were no significant differences between
the two groups (P>0.05). After treatment, serum Cys C levels in
the study group and control group were 0.780.34 and 1.210.25 mg/l,
respectively. The serum Hcy levels were 7.212.67 and 103.48 µmol/l,
respectively. After treatment, the levels of both groups were lower
than those before treatment, but the serum levels of Cys C and Hcy
in the study group were significantly lower than those in the
control group, with statistically significant differences
(P<0.05) (Fig. 2).
Comparison of urinary protein levels
before and after treatment in the two groups
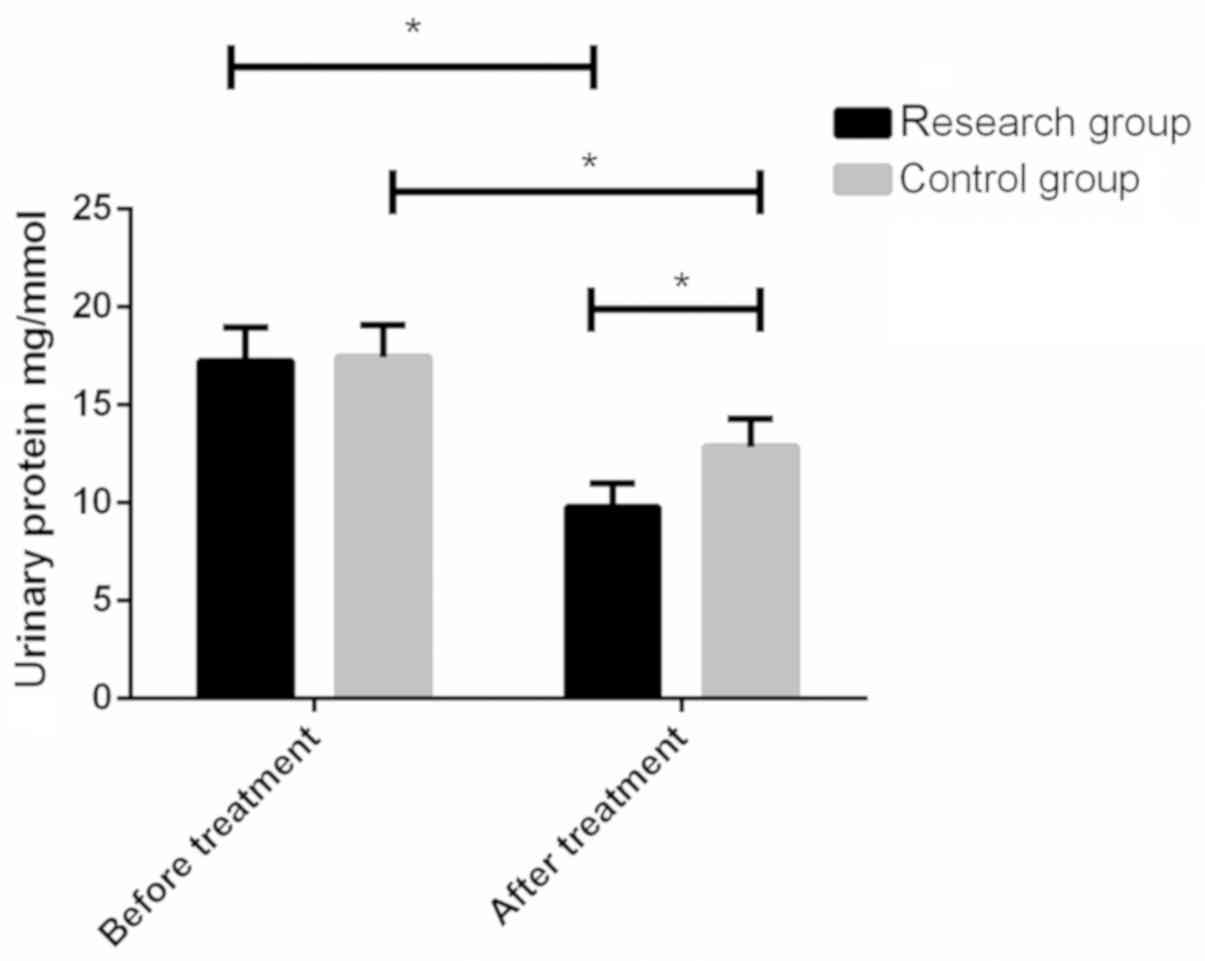
Urine protein levels in the study group and control
group before treatment were 17.271.68 and 17.451.64 mg/mmol,
respectively, and there were no significant differences between the
two groups (P>0.05). After treatment, the urine protein levels
of the study group and the control group were 9.781.21 and
12.861.45 mg/mmol, respectively. After treatment, the urine protein
levels of both groups were lower than those before treatment, and
the level in the study group was significantly lower than that in
the control group, with statistically significant differences
(P<0.05) (Fig. 3).
Comparison of adverse reactions during
pregnancy between the two groups
The number of patients with nausea and vomiting,
dizziness, diarrhea and hypertension in the study group were 2, 1,
2 and 1, respectively. The number of patients with nausea and
vomiting, dizziness, diarrhea and hypertension in the control group
were 1, 1, 0 and 1, respectively. There were no significant
differences in the total number of adverse reactions between the
two groups (P>0.05) (Table II).
All adverse reactions were relieved after symptomatic
treatment.
 | Table II.Comparison of adverse reactions during
pregnancy in the two groups. |
Table II.
Comparison of adverse reactions during
pregnancy in the two groups.
| Adverse
reactions | Research group
(n=42) | Control group
(n=38) | χ2
value | P-value |
|---|
| Nausea and
vomiting | 2 (4.76) | 1 (2.63) | 0.251 | 0.617 |
| Dizziness | 1 (2.38) | 1 (2.63) | 0.005 | 0.942 |
| Diarrhea | 2 (4.76) | 0 (0) | 1.856 | 0.173 |
| Hypertension | 1 (2.38) | 2 (5.36) | 0.459 | 0.498 |
| Total number | 6 (14.27) | 4 (10.53) | 0.612 | 0.258 |
Comparison of neonatal outcomes
between the two groups
When pregnancy ended, the number of neonates with
hypoglycemia, neonatal jaundice, macrosomia and fetal abnormalities
in the study group were 1, 2, 2 and 1, respectively. The number of
the neonates with those symptoms in the control group were 3, 5, 3
and 2, respectively. The incidence of neonatal adverse outcomes in
the study group were significantly lower than those in the control
group (P<0.05) (Table III).
 | Table III.Comparison of neonatal outcomes
between the two groups. |
Table III.
Comparison of neonatal outcomes
between the two groups.
| Groups | Research group
(n=42) | Control group
(n=38) | χ2
value | P-value |
|---|
| Hypoglycemia | 1 (2.08) | 3 (6.25) | 1.277 | 0.259 |
| Neonatal
jaundice | 2 (4.16) | 3 (7.89) | 1.761 | 0.185 |
| Macrosomia | 3 (7.14) | 5 (13.15) | 0.334 | 0.563 |
| Fetal
abnormalities | 1 (2.08) | 2 (5.26) | 0.459 | 0.498 |
| Total number | 7 (16.67) | 13 (34.21) | 4.373 | 0.037 |
Comparison of adverse pregnancy
outcomes in the two groups
The number of adverse pregnancies in the study group
was 10, and the number of adverse pregnancies in the control group
was 17. The number of adverse pregnancies in the study group was
significantly lower than that in the control group (P<0.05)
(Table IV).
 | Table IV.Comparison of adverse pregnancy
outcomes in the two groups. |
Table IV.
Comparison of adverse pregnancy
outcomes in the two groups.
| Groups | Research group
(n=42) | Control group
(n=38) | χ2
value | P-value |
|---|
| Cesarean
section | 2 (4.76) | 4 (10.53) | 0.956 | 0.328 |
| Premature
birth | 4 (9.52) | 5 (13.16) | 0.264 | 0.608 |
| Polyhydramnios | 2 (4.76) | 4 (10.53) | 0.956 | 0.328 |
| Premature rupture
of membrane | 1 (2.38) | 2 (5.23) | 0.460 | 0.498 |
| Postpartum
hemorrhage | 1 (2.38) | 2 (5.23) | 0.460 | 0.498 |
| Total number | 10 (23.81) | 17 (44.73) | 3.908 | 0.048 |
Discussion
With the development of the society, people's
quality of life has improved, and the incidence of GDM (14) has increased with the constant change
of dietary forms. A high proportion of the incidence among young
people has lead to an impact on human health. However, the
pathogenesis of GDM is still unknown. GDM is known to cause great
harm to pregnant women and infants (15). Thus, the primary principle of GDM
treatment is to control the blood glucose level of GDM patients to
a normal level, so as to reduce the occurrence of related
complications of pregnant women and infants.
Metformin is the primary choice for the treatment of
type 2 diabetes for its significant efficacy in controlling blood
glucose level, with low incidence of hypoglycemia and protective
effect on cardiovascular system (16). It is therefore widely used in
clinical practice. In addition, metformin is often used alone or in
combination with other drugs in clinical practice (17). Therefore, in the present study, we
used insulin combined with metformin to treat GDM patients.
Comparisons of the levels of FBG and 2hPG before and after
treatment in the study group and the control group showed reduced
level in the study group treated with insulin combined with
metformin was greater than that in the control group treated with
insulin alone. There were no significant differences in adverse
reactions during pregnancy in the two groups, suggesting that
insulin combined with metformin was more effective in lowering
blood sugar levels and did not increase the incidence of adverse
reactions during pregnancy. It confirmed the safety of the two
drugs. Studies have also confirmed that insulin combined with
insulin sensitizers has a relatively significant effect on the
control of blood glucose level in the body, and can also reduce the
incidence of maternal and neonatal complications (18). Other studies have shown that insulin
and metformin are safe and effective during pregnancy (19). Those findings are consistent with our
results.
Cys C is mainly filtered and cleared in the kidneys
(20,21). If the glomerulus of the body is
damaged, its level will increase rapidly and be positively
correlated with the degree of injury (20,21). Hcy
is one of the risk factors of vasculopathy (22). When vascular endothelial injury
occurs, the risk of diabetic nephropathy increases. Studies have
shown that serum levels of Cys C and Hcy are somewhat correlated
with disease progression and maternal and neonatal complications
(23). Therefore, we compared the
levels of the patients in the study and control groups before and
after treatment. The results showed that there were no significant
differences in serum Cys C and Hcy levels between the two groups
before treatment, and the levels of patients in the study group
after treatment were lower than those in the control group. At the
same time, in order to study its correlation with renal injury, we
also compared the urine protein levels of the two groups before and
after treatment. It was found that there were no significant
differences between the urine protein levels of the two groups
before treatment. However, the urine protein levels of the
treatment group were significantly lower than those of the control
group. Therefore, it was speculated that the increase of serum Cys
C and Hcy levels would lead to an increase of urinary protein
levels, which may affect the kidneys of GMD pregnant women to some
extent. The above results suggest that insulin combined with
metformin can effectively reduce the serum Cys C and Hcy levels of
patients, thus improving the patient's condition. Previous findings
have indicated that the therapeutic effect and complications of
patients with GDM could be predicted by monitoring factors in serum
of patients with GDM (24).
GDM not only affects the normal development of
infants, but also greatly increases the incidence of adverse
pregnancy outcomes (25). In
addition, maternal and neonatal outcomes of GDM depend on blood
glucose control, and good blood glucose control can reduce the
incidence of maternal and neonatal complications (26). Therefore, we compared the number of
neonatal hypoglycemia, neonatal jaundice, macrosomia and fetal
malformation in the two groups, and found that the total number of
adverse outcomes in the study group was lower than that in the
control group, and the total number of adverse pregnancies in the
GDM patients in the study group was significantly lower than that
in the control group. This finding indicated that insulin combined
with metformin had a significant effect on reducing blood glucose
level in GDM patients, which could reduce adverse pregnancy and
fetal outcomes in pregnant women, and it was conducive to improving
maternal and neonatal quality of life. Therefore, effective control
of blood glucose level is necessary in GDM.
In conclusion, the use of insulin combined with
metformin can effectively reduce the levels of FBG, 2hPG and serum
Cys C and Hcy in GDM patients, and can effectively improve maternal
and neonatal outcomes without increasing adverse reactions during
pregnancy. However, the current study did not make a comparison,
for example, with other types of insulin, making further studies
necessary.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and JX conceived and designed the study, and
drafted the manuscript. JZ, JX, YZ and NZ collected, analyzed and
interpreted the experimental data. JZ revised the manuscript for
important intellectual content. JZ wrote the manuscript. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Liaocheng Third People's Hospital (Liaocheng, China). Signed
informed consents were obtained from the patients and their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Szmuilowicz ED, Josefson JL and Metzger
BE: Gestational diabetes mellitus. Endocrinol Metab Clin North Am.
48:479–493. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu X, Wu F, Jiang M, Sun X and Tian G:
Curcumin ameliorates gestational diabetes in mice partly through
activating AMPK. Pharm Biol. 57:250–254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ustebay S, Baykus Y, Deniz R, Ugur K,
Yavuzkir S, Yardim M, Kalayci M, Çaglar M and Aydin S: Chemerin and
dermcidin in human milk and their alteration in gestational
diabetes. J Hum Lact. 35:550–558. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Budak MS, Kahramanoglu I, Vitale SG, Akgol
S, Dilek ME, Kartal S, Caruso S, Kahveci B, Obut M, Bademkiran MH,
et al: Maternal abdominal subcutaneous fat thickness as a simple
predictor for gestational diabetes mellitus. J Perinat Med.
47:605–610. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng H, Shen H, Oprea I, Worrall C,
Stefanescu R, Girnita A and Girnita L: β-Arrestin-biased agonism as
the central mechanism of action for insulin-like growth factor 1
receptor-targeting antibodies in Ewing's sarcoma. Proc Natl Acad
Sci USA. 109:20620–20625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitchell PL, Nachbar R, Lachance D,
St-Pierre P, Trottier J, Barbier O and Marette A: Treatment with a
novel agent combining docosahexaenoate and metformin increases
protectin DX and IL-6 production in skeletal muscle and reduces
insulin resistance in obese diabetic db/db mice. Diabetes Obes
Metab. 19:313–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan J, Hu Z, Zeng K, Yin Y, Zhao M, Chen M
and Chen Q: The reduction in circulating levels of estrogen and
progesterone in women with preeclampsia. Pregnancy Hypertens.
11:18–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heerspink HJ, Andress DL, Bakris G,
Brennan JJ, Correa-Rotter R, Dey J, Hou FF, Kitzman DW, Kohan D,
Makino H, et al: Rationale and protocol of the Study Of diabetic
Nephropathy with AtRasentan (SONAR) trial: A clinical trial design
novel to diabetic nephropathy. Diabetes Obes Metab. 20:1369–1376.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia-Carretero R, Vigil-Medina L,
Mora-Jimenez I, Soguero-Ruiz C, Goya-Esteban R, Ramos-Lopez J and
Barquero-Perez O: Cardiovascular risk assessment in prediabetic
patients in a hypertensive population: The role of cystatin C.
Diabetes Metab Syndr. 12:625–629. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong T, Wang J, Yang M, Shao Y, Liu J, Wu
Q, Xu Q, Wang H, He X, Chen Y, et al: Serum homocysteine level and
gestational diabetes mellitus: A meta-analysis. J Diabetes
Investig. 7:622–628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Wu J, Jiang P, Li M, Liu Q, Cao Y
and Wang S: Serum fatty acid binding protein 4 is positively
associated with early stroke recurrence in nondiabetic ischemic
stroke. Aging (Albany NY). 11:1977–1989. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maesa JM, Fernandez-Riejos P,
Gonzalez-Rodriguez C and Sanchez-Margalet V: Screening for
gestational diabetes mellitus by measuring glycated hemoglobin can
reduce the use of the glucose challenge test. Ann Lab Med.
39:524–529. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salman L, Pardo A, Krispin E, Oron G,
Toledano Y and Hadar E: Perinatal outcome in gestational diabetes
according to different diagnostic criteria. J Perinat Med.
47:553–557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pheiffer C, Dias S, Rheeder P and Adam S:
MicroRNA profiling in HIV-infected South African women with
gestational diabetes mellitus. Mol Diagn Ther. 23:499–505. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Catalano PM, McIntyre HD, Cruickshank JK,
McCance DR, Dyer AR, Metzger BE, Lowe LP, Trimble ER, Coustan DR,
Hadden DR, et al HAPO Study Cooperative Research Group, : The
hyperglycemia and adverse pregnancy outcome study: Associations of
GDM and obesity with pregnancy outcomes. Diabetes Care. 35:780–786.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qian D, Zhang T, Zheng P, Liang Z, Wang S,
Xie J, Zhao L, Zhang Y and Situ B: Comparison of oral antidiabetic
drugs as add-in treatments in patients with type 2 diabetes
uncontrolled on metformin: A Network Meta-Analysis. Diabetes Ther.
9:1945–1958. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaturvedi R, Desai C, Patel P, Shah A and
Dikshit RK: An evaluation of the impact of antidiabetic medication
on treatment satisfaction and quality of life in patients of
diabetes mellitus. Perspect Clin Res. 9:15–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li YT, Kuo TC and Wang PH: Low serum level
of irisin and gestational diabetes mellitus. Taiwan J Obstet
Gynecol. 58:443–444. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Formoso G, Ginestra F, Di Dalmazi G and
Consoli A: Empagliflozin, metformin and insulin degludec, during
pregnancy: A case report. Acta Diabetol. 55:759–761. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chong PL, Pisharam J, Abdullah A and Chong
VH: Gestational diabetes insipidus. QJM. 112:123–124. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tavakoli R and Lebreton G: Biomarkers for
early detection of cardiac surgery-associated acute kidney injury.
J Thorac Dis. 10 (Suppl 33):S3914–S3918. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma L, Liu Q, Jiang Y, Zhao H, Zhao T, Cao
Y, Li P and Niu W: Genetically elevated circulating homocysteine
concentrations increase the risk of diabetic kidney disease in
Chinese diabetic patients. J Cell Mol Med. 23:2794–2800. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawasaki M, Arata N and Ogawa Y: Obesity
and abnormal glucose tolerance in the offspring of mothers with
diabetes. Curr Opin Obstet Gynecol. 30:361–368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu Z, Yang X, Shen M, Xue H, Qian G, Cao
F, Guo J, Dong W and Chen Y: Prognostic ability of cystatin C and
homocysteine plasma levels for long-term outcomes in very old acute
myocardial infarction patients. Clin Interv Aging. 13:1201–1209.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hiersch L and Yogev Y: Impact of
gestational hyperglycemia on maternal and child health. Curr Opin
Clin Nutr Metab Care. 17:255–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guillén-Sacoto MA, Barquiel B, Hillman N,
Burgos MÁ and Herranz L: Gestational diabetes mellitus: Glycemic
control during pregnancy and neonatal outcomes of twin and
singleton pregnancies. Endocrinol Diabetes Nutr. 65:319–327. 2018.
View Article : Google Scholar : PubMed/NCBI
|