Introduction
Acute stroke is a common type of acute
cerebrovascular dysfunction. It is mainly caused by ischemia
resulting from occluded or ruptured cerebral vessels or by tissue
damage from hemorrhagic acute cerebral circulation disorder, making
patients susceptible to long-term disability or multi-functional
disorders (1–3). Data have revealed a sharp increase in
the number of patients with acute stroke due to the changing living
environment and the aging population in recent years. Most acute
stroke patients have recurrent disease during the treatment, facing
a higher risk of dementia, disability, and even death (4,5). Nerve
damage is a frequent complication of the common treatment of
various neurological diseases. Early prediction of injuries is
crucial for the prediction and improvement of the prognosis of
acute stroke. Therefore, the search for sensitive biomarkers is
essential for the accurate diagnosis and prognosis assessment of
acute stroke.
MicroRNAs (miRNAs) are single-stranded endogenous
non-coding RNAs with 21–25 nucleotides in eukaryotes, which can
regulate gene expression through the specific recognition of target
genes (6). miRNAs play a role in the
development, progression, and pathophysiological processes of acute
stroke by regulating the post-transcriptional level of target genes
(7,8). Plasma expression of miRNAs varies in
patients with different cardiovascular and cerebrovascular diseases
such as stroke, coronary heart disease and heart failure (9–11).
miR-126 can inhibit the proliferation of vascular smooth muscle
cells by regulating the expression of target genes such as vascular
cell adhesion molecule 1 and monocyte chemoattractant protein
(12). miR-182 is a recently
discovered microRNA that is involved in the development of ischemic
encephalopathy (13). However, there
are few reports on the expression and prognostic value of miR-126
and miR-182 in patients in acute stroke. This study evaluated the
serum expression of miR-126 and miR-182 in patients with different
types of acute strokes to investigate the relationship between the
two genes and the neurological function and prognosis of
patients.
Patients and methods
Patient data
In total, 153 patients with acute stroke who were
admitted to the Second Affiliated Hospital of Soochow University
(Suzhou, China) from February 2016 to February 2018 were enrolled
into the observation group and assigned as group A (88 patients
with AIS) or group B (65 patients with ICH). The observation group
was comprised of 97 males and 56 females with an average age of
62.68±13.32 years. Moreover, 69 healthy people with normal physical
examination results in the hospital were enrolled as the control
group, including 44 males and 25 females, with an average age of
61.92±13.42 years.
The patients were followed up for 3 months after
discharge from the hospital to record the prognosis. Patients were
divided into the good prognosis group and the poor prognosis group
according to the MRS score, and the relative expression of miR-126
and miR-182 in patients before the discharge were observed.
Inclusion and exclusion criteria
Inclusion criteria: i) Patients diagnosed with acute
stroke by CT scan or MRI for the head; ii) patients admitted to the
hospital within 6 h after the onset of stroke; iii) patients with a
National Institutes of Health Stroke Scale (NIHSS) score of no less
than 4 points but less than 20 points (14). Exclusion criteria: i) Patients with a
history of stroke; ii) patients with other lesions in the brain;
iii) patients with disorders or functional impairments in the blood
or coagulation system; iv) patients with poor communication and
poor compliance. The patients and their families signed an informed
consent. This study was approved by the Ethics Committee of The
Second Affiliated Hospital of Soochow University.
Experimental reagents and
materials
The RNA extraction kit was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. The TRIzol reagent and
reverse transcription kit were provided by Beijing ComWin Biotech
Co., Ltd. The SYBR-G reen PCR Master Mix was from Takara. All
primers and sequencing were designed by Sangon Biotech (Shanghai)
Co., Ltd.
Experimental methods
The extraction of plasma miRNA from all subjects and
the reverse transcription were performed in strict accordance with
the reagent manual. RNA purity and concentration were measured
using an ultraviolet spectrophotometer. The operations were
performed on ice to prevent degradation of the RNA. The
amplification by polymerase reaction was performed with U6 as the
standard internal reference. The reaction system: 2 μl of
reverse transcription primer, 10 μl of 2X SYBR-Green PCR
Master Mix, 6 μl of ddH2O, 1 μl of forward
primer and 1 μl of reverse primer. The reaction condition:
45 cycles of 95°C for 10 min, 95°C for 15 sec, and 60°C for 1 min.
Three replicate wells were set for each sample. The relative
expression levels of miR-126 and miR-182 were analyzed by
2−ΔCt. Primer sequence: miR-126 F:
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCGCATT-3′ and R:
5′-GTGCAGGGTCCGAGGT-3′; miR-182 F:
5′-ACACTCCAGCTGGGTTTGGCAATGGTAGAACT-3′ and R:
5′-TGGTGTCGTGGAGTCG-3′; U6 F: 5′-CTCGCTTCGGCAGCACA-3′ and R:
5′-AACGCTTCACGAATTTGCGT-3′.
Outcome measures
i) Analysis of the differences in the basic data and
the relative expression of miR-126 and miR-182 between the
observation group and the control group. ii) Comparison of the
expression of miR-126 and miR-182 between patients with different
NIHSS, ADL and MRS scores and analysis of the correlation of
miR-126 and miR-182 with NIHSS, ADL and MRS scores. The NIHSS score
was used to assess the degree of neurological deficits in patients
(higher scores indicate more severe neurological deficits), the ADL
score to judge the patient's self-care ability (higher scores
indicate stronger self-care ability), and the MRS score to measure
the neurological recovery of patients (higher scores indicate
poorer neurological recovery). iii) Assessment of the predictive
value of miR-126 and miR-182, alone or in combination, for the
short-term prognosis in patients with acute stroke.
Statistical analysis
Statistical analysis of data was performed on SPSS
19.0 statistical software (Beijing NDTimes Technology Co., Ltd.).
The enumeration data were analyzed by chi-square test. The
measurement data were expressed as the mean ± standard deviation
and compared between two groups by the independent t-test.
Pearson's correlation efficient was used to analyze the correlation
of miR-126 and miR-182 with NIHSS, ADL and MRS scores. ROC curve
was plotted to assess the predictive value of miR-126 and miR-182,
alone or in combination, for the short-term prognosis in patients
with acute stroke. The experiment data were visualized by GraphPad
Prism8. A statistical difference was recognized at P<0.05.
Results
Comparison of basic information
Group A and B were not notably different in sex,
age, diabetes or family history of stroke (P>0.05). The time
from onset to treatment in group A was significantly shorter than
in group B, and the systolic, diastolic, and neurological scores
were lower in group B than in group A (P<0.05). The incidence of
hypertension was higher in group B, and the incidence of
hyperlipidemia and heart disease was higher in group A (P<0.05).
The observation group and the control group were not notably
different relating to sex, age and medical history (P>0.05)
(Tables I and II).
 | Table I.Comparison of basic information
between patients with acute stroke. |
Table I.
Comparison of basic information
between patients with acute stroke.
| Groups | Group A (n=88) | Group B (n=65) | χ2/t
value | P-value |
|---|
| Sex (case) |
|
| 0.169 | 0.681 |
| Male | 57 (64.77) | 40 (61.54) |
|
|
|
Female | 31 (35.23) | 25 (38.46) |
|
|
| Age (years) | 62.55±13.34 | 62.93±13.29 | 0.174 | 0.862 |
| Time from onset to
treatment (min) | 259.35±21.32 | 145.35±15.42 | 36.600 | <0.001 |
| Systolic blood
pressure (mmHg) | 159.34±9.24 | 186.24±9.96 | 17.220 | <0.001 |
| Diastolic blood
pressure (mmHg) | 89.92±6.93 | 103.93±7.82 | 11.700 | <0.001 |
| NIHSS score | 13.34±1.23 | 14.94±2.82 | 4.750 | <0.001 |
| Hypertension |
|
| 5.979 | 0.015 |
| Yes | 45 (51.14) | 46 (70.77) |
|
|
| No | 43 (48.86) | 19 (29.23) |
|
|
| Diabetes |
|
| 0.013 | 0.910 |
| Yes | 36 (40.91) | 26 (40.00) |
|
|
| No | 52 (59.09) | 39 (60.00) |
|
|
| Hyperlipemia |
|
| 4.046 | 0.044 |
| Yes | 55 (62.50) | 30 (46.15) |
|
|
| No | 33 (37.50) | 35 (53.85) |
|
|
| Heart disease |
|
| 5.350 | 0.021 |
| Yes | 36 (40.91) | 15 (23.08) |
|
|
| No | 52 (59.09) | 50 (76.92) |
|
|
| Family history of
stroke |
|
| 0.070 | 0.792 |
|
Yes | 28 (31.82) | 22 (33.85) |
|
|
| No | 60 (68.18) | 43 (66.15) |
|
|
| Smoking |
|
| 0.003 | 0.957 |
|
Yes | 47 (53.41) | 35 (53.85) |
|
|
| No | 41 (46.59) | 30 (46.15) |
|
|
| Drinking |
|
| 0.188 | 0.665 |
|
Yes | 58 (65.91) | 45 (69.23) |
|
|
| No | 30 (34.09) | 20 (30.77) |
|
|
 | Table II.Comparison of basic information
between all research subjects. |
Table II.
Comparison of basic information
between all research subjects.
| Groups | Observation group
(n=153) | Control group
(n=69) | χ2/t
value | P-value |
|---|
| Sex (case) |
|
| 0.003 | 0.958 |
| Male | 97 (63.40) | 44 (63.77) |
|
|
| Female | 56 (36.60) | 25 (36.23) |
|
|
| Age (year) | 62.68±13.32 | 61.92±13.42 | 0.348 | 0.729 |
| Hypertension |
|
| 0.161 | 0.689 |
|
Yes | 91 (59.48) | 43 (62.32) |
|
|
| No | 62 (40.52) | 26 (37.68) |
|
|
| Diabetes |
|
| 0.038 | 0.845 |
|
Yes | 62 (40.52) | 27 (39.13) |
|
|
| No | 91 (59.48) | 42 (60.87) |
|
|
| Hyperlipemia |
|
| 0.072 | 0.789 |
|
Yes | 85 (55.56) | 37 (53.62) |
|
|
| No | 68 (44.44) | 32 (46.38) |
|
|
| Heart disease |
|
| 0.045 | 0.832 |
|
Yes | 51 (33.33) | 24 (53.33) |
|
|
| No | 102 (66.67) | 45 (65.22) |
|
|
| Family history of
stroke |
|
| 0.110 | 0.740 |
|
Yes | 50 (32.68) | 21 (30.43) |
|
|
| No | 103 (67.32) | 48 (69.57) |
|
|
| Smoking |
|
| 0.039 | 0.844 |
|
Yes | 82 (53.59) | 36 (52.17) |
|
|
| No | 71 (46.41) | 33 (47.83) |
|
|
| Drinking |
|
| 0.014 | 0.907 |
|
Yes | 103 (67.32) | 47 (68.12) |
|
|
| No | 50 (32.68) | 22 (31.88) |
|
|
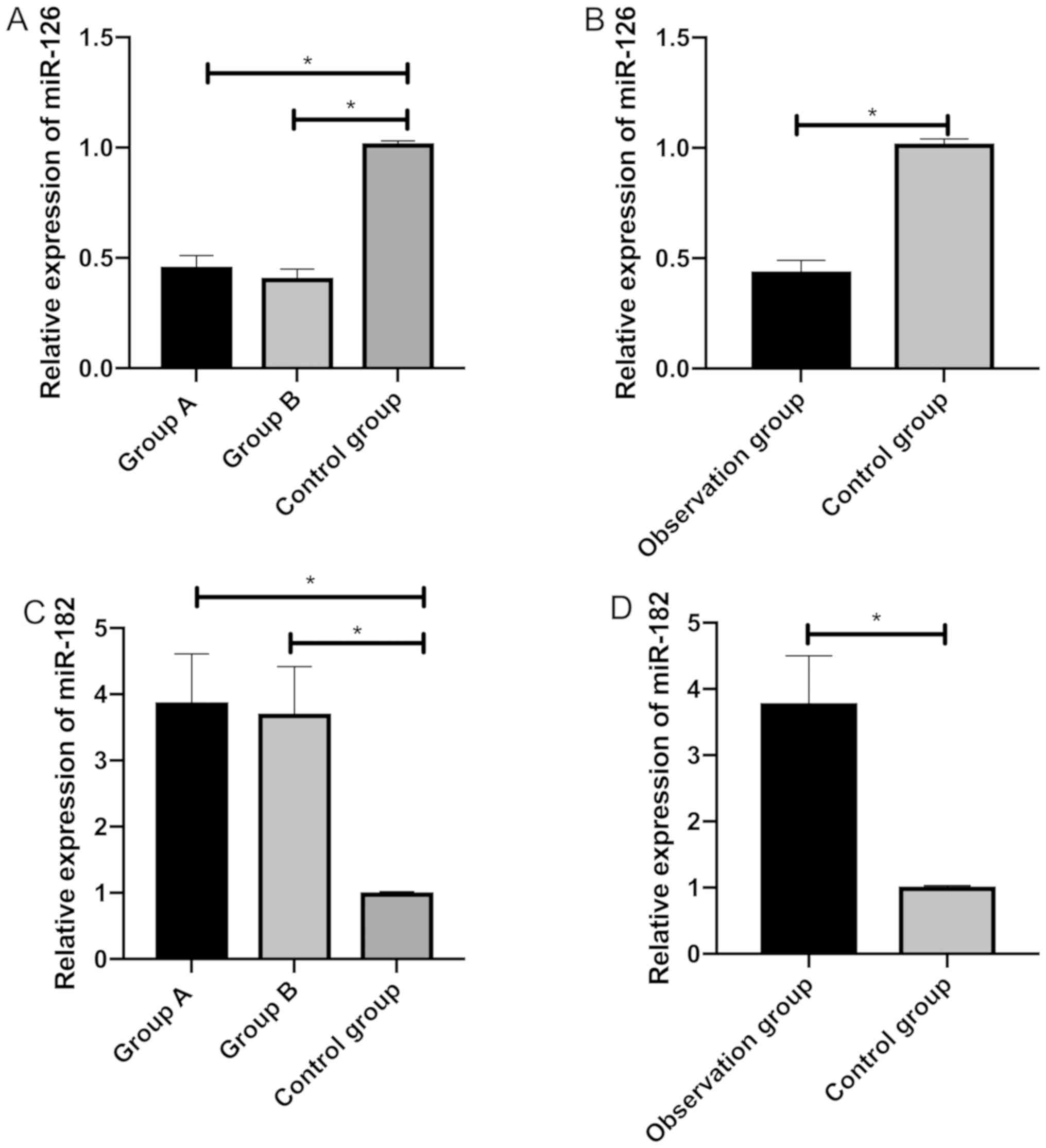
Comparison of the relative expression
of miR-126 and miR-182
The relative expression of miR-126 was markedly
higher in the control group than in group A, group B and the
observation group, and group A and group B were different in the
miR-126 expression (P<0.05). The relative expression of miR-182
was markedly lower in the control group than in group A and B.
Furthermore, the control group, and group A and B were different in
the miR-182 expression (P<0.05) (Fig.
1).
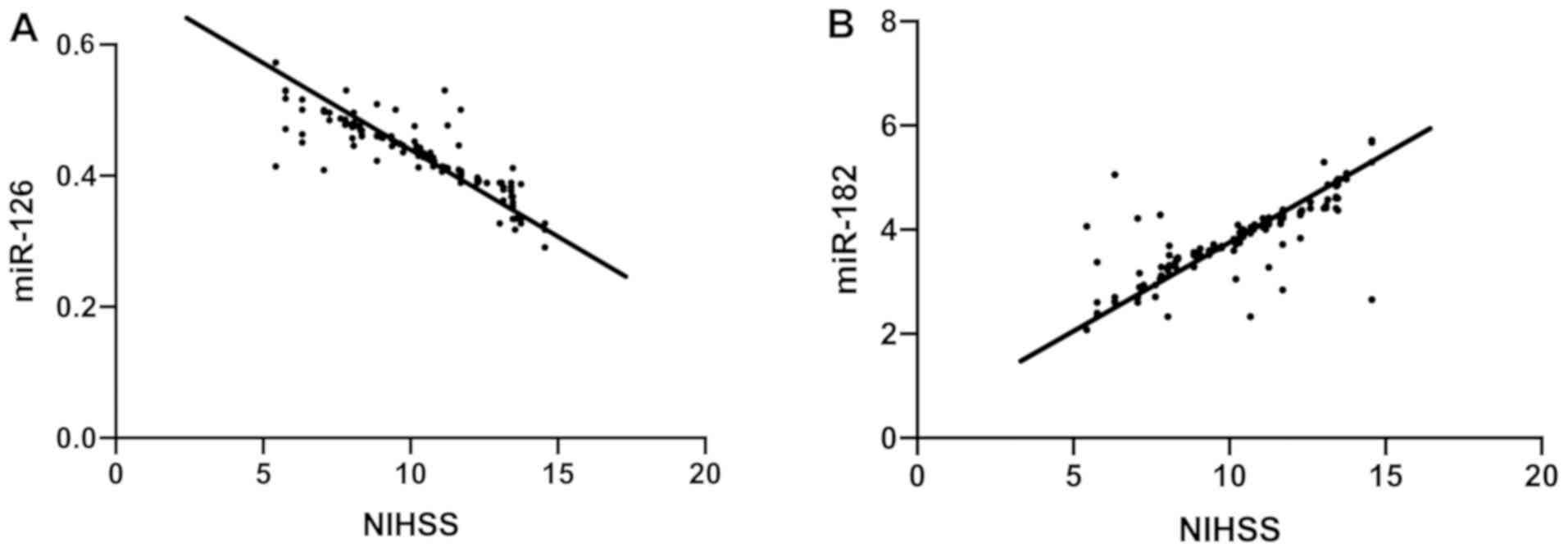
Correlation of miR-126 and miR-182
with NIHSS scores in the observation group
According to the degree of neurological deficits, 83
patients with an NIHSS score of no more than 10 points were divided
into the mild condition group, while 70 patients with an NIHSS
score of more than 10 points were divided into the severe condition
group. The relative expression of miR-126 was markedly higher in
the mild condition group than in the severe condition group, while
the relative expression of miR-182 was markedly lower in the mild
condition group than in the severe condition group (P<0.05).
Pearson's correlation analysis suggested that in the observation
group, NIHSS scores were negatively correlated with miR-126
(r=−8749, P<0.001) and positively correlated with miR-182
(r=8083, P<0.001) (Table III
and Fig. 2).
 | Table III.Correlation of miR-126 with NIHSS
scores in the observation group. |
Table III.
Correlation of miR-126 with NIHSS
scores in the observation group.
| Groups | Mild condition
group (n=83) | Severe condition
group (n=70) | t value | P-value |
|---|
| Relative expression
of miR-126 | 0.52±0.05 | 0.41±0.03 | 16.120 | <0.001 |
| Relative expression
of miR-182 | 3.24±0.68 | 4.32±0.81 | 8.967 | <0.001 |
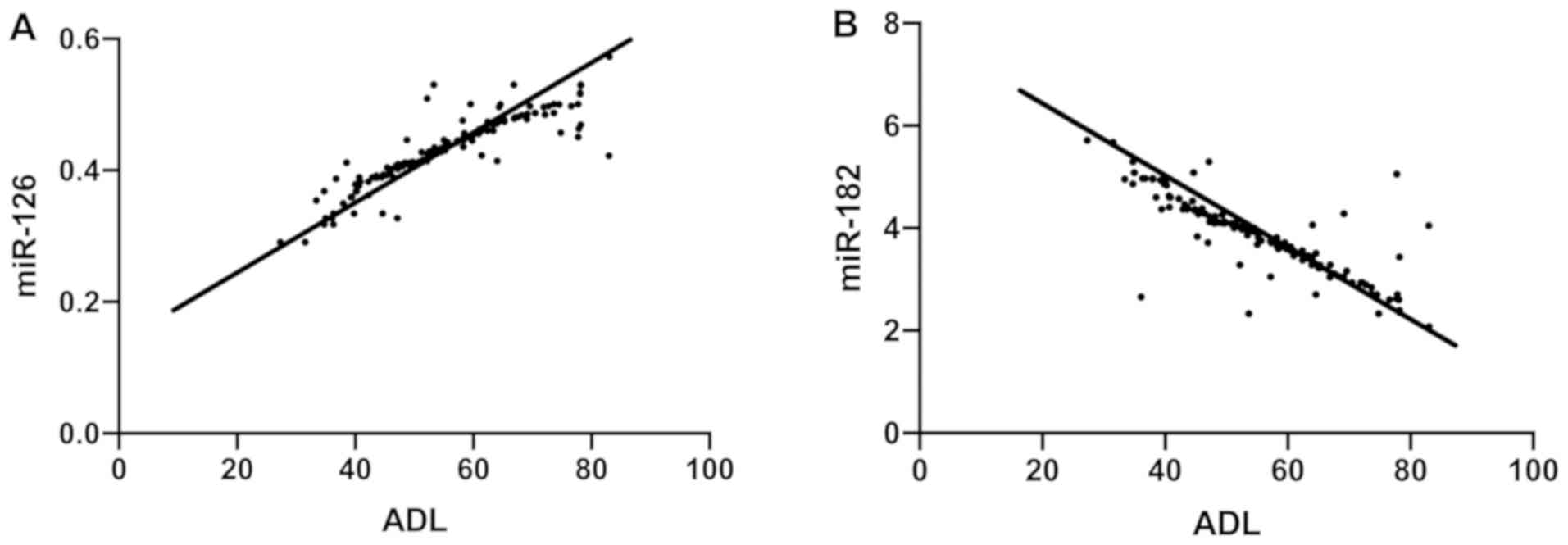
Correlation of miR-126 and miR-182
with ADL scores in the observation group
Pearson's correlation analysis suggested that in the
observation group, ADL scores were positively correlated with
miR-126 (r=0.8876, P<0.001) and negatively correlated with
miR-182 (r=−0.8375, P<0.001) (Fig.
3).
Relationship between the relative
expression of miR-126 and miR-182 and MRS scores in the observation
group
In the observation group, 97 patients with an MRS
score of no more than 2 points were divided into the good prognosis
group, while 56 patients with an MRS score of more than 2 points
were divided into the poor prognosis group. The relative expression
of miR-126 was markedly higher in the good prognosis group than in
the poor prognosis group, while the relative expression of miR-182
was markedly lower in the good prognosis group than in the poor
prognosis group (P<0.05) (Table
IV).
 | Table IV.Comparison of relative expression of
miR-126 and miR-182 between patients with different prognosis. |
Table IV.
Comparison of relative expression of
miR-126 and miR-182 between patients with different prognosis.
| Groups | Good prognosis
group (n=97) | Poor prognosis
group (n=56) | t value | P-value |
|---|
| Relative expression
of miR-126 | 0.51±0.11 | 0.39±0.04 | 8.654 | <0.001 |
| Relative expression
of miR-182 | 3.30±0.67 | 4.53±0.83 | 10.140 | <0.001 |
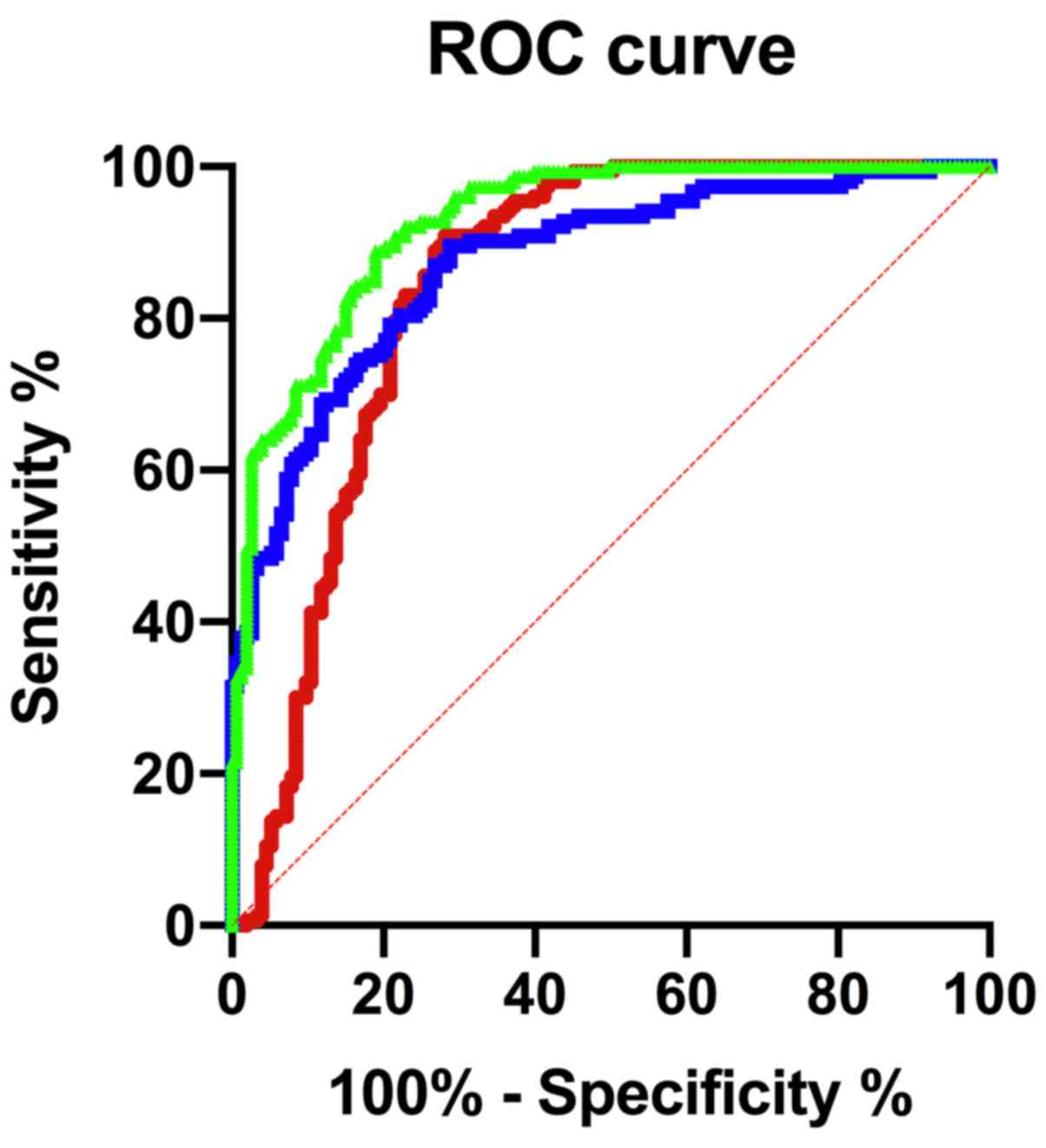
Predictive value of miR-126 and
miR-182, alone or in combination, for the prognosis of patients in
the observation group
ROC curve was used to assess the predictive value of
miR-126 and miR-182, alone or in combination, for the prognosis of
patients with acute stroke. The sensitivity of miR-126 was 90.85%,
the specificity was 71.90%, and the area under the curve (AUC) was
0.8411 at the optimal cut-off point. The sensitivity of miR-182 was
89.54%, the specificity was 71.24%, and the AUC was 0.8733 at the
optimal cut-off point. The sensitivity of miR-126 combined with
miR-182 was 88.89%, the specificity was 81.05%, and the AUC was
0.9273 at the optimal cut-off point. More details are shown in
Table V and Fig. 4.
Discussion
Acute stroke, characterized by high morbidity,
mortality and disability rate, greatly threatens the normal life
and health of patients. Acute stroke induced by intracerebral
ischemia or hemorrhage possibly leads to an inflammatory reaction,
free radical damage, and brain tissue damage. Patients with stroke
onset failing to receive timely treatment are at risk of
neurological impairment or death (15,16). The
existing diagnosis for acute stroke is mainly by imaging methods,
which have limitations in accuracy and operation (17). Identifying biomarkers with high
specificity and high effectiveness for detecting acute stroke is
important. Advancements in the chip science promoted the stability
of circulating miRNAs, enabling miRNAs to be used as new biomarkers
for the early diagnosis and prognosis evaluation of many diseases
(18). This study explored the
relationship between miRNAs and the prognosis of patients with
acute stroke.
We analyzed the basic clinical information of all
subjects and found that subjects from the control group were not
different from patients in the observation group in basic
information, but patients with different medical histories, living
habits, and causes of stroke onset had different symptoms. Then the
expression of miR-126 and miR-182 was evaluated in healthy subjects
and patients with different conditions. The relative expression of
miR-126 was markedly higher in the control group than in group A
and group B of the observation group, while the relative expression
of miR-182 was markedly lower in the control group than in group A
and group B of the observation group. Group A and group B were
statistically different in miR-126 and miR-182 expression. A
previous study (19) showed that
serum miR-126 expression was significantly reduced in the rat
models of middle cerebral artery occlusion. A study (20) discovered a marked increase in miR-182
expression in the cerebral cortex of mouse models of cerebral
ischemia and hypoxia, and speculated that miR-182 was involved in
the process of cerebral ischemia and hypoxia. Such findings suggest
that miR-126 and miR-182 are involved in the course of stroke
development, and their expression is related to the severity of
disease condition. The expression of miR-126 and miR-182 varies
among patients with different types of stroke. It has been reported
(21,22) that an increase in blood flow resulted
in high wall shear stress and vascular fragility, thinner blood
vessel diameter, and a higher risk of cerebral infarction, besides,
atherosclerosis caused by long-term diabetes and heart disease can
lead to the occurrence of cerebral infarction or cerebral
hemorrhage to a certain extent. miR-126 induces angiogenesis by
regulating vascular endothelial cells and angiogenic growth factors
to promote Akt signaling pathway activation and cell apoptosis
(23–25). miR-182 plays an important role in the
regulation of sugar and lipid metabolism. Abnormally expressed
miR-182 may induce metabolic diseases and diseases such as
atherosclerosis (26,27). The above-listed literature proves
that both miR-126 and miR-182 can regulate angiogenesis, and their
expression changes with the changes in vessels in different body
environments according to the severity of disin summarease
condition, which is consistent with the results of this study. We
explored the relationship between the two genes and the
neurological function and self-care ability of acute stroke and
came to the conclusion that patients with more severe neurological
damage and worse self-care ability had lower miR-126 expression and
higher miR-182 expression. Such results indicate that both genes
may have a predictive value for neurological function and self-care
ability. MiR-126 is an intronic miRNA, which can inhibit the host
gene Egfl7 during the differentiation of neural stem cells
(28). A previous study (29) demonstrated that miR-182 could
aggravate neuronal damage by down-regulating the expression of the
target gene APLN and inhibiting the PI3k/p-Akt pathway to protect
nerve function. The regulation of neurological function in patients
by the two genes may result in changes in the prognosis. Few
studies have been made on the effect of the two genes on the
prognosis of patients with acute stroke. This study explored the
prognostic value of the two genes in patients with acute stroke and
found that patients with good prognosis had higher miR-126
expression and lower miR-182 expression. Both miR-126 and miR-182
could predict the prognosis of acute stroke, and the combination of
miR-126 and miR-182 presented better accuracy. Such findings
suggest that the prediction by miR-126 combined with miR-182 for
the prognosis of patients within 3 months after the treatment is
highly accurate.
In conclusion, the expression levels of miR-126 and
miR-182 are associated with the neurological function, self-care
ability, and prognosis in patients with acute stroke, and are
highly valuable for predicting the prognosis of patients. However,
we only studied the correlation of miR-126 and miR-182 with
neurological function and self-care ability in the included
patients with acute stroke. Thus comparison studies are still
required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RQ conceived the study and drafted the manuscript.
RQ and HL were responsible for the extraction of plasma miRNA.
ChenglongL and YX measured RNA purity and concentration. ChunfengL
was responsible for the PCR. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Second Affiliated Hospital of Soochow University (Suzhou,
China). Patients who participated in this research, signed an
informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Slivka AP, Notestine MA, Li J and
Christoforidis GA: Clinical predictors of cerebrovascular occlusion
for patients presenting with acute stroke. J Stroke Cerebrovasc
Dis. 15:30–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Appiah KO, Minhas JS and Robinson TG:
Managing high blood pressure during acute ischemic stroke and
intracerebral hemorrhage. Curr Opin Neurol. 31:8–13. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lawrence ES, Coshall C, Dundas R, Stewart
J, Rudd AG, Howard R and Wolfe CD: Estimates of the prevalence of
acute stroke impairments and disability in a multiethnic
population. Stroke. 32:1279–1284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bertog SC, Grunwald IQ, Kühn AL, Vaskelyte
L, Hofmann I, Gafoor S, Reinartz M, Matic P and Sievert H: Acute
stroke intervention. Interventional Cardiology: Principles and
Practice. Second. Dangas GD, Di Mario C, Kipshidze NN, Barlis P,
Addo T and Serruys PW: Wiley Online Library; pp. 641–652. 2017
|
|
5
|
Sicras-Mainar A, Planas-Comes A,
Frias-Garrido X, Navarro-Artieda R, de Salas-Cansado M and
Rejas-Gutiérrez J: Statins after recent stroke reduces recurrence
and improves survival in an aging Mediterranean population without
known coronary heart disease. J Clin Pharm Ther. 37:441–447. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao Q, Liu F, Ji K, Liu N, He Y, Zhang W
and Wang L: MicroRNA-381 inhibits the metastasis of gastric cancer
by targeting TMEM16A expression. J Exp Clin Cancer Res. 36:292017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Song Y, Huang J, Qu M, Zhang Y,
Geng J, Zhang Z, Liu J and Yang GY: Increased circulating exosomal
miRNA-223 is associated with acute ischemic stroke. Front Neurol.
8:572017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji B, Wang J, Ma X, Yi YB and Chen ZY:
Exosome and miRNA in stroke. Cellular and Molecular Approaches to
Regeneration and Repair. Springer; New York, NY: pp. 325–361.
2018
|
|
9
|
Florijn BW, Bijkerk R, van der Veer EP and
van Zonneveld AJ: Gender and cardiovascular disease: Are sex-biased
microRNA networks a driving force behind heart failure with
preserved ejection fraction in women? Cardiovasc Res. 114:210–225.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng HL, Fu CY, Kuo WC, Chen YW, Chen YS,
Lee YM, Li KH, Chen C, Ma HP, Huang PC, et al: Detecting miRNA
biomarkers from extracellular vesicles for cardiovascular disease
with a microfluidic system. Lab Chip. 18:2917–2925. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giridharan VV, Quevedo J, Krishnamurthy P
and Thandavarayan RA: Editorial commentary: miRNA a tiny genetic
tool: Key to the puzzle of cardiovascular disease. Trends
Cardiovasc Med. 26:420–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maitrias P, Metzinger-Le Meuth V, Nader J,
Reix T, Caus T and Metzinger L: The involvement of miRNA in
carotid-related stroke. Arterioscler Thromb Vasc Biol.
37:1608–1617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D,
Kang KM, Park KH, Bae EK, Kim M, Lee SK, et al: MicroRNAs induced
during ischemic preconditioning. Stroke. 41:1646–1651. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dunning K: National Institutes of Health
Stroke Scale. Encyclopedia of Clinical Neuropsychology. Kreutzer
JS, DeLuca J and Caplan B: Springer; New York, NY: pp. 1714–1715.
2011, View Article : Google Scholar
|
|
15
|
Bramlett HM and Dietrich WD:
Pathophysiology of cerebral ischemia and brain trauma: Similarities
and differences. J Cereb Blood Flow Metab. 24:133–150. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suda S, Katsura K, Kanamaru T, Saito M and
Katayama Y: Valproic acid attenuates ischemia-reperfusion injury in
the rat brain through inhibition of oxidative stress and
inflammation. Eur J Pharmacol. 707:26–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liebeskind DS, Sanossian N, Yong WH,
Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK,
et al: CT and MRI early vessel signs reflect clot composition in
acute stroke. Stroke. 42:1237–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Yan CC, Zhang X, You ZH, Deng L,
Liu Y, Zhang Y and Dai Q: WBSMDA: Within and between score for
miRNA-disease association prediction. Sci Rep. 6:211062016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JM, Jung KH, Chu K, Lee ST, Ban J,
Moon J, Kim M, Lee SK and Roh JK: Atherosclerosis-related
circulating MicroRNAs as a predictor of stroke recurrence. Transl
Stroke Res. 6:191–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui H and Yang L: Analysis of microRNA
expression detected by microarray of the cerebral cortex after
hypoxic-ischemic brain injury. J Craniofac Surg. 24:2147–2152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leung LY, Bartz TM, Rice K, Floyd J, Psaty
B, Gutierrez J, Longstreth WT Jr and Mukamal KJ: Blood pressure and
heart rate measures associated with increased risk of covert brain
infarction and worsening leukoaraiosis in older adults.
Arterioscler Thromb Vasc Biol. 37:1579–1586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sha RJ, Zhang Z, Yu TH, Yuan HL and Icu
DO: Etiological factor, risk factor and prognosis in young patients
with cerebral infarction and cerebral hemorrhage: comparative
analysis among 293 cases. Chin J Clin Rehabil. 26:794–798. 2004.(In
Chinese).
|
|
23
|
Wang S, Aurora AB, Johnson BA, Qi X,
McAnally J, Hill JA, Richardson JA, Bassel-Duby R and Olson EN: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radom-Aizik S, Zaldivar FP Jr, Haddad F
and Cooper DM: Impact of brief exercise on circulating monocyte
gene and microRNA expression: Implications for atherosclerotic
vascular disease. Brain Behav Immun. 39:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang M, Wang W, Wang J and Zhang J:
MiR-182 promotes glucose metabolism by upregulating
hypoxia-inducible factor 1α in NSCLC cells. Biochem Biophys Res
Commun. 504:400–405. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng HP, Gong D, Zhao ZW, He PP, Yu XH,
Ye Q, Huang C, Zhang X, Chen LY, Xie W, et al: MicroRNA-182
promotes lipoprotein lipase expression and atherogenesis by
targeting histone deacetylase 9 in apolipoprotein E-knockout mice.
Circ J. 82:28–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmidt MHH, Bicker F, Nikolic I, Meister
J, Babuke T, Picuric S, Müller-Esterl W, Plate KH and Dikic I:
Epidermal growth factor-like domain 7 (EGFL7) modulates Notch
signalling and affects neural stem cell renewal. Nat Cell Biol.
11:873–880. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee YJ, Bernstock JD, Klimanis D and
Hallenbeck JM: Akt protein kinase, miR-200/miR-182 expression and
epithelialmesenchymal transition proteins in hibernating ground
squirrels. Front Mol Neurosci. 11:222018. View Article : Google Scholar : PubMed/NCBI
|