Introduction
Depression, also known as depressive disorder, is
one of the most common mental diseases. It is a sustained and
persistent emotional disorder syndrome, clinically characterized by
high disability and suicide rates (1,2). With
the development of science and technology, the pathogenesis of
depression has been deeply studied from biology, neurology and
imaging science and a number of achievements have been recorded.
However, there has been no complete theory that can reveal the
occurrence and development of depression, and thus, its
pathogenesis remains unclear (3,4).
Therefore, exploring the prevention and treatment of depression is
the research focus worldwide (5,6).
Micro ribonucleic acids (miRs) are a type of
non-coding RNAs with ~21–23 nucleotides in length, which are highly
conserved in the biosynthetic pathway. Each miR can regulate one or
more targets, and regulate the degradation of target genes or
inhibit their translation into proteins (7,8).
According to the miR chip results, there is an abnormal expression
of miRs in the brain cortex of patients who commit suicide due to
depression (9). The miR-9 gene
family is ubiquitous in different species, and the number and
sequences of miR-9 vary from species to species. A previous study
has shown that miR-9 is involved in numerous physiological and
pathological processes in the body (10). Moreover, the research results of
numerous studies have confirmed that miR-9 has important
significance in regulating the cranial nerve and brain development
(11,12). Therefore, understanding and exploring
the role of miRs in depression and their regulatory mechanism can
provide an experimental basis for the prevention and treatment of
depression.
Recently, it was demonstrated that neuronal
apoptosis plays an important role in the pathogenesis of
depression. The Notch signaling pathway has obvious persistent
signals in the hippocampus of adult vertebrates and human, and it
plays a key role in promoting neurogenesis and remodeling (13). The Notch signaling pathway is
composed of the Notch receptor, Notch ligand and other regulatory
molecules. When stimulated, Notch activates the downstream target
basic helix-loop-helix transcription factor family, such as Hes1,
Mash1 and Neurogenins, and further regulates the expression of
transcription factors (14).
However, there are few studies on whether miR-9 can improve the
neuronal apoptosis in depression rats through regulating the Notch
signaling pathway.
In the present study, a rat model of depression was
established using the chronic stress method, and the regulatory
effect of miR-9 on the neuronal apoptosis in depression rats, as
well as its mechanism of action, were explored.
Materials and methods
Reagents
miR-9 inhibitor was purchased from Guangzhou RiboBio
Co., Ltd., hematoxylin and eosin (H&E) staining kit from
Beijing Solarbio Science & Technology Co., Ltd., enzyme-linked
immunosorbent assay (ELISA) kit from R&D Systems, sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
loading buffer from Shanghai Beyotime Institute of Biotechnology,
terminal deoxynucleotidyl transferase-mediated dUTP nick end
labeling (TUNEL) kit from Nanjing KeyGen Biotech Co., Ltd.
Instruments
Water bath kettle was purchased from Thermo Fisher
Scientific, Inc., the ice machine from Grant Ice Systems (NingBo),
the inverted fluorescence microscope from Nikon Corp., the water
maze from Jiangsu SANS Biological Technology Co. Ltd., the western
blot electrophoresis apparatus and the membrane transfer machine
from Bio-Rad Laboratories, Inc.
Rats
A total of 30 specific pathogen-free male
Sprague-Dawley rats, aged 12 weeks old, and weighing 220–260 g,
were purchased from the Animal Center of Jining Psychiatric
Hospital (Jining, China). They had good general conditions, and
were adaptively fed in separate cages for 7 days under 12/12 h
light/dark cycle. The rat model of depression was established using
the chronic stress method, as follows: Deprivation of food for 24
h, deprivation of water for 24 h, swimming in ice water at 4°C for
5 min, tail clamping for 5 min, cage tilt for 45° for 12 h, and
flash stimulation for 2 h. Two to three types of stress were
induced every day for 21 consecutive days. The miR-9 inhibitor was
injected via caudal vein in the rats of the miR-9 inhibitor group,
while normal saline was given to the rats of the control group. The
study was approved by the Animal Ethics Committee of Jining
Psychiatric Hospital.
Detection of learning and memory
abilities via water maze test
The learning and memory abilities of rats in each
group were detected via water maze test for a total of 5 days. All
rats were subjected to positioning training and space exploration
on the first 4 days, and those who failed to find the platform
within 60 sec were guided. Then, on day 5, the time of crossing the
original platform within 60 sec, and the residence time in the
original quadrant were recorded.
Detection of neuronal morphology of
brain tissues via H&E staining
Brain tissues were collected from rats and were
immersed in 80% ethanol overnight, fixed with 4% paraformaldehyde,
embedded in paraffin, and sliced into 8 µm-thick sections.
According to the manufacturer's instructions of the H&E
staining kit, the sections were permeabilized with xylene,
deparaffinized with ethanol at different concentrations, added
dropwise with H&E dye and resin adhesive, and after being
covered with the cover glass, observation of staining was
followed.
Detection of neuronal apoptosis in the
brain using TUNEL staining
Brain tissues of rats were collected and treated as
aforementioned. The sections were fixed using 4% paraformaldehyde
at 20°C for 30 min. Next, the sections were added drop-wise with
TdT fluorescein-labeled dUTP solution and were incubated in the
dark. A total of 50 µl TUNEL reagent (cat. no. C1088; Beyotime
Institute of Biotechnology) was added to each section for
incubation at 37°C for 60 min. Nuclear staining reagent and
mounting medium were used according to the manufacturer's
instructions of the TUNEL kit. After staining, the cells were
observed in five randomly-selected fields under a fluorescent
microscope (IX70; Olympus Corp.). The cells were stained green if
apoptosis occurred, and the apoptosis rate was calculated according
to the fluorescence.
Determination of serum B-cell
lymphoma-2 (Bcl-2) and Bcl-2 associated X protein (Bax) levels via
ELISA
The kits were taken and equilibrated at room
temperature for 30 min. Blood samples were collected and placed at
room temperature. The standards were diluted at 1:5 into standard
solution, and the standard curves were plotted. The samples were
added into each well repeatedly for 3 times, and 50 µl of
streptavidin solution were also added, followed by incubation at
37°C for 1 h. Then, the plate was washed with washing liquid and
patted dry. Fifty microliters of developing solution A and 50 µl of
developing solution B were added for incubation at 37°C for 10 min
in the dark, and the reaction was terminated using stop buffer.
Finally, the absorbance of each well was measured. Rat Bcl-2 ELISA
kit (cat. no. E-EL-R0096c) and rat Bax ELISA kit (cat. no.
E-EL-R0098c) were purchased from Elabscience Biotechnology Co.,
Ltd.
Determination of protein levels of
Notch1 and Hes1 in brain tissues using western blot analysis
The brain tissues of rats were lysed using a protein
lysis buffer (cat. no. QC25-05099; Shanghai Qincheng Biotechnology,
Co. Ltd.) and the protein concentration was determined. BSA
blocking buffer (5%) was used as the blocking reagent. 5X protein
loading buffer was added for water bath at 100°C for 5 min. Total
protein concentration was calculated by BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). After 10% SDS-PAGE, 30 µg
of protein were transferred onto a polyvinylidene difluoride
membrane via a wet process for 1 h under 120 V, and were sealed
with 5% skim milk powder for 1 h. The membranes were incubated with
rabbit anti-Notch1 (1:1,000) and Hes1 (1:1,000) primary antibodies
at 4°C overnight. Following the primary incubation, membranes were
incubated with secondary goat anti-rabbit (HRP) IgG antibody for 1
h. Immunoreactive bands were visualized by an enhanced
chemiluminescence detection kit (Amersham; GE Healthcare). The
membranes were scanned for image development, and the optical
density was analyzed using ImageJ software (National Institutes of
Health). Primary rabbit monoclonal anti-Notch1 antibody (dilution:
1/1,000; cat. no. ab194123), rabbit monoclonal Hes1 antibody
(dilution: 1/1,000; cat. no. ab221788), rabbit polyclonal β-actin
antibody (dilution: 1/1,000; cat. no. ab8227), and secondary goat
anti-rabbit (HRP) IgG antibody (dilution: 1/2,000; cat. no. ab6721)
were all purchased from Abcam.
Statistical analysis
GraphPad 5.0 software (GraphPad Software, Inc.) was
used for statistical analysis. The experimental data were expressed
as mean ± standard deviation. Differences between two groups were
analyzed using the Student's t-test. Comparison between multiple
groups was made using one-way ANOVA followed by a post hoc test
(Least Significant Difference). P<0.05 was considered to
indicate a statistically significant difference.
Results
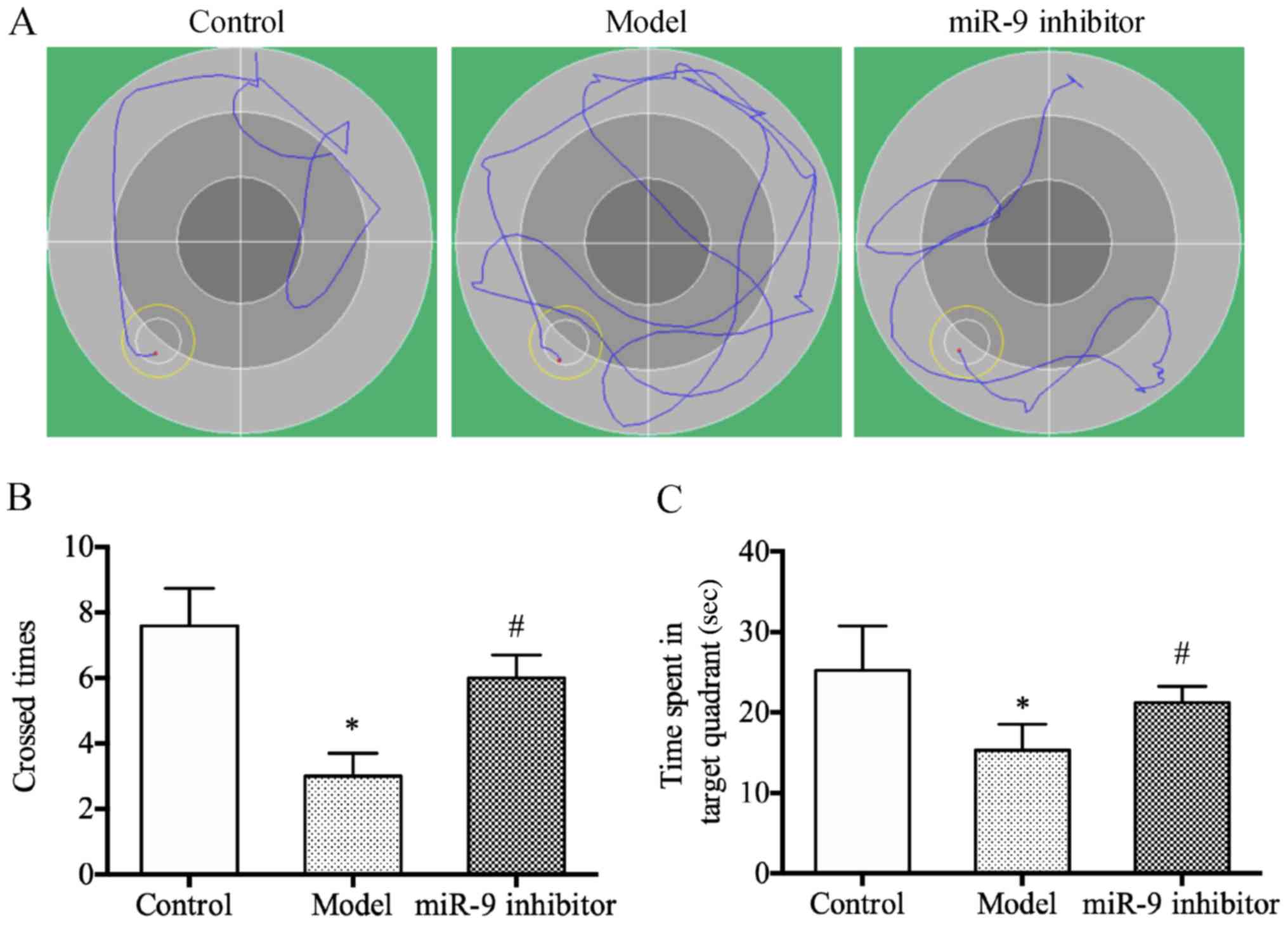
miR-9 inhibitor could improve the
learning and memory abilities of depression rats
The results of water maze test revealed that the
times of crossing the original platform and the residence time in
the original quadrant were significantly decreased in the model
group compared with those in the control group (P<0.05)
(Fig. 1A); whereas, they were
significantly increased in miR-9 inhibitor group compared with
those in the model group (P<0.05) (Fig. 1B and C). The above results indicate
that miR-9 inhibitor can improve the learning and memory abilities
of depression rats.
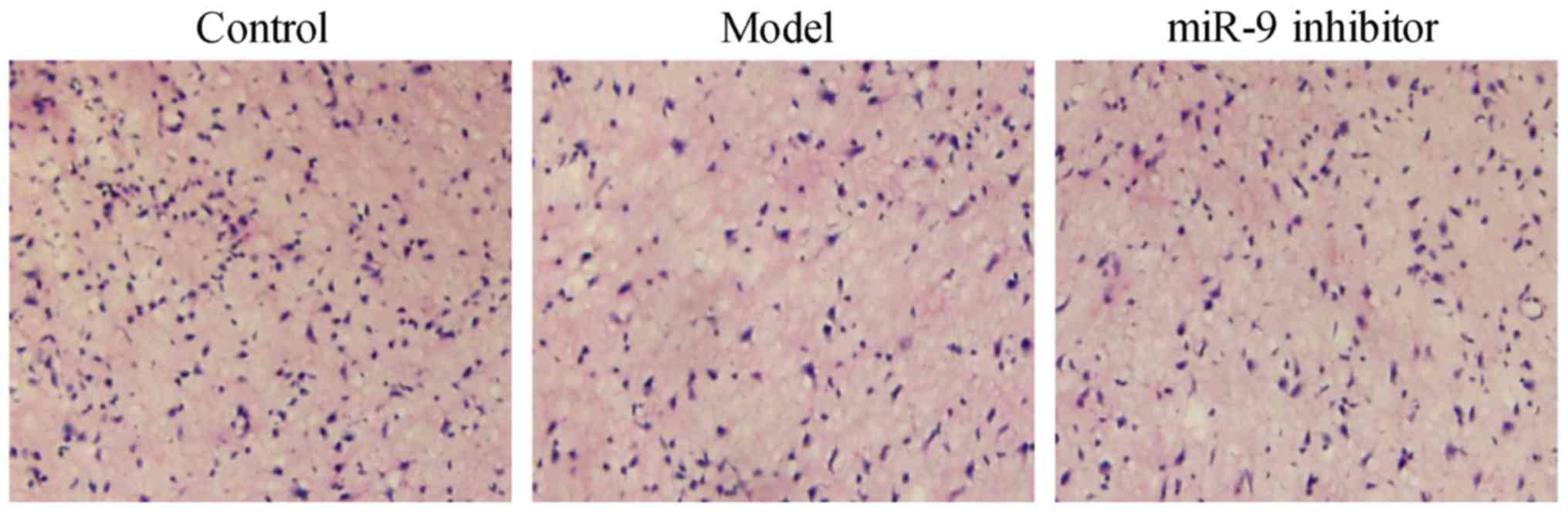
miR-9 inhibitor could ameliorate the
neuronal morphology of the brain of depression rats
According to the results of H&E staining
(Fig. 2), the neurons in cortical
tissues shrunk, the number of neurons was reduced, and they were
arranged disorderly in the model group compared with those in the
control group. Compared with the model group, the neurons in
cortical tissues were relaxed, the number of neurons was increased,
and they were arranged orderly in the miR-9 inhibitor group. The
above findings suggest that miR-9 inhibitor can ameliorate the
neuronal morphology of the brain of depression rats.
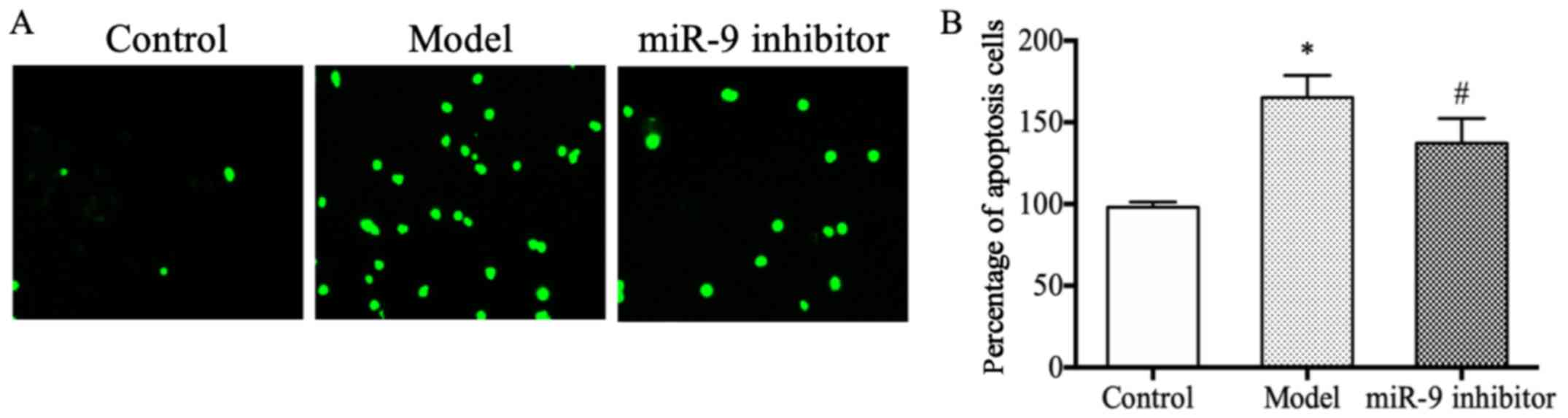
miR-9 inhibitor could inhibit the
neuronal apoptosis in depression rats
The results of TUNEL staining revealed that the
number of apoptotis neurons was obviously larger in the model group
than that in the control group (P<0.05) (Fig. 3A); whereas, it was obviously smaller
in the miR-9 inhibitor group compared with than in the model group
(P<0.05) (Fig. 3B), suggesting
that miR-9 inhibitor can inhibit neuronal apoptosis in depression
rats.
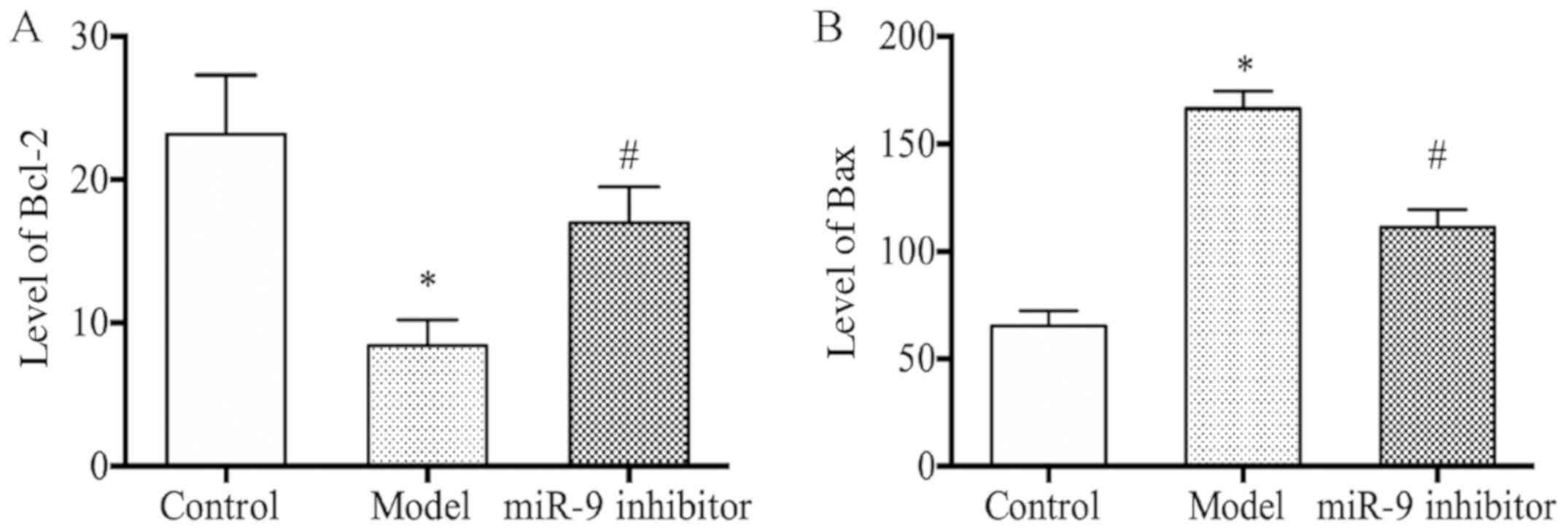
miR-9 inhibitor could upregulate the
serum Bcl-2 level and downregulate the Bax level in depression
rats
As shown in Fig. 4A and
B, the model group presented a decreased level of serum Bcl-2
and an increased level of serum Bax compared with those in the
control group (P<0.05); whereas, in the miR-9 inhibitor group
there was observed an increased level of serum Bcl-2 and a
decreased level of serum Bax compared with those in the model group
(P<0.05).
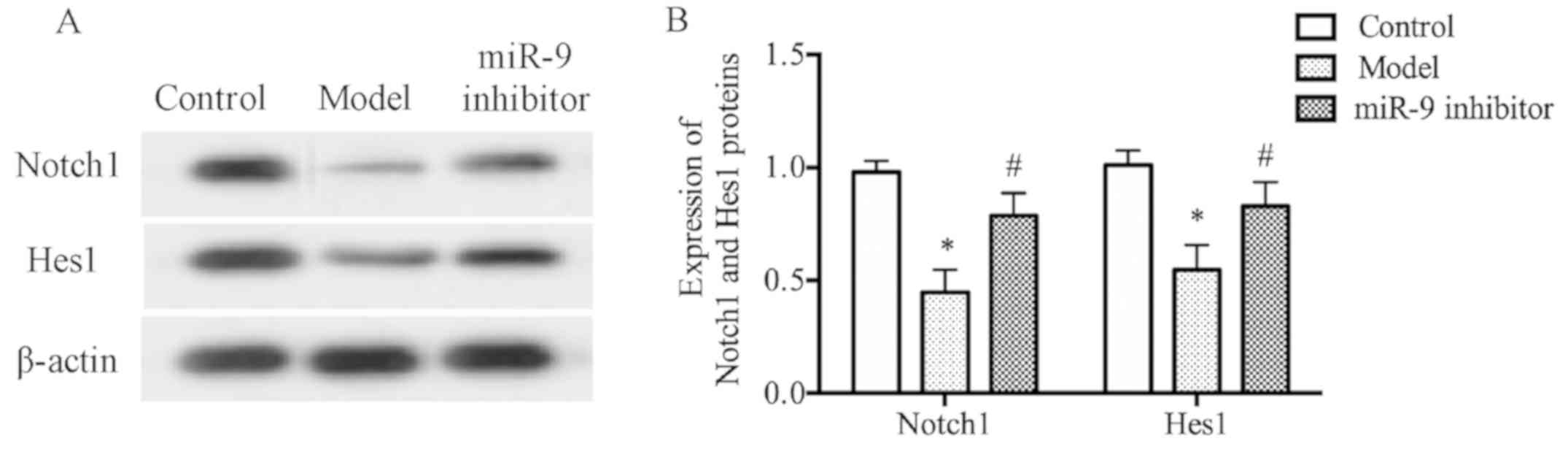
miR-9 inhibitor could increase the
protein levels of Notch1 and Hes1 in brain tissues of depression
rats
The results of western blot analysis revealed that
model group had evidently decreased protein levels of Notch1 and
Hes1 in brain tissues compared with those in the control group
(P<0.05) (Fig. 5A); whereas,
miR-9 inhibitor group had evidently increased protein levels of
Notch1 and Hes1 in brain tissues compared with those in the model
group (P<0.05) (Fig. 5B),
demonstrating that the miR-9 inhibitor can increase the protein
levels of Notch1 and Hes1 in brain tissues of depression rats.
Discussion
Depression is known to severely affect the human
health around the world. According to the report of the World
Health Organization, the depression patients account for ~20% of
the global population, with a dramatically increasing annual
incidence rate (15,16). Depression patients often live their
life in a depressive mood, often have low spirits, and even feel
inconsolable and profoundly pessimistic. Depression affects
patients and their families in different ways, seriously troubling
the patients' lives, learning and work, bringing a heavy burden to
families and society. Therefore, finding appropriate and safe
treatment means and solutions is the only way to solve the problem
of depression.
In recent years, it has been shown that miRs may
play an important regulatory role in the occurrence of neurological
diseases, such as depression. miR-9 is a type of abnormally
expressed miR. Katz et al (17) have shown that miR-9 can stimulate the
proliferation of neural stem cells. Coolen et al (18) have also reported that miR-9 plays a
key role in nerve regeneration and nerve repair. Moreover, Li et
al (19) showed that miR-9
expression was downregulated in an in vitro cell model of
Alzheimer's disease, and miR-9 was shown to significantly inhibit
the differentiation of neural stem cells whose mechanism may be
related to the regulation on Notch signaling pathway. The above
results confirm that miR-9 is closely related to the development of
cranial nerves; however, the regulatory effect and regulatory
mechanism of miR-9 in depression are still unclear.
In the present study, a rat model of depression was
established using the chronic stress method, and the miR-9
inhibitor was used for intervention. First, the water maze test, an
experimental method to detect the learning and memory abilities of
rats or mice (20), was performed.
It was found that the escape latency and residence time
significantly declined in the model group compared with those in
the control group; whereas, they were significantly increased in
the miR-9 inhibitor group compared with those in the model group,
indicating that miR-9 inhibitor can improve the learning and memory
abilities of depression rats. Then, the changes in the neuronal
morphology of the brain and the neuronal apoptosis were determined.
The results revealed that in miR-9 inhibitor group, the neuronal
morphology in the brain was obviously improved, the number of
neurons was increased and they were arranged orderly, and the
number of apoptosis neurons was obviously reduced, suggesting that
the miR-9 inhibitor can remarkably improve the cranial nerve
function and suppress the neuronal apoptosis in depression rats. In
addition, the levels of serum Bcl-2 and Bax were detected. It was
confirmed that miR-9 inhibitor group had an increased level of
serum Bcl-2 and a decreased level of serum Bax, proving that miR-9
inhibitor can inhibit apoptosis. To further explore the mechanism
of action of miR-9 inhibitor, the protein levels of Notch1 and Hes1
in the brain were determined using western blot analysis. The
results revealed that miR-9 inhibitor group had evidently increased
protein levels of Notch1 and Hes1 in the brain compared with those
in the model group, indicating that miR-9 inhibitor can exert a
therapeutic effect on depression rats through activating the Notch
signaling pathway.
In conclusion, the results of this study demonstrate
that miR-9 inhibitor can improve the neuronal morphology,
ameliorate the neurological function and inhibit the apoptosis in
depression rats, and the mechanism may be related to the activation
of the Notch signaling pathway. The present study provides a new
perspective for the treatment of depression and an experimental
basis for the application of miR-9 inhibitor in the prevention and
diagnosis of depression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PX, XG and YL designed the study and performed the
experiments. PX and XZ established the animal models. ZM and SS
analyzed the data. PX and XG prepared the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Ethics
Committee of Jining Psychiatric Hospital (Jining, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith K: Mental health: A world of
depression. Nature. 515:1812014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu J, Xu ZQ, Yi X, Wang YJ and Zhou HD: A
study on related factors of hemodynamic depression in carotid
artery stenting. Eur Rev Med Pharmacol Sci. 22:5255–5263.
2018.PubMed/NCBI
|
|
3
|
Anthes E: Depression: A change of mind.
Nature. 515:185–187. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahim T and Rashid R: Comparison of
depression symptoms between primary depression and
secondary-to-schizophrenia depression. Int J Psychiatry Clin Pract.
21:314–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Monteggia LM, Malenka RC and Deisseroth K:
Depression: The best way forward. Nature. 515:200–201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammen C: Risk factors for depression: An
autobiographical review. Annu Rev Clin Psychol. 14:1–28. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Z, Jiang Y, Huo X, Yang Y, Davies H,
Botchway BO and Fang M: Prospective role of MicroRNAs in
depression. Curr Med Chem. 24:3508–3521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tavakolizadeh J, Roshanaei K, Salmaninejad
A, Yari R, Nahand JS, Sarkarizi HK, Mousavi SM, Salarinia R,
Rahmati M, Mousavi SF, et al: MicroRNAs and exosomes in depression:
Potential diagnostic biomarkers. J Cell Biochem. 119:3783–3797.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez JP, Kos A and Turecki G: Major
depression and its treatment: microRNAs as peripheral biomarkers of
diagnosis and treatment response. Curr Opin Psychiatry. 31:7–16.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma ZY, Chen F, Xiao P, Zhang XM and Gao
XX: Silence of miR-9 protects depression mice through Notch
signaling pathway. Eur Rev Med Pharmacol Sci. 23:4961–4970.
2019.PubMed/NCBI
|
|
11
|
Sim SE, Lim CS, Kim JI, Seo D, Chun H, Yu
NK, Lee J, Kang SJ, Ko HG, Choi JH, et al: The brain-enriched
MicroRNA miR-9-3p regulates synaptic plasticity and memory. J
Neurosci. 36:8641–8652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Madelaine R, Sloan SA, Huber N, Notwell
JH, Leung LC, Skariah G, Halluin C, Paşca SP, Bejerano G, Krasnow
MA, et al: MicroRNA-9 couples brain neurogenesis and angiogenesis.
Cell Rep. 20:1533–1542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ables JL, Breunig JJ, Eisch AJ and Rakic
P: Not(ch) just development: Notch signalling in the adult brain.
Nat Rev Neurosci. 12:269–283. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du M, Tan Y, Liu G, Liu L, Cao F, Liu J,
Jiang P and Xu Y: Effects of the Notch signalling pathway on
hyperoxia-induced immature brain damage in newborn mice. Neurosci
Lett. 653:220–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cepeda MS, Katz EG and Blacketer C:
Microbiome-Gut-Brain Axis: Probiotics and their association with
depression. J Neuropsychiatry Clin Neurosci. 29:39–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dusi N, Barlati S, Vita A and Brambilla P:
Brain structural effects of antidepressant treatment in major
depression. Curr Neuropharmacol. 13:458–465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katz S, Cussigh D, Urbán N, Blomfield I,
Guillemot F, Bally-Cuif L and Coolen M: A nuclear role for miR-9
and argonaute proteins in balancing quiescent and activated neural
stem cell states. Cell Rep. 17:1383–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coolen M, Katz S and Bally-Cuif L: miR-9:
A versatile regulator of neurogenesis. Front Cell Neurosci.
7:2202013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li F, Chen A and Zhang J: miR-9
stimulation enhances the differentiation of neural stem cells with
zoanthamine by regulating Notch signaling. Am J Transl Res.
11:1780–1788. 2019.PubMed/NCBI
|
|
20
|
Guo J, Chang L, Li C, Li M, Yan P, Guo Z,
Wang C, Zha Q and Wang Q: SB203580 reverses memory deficits and
depression-like behavior induced by microinjection of Aβ1-42 into
hippocampus of mice. Metab Brain Dis. 32:57–68. 2017. View Article : Google Scholar : PubMed/NCBI
|