Introduction
Gestational diabetes mellitus (GDM) is a temporary
diabetes mellitus with glucose intolerance during pregnancy. The
difference between GDM and diabetes mellitus is sex specificity and
temporality, while insulin resistance (IR) is a similar feature
between GDM and diabetes mellitus (1,2).
According to statistics, GDM accounts for ~2–6% of the total
pregnancies in Europe and nearly half of GDM patients are highly
likely to develop diabetes within 10 years (3). In recent years, the methods of early
diagnosis and treatment of GDM have been continuously optimized,
but early GDM may still lead to poor pregnancy outcomes (4). The pathophysiological mechanism of GDM
involves chronic low-grade inflammation, insulin secretion
deficiency and abnormal glucose and lipid metabolism caused by
obesity (5–7). Therefore, we can explore new early
diagnostic tools and potential therapeutic targets for GDM from
three angles of improving chronic inflammatory response, increasing
insulin sensitivity and maintaining glucose and lipid metabolism
balance, which is of great significance for improving pregnancy
outcomes of GDM patients.
Chemerin is an inflammatory adipocyte factor and
chemoattractant protein secreted by adipocytes. Chemerin has
regulatory functions on inflammatory state, fat formation and
glycolipid homeostasis and is closely related to IR (8–10).
Studies have shown that high levels of Chemerin are associated with
poor prognosis of preeclampsia, polycystic ovary syndrome, GDM and
other pregnancy diseases (11–13). In
the studies of Yang et al (14), serum Chemerin level was upregulated
in GDM patients. The higher the Chemerin level of GDM patients in
early pregnancy, the greater the risk of GDM development. It
suggested that Chemerin can be used as a predictive marker of GDM
development risk. The fatty acid-binding protein (FABP) family is a
group of small molecular proteins that act as fatty acid
transporters in cells, while FABP4 plays a key role in lipid
metabolism as a member of FABP family (15). Studies have shown that FABP4 can
enhance insulin sensitivity and reduce atherosclerosis. Knockdown
of FABP4 gene can reduce the expression of inflammation-driven
macrophage receptor (16). Ning
et al (17), reported that
FABP4 was overexpressed in GDM patients and has a significant
positive correlation with IR and inflammatory factor TNF-α,
suggesting that FABP4 can be used as a new biomarker for GDM.
Chemerin and FABP4 are both inflammatory adipocyte factors
expressed in adipocytes, both of which are related to the
development and progression of GDM (18).
At present, there are few reports on the expression
and correlation of Chemerin and FABP4 in the peripheral blood of
GDM patients. Therefore, we explored the diagnostic value and
potential therapeutic methods of Chemerin and FABP4 in GDM patients
by detecting the expression of Chemerin and FABP4 in the peripheral
blood of GDM patients.
Patients and methods
Baseline data
Sixty patients with GDM admitted to the People's
Hospital of Zhangqiu Area (Jinan, China) from March 2018 to March
2019 were selected as the SG and another 50 healthy pregnant women
corresponding in age and pregnancy were selected as the CG. In the
SG, the age was 20–35 years with an average age of 27.33±4.75
years. In the CG, the age was 20–35 years with an average age of
27.80±5.27 years. The study was approved by the Ethics Committee of
the People's Hospital of Zhangqiu Area. The subjects and family
members signed an informed consent form.
Inclusion and exclusion criteria
Inclusion criteria: Patients who met the standards
formulated by the American Diabetes Association (19) in 2012. After 75 g glucose tolerance
test at 24–28 weeks of gestation, GDM was diagnosed if any one of
following was present: fasting blood glucose over 5.1 mmol/l, blood
glucose over 10.0 mmol/l for 1 h, blood glucose over 8.5 mmol/l for
2 h. The age range was 20–35 years. The patient was informed and
agreed to cooperate with the study.
Exclusion criteria: Patients with communication
barrier or severe mental disorder; patients comorbid with malignant
tumor or serious cardiac, lung, liver, kidney and other
dysfunction; pregnant women; patients with hypertension or
endocrine and metabolic diseases before pregnancy.
Detection methods
Elbow venous blood (5 ml) was extracted from
subjects on an empty stomach in the morning and then placed in a
vacuum tube without anticoagulant and centrifuged at 2,600 × g for
10 min at 4°C. The serum was stored in EP tube for later use and
placed in a low temperature refrigerator at −75°C. The serum was
taken from the freezer, placed it in a refrigerator at 4°C for
dissolution, and then placed it at room temperature for complete
dissolution. Enzyme linked immunosorbent assay (ELISA) (20) was used to detect the expression of
Chemerin, FABP4, interleukin-6 (IL-6) and tumor necrosis factor-α
(TNF-α) in serum. The tests were carried out in strict accordance
with the specifications of human Chemerin ELISA kit, human FABP4
ELISA kit, human IL-6 ELISA kit and human TNF-α ELISA kit (Shanghai
Zhenyu Biotechnology Co., Ltd.; CSB-E10398h, CSB-E12995h,
E-EL-H0102km, E-EL-H0109km). The sample well, standard sample well
and blank well were set up. Sample (50 µl) to be tested was added
to the sample well. The standard sample (50 µl) was added to the
standard sample well. No reagent was added to blank well.
Horseradish peroxidase labeled detection antibody (100 µl) was
added to the sample well and the standard sample well, then the
plate was sealed and incubated at 37°C for 60 min. The liquid was
discarded, shaken off and repeatedly washed 5 times. The substrates
A and B were fully mixed to volume of 1:1. Then (100 µl) of
substrate mixed solution was added to each well. The plates were
sealed and incubated at 37°C for 15 min. Terminal liquid (50 µl)
was added to each well. The absorbance (OD value) at 450 nm of each
well was read by a fully-automatic enzyme-labeled analyzer (M15;
Shanghai Chenlian Biotechnology Development Co., Ltd.). The
expression of Chemerin, FABP4, IL-6 and TNF-α were calculated.
Statistical analysis
SPSS 19.0 (IBM Corp.) statistical data software was
used for statistical analysis. GraphPad Prism6 (GraphPad Software)
was used to draw the data. Enumeration data was expressed by the
number of samples/percentage [n(%)]. The Chi-square test was used
for comparison of enumeration data between groups. The measurement
data were expressed as mean number ± standard deviation (mean ±
SD). The independent-sample t-test was used to compare the
measurement data between groups. Receiver operating characteristic
(ROC) curve was used to evaluate the diagnostic value of peripheral
blood Chemerin and FABP4 in GDM patients. Pearson's correlation
coefficient was used to analyze the correlation between Chemerin
and FABP4 as well as the correlation with inflammatory factors IL-6
and TNF-α. Logistic multivariate regression analysis was used to
analyze the independent risk factors affecting GDN. P<0.05 was
considered statistically significant.
Results
Baseline data
There was no significant difference between the two
groups in baseline data of height, gestational age, abdominal
circumference, systolic blood pressure, diastolic blood pressure,
postprandial insulin for 0.5 h, postprandial insulin for 1 h,
postprandial insulin for 2 h, total cholesterol (P>0.05), but
there was a significant difference in baseline data of age,
diabetes history, hyperlipidemia, pre-pregnancy BMI, increase of
body mass during pregnancy, fasting blood glucose, fasting insulin,
IR index (HOMA-IR), Chemerin or FABP4 (P<0.05) (Table I).
 | Table I.Comparison of baseline data of
patients between the two groups [n(%), mean ± SD]. |
Table I.
Comparison of baseline data of
patients between the two groups [n(%), mean ± SD].
| Category | CG (n=50) | SG (n=60) | χ2/t
value | P-value |
|---|
| Age/years |
|
| 3.976 | 0.046 |
| ≥35 | 7 (14.00) | 18 (28.33) |
|
|
|
<35 | 43 (86.00) | 42 (71.67) |
|
|
| Diabetes history |
|
| 13.943 | 0.002 |
| Yes | 8 (16.00) | 30 (50.00) |
|
|
| No | 42 (84.00) | 30 (50.00) |
|
|
| Hyperlipidemia |
|
| 4.125 | 0.042 |
| Yes | 5 (10.00) | 15 (25.00) |
|
|
| No | 45 (90.00) | 45 (75.00) |
|
|
| Height (cm) | 161.54±5.23 | 162.01±5.12 | 0.637 | 0.526 |
| Pre-pregnancy BMI
(kg/m2) |
|
| 4.073 | 0.044 |
| ≥23 | 11 (26.00) | 24 (35.00) |
|
|
|
<23 | 39 (74.00) | 36 (65.00) |
|
|
| Increase of body mass
during pregnancy (kg) | 13.52±4.26 | 15.61±4.53 | 2.475 | 0.015 |
| Gestational age
(week) | 23.85±1.85 | 24.45±1.55 | 1.851 | 0.067 |
| Abdominal
circumference (cm) | 99.98±6.36 | 101.87±6.65 | 1.514 | 0.133 |
| Systolic blood
pressure (mmHg) | 114.12±9.06 | 115.59±8.99 | 0.851 | 0.397 |
| Diastolic blood
pressure (mmHg) | 72.98±7.16 | 75.04±6.88 | 1.535 | 0.128 |
| Fasting blood
glucose (mmol/l) | 4.58±0.35 | 6.13±0.89 | 11.580 | <0.001 |
| Fasting insulin
(mU/l) | 9.12±4.67 | 13.19±5.15 | 4.304 | <0.001 |
| Postprandial
insulin for 0.5 h (mU/l) | 71.39±37.85 | 67.88±24.05 | 0.590 | 0.557 |
| Postprandial
insulin for 1 h (mU/l) | 90.87±34.58 | 83.81±23.66 | 1.266 | 0.208 |
| Postprandial
insulin for 2 h (mU/l) | 70.27±22.64 | 80.85±36.09 | 1.798 | 0.075 |
| HOMA-IR | 6.42±1.81 | 22.35±13.88 | 8.053 | <0.001 |
| Total cholesterol
(mmol/l) | 5.92±1.43 | 6.03±1.28 | 0.426 | 0.671 |
| Chemerin
(µg/l) | 5.78±1.35 | 7.71±2.23 | 5.354 | <0.001 |
| FABP4 (µg/l) | 21.53±8.89 | 35.14±11.39 | 6.880 | <0.001 |
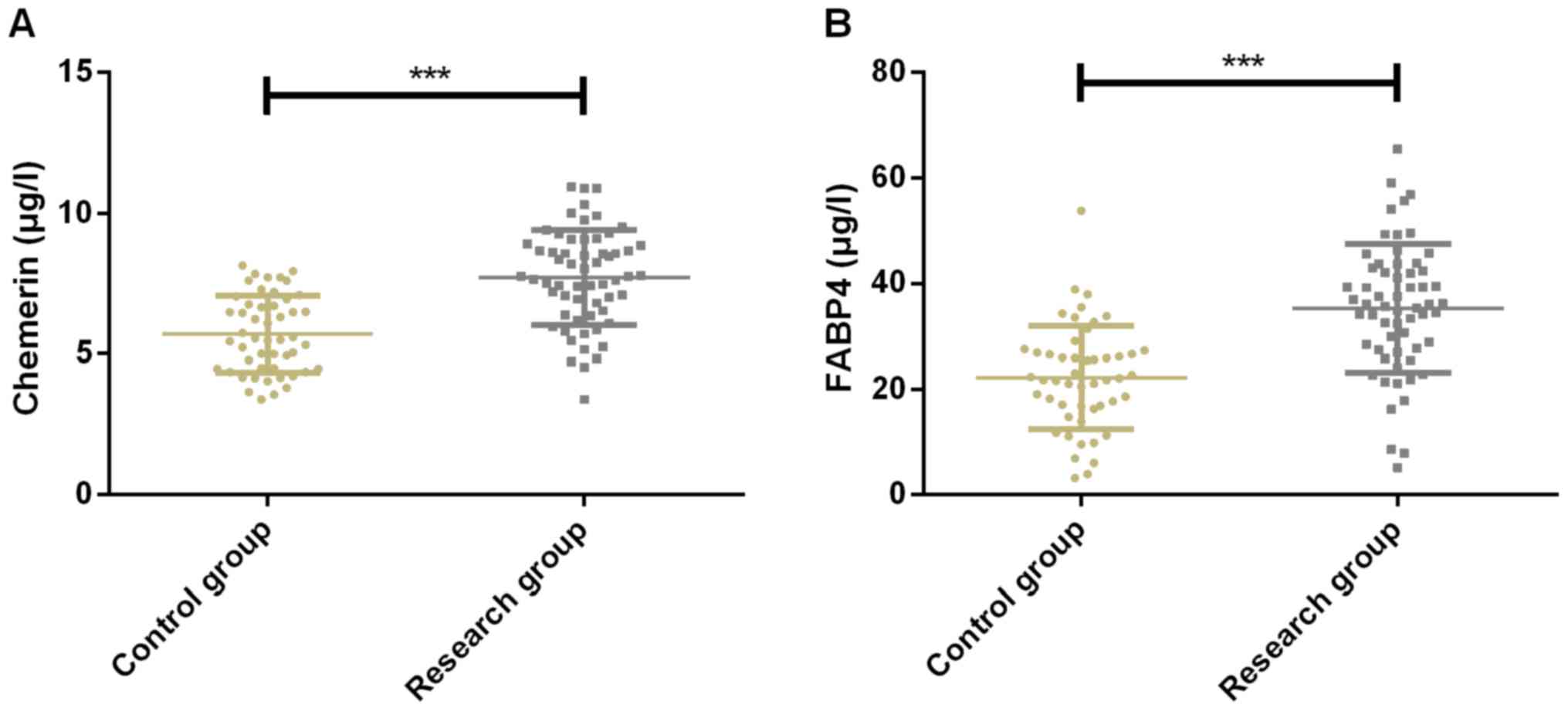
Expression of Chemerin and FABP4 of
patients in the two groups
Expression of Chemerin was 5.78±1.35 and 7.71±2.23
µg/l in CG and SG, respectively, while expression of FABP4 was
21.53±8.89 and 35.14±11.39 µg/l in CG and SG, respectively.
Expression of Chemerin in CG was significantly lower than that in
SG (P<0.001). The expression of FABP4 in CG was significantly
lower than that in SG (P<0.001) (Fig.
1).
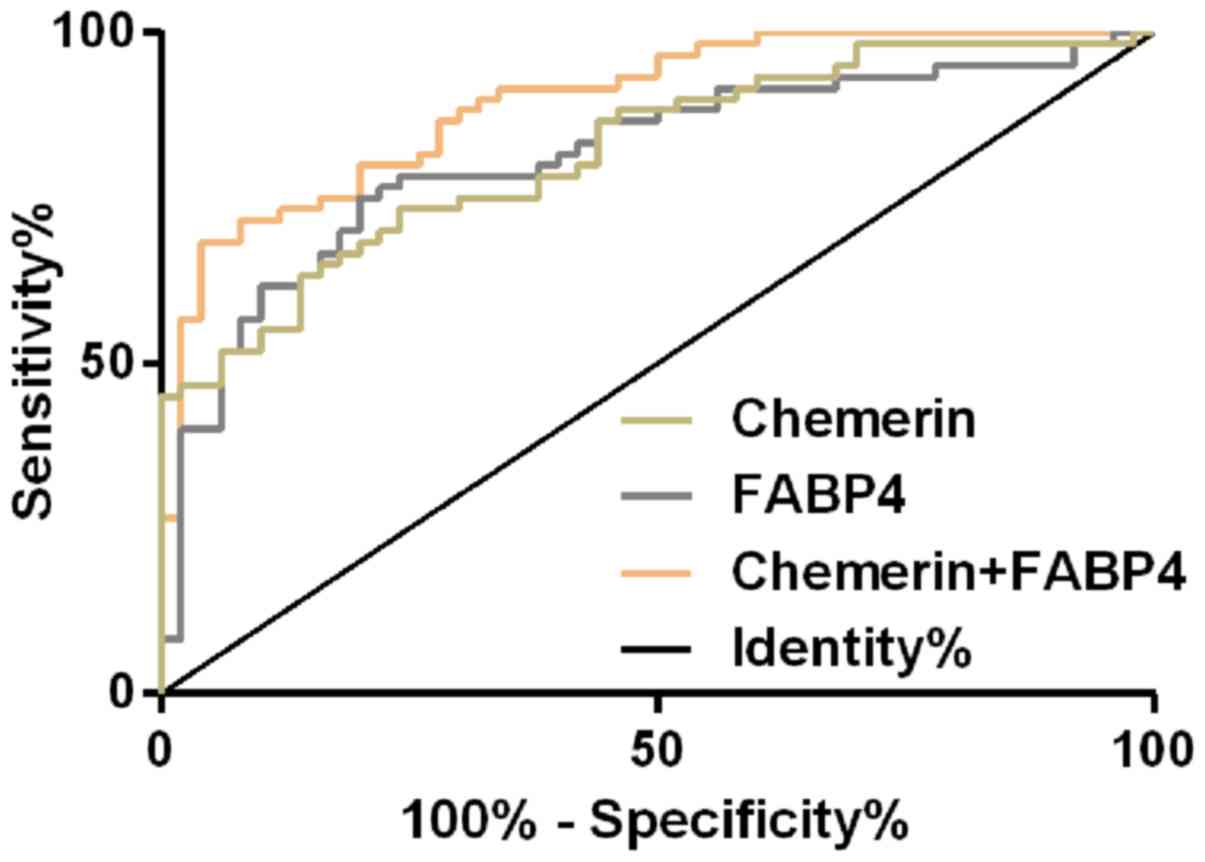
Diagnostic value of Chemerin and FABP4
in GDM patients
The ROC curve of peripheral blood of Chemerin in the
diagnosis of GDM patients was plotted and it was found that the AUC
of peripheral blood of Chemerin in the diagnosis of GDM patients
was 0.820 (95% CI, 0.744–0.896), the cut-off value was 6.78, the
sensitivity was 73.33% and the specificity was 76.00%. AUC of
peripheral blood FABP4 in the diagnosis of GDM patients was 0.814
(95% CI, 0.733–0.895), the cut-off value was 27.64, the sensitivity
was 75.00% and the specificity was 80.00%. Then, the two single
factors, Chemerin and FABP4, were used as independent variables to
conduct binomial Logistic regression analysis. Logistic regression
model was obtained: Logit (p) =−8.73+1.663 chemerin+27.574 FABP4.
The AUC of the model for diagnosis of GDM patients was 0.904 (95%
CI, 0.837–0.952), the cut-off value was 0.71, the sensitivity was
80.00% and the specificity was 96.00% (Fig. 2 and Table
II).
 | Table II.ROC parameters of Chemerin and FABP4
in diagnosis of GDM patients. |
Table II.
ROC parameters of Chemerin and FABP4
in diagnosis of GDM patients.
| Grouping | AUC | 95% CI | SE | Cut-off | Sensitivity
(%) | Specificity
(%) |
|---|
| Chemerin | 0.820 | 0.744–0.896 | 0.039 | 6.78 | 73.33 | 76.00 |
| FABP4 | 0.814 | 0.733–0.895 | 0.041 | 27.64 | 75.00 | 80.00 |
| Chemerin +
FABP4 | 0.904 | 0.837–0.952 | 0.029 | 0.71 | 80.00 | 96.00 |
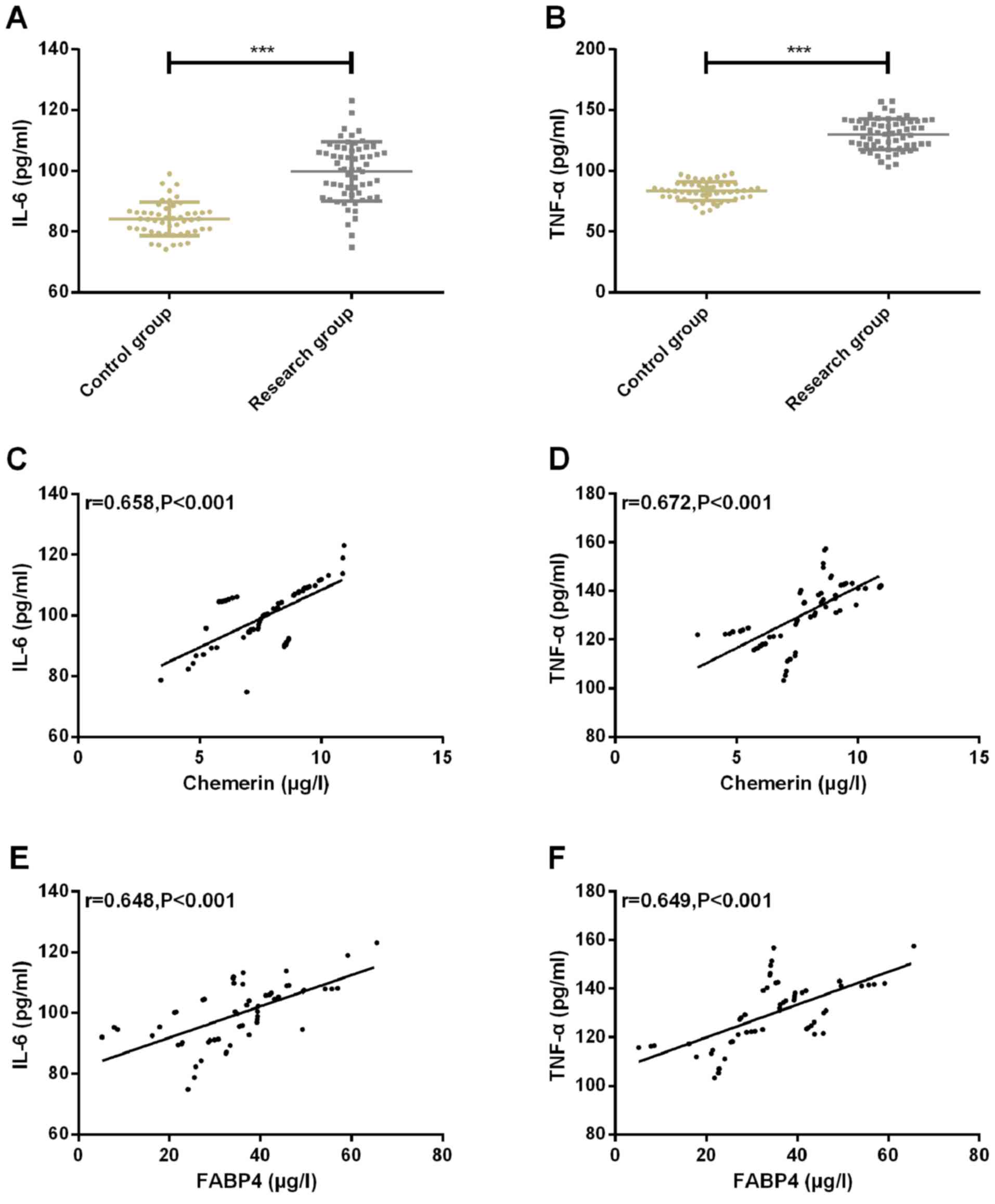
Expression of inflammatory factors
IL-6 and TNF-α of patients in the two groups and the correlation
with Chemerin and FABP4
The expression of inflammatory factor IL-6 in CG and
SG was 83.89±5.74 and 98.34±8.98, respectively. The expression of
inflammatory factor TNF-α in CG and SG were 84.58±7.38 and
130.24±12.02, respectively. The expression of inflammatory factors
IL-6 and TNF-α in CG was significantly lower than that in SG
(P<0.001). Pearson correlation coefficient was used to analyze
the correlation between chemerin, FABP4 and inflammatory factors
IL-6, TNF-α. The results showed that chemerin and FABP4 were
positively correlated with inflammatory factors IL-6 and TNF-α
(r=0.658, P<0.001; r=0.672, P<0.001; r=0.648, P<0.001;
r=0.649, P<0.001) (Fig. 3).
Multiple Logistic regression analysis
of GDM
Multivariate Logistic regression analysis was
conducted on the factors with differences. The results showed that
age (P=0.002), diabetes history (P=0.007), hyperlipidemia
(P=0.021), pre-pregnancy BMI (P=0.010), fasting blood glucose
(P=0.002), Chemerin (P=0.004) and FABP4 (P=0.001) were independent
risk factors for affecting GDM. Patients with advanced age (≥35
years), family history of diabetes, hyperlipidemia, high
pre-pregnancy BMI, high fasting blood glucose, high Chemerin and
high FABP4 expression have increased risk of GDM (Tables III and IV).
 | Table III.Assignment of logistic multivariate
regression analysis. |
Table III.
Assignment of logistic multivariate
regression analysis.
| Factors | Variables | Assignment |
|---|
| Age | X1 | <35=0,
≥35=1 |
| Family history of
diabetes | X2 | No=0; Yes=1 |
| Hyperlipidemia | X3 | No=0; Yes=1 |
| Pre-pregnancy
BMI | X4 | Continuous
variables |
| Increase of body
mass during pregnancy | X5 | Continuous
variables |
| Fasting blood
glucose | X6 | Continuous
variables |
| Fasting
insulin | X7 | Continuous
variables |
| HOMA-IR | X8 | Continuous
variables |
| Chemerin | X9 | Continuous
variables |
| FABP4 | X10 | Continuous
variables |
 | Table IV.Multiple Logistic regression analysis
of GDM. |
Table IV.
Multiple Logistic regression analysis
of GDM.
| Variables | B | SE | Wals | P-value | OR | 95% CI |
|---|
| Age | 0.143 | 0.048 | 9.394 | 0.002 | 1.153 | 1.054–1.619 |
| Family history of
diabetes | 0.579 | 0.192 | 7.040 | 0.007 | 1.784 | 1.165–2.751 |
| Hyperlipidemia | 0.174 | 0.080 | 5.001 | 0.021 | 1.897 | 1.003–1.343 |
| Pre-pregnancy
BMI | 0.742 | 0.305 | 6.622 | 0.010 | 2.118 | 1.189–3.734 |
| Increase of body
mass during pregnancy | 0.101 | 0.473 | 0.045 | 0.829 | 1.106 | 0.429–2.819 |
| Fasting blood
glucose | 0.338 | 0.108 | 9.935 | 0.002 | 1.399 | 1.137–1.736 |
| Fasting
insulin | 0.634 | 0.599 | 1.145 | 0.280 | 1.901 | 0.571–6.152 |
| HOMA-IR | 0.945 | 0.703 | 1.732 | 0.179 | 2.563 | 0.624–10.105 |
| Chemerin | 1.345 | 0.475 | 8.617 | 0.004 | 4.029 | 1.598–10.217 |
| FABP4 | 1.612 | 0.471 | 11.658 | 0.001 | 5.005 | 1.942–12.618 |
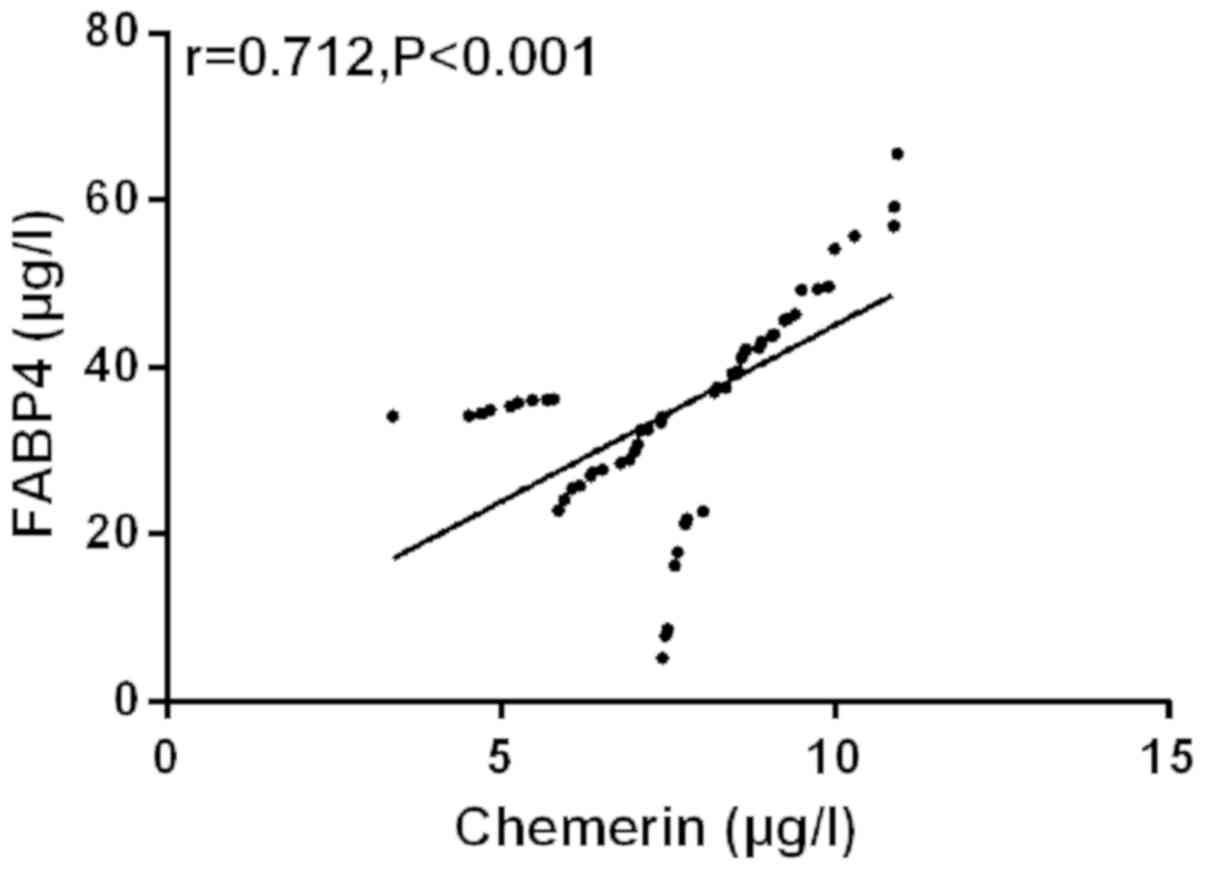
Correlation analysis of Chemerin and
FABP4
The correlation between Chemerin and FABP4 was
analyzed by Pearson's correlation coefficient. The results showed
that peripheral blood of Chemerin was positively correlated with
FABP4 in SG (r=0.712, P<0.001) (Fig.
4).
Discussion
GDM is a heterogeneous multivariate pregnancy
disease with complicated pathological mechanism. In addition to
inflammatory reaction, IR and abnormality of lipid and glucose
metabolism, GDM also involves DNA methylation and oxidative stress
signal transduction that affect cardiac function (21). Statistics showed that the increase in
the prevalence of GDM is global and 33.33% of GDM pregnant women
will suffer from postpartum depression (22,23). In
order to avoid the possible serious impact of GDM on the health of
pregnant women and newborns, we advocate healthy diet and
reasonable physical exercise for women during pregnancy. Some
studies have shown that this has preventive effect on GDM (24).
In this study, the expression of peripheral blood of
Chemerin and FABP4 in GDM patients was significantly upregulated
compared with the CG. The AUC of peripheral blood of Chemerin and
FABP4 for diagnosis of GDM patients was 0.820 and 0.814, while the
AUC of peripheral blood of Chemerin combined with FABP4 for
diagnosis of GDM patients was 0.904, indicating that peripheral
blood of Chemerin combined with FABP4 has excellent diagnostic
value for diagnosis of GDM patients and can be used as biomarker
for prediction of GDM. In the study of Francis et al
(25) on adipocyte factors and GDM
risks, the concentrations of chemerin and FABP4 in GDM patients
were significantly higher than those in CG and both were
significantly positively correlated with GDM risks, indicating that
Chemerin and FABP4 have certain diagnostic value for GDM and are
important risk factors for GDM development. This is similar to the
results of the present study. In the study of Zhang et al
(26) on inflammation and metabolism
of GDM patients in Inner Mongolia, the distribution frequency of
inflammatory factors IL-6 and TNF-α in placenta in GDM women was
significantly higher than that in healthy pregnant women and IL-6
was significantly correlated with GDM disease, suggesting that
overexpression of these two inflammatory mediators may aggravate
the progression of GDM disease by activating inflammatory cascade
reaction in placenta. Other studies have shown that elevated levels
of IL-6 and TNF-α in amniotic fluid of GDM patients may play an
important role in the process of GDM (27). The results of this study on
inflammatory factors showed that the expression of inflammatory
factors IL-6 and TNF-α in SG was significantly higher than those in
CG. Chemerin and FABP4 were significantly positively correlated
with inflammatory factors IL-6 and TNF-α, suggesting that the high
expression of Chemerin and FABP4 in inflammatory environment may be
related to the development and progression of GDM. In studies of
Feng et al (28) on risk
factors of GDM, advanced age, hepatitis B virus, family history of
diabetes, high BMI before pregnancy and a large increase of weight
before 24 weeks of pregnancy may all increase the risk of GDM. In
this study, the results of Logistic multivariate regression
analysis of affecting GDM showed that advanced age (≥35 years),
family history of diabetes, hyperlipidemia, high pre-pregnancy BMI,
high fasting blood glucose, high Chemerin and high FABP4 expression
are risk factors of GDM patients. Among them, high Chemerin and
high FABP4 expression have the greatest risk multiple, indicating
that knockdown of Chemerin and FABP4 expression may reduce the
onset risk of GDM patients. In the report of Chung et al
(29), a CRISPR system of
interfering with FABP4 expression was directionally transmitted to
white adipocytes, which showed improvement effect on obesity,
inflammation and IR. In studies of Josephrajan et al
(30) on the secretion mechanism of
FABP4, the main secretion of FABP4 is the selective secretion
mediated by autophagy, suggesting inhibition of the secretion of
FABP4 by autophagy inhibitor to reduce the influence of FABP4
expression on IR in GDM patients. Chemerin and FABP4 in SG have
significant positive correlation, which indicated that Chemerin and
FABP4 may play a synergistic role in GDM, but the specific
regulatory mechanism needs to be further determined by cytological
function research.
This study confirmed the positive correlation
between Chemerin and FABP4, both of which are overexpressed in GDM
patients and have positive correlation with inflammatory factors
IL-6 and TNF-α.
In conclusion, Chemerin and FABP4 have satisfactory
diagnostic value for GDM patients and inhibition of chemorin and
FABP4 expression can be used as potential therapeutic targets for
GDM patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, JL, DW and JJ led the conception and design of
this study. XW, JL, DW, HZ and LK were responsible for the data
collection and analysis. XW, JL and JJ were in charge of
interpreting the data and drafting the manuscript. HZ and LK made
revision from critical perspective for important intellectual
content. The final version was read and adopted by all the
authors.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the People's Hospital of Zhangqiu Area (Jinan, China). Signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vokalova L, van Breda SV, Ye XL, Huhn EA,
Than NG, Hasler P, Lapaire O, Hoesli I, Rossi SW and Hahn S:
Excessive neutrophil activity in gestational diabetes mellitus:
Could it contribute to the development of preeclampsia? Front
Endocrinol (Lausanne). 9:5422018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaefer-Graf U, Napoli A and Nolan CJ;
Diabetic Pregnancy Study Group, : Diabetes in pregnancy: A new
decade of challenges ahead. Diabetologia. 61:1012–1021.
2018.PubMed/NCBI
|
|
3
|
Damm P, Houshmand-Oeregaard A, Kelstrup L,
Lauenborg J, Mathiesen ER and Clausen TD: Gestational diabetes
mellitus and long-term consequences for mother and offspring: A
view from Denmark. Diabetologia. 59:1396–1399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sweeting AN, Ross GP, Hyett J, Molyneaux
L, Constantino M, Harding AJ and Wong J: Gestational diabetes
mellitus in early pregnancy: Evidence for poor pregnancy outcomes
despite treatment. Diabetes Care. 39:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pantham P, Aye IL and Powell TL:
Inflammation in maternal obesity and gestational diabetes mellitus.
Placenta. 36:709–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Powe CE, Allard C, Battista MC, Doyon M,
Bouchard L, Ecker JL, Perron P, Florez JC, Thadhani R and Hivert
MF: Heterogeneous contribution of insulin sensitivity and secretion
defects to gestational diabetes mellitus. Diabetes Care.
39:1052–1055. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Du J, Zhu YL and Gao XM: Expressions of
inflammatory cytokines and fat factors in placentas of patients
with gestational diabetes mellitus and their relationship with
glucose and lipid metabolism. Hainan Yixueyuan Xuebao. 22:39–42.
2016.(In Chinese).
|
|
8
|
Mariani F and Roncucci L: Chemerin/chemR23
axis in inflammation onset and resolution. Inflamm Res. 64:85–95.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kennedy AJ and Davenport AP: International
union of basic and clinical pharmacology CIII: Chemerin receptors
CMKLR1 (Chemerin1) and GPR1 (Chemerin2) nomenclature, pharmacology,
and function. Pharmacol Rev. 70:174–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haberl EM, Pohl R, Rein-Fischboeck L,
Feder S, Eisinger K, Krautbauer S, Sinal CJ and Buechler C: Ex vivo
analysis of serum chemerin activity in murine models of obesity.
Cytokine. 104:42–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cetin O, Kurdoglu Z, Kurdoglu M and Sahin
HG: Chemerin level in pregnancies complicated by preeclampsia and
its relation with disease severity and neonatal outcomes. J Obstet
Gynaecol. 37:195–199. 2017.PubMed/NCBI
|
|
12
|
Yang X, Quan X, Lan Y, Wei Q, Ye J, Yin X,
Ji Z, Xing H and Yang Y: Serum chemerin level in women with PCOS
and its relation with the risk of spontaneous abortion. Gynecol
Endocrinol. 34:864–867. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ademoglu E, Berberoglu Z, Dellal FD and
Ariel KM: Higher levels of circulating chemerin in obese women with
gestational diabetes mellitus. Acta Endocrinol (Bucur).
11:1841–0987. 2015.
|
|
14
|
Yang X, Quan X, Lan Y, Ye J, Wei Q, Yin X,
Fan F and Xing H: Serum chemerin level during the first trimester
of pregnancy and the risk of gestational diabetes mellitus. Gynecol
Endocrinol. 33:770–773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guaita-Esteruelas S, Gumà J, Masana L and
Borràs J: The peritumoural adipose tissue microenvironment and
cancer. The roles of fatty acid binding protein 4 and fatty acid
binding protein 5. Mol Cell Endocrinol. 462:107–118. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hertzel AV, Xu H, Downey M, Kvalheim N and
Bernlohr DA: Fatty acid binding protein 4/aP2-dependent BLT1R
expression and signaling. J Lipid Res. 58:1354–1361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ning H, Tao H, Weng Z and Zhao X: Plasma
fatty acid-binding protein 4 (FABP4) as a novel biomarker to
predict gestational diabetes mellitus. Acta Diabetol. 53:891–898.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Gennaro G, Palla G, Battini L,
Simoncini T, Del Prato S, Bertolotto A and Bianchi C: The role of
adipokines in the pathogenesis of gestational diabetes mellitus.
Gynecol Endocrinol. 35:737–751. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
American Diabetes Association:
Classification and diagnosis of diabetes. Diabetes Care. 40 (Suppl
1):S11–S24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang L, Rissin DM, Fournier DR, Piech T,
Patel PP, Wilson DH and Duffy DC: Single molecule enzyme-linked
immunosorbent assays: theoretical considerations. J Immunol
Methods. 378:102–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Z, Gong L, Zhang P, Li Y, Liu B,
Zhang L, Zhuang J and Xiao D: Epigenetic down-regulation of Sirt 1
via DNA methylation and oxidative stress signaling contributes to
the gestational diabetes mellitus-induced fetal programming of
heart ischemia-sensitive phenotype in late life. Int J Biol Sci.
15:1240–1251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muller PS and Nirmala M: Effects of pre
pregnancy maternal body mass index on gestational diabetes
mellitus. IACSIT Int J Eng Technol. 7:279–282. 2018. View Article : Google Scholar
|
|
23
|
Gilbert L, Gross J, Lanzi S, Quansah DY,
Puder J and Horsch A: How diet, physical activity and psychosocial
well-being interact in women with gestational diabetes mellitus: An
integrative review. BMC Pregnancy Childbirth. 19:602019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Artal R, Catanzaro RB, Gavard JA, Mostello
DJ and Friganza JC: A lifestyle intervention of weight-gain
restriction: diet and exercise in obese women with gestational
diabetes mellitus. Appl Physiol Nutr Metab. 32:596–601. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roca-Rodríguez MM, López-Tinoco C,
Fernández-Deudero A, Murri M, García-Palacios MV, García-Valero MA,
Tinahones-Madueño FJ and Aguilar-Diosdado M: Adipokines and
metabolic syndrome risk factors in women with previous gestational
diabetes mellitus. Diabetes Metab Res Rev. 28:542–548. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Chi H, Xiao H, Tian X, Wang Y,
Yun X and Xu Y: Interleukin-6 (IL-6) and tumor necrosis factor-α
(TNF-α) single nucleotide polymorphisms (SNPs), inflammation and
metabolism in gestational diabetes mellitus in Inner Mongolia. Med
Sci Monit. 23:4149–4157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Melekoglu R, Ciftci O, Celik E, Yilmaz E
and Bastemur AG: Evaluation of second trimester amniotic fluid
ADAMTS4, ADAMTS5, interleukin-6 and tumor necrosis factor-α levels
in patients with gestational diabetes mellitus. J Obstet Gynaecol
Res. 45:824–829. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng Y, Jiang CD, Chang AM, Shi Y, Gao J,
Zhu L and Zhang Z: Interactions among insulin resistance,
inflammation factors, obesity-related gene polymorphisms,
environmental risk factors, and diet in the development of
gestational diabetes mellitus. J Matern Fetal Neonatal Med.
32:339–347. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chung JY, Ain QU, Song Y, Yong SB and Kim
YH: Targeted delivery of CRISPR interference system against Fabp4
to white adipocytes ameliorates obesity, inflammation, hepatic
steatosis, and insulin resistance. Genome Res. 29:1442–1452. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Josephrajan A, Hertzel AV, Bohm EK,
McBurney MW, Imai SI, Mashek DG, Kim DH and Bernlohr DA:
Unconventional secretion of adipocyte fatty acid binding protein
(FABP4) by adipocytes. FASEB J. 32:814.11. 2018.
|