Introduction
Neonatal hypoxic ischemic encephalopathy (NHIE) can
be caused by various perinatal factors, which mainly leads to
neonatal ischemia and hypoxia in the central nervous system, and
decline or even suspension of cerebral blood flow, as well as
secondary fetal or neonatal hypoxic injury in the central nervous
system (1). NHIE is the most common
complication of neonatal asphyxia, and also the major cause of
mental retardation in children. Studies have shown that ~30% of
NHIE patients suffer from disorders of mental development (2). The moderate-severe NHIE patients,
although survived, have severe neurological sequelae, such as
cerebral palsy, epilepsy and dysgnosia, causing serious
psychological and economic burden on the families of the child
patient and society (3).
Asphyxia is considered as the most direct cause of
NHIE (4), during which central
nervous system lesions are caused by the decline in the neonatal
blood oxygen saturation mostly due to fetal-maternal circulation
and gas exchange disorders (5). A
study proved (6) that NHIE may be
complicated with multiple organ dysfunctions after onset, and the
organ dysfunction becomes more severe with the increasing severity
of asphyxia, exerting a more serious negative impact on the
long-term prognosis, and even affecting the quality of life of the
child patients. Therefore, early detection, diagnosis and treatment
of NHIE are of important significance for reducing the morbidity
and mortality rates of the disease, which is also an important
method to improve the neonatal birth quality in China. The present
study explored the associations of serum S-100β, cystatin C (Cys-C)
and C-reactive protein (CRP) with umbilical cord blood troponin I
(TnI), myoglobin (Mb) and creatine kinase-MB (CK-MB) in NHIE
patients.
Patients and methods
General data
A total of 40 patients with NHIE treated in the
Binzhou Medical University Hospital (Binzhou, China) from March
2017 to June 2019 were selected as observation group, while another
40 healthy neonates in the same period were selected as control
group. All child patients met the diagnostic criteria for NHIE of
the Society of Pediatrics, Chinese Medical Association in 2015, and
they had high-risk factors for perinatal asphyxia. According to the
neonatal birth mode, gestational weeks, Apgar scores immediately
after birth and at 1 and 5 min after birth, the history of birth
asphyxia, and diagnosis of neurological symptoms, the following
subjects were excluded: Mothers complicated with severe systemic
infection during pregnancy or taking immunosuppressive drugs and/or
glucocorticoids during pregnancy, neonates definitely with
congenital genetic diseases and/or deformity after birth, mothers
complicated with severe respiratory system, digestive system or
urinary system infection, or premature rupture of membranes for
more than 24 h during enrollment, or mothers who used analgesic
sedative drugs in the perinatal period or definitely had
hemorrhagic shock. In observation group, there were 26 males and 14
females, the gestational age at birth was 32–42 weeks with an
average of 37.6±0.4 weeks, and the birth weight was 2,000–4,500 g
with an average of 3,200.0±100.0 g. In control group, there were 25
males and 15 females, the gestational age at birth was 32–42 weeks
with an average of 37.7±0.5 weeks, and the birth weight was
2,000–4,500 g with an average of 3,200.5±100.5 g. The sex,
gestational age at birth and birth weight had no statistically
significant differences between the two groups (P>0.05). This
study was approved by the Ethics Committee of the Hospital Attached
to the Binzhou Medical University. Signed informed consents were
obtained from all the parents of the child participants before the
study.
Methods
The related data of all subjects were collected, and
the levels of serum S-100β protein, CRP and Cys-C, and umbilical
cord blood TnI, Mb and CK-MB were compared between the two groups.
Moreover, the associations of the neonatal behavioral neurological
assessment (NBNA) score with the changes in serum S-100β protein,
CRP and Cys-C and umbilical cord blood TnI, Mb and CK-MB were
analyzed. The univariate and multivariate logistic regression
analyses were performed to determine the risk factors for NHIE.
Evaluation criteria
The specimens of serum S-100β protein [enzyme-linked
immunosorbent assay (ELISA) (R&D Systems), <0.5 µg/l], CRP
(ELISA, <10 mg/l) and Cys-C (ELISA, 0.51–1.09 mg/l) were
obtained from the venous blood of neonates. The specimens of TnI
(ELISA, 0–0.034 ng/ml), Mb (ELISA, 0–121 ng/ml) and CK-MB (ELISA,
109–245 ng/ml) were obtained from the umbilical cord blood via
puncture after the umbilical cord was cut at 1–2 min postnatally,
and they were sent for detection within 15 min. The neurobehavioral
ability of neonates was examined using the NBNA under a quiet, warm
and semi-dark environment, and the neonates were breast-fed and
helped to sleep at 1 h before assessment. The NBNA score includes
the behavioral ability (6 major items), passive muscle tension (4
major items), active muscle tension (4 major items) and primary
reflex (3 major items). The score of ≥37 points indicates normal
ability, score of 35–36 points indicates the suspected brain
injury, and score of <35 points indicates brain injury. The
lower the score is, the more severe the brain injury will be.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
20.0 software (IBM Corp.) was used for statistical processing.
Measurement data were expressed as mean ± standard deviation (mean
± SD). t-test was performed for the comparison of means between two
groups, and χ2 test for the comparison of rates between
two groups. P<0.05 indicates statistically significant
difference.
Results
Comparison of the levels of serum
S-100β protein, CRP and Cys-C between the two groups
In observation group, the levels of serum S-100β
protein, CRP and Cys-C were significantly higher than those in
control group (P<0.05) (Table
I).
 | Table I.Comparison of levels of serum S-100β
protein, CRP and Cys-C between the two groups (mean ± SD). |
Table I.
Comparison of levels of serum S-100β
protein, CRP and Cys-C between the two groups (mean ± SD).
|
| S-100β (µg/l) | CRP (mg/l) | Cys-C (mg/l) |
|---|
| Observation
group | 1.3±0.2 | 24.4±1.6 | 2.1±0.1 |
| Control group | 0.4±0.1 | 7.4±0.3 | 0.8±0.1 |
| t-value | 25.456 | 66.047 | 58.138 |
| P-value | <0.001 | <0.001 | <0.001 |
Comparison of the levels of umbilical
cord blood TnI, Mb and CK-MB between the two groups
In observation group, the levels of umbilical cord
blood TnI, Mb and CK-MB were also obviously higher than those in
control group (P<0.05) (Table
II).
 | Table II.Comparison of levels of umbilical cord
blood TnI, Mb and CK-MB between the two groups (ng/ml, mean ±
SD). |
Table II.
Comparison of levels of umbilical cord
blood TnI, Mb and CK-MB between the two groups (ng/ml, mean ±
SD).
|
| TnI | Mb | CK-MB |
|---|
| Observation
group | 0.057±0.002 | 145.3±3.5 | 287.5±15.3 |
| Control group | 0.023±0.001 | 102.3±1.8 | 189.2±9.8 |
| t-value | 96.167 | 69.099 | 34.217 |
| P-value | <0.001 | <0.001 | <0.001 |
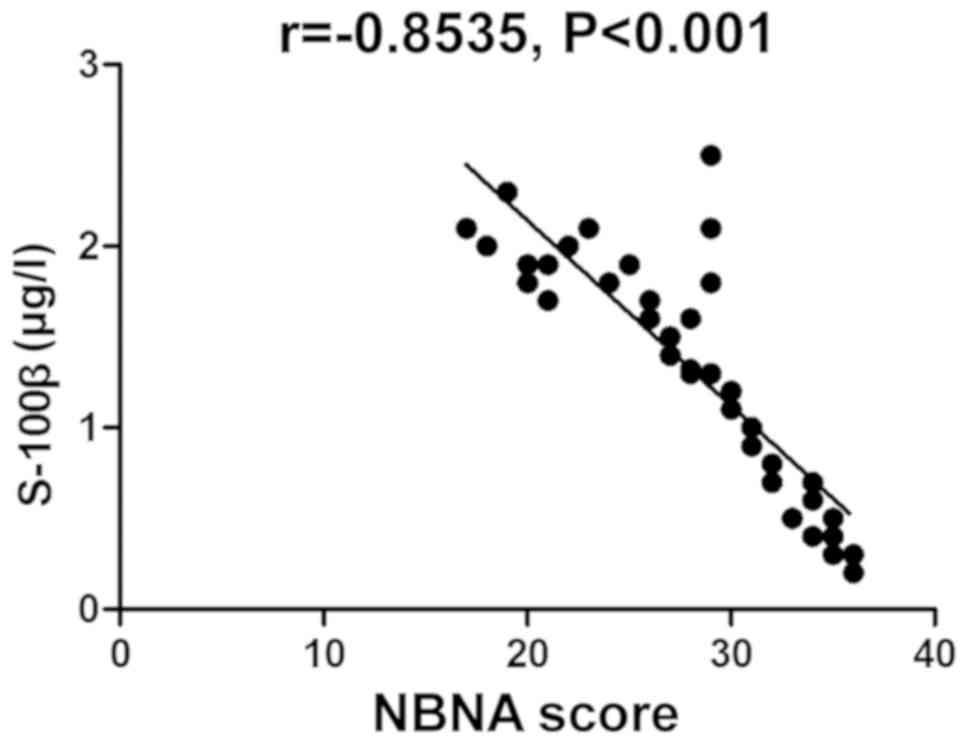
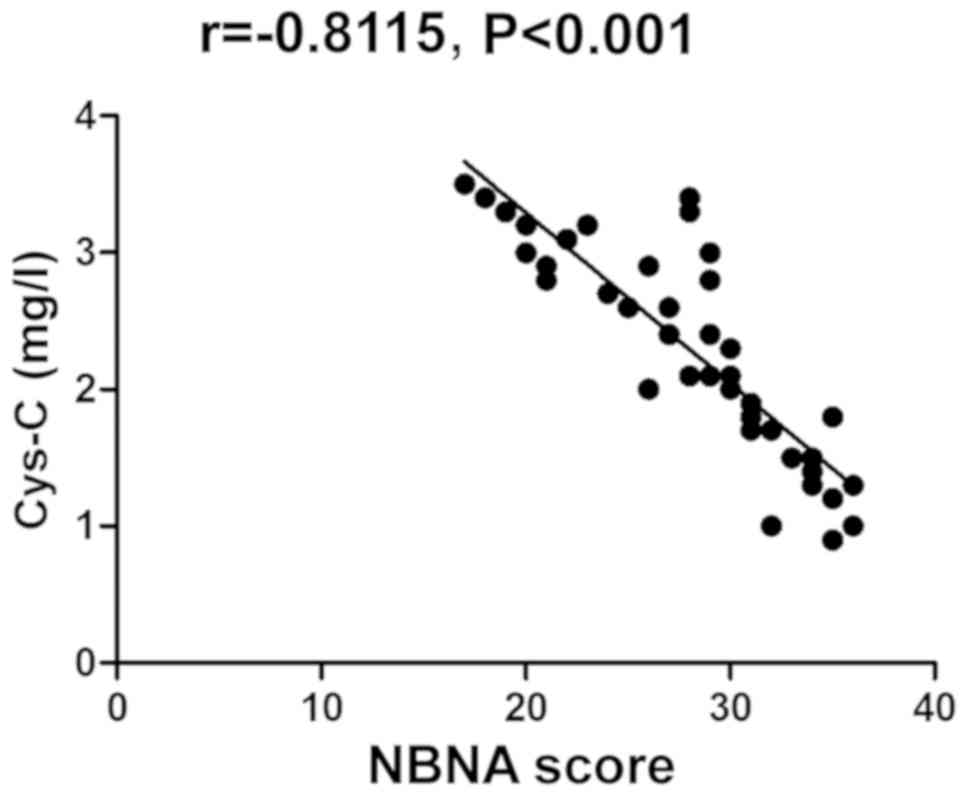
Correlation analysis between NBNA
score and changes in serum S-100β protein, CRP and Cys-C
The NBNA score was negatively correlated with the
changes in serum S-100β protein, CRP and Cys-C (P<0.05)
(Table III and Figs. 1–3).
 | Table III.Correlation analysis between NBNA
score and changes in serum S-100β protein, CRP and Cys-C. |
Table III.
Correlation analysis between NBNA
score and changes in serum S-100β protein, CRP and Cys-C.
|
| r-value | P-value |
|---|
| Serum S-100β protein
level | −0.8535 | <0.001 |
| Serum CRP level | −0.7371 | <0.001 |
| Serum Cys-C
level | −0.8115 | <0.001 |
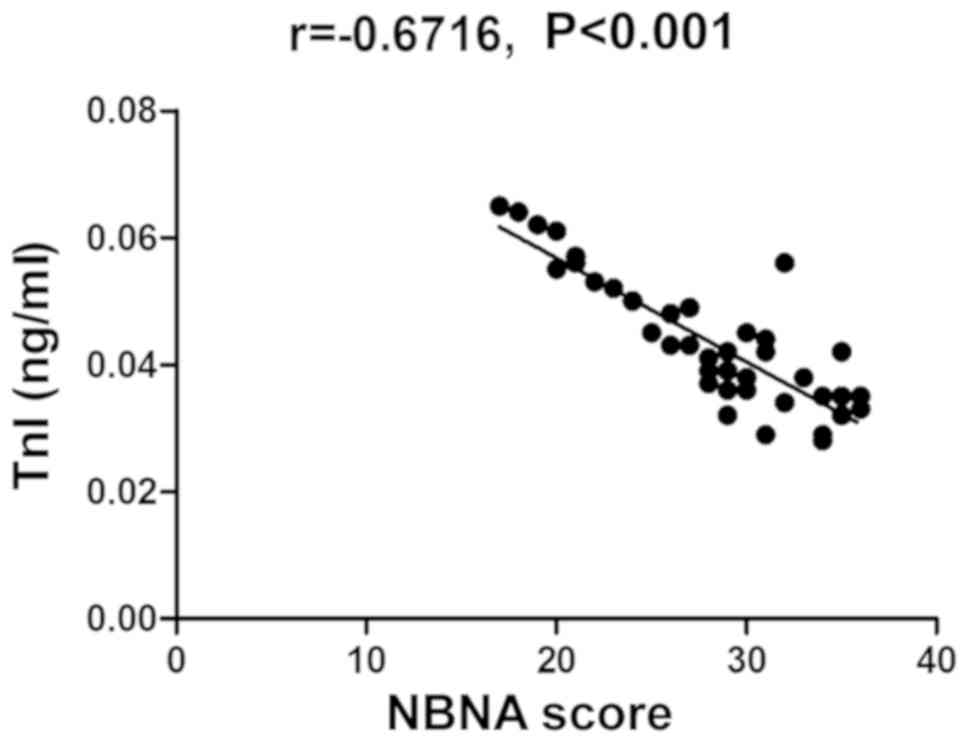
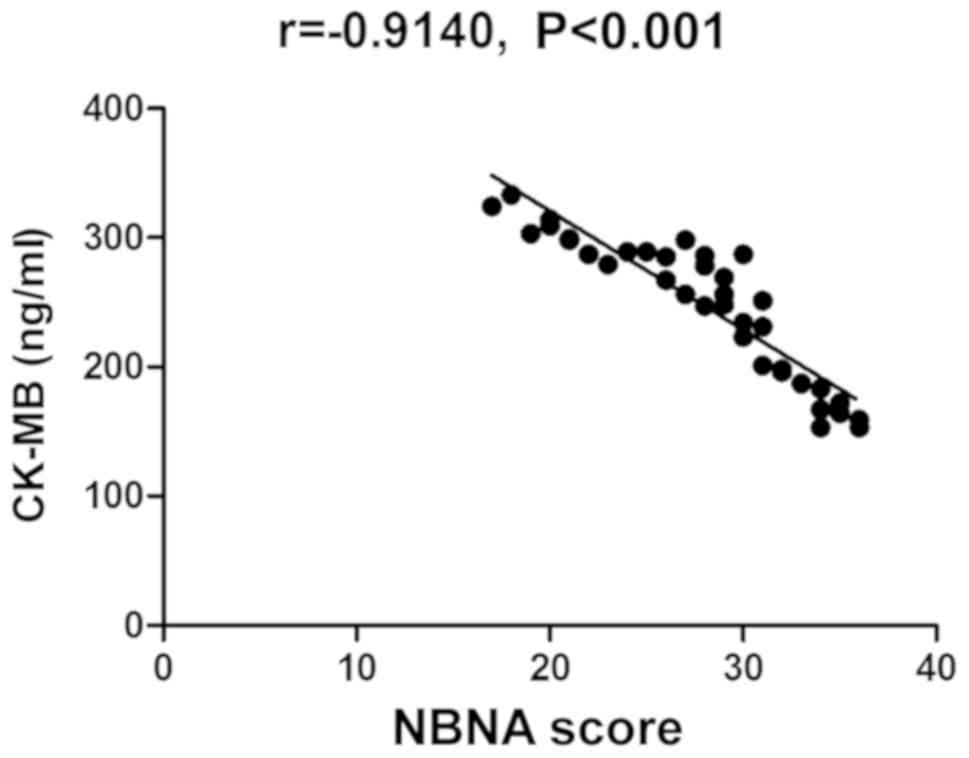
Correlation analysis between NBNA
score and changes in umbilical cord blood TnI, Mb and CK-MB
The NBNA score was also negatively correlated with
the changes in umbilical cord blood TnI, Mb and CK-MB
(P<0.05)(Table IV and Figs. 4–6).
 | Table IV.Correlation analysis between NBNA
score and changes in umbilical cord blood TnI, Mb and CK-MB. |
Table IV.
Correlation analysis between NBNA
score and changes in umbilical cord blood TnI, Mb and CK-MB.
|
| r-value | P-value |
|---|
| Umbilical cord blood
TnI level | −0.6716 | <0.001 |
| Umbilical cord blood
Mb level | −0.7967 | <0.001 |
| Umbilical cord blood
CK-MB level | −0.9140 | <0.001 |
Univariate analysis for risk factors
for NHIE
According to the univariate analysis, the levels of
serum S-100β protein, CRP and Cys-C, and umbilical cord blood TnI,
Mb and CK-MB were related risk factors for NHIE (Table V).
 | Table V.Univariate analysis for risk factors
for NHIE (n). |
Table V.
Univariate analysis for risk factors
for NHIE (n).
|
| Onset | Normal | χ2
value | P-value |
|---|
| Serum S-100β protein
level |
| Rise | 36 | 4 | 54.586 | <0.001 |
|
Normal | 2 | 38 |
|
|
| Serum CRP level |
| Rise | 35 | 5 | 55.000 | <0.001 |
|
Normal | 1 | 39 |
|
|
| Serum Cys-C
level |
| Rise | 34 | 6 | 42.155 | 0.001 |
|
Normal | 4 | 36 |
|
|
| Umbilical cord blood
TnI level |
| Rise | 37 | 3 | 45.255 | 0.011 |
|
Normal | 6 | 34 |
|
|
| Umbilical cord blood
Mb level |
| Rise | 36 | 4 | 54.586 | <0.001 |
|
Normal | 2 | 38 |
|
|
| Umbilical cord blood
CK-MB level |
| Rise | 38 | 2 | 57.836 | <0.001 |
|
Normal | 3 | 37 |
|
|
Multivariate logistic regression
analysis for risk factors for NHIE
According to the multivariate logistic regression
analysis, the increased levels of serum S-100β protein, CRP and
Cys-C, and umbilical cord blood TnI, Mb and CK-MB were independent
risk factors for NHIE (Table
VI).
 | Table VI.Multivariate logistic regression
analysis for risk factors for NHIE. |
Table VI.
Multivariate logistic regression
analysis for risk factors for NHIE.
|
| β | SE | W | OR | P-value | 95% CI |
|---|
| Increased level of
S-100β | 0.89 | 0.53 | 9.47 | 2.44 | <0.001 | 1.38–4.31 |
| Increased level of
CRP | 1.83 | 0.57 | 4.82 | 1.96 | 0.04 | 1.03–7.45 |
| Increased level of
Cys-C | 1.71 | 33.60 | 7.11 | 5.52 | <0.001 | 3.10–9.83 |
| Increased level of
TnI | 2.92 | 0.54 | 4.46 | 6.86 | 0.04 | 1.59–9.24 |
| Increased level of
Mb | 1.12 | 5.50 | 6.38 | 3.07 | <0.001 | 1.76–5.34 |
| Increased level of
CK-MB | 2.34 | 0.46 | 9.92 | 2.81 | 0.01 | 1.21–4.40 |
Discussion
The most important cause of NHIE is neonatal
perinatal asphyxia, which results in neonatal hypoxic-ischemic
injury in the central nervous system (7). In the case of delayed treatment, most
child patients will die clinically (8), and there will be different degrees of
neurological sequelae even if they survive, seriously affecting the
prognosis of patients (9). NHIE is
considered as a disease seriously threatening the quality of life
and even life health of neonates, as well as one of the most
important complications of perinatal asphyxia (10). Studies have proved that the main
causes of NHIE are maternal factors (11), such as pregnancy-induced
hypertension, anemia during pregnancy, postpartum hemorrhage and
placental abnormalities (12)
(placenta previa, placental abruption and placental dysfunction),
as well as fetal factors (13), such
as intrauterine hypoxia and developmental malformation. The above
high-risk factors have been clinically recognized, but not only
central nervous system injury but also multiple organ dysfunctions
occur in NHIE, especially common in the kidneys, cardiovascular
system and respiratory system, which then lead to changes in
various inflammatory factors, nerve injury-related factors and
myocardial enzymes in vivo. Therefore, the early effective
diagnosis and treatment of NHIE is of important significance for
reducing the mortality rate and improving the prognosis of child
patients (14).
In the present study, the levels of serum S-100β,
CRP and Cys-C, umbilical cord blood TnI, Mb and CK-MB in NHIE
patients were analyzed. It was found in the comparison of levels of
serum S-100β protein, CRP and Cys-C between the two groups that
their levels in observation group were significantly higher than
those in control group, indicating that the levels of serum S-100β
protein, CRP and Cys-C in NHIE patients are significantly higher
than those in normal children. The comparison of the levels of
umbilical cord blood TnI, Mb and CK-MB between the two groups
showed that their levels in observation group were also
significantly higher than those in control group, suggesting that
the levels of umbilical cord blood TnI, Mb and CK-MB obviously rise
in NHIE patients. At the same time, the correlations between NBNA
score and changes in serum S-100β protein, CRP and Cys-C, and
umbilical cord blood TnI, Mb and CK-MB were analyzed, and the
results revealed that the NBNA score was negatively correlated with
the changes in serum S-100β protein, CRP and Cys-C as well as the
umbilical cord blood TnI, Mb and CK-MB, further suggesting that
with the increasing severity of nervous system dysfunction and
disease, the levels of nerve injury-related factors and
inflammation are higher and the myocardial damage is more severe in
NHIE patients. Finally, the related and independent risk factors
for NHIE were analyzed. The results manifested that the levels of
serum S-100β protein, CRP and Cys-C, and umbilical cord blood TnI,
Mb and CK-MB were related risk factors for NHIE. The increased
levels of serum S-100β protein, CRP and Cys-C, and umbilical cord
blood TnI, Mb and CK-MB were independent risk factors for NHIE.
In neonatal hypoxia-ischemia, S-100β protein enters
the blood through the blood-brain barrier, increasing the level of
serum S-100β protein. In particular, after central nervous system
injury definitely occurs in NHIE, the blood-brain barrier is
further destroyed, so the level of serum S-100β protein
significantly increases (15). At
the same time, the level of Cys-C is also increased, and it has a
certain correlation with the local cerebrovascular stimuli in the
central nervous system (16), which
will aggravate vasospasm (17) and
metabolic disorders in the body, induce microcirculation
disturbance and platelet aggregation, worsen ischemia and hypoxia
in brain tissues, and lead to the increase of CRP level (18). In addition, when asphyxia occurs,
there will be obvious hypoxic-ischemic changes in the central
nervous system, and the aerobic metabolism is replaced with
anaerobic glycolysis to cause massive accumulation of lactic acid,
thereby resulting in myocardial injury, competitive inhibition of
intracellular calcium iononproteases and excitation-contraction
coupling (19), and aggravating the
degradation of binding troponin in the cytoplasm. In addition,
myocardial injury is further aggravated due to the effect of lipid
peroxides, so the levels of umbilical cord blood TnI, Mb and CK-MB
all obviously rise (20).
In conclusion, in NHIE patients, the levels of serum
S-100β protein, CRP and Cys-C, and umbilical cord blood TnI, Mb and
CK-MB all significantly rise, and they have negative correlations
with the nervous system function after onset.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BW and GZ designed the study and performed the
experiments, BW and XP established the animal models, GZ and JM
collected the data, YL and JL analyzed the data, BW and GZ prepared
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Hospital Attached to the Binzhou Medical University (Binzhou,
China). Signed informed consents were obtained from the parents
and/or guardians of the child participants before the study.
Patient consent for publication
Not available.
Competing interests
The authors declare no competing interests.
References
|
1
|
Maggiotto LV, Sondhi M, Shin BC, Garg M
and Devaskar SU: Circulating blood cellular glucose transporters -
Surrogate biomarkers for neonatal hypoxic-ischemic encephalopathy
assessed by novel scoring systems. Mol Genet Metab. 127:166–173.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen HM, Gao LX, Wang JJ and Gao C: The
correlation between AGT gene polymorphism and neonatal
hypoxic-ischemic encephalopathy (HIE). Eur Rev Med Pharmacol Sci.
23:2194–2199. 2019.PubMed/NCBI
|
|
3
|
Davies A, Wassink G, Bennet L, Gunn AJ and
Davidson JO: Can we further optimize therapeutic hypothermia for
hypoxic-ischemic encephalopathy? Neural Regen Res. 14:1678–1683.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enweronu-Laryea C, Martinello KA, Rose M,
Manu S, Tann CJ, Meek J, Ahor-Essel K, Boylan GB and Robertson NJ:
Core temperature after birth in babies with neonatal encephalopathy
in a sub-Saharan African hospital setting. J Physiol.
597:4013–4024. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chin EM, Jayakumar S, Ramos E, Gerner G,
Soares BP, Cristofalo E, Leppert M, Allen M, Parkinson C, Johnston
M, et al: Preschool language outcomes following perinatal
hypoxic-ischemic encephalopathy in the age of therapeutic
hypothermia. Dev Neurosci. Jun 5–2019.(Epub ahead of print). doi:
10.1159/000499562. PubMed/NCBI
|
|
6
|
Kovacs K, Szakmar E, Meder U, Szakacs L,
Cseko A, Vatai B, Szabo AJ, McNamara PJ, Szabo M and Jermendy A: A
randomized controlled study of low-dose hydrocortisone versus
placebo in dopamine-treated hypotensive neonates undergoing
hypothermia treatment for hypoxic-ischemic encephalopathy. J
Pediatr. 211:13–19.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang M, Liu C, Li X, Liu H, Jin C, Tao X,
Wang X, Zhao H, Cheng Y, Wu F, et al: Isolated periventricular
pseudocysts do not affect white matter microstructure development
in neonatal stage: A retrospective case-control diffusion tensor
imaging study. Eur J Radiol. 116:152–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang XL, Wang X, Shao L, Jiang GT, Min JW,
Mei XY, He XH, Liu WH, Huang WX and Peng BW: TRPV1 mediates
astrocyte activation and interleukin-1β release induced by hypoxic
ischemia (HI). J Neuroinflammation. 16:1142019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YE, Sung SI, Chang YS, Ahn SY, Sung DK
and Park WS: Thrombin preconditioning enhances therapeutic efficacy
of human Wharton's Jelly-derived mesenchymal stem cells in severe
neonatal hypoxic ischemic encephalopathy. Int J Mol Sci.
20:24772019. View Article : Google Scholar
|
|
10
|
Jiang F, Yang M, Wu C and Wang J:
Potential roles of miR-374a-5p in mediating neuroprotective effects
and related molecular mechanism. J Mol Neurosci. 69:123–132. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scheidegger S, Held U, Grass B, Latal B,
Hagmann C and Brotschi B; National Asphyxia and Cooling Register
Group, : Association of perinatal risk factors with neurological
outcome in neonates with hypoxic ischemic encephalopathy. J Matern
Fetal Neonatal Med. Jun 4–2019.(Epub ahead of print). doi:
10.1080/14767058.2019.1623196. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakhuja P, More K, Ting JY, Sheth J,
Lapointe A, Jain A, McNamara PJ and Moore AM: Gastrointestinal
hemodynamic changes during therapeutic hypothermia and after
rewarming in neonatal hypoxic-ischemic encephalopathy. Pediatr
Neonatol. Apr 16–2019.(Epub ahead of print). doi:
10.1016/j.pedneo.2019.04.003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cánovas-Ahedo M and Alonso-Alconada D:
Combined therapy in neonatal hypoxic-ischaemic encephalopathy. An
Pediatr (Barc). 91:59.e1–59.e7. 2019.(In Spanish). View Article : Google Scholar
|
|
14
|
O'Brien CE, Santos PT, Kulikowicz E, Reyes
M, Koehler RC, Martin LJ and Lee JK: Hypoxia-ischemia and
hypothermia independently and interactively affect neuronal
pathology in neonatal piglets with short-term recovery. Dev
Neurosci. 41:17–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tani N, Ikeda T, Shida A, Aoki Y, Oritani
S and Ishikawa T: Postmortem water contents of major organs with
regard to the cause of death. J Forensic Leg Med. 65:48–54. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu X, Cao L, Yu Z, Xin D, Li T, Ma W,
Zhou X, Chen W, Liu D and Wang Z: Hydrogen-rich saline promotes
microglia M2 polarization and complement-mediated synapse loss to
restore behavioral deficits following hypoxia-ischemic in neonatal
mice via AMPK activation. J Neuroinflammation. 16:1042019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Wang H, Liu N, Du J, Lan X, Qi X,
Zhuang C, Sun T, Li Y and Yu J: Oxymatrine protects neonatal rat
against hypoxic-ischemic brain damage via PI3K/Akt/GSK3β pathway.
Life Sci. May 15–2019.(Epub ahead of print). doi:
10.1016/j.lfs.2019.04.070.
|
|
18
|
Zou L, Yuan H, Liu Q, Lu C and Wang L:
Potential protective effects of bilirubin following the treatment
of neonatal hypoxic-ischemic encephalopathy with hypothermia
therapy. Biosci Rep. Jun 4–2019.(Epub ahead of print). doi:
10.1042/BSR20182332.
|
|
19
|
Ciobanou A, Jabak S, De Castro H, Frei L,
Akolekar R and Nicolaides KH: Biomarkers of impaired placentation
at 35–37 weeks' gestation in the prediction of adverse perinatal
outcome. Ultrasound Obstet Gynecol. 54:79–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arriagada S, Huang H, Fletcher K and
Giannone P: Prevention of excessive hypothermia in infants with
hypoxic ischemic encephalopathy prior to admission to a quaternary
care center: A neonatal outreach educational project. J Perinatol.
39:1417–1427. 2019. View Article : Google Scholar : PubMed/NCBI
|