Introduction
Glaucoma, a group of diseases of the optic nerve
(ON) causing axon damage and permanent visual function impairment,
is the leading cause of irreversible blindness worldwide (1–3). The
glaucomatous optic neuropathy involves both anterograde (retina to
visual cortex) (4) and retrograde
(visual cortex to retina) degeneration spreading under various
pathological conditions (4). The
eye-to-brain pathway is composed of retinal ganglion cell (RGC)
connections to corresponding subcortical targets (5,6). Damage
of the adult mammalian central nervous system (CNS) leads to
irreversible neuron loss thereby preventing recovery of numerous
neural functions (7,8).
An important target of neuroprotection is to
identify the restricting elements that regulate CNS repair
(6). Leucine-rich repeat and
immunoglobulin-like domain-containing nogo receptor-interacting
protein 1 (lingo-1), a transmembrane protein, is expressed on
neurons and oligodendrocytes in the CNS and functions as a
component of the negative growth regulatory protein NGR1 (NgR1)/p75
and NgR1/tumor necrosis factor receptor superfamily member 19
signaling complexes (9–12). Lingo-1 negatively regulates axonal
sprouting and myelination in the CNS by binding to Nogo-A, myelin
associated glycoprotein (13), and
oligodendrocyte myelin glycoprotein to inhibit the function of
growth factors. Lingo-1 expression is upregulated in degenerative
diseases and CNS injuries including spinal cord injury, multiple
sclerosis, Parkinson's disease and glaucoma (3,12).
Lingo-1 was reported to bind to epidermal growth factor receptor
(EGFR) or brain-derived neurotrophic factor (BDNF)/NT-3 growth
factors receptor to inhibit survival pathways in neurons (10–12,14–16) and
oligodendrocyte differentiation by binding to erythroblastic
leukemia viral oncogene homolog 2 (12). Inhibition of lingo-1 function using
RNA interference, dominant negative lingo-1, soluble lingo-1
lacking the cytoplasmic domain (10,11,15,16) and
anti-lingo-1 antibodies (15,17,18)
revealed promising effects on promoting neuronal survival, axon
regeneration and oligodendrocyte differentiation in animal models
of degenerative diseases and CNS injuries (9,12,16,19,20).
Experimental ON lesion is a model frequently used to
study the molecular mechanisms underlying CNS neuronal death and
axonal growth in vivo (21–24). The
ON crush (ONC) mimics certain responses of neurons in the CNS to
injury, including glaucomatous optic neuropathy and optic
neurotrauma (5). In animal models of
ONC, injured RGC axons fail to regenerate following mechanical
crush, eventually leading to RGC death (25). A study using ON transection models
revealed that lingo-1 was upregulated following ON transfection,
and inhibition of the function of lingo-1 with lingo-1 antagonist
rescued RGCs from cell death (14).
In the present study, the authors delineated the protein kinase B
(Akt) pathways as the predominant effectors in the ON transection
procedure. A previous study also suggested that some leucine-rich
repeat (LRR) Ig-containing proteins can influence growth factors by
modulating EGFR signaling-associated pathways (15). Lingo-1 gene expression is increased
when adult neurons are exposed to traumatic injuries (12,14–16,26).
These results indicate that lingo-1 may be involved in neuron
injury responses. As observed in the current study, lingo-1 may
impede axon maintenance and the structural integrity of RGCs.
However, whether inhibition of lingo-1 may enhance RGC survival
during ONC and the underlying mechanism in vivo, remain
unknown.
The current study hypothesized that lingo-1 short
hairpin RNA (shRNA) may exhibit neuroprotective effects for the ON
and RGCs, resulting in enhanced RGC survival and preserved visual
function. The current study used an adeno-associated virus serotype
2 (AAV2) vector encoding lingo-1 shRNA for the targeted inhibition
of lingo-1 in RGCs. AAV2-lingo-1-shRNA constructs were injected
into the vitreous bodies in rats. Following the viral transfer, the
ONC injury was performed to investigate the effects of this
treatment on neurodegeneration in vivo. The current study
subsequently investigated the potential mechanisms underlying these
effects. The results indicate that the targeted inhibition of
lingo-1 may promote RGC survival and axon integrity, and prompt the
recovery of neurological functions via Akt phosphorylation at
Ser473.
Materials and methods
Animals
A total of 240 Sprague-Dawley rats of both sexes
(age, 8–10 weeks; sex ratio: 1:1; weight, 200±20 g; Experimental
Animal Center of Sun Yat-sen University, Guangzhou, China) were
handled in accordance with the Association for Research in Vision
and Ophthalmology statement on the use of animals in research. All
experimental protocols and the ethical care of the rats were
reviewed and approved by the Institutional Animal Care and Use
Committee of the Zhongshan Ophthalmic Center, Sun Yat-sen
University (approval no. 2016187). The rats had free access to food
and water in an environmentally controlled room at a temperature of
23°C and 55% humidity with a 12-h light/dark cycle. The rats were
randomly divided into 3 groups: Sham operation group, negative
control shRNA (NC-shRNA) group and lingo-1-shRNA group as described
previously (6,7,26).
AAV2 production
The lingo-1 shRNA AAV2 vectors were constructed to
specifically silence the lingo-1 gene (Shanghai GeneChem Co., Ltd.,
Shanghai, China). The following sequences were used: Lingo-1-shRNA,
5′-TAAGCACAACATCGAAATTGAATTCAAGAGATTCAATTTCGATGTTGTGCTTTTTTTTC-3′
and NC-shRNA,
5′-CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAA-3′. A hybrid
of the cytomegalovirus and chicken β-actin promoters was used to
control the expression of lingo-1. The reporter green fluorescent
protein (GFP) gene was linked to the shRNA via an internal ribosome
entry site. The lingo-1 shRNA and NC sequences were packaged into
the AAV2 supplied by Shanghai GeneChem Co., Ltd. The final titer of
AAV2-lingo-1 shRNA was ~1012 TU/ml.
Intravitreal injections
The intravitreal injections were conducted as
described previously (7,27). Following anesthesia with inhalant
isoflurane, a puncturing hole was made near the ora serrata of the
right eye. Vectors (3 µl) were injected into the vitreal chamber
with a 33 gauge Hamilton™ needle (Hamilton Company, Reno, NV, USA)
under a dissecting microscope. The left eye was left untreated.
Care was taken to avoid inflammation, damage to the lens or
induction of cataracts (28). Any
rats with cataract or inflammation induced by injection were
euthanized and were not included in the dataset. Subsequent
experimentation (sham operation group, n=40; negative control shRNA
(NC-shRNA) group, n=44; lingo-1-shRNA group, n=50) was performed 2
weeks following the injections to allow viral expression. All
injections were performed by a researcher blind to the treatment
conditions.
ONC surgery
Prior to surgery, animals were anesthetized by
intraperitoneal injection of 50 mg kg−1 sodium
pentobarbital (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China), and the right eye was numbed with a drop of 0.5%
proparacaine hydrochloride (Alcon Laboratories, Inc., Fort Worth,
TX, USA). ONC surgery was performed as previously described with
minor modifications (29). Briefly,
an incision was made through the conjunctiva at the upper
conjunctival fornix to expose the sclera. Following exposure of the
ON, self-closing Jeweler's fine forceps (cat. no. 11254-20; Dumont
#5; Fine Science Tools, Inc., Foster City, CA, USA) were used to
crush the ON at a distance ~2 mm behind the posterior pole of the
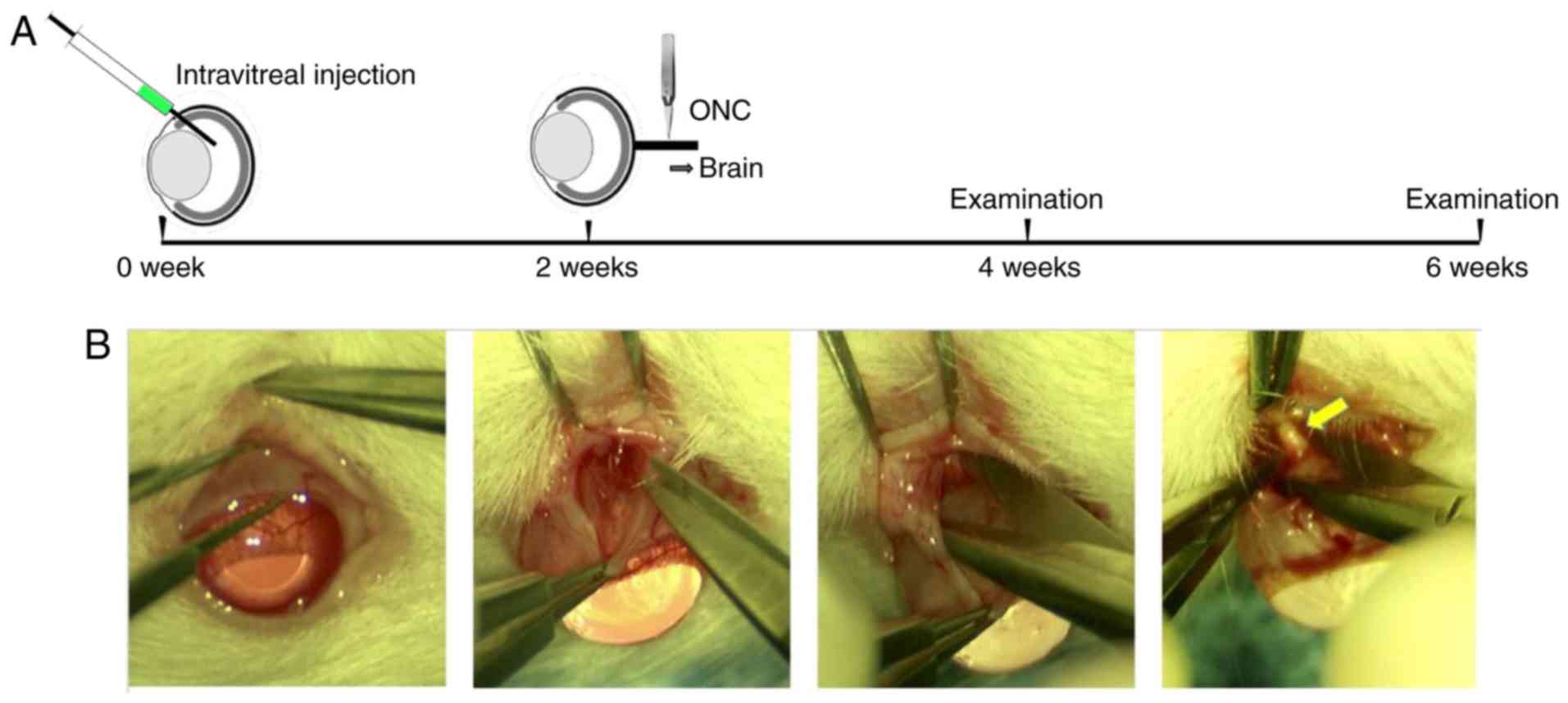
eye for 10 sec (Fig. 1) (6). Following surgery, eyes subjected to the
ONC were closely monitored for several days for any signs of
bleeding. Any rats with eyes with vascular damage or abnormalities
of the optic fundus following surgery were excluded from the
following examination and data analysis. A total of 18 rats were
excluded due to vascular damage or abnormalities of the optic
fundus following surgery.
Flash visual evoked potential (F-VEP)
recording
Rats were examined for functional recovery based on
the measurement of F-VEP. An electrophysiological diagnostic
apparatus (RETI-port/scan 21; Roland Consult Stasche & Finger
GmbH, Brandenburg an der Havel, Germany) was used, in accordance
with the International Society for the Clinical Electrophysiology
of Vision standard for electrophysiological studies (7). The F-VEP was recorded under deep
anesthesia with inhalant isoflurane (verified by the absence of a
tail-pinch reaction). Following 20 min of dark adaptation, F-VEP
was recorded with silver needle electrodes, which were implanted
supraperiosteally over the bilateral visual cortex (V1). A
reference electrode was implanted subcutaneously at the midpoint of
the binoculus and ground electrode was implanted into the tail of
each rat. The test room was illuminated with a dim red safelight.
White flash stimuli were delivered at a frequency of 2 Hz, 250 ms
for analysis. The responses were amplified 10,000 times and
band-pass-filtered from 1 to 1,000 Hz, and superposition was
conducted 100 times (18,30). Recordings of evoked potentials were
taken from bilateral cortices for each experiment; simulation was
unilateral, and the other eye was covered with an opaque
eyeshade.
The parameters recorded were the latency of N1 waves
and N1 amplitude (measured from N1 wave peak to P1 wave trough).
All of the parameter values were measured automatically by the
reto-port/scan 21 computer output, and the average of the three
successive measurements was calculated.
Immunostaining
Rats were sacrificed 4 weeks after ONC surgery.
After anesthesia and transcardial perfusion with normal saline
followed by 4% paraformaldehyde (PFA), the eyes and ONs were
harvested and post-fixed in 4% PFA overnight. The ONs were then
transferred to PBS then placed in 30% sucrose and frozen at −80°C
in Optimal Cutting Temperature compound (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The ON was sectioned using a sledge
microtome, cutting longitudinally or transversely at 10 µm.
Immunofluorescent staining was performed in a blocking solution at
room temperature for 1 h (20% normal donkey serum, cat. no. ab7475;
Abcam, Cambridge, UK; 0.1% Triton-X-100 in PBS, Solarbio, Shanghai,
China). Primary antibodies were applied overnight at 4°C and, after
PBS washes, sections were incubated with the appropriate primary
antibodies overnight, and the secondary antibody for 1 h at room
temperature. Primary antibodies were as follows:
Rabbit-anti-RNA-binding protein with multiple splicing (RBPMS, 1:
200; cat. no. ABN1376; EMD Millipore, Billerica, MA, USA), to label
RGCs; rabbit-anti-GFP (1: 300; cat. no. ab2556; Abcam), to detect
GFP; rabbit-anti-p-Ser473 (1:200; cat. no. D9E; Cell Signaling
Technology, Inc., Danvers, MA, USA), to label phosphorylated Akt
Ser473; and rabbit anti-Lingo-1 (1:200; cat. no. ab23631, Abcam),
to detect lingo-1 expression. The Alexa Fluor 488 goat anti-rabbit
or anti-guinea pig (cat. nos. A-11008, A-11073, Invitrogen; Thermo
Fisher Scientific, Inc.), or Alexa Fluor 594 goat anti-rabbit (cat.
no. A-11012, Invitrogen; Thermo Fisher Scientific, Inc.) were used
as secondary antibodies. Immunofluorescent labeling was analyzed
with a fluorescence microscope (Axio Observer Z1; Zeiss AG,
Oberkochen, Germany).
Hematoxylin-eosin (HE) staining
The rat eyes and the ONs were collected as
aforementioned and used for HE staining (Fig. 1A). The tissue specimens were
dehydrated in gradient alcohol after 4% PFA fixation for 2 h at
room temperature and immersed for 5 min in xylene before embedding
in wax. The specimens were incubated at 60°C overnight then
embedded in liquid paraffin, immersed in water and cut into 10-µm
thick slices. After deparaffinization and gradient rehydration, the
slices were stained with hematoxylin for 10 min at room temperature
and washed with running water for 5 min. Depigmentation was
performed in 1% hydrochloric acid alcohol. Sections were washed
with running water for 5 min, then washed with 50, 70 and 80%
alcohol for 3 min at each concentration and stained with 0.5% eosin
for 5 min at room temperature. Subsequently, samples were washed
with 95 and 100% alcohol for 3 min each and treated with xylene for
10 min, o-xylene for 2 min and m-xylene for 2 min. The slices were
sealed by neutral resin and observed under a light microscope
(Zeiss AG).
Cell number quantification
After euthanasia and perfusion of the rat (as
described above), the superior portion of the eye was marked with a
marking pen and then the whole eye was enucleated and fixed in 4%
PFA at 4°C. After 1 h, the eye was rinsed in PBS and the anterior
segment removed to create an eye cup. The retina was removed from
the eye cup and placed with the ganglion cell layer facing up into
a culture dish; four cuts were made to allow the retina to lay
flat. Flattened retinae were fixed in 4% PFA at 4°C overnight,
subsequently blocked and incubated at 4°C for 1 h in a solution
(0.3% Triton-X-100, 10% donkey serum and 0.01% sodium azide in PBS;
Sorlarbio Science & Technology Co., Ltd.) containing a primary
antibody (EMD Millipore) against RBPMS, an RGC marker used for RGC
quantification (31). RGC intensity
was compared in the flat mount retinas of AAV2-lingo-1
shRNA-injected animals and control animals injected with saline.
Each RBPMS+ cell was subsequently counted in the four
quadrants of the retina using an epifluorescence microscope and
included in the quantification of the RGC numbers in different
experimental conditions. The number of RGC bodies was quantified at
1 mm from the optic disc in four quadrants. The density of
surviving RGCs was calculated per mm2 (n=5 rats per
group) manually.
Western blotting
ON protein lysates were extracted from tissues (n=5
per group) by incubating in RIPA buffer (EMD Millipore)
supplemented with PMSF (EMD Millipore). Protein samples were
separated by SDS-PAGE (NuPAGE 4–12% Bis-Tris gel) and transferred
to PVDF membranes (EMD Millipore). The membranes were blocked with
5% nonfat dry milk containing 0.1% Tween-20 in PBS for 1 h at room
temperature and incubated with primary antibodies at 4°C overnight.
The following primary antibodies were used at 1:1,000 dilution:
Lingo-1, p-Akt (p-Ser473; cat. no. D9E, Cell Signaling Technology,
Inc.), Akt (Cell Signaling Technology, Inc.), GAPDH (cat. no.
PA-987; Thermo Fisher Scientific, Inc.). Super Signal West Pico
Chemiluminescent Substrate kit (cat. no. 34580; Thermo Fisher
Scientific, Inc.) was applied to visualize protein bands, band
intensity was analyzed with ImageJ v6.0 software (National
Institutes of Health, Bethesda, MD, USA). Protein levels in tissues
were quantified by densitometry and normalized to GAPDH,
respectively (phospho-Akt levels were normalized to total Akt
levels).
Reverse transcription-quantitative PCR
(RT-qPCR)
To investigate molecular events associated with
lingo-1 inhibition, changes in gene expression were evaluated by
qPCR. The following primers were used for qPCR: Lingo-1,
5′-CTTTCCCCTTCGACATCAAGAC-3′ and 3′-CAGCAGCACCAGGCAGAA-5′; GAPDH,
5′-ACAGTCAGCCGCATCTTCTT-3′ and 3′-GACAAGCTTCCCGTTCTCAG-5′. GAPDH
was used for normalization. qPCR was performed using unfixed ONs 28
days after ONC in the lingo-1-shRNA and NC-shRNA groups. ONs were
dissected in ice-cold PBS and immediately immersed into
RNAlater reagent (Qiagen GmbH, Hilden, Germany). RNA was
extracted using a RNeasy kit (Qiagen GmbH) and reverse-transcribed
using iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) to obtain cDNA. qPCR was performed using the iQ™
SYBR® Green Supermix kit according to manufacturer's
protocol (Bio-Rad Laboratories, Inc.). The following thermocycling
conditions were used: Initial denaturation at 95°C for 10 min; 40
cycles of 95°C for 30 sec and 60°C for 1 min; and a final extension
at 72°C for 1.5 min. The 2−ΔΔCq method was used to
quantify the relative changes in gene expression (32). The average Cq was
calculated for the target gene and GAPDH and the ΔCq
(Cq,target-Cq,GAPDH) values were analyzed.
All qPCR experiments were performed with three technical
replicates.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Normality
tests and variance heterogeneity tests were performed on all
datasets. Statistical analysis was performed using Student's t-test
for comparisons between two groups or by one-way analysis of
variance followed by Tukey's post-hoc tests for comparisons of more
than two groups. Error bars are presented as mean ± standard error
(S.E.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Lingo-1 shRNA knocks down lingo-1
expression in RGCs
It has been reported that lingo-1 is detected in the
retina and ON of adult rats (12,16). To
analyze the role of lingo-1 in RGCs, RGCs were transduced with a
GFP-expressing lingo-1 shRNA vectors via intravitreal injections.
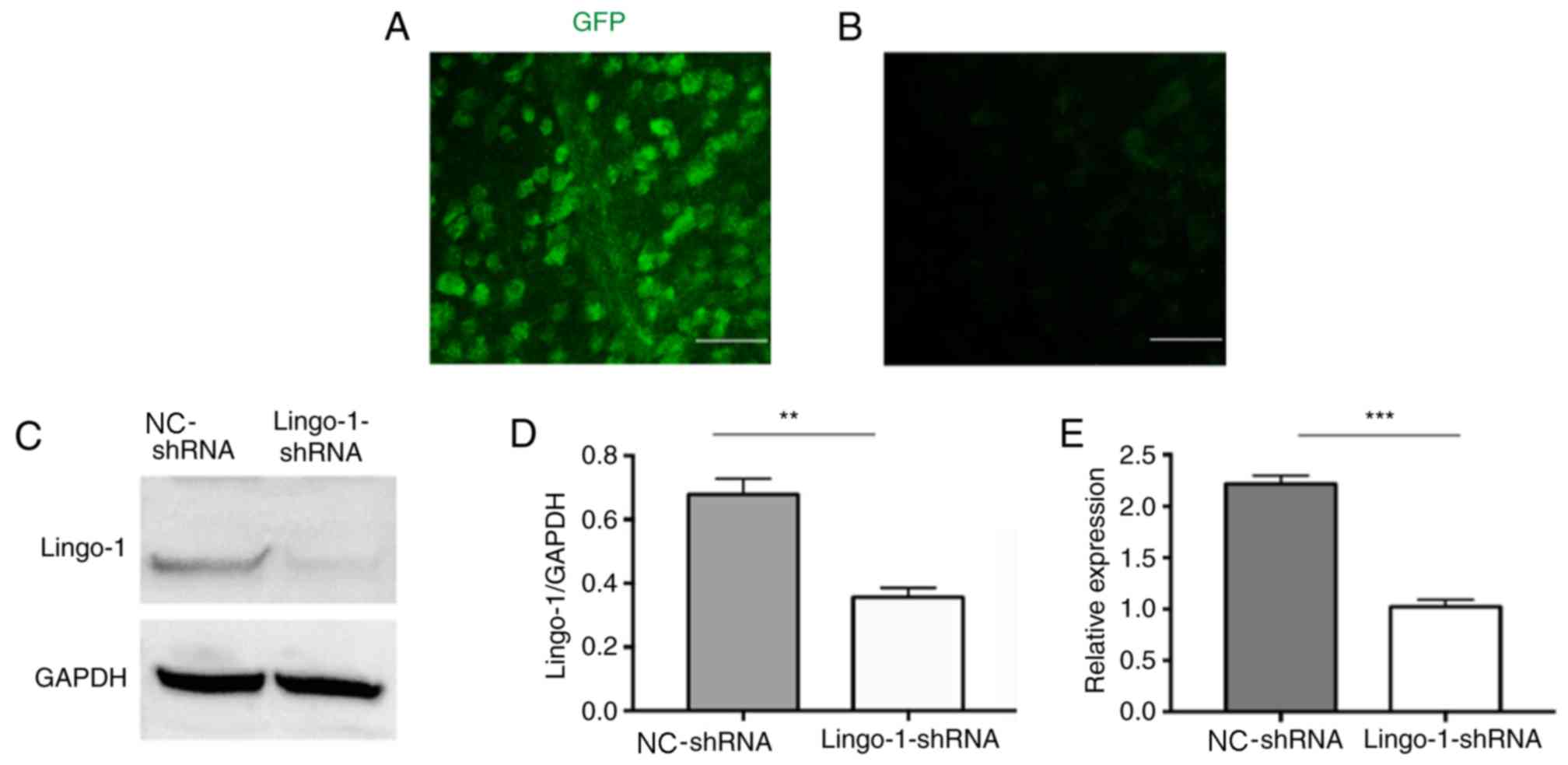
Two weeks after the injection, GFP expression was observed in flat
mount retinas (Fig. 2A and B), and
the results suggested that the transfection was successful.
Furthermore, western blot analysis 2 weeks after AAV2 injection
revealed that the expression of lingo-1 was knocked down by
AAV2-lingo-1-shRNA compared with AAV2 NC-shRNA (P<0.01; Fig. 2C-E). Taken together, these results
indicate that shRNA-mediated knockdown in vivo lead to
significant alterations in lingo-1 expression in RGCs in rats.
Inhibiting lingo-1 expression
increases the RGC cell density
To investigate whether changes in lingo-1 expression
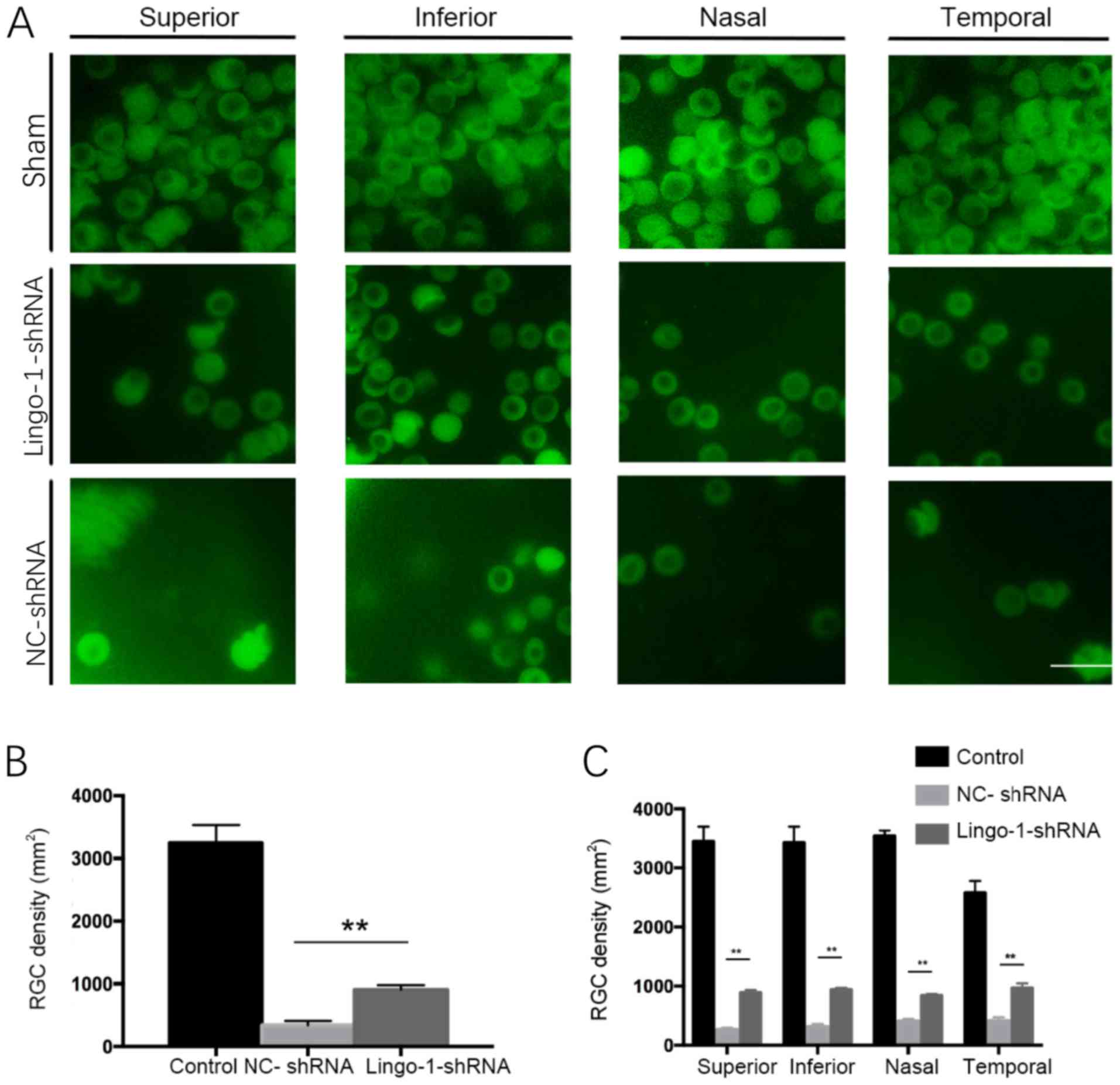
levels affect RGCs survival in rats with ON-injury, the densities
of RGCs were quantified in whole-mount retinas of sham ONC,
NC-shRNA and AAV2-lingo-1-shRNA injected rats. RGC densities were
quantified by RBPMS-positive cell counts on flat-mounted retinas.
The RGC cell density of each group is presented in Fig. 3. Four weeks after ONC, the RGC
density was significantly higher in the lingo-1-shRNA-injected
group compared with the control group (908.3 cells/mm2
vs. 338.3 cells/mm2, respectively). There was a
significant increase in RGC density in the lingo-1-shRNA-treated
groups compared with NC-shRNA in all four quadrants of retina
(P<0.01; Fig. 3). Together, these
results revealed that transfection of lingo-1-shRNA enhanced the
survival rate of RGCs after ONC (P<0.01; Fig. 3). Together, these data suggested that
lingo-1-shRNA can reduce RGC loss during ON injury.
Lingo-1-shRNA reduces the lesion
volume of the injured ON
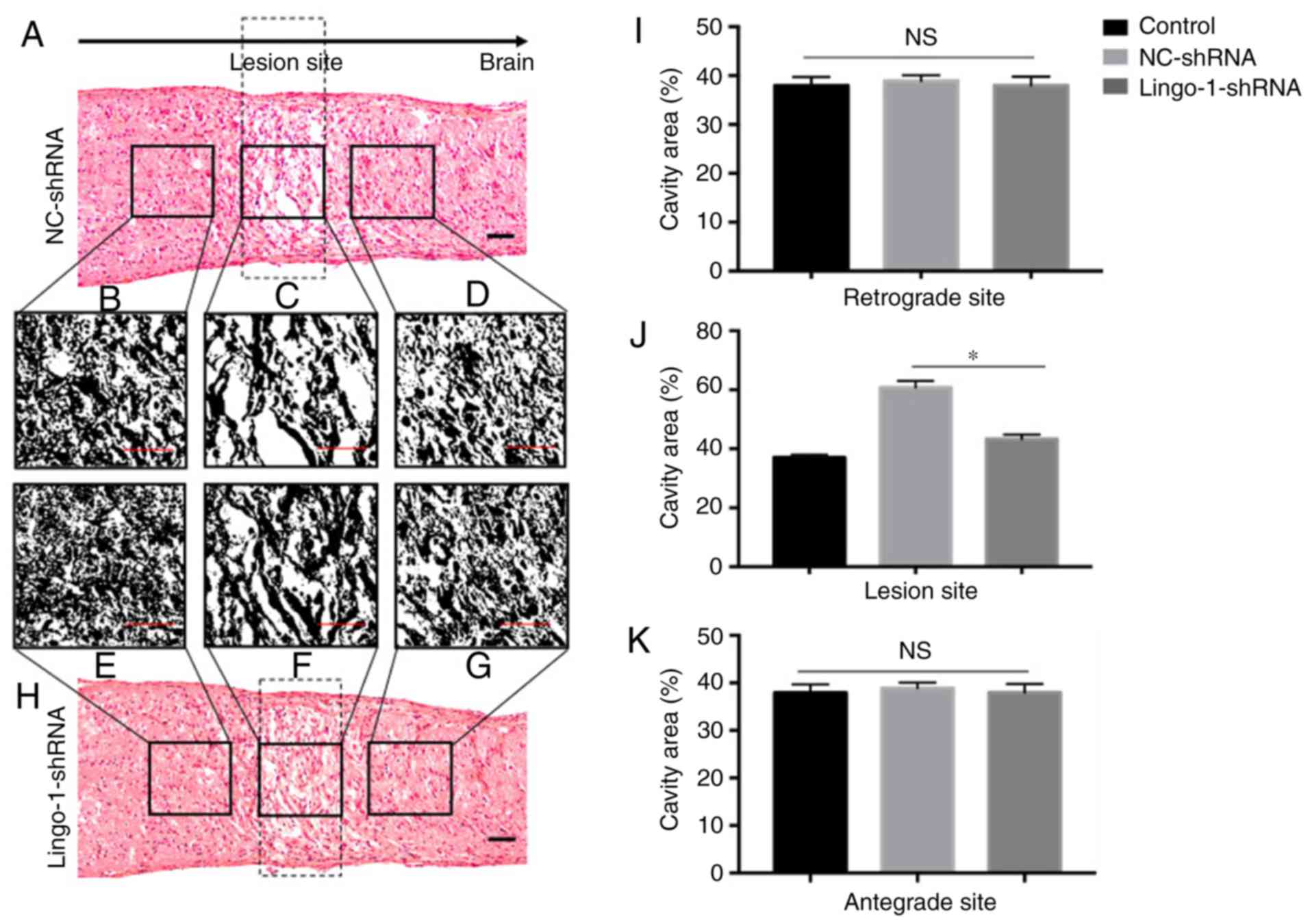
Tissue repair was estimated by calculating the size
of the lesion cavities in injured ONs. The size of the lesion
cavity was calculated in HE-stained sections (longitudinal and
transverse ON sections) at 4 weeks post-injury to detect tissue
repair (Fig. 4). The tissue did not
narrow from the outer edge of the lesioned ON. The outer margins
near the lesion site relatively retained structural integrity,
however, multiple cavities were observed in HE staining of ONs
(Fig. 4A and H). In rats treated
with lingo-1-shRNA, the total cavity area in the longitudinal plane
was 21.9% smaller compared with the NC-shRNA group (27930.0 pixels
vs. 34034.7 pixels, respectively; P<0.05; Fig. 4C, F and J). The differences of total
cavity area between the lingo-1-shRNA group and the NC-shRNA group
at the retrograde and antegrade sites were insignificant (Fig. 4B, D, E, G, I and K). Differences in
lesion volume were also observed in transverse sections (Fig. 5), the cavities in
lingo-1-shRNA-treated rats were significantly smaller and less
numerous than those in the NC-shRNA group.
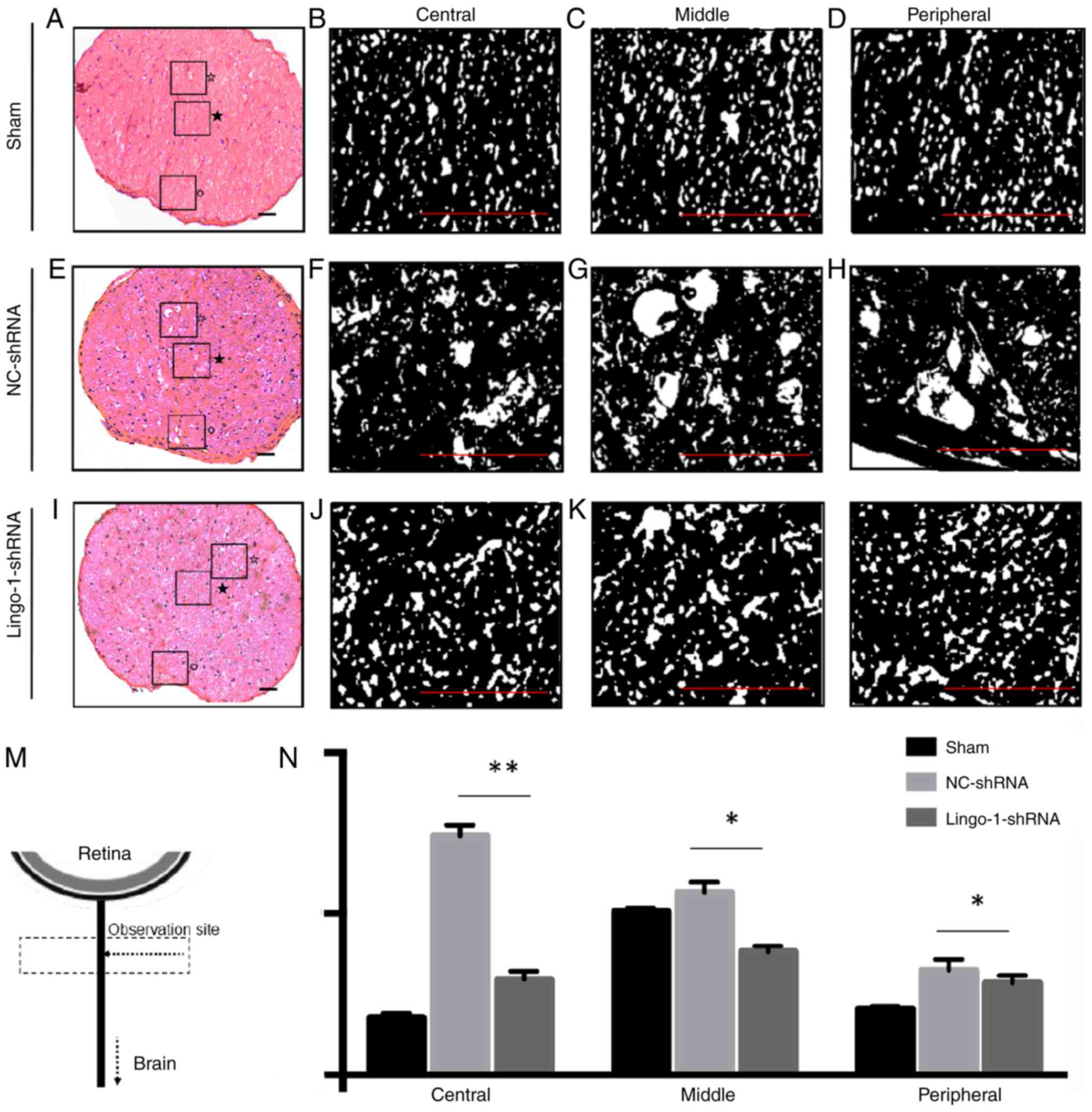 | Figure 5.Transverse histological sections of
optic nerves. (A) Representative histological section of the sham
ONC group. Details of histological alterations of the (B) central,
(C) middle and (D) peripheral part of the optic nerve of the sham
ONC group. (E) Representative histological section of the NC-shRNA
group. Details of histological alterations of the (F) central, (G)
middle and (H) peripheral part of the optic nerve of the NC-shRNA
group. (I) Representative histological section of the lingo-1-shRNA
group. Details of histological alterations of the (J) central, (K)
middle and (L) peripheral part of the optic nerve of the
lingo-1-shRNA group. ★, central area; ☆,
middle area; °, peripheral area. Scale bar=100 µm. (M) Schematic
drawing showing the observation site of transverse histological
sections of optic nerves. (N) Quantitative analysis of nerve cavity
areas in the three groups revealed that the injury damaged the
central, middle and the peripheral parts of optic nerves, and the
damage was most severe in the central parts of the optic nerves.
n=5. *P<0.05 and **P<0.01. Lingo-1, leucine-rich repeat and
immunoglobulin-like domain-containing nogo receptor-interacting
protein 1; ONC, optic nerve crush; NC-shRNA, negative control
shRNA; shRNA, short hairpin RNA. |
Targeted inhibition of lingo-1
preserves F-VEP after ONC
F-VEPs were measured to test the functional recovery
after ONC. The N1 waves were detected before, and 2 and 4 weeks
post-injury (Fig. 6A). The ONC
procedure lead to a delay in peak latencies of N1 waves. Both in
lingo-1-shRNA and NC-shRNA group, longer N1 wave latencies were
observed at 2 weeks (P<0.05) and 4 weeks post-injury compared
with the respective control groups (P<0.01; Fig. 6C and D). The P1-N2 amplitudes 4 weeks
post-injury in the sham, NC-shRNA and lingo-1-shRNA groups were
36.27±7.81, 5.27±3.56, 17.06±2.89 µV, respectively (Fig. 6C; Table
I). Compared with the NC-shRNA group, the lingo-1-shRNA-treated
group exhibited a decreased latency of N1 waves at 2 weeks
(P<0.01) and 4 weeks post-injury (P<0.001; Fig. 6D). No complete restoration of N1
waves was observed in the current study. The results suggest that
lingo-1-shRNA treatment can partially preserve the visual function
in the ONC model. Detailed data of F-VEP recording are listed in
Table I.
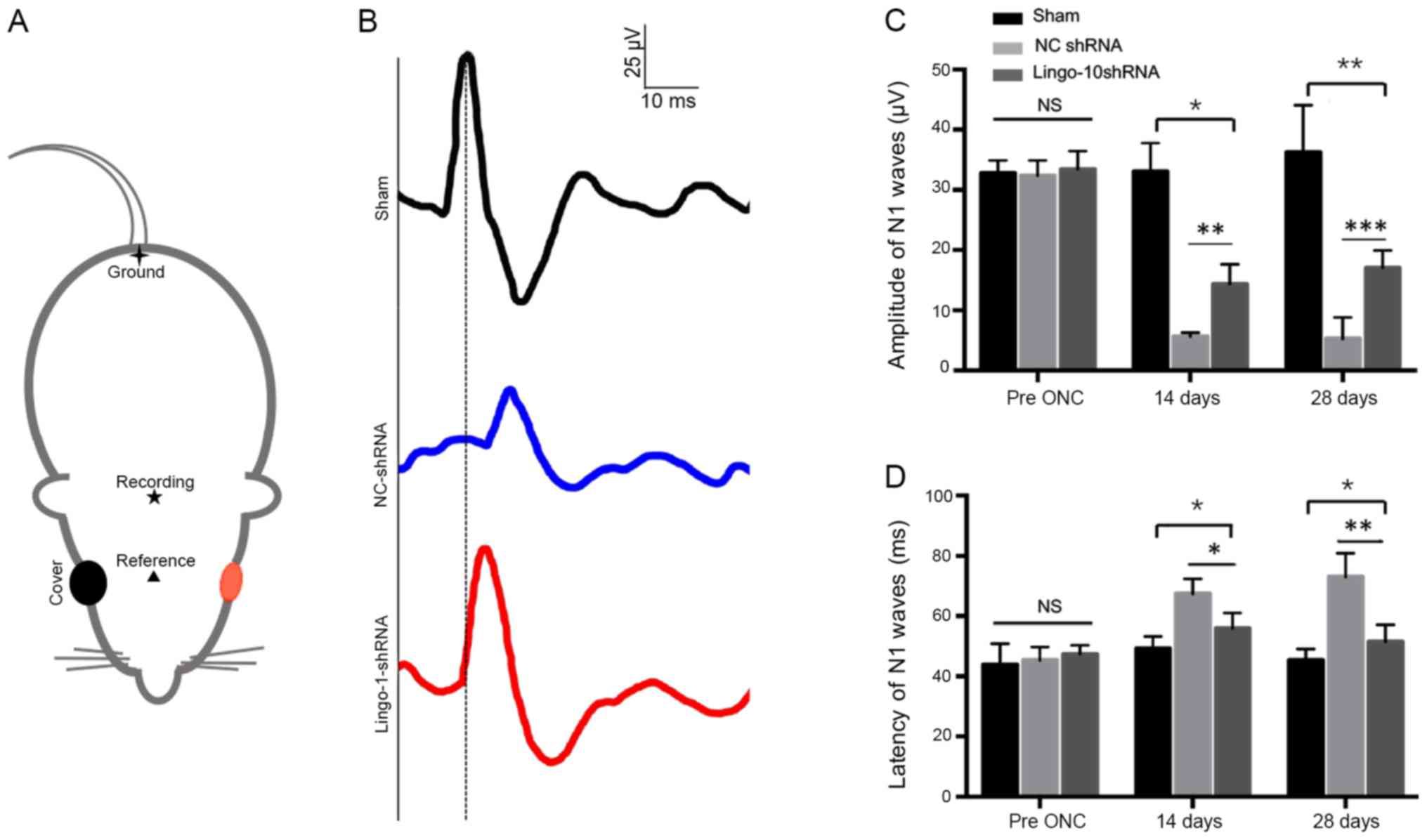 | Figure 6.Evaluation of the recovery of injured
optic nerves using the F-VEP wave pattern. (A) Locations of silver
needle electrodes. ▲, the reference electrode;
★, the recording electrode; ✦, the ground
electrode. The red color indicated the testing eye of the rat. (B)
Representative F-VEP tracings 4 weeks following ONC in the sham
surgery, NC-shRNA and lingo-1-shRNA groups. Y-axis scale, 25 µV;
x-axis scale, 10 ms. (C) N1 amplitude 2 and 4 weeks following ONC.
(D) N1 latency 2 and 4 weeks following ONC. Error bars represent
standard error of the mean, n=10. *P<0.05, **P<0.01 and
***P<0.001. Lingo-1, leucine-rich repeat and immunoglobulin-like
domain-containing nogo receptor-interacting protein 1; NC-shRNA,
negative control shRNA; shRNA, short hairpin RNA; F-VEP, flash
visual evoked potential; NS, not significant. |
 | Table I.Amplitude and latency of flash-visual
evoked potential. |
Table I.
Amplitude and latency of flash-visual
evoked potential.
| A, Latency of N1-P1
waves, µV (n=10) |
|---|
|
|---|
| Timepoint | Sham ONC | NC-shRNA | Lingo-shRNA | P-value |
|---|
| Pre ONC | 43.97±6.93 | 45.37±4.35 | 47.33±2.99 | 0.662 |
| 2 weeks | 45.23±4.07 | 65.86±6.37 | 55.98±5.04 | 0.002 |
| 4 weeks | 49.23±3.69 | 59.03±3.94 | 50.88±6.45 | 0.0004 |
|
| B, Amplitude of
N1 waves, ms (n=10) |
|
| Timepoint | Sham ONC | NC-shRNA | Lingo-shRNA | P-value |
| Pre ONC | 32.7±2.14 | 32.37±2.53 | 33.43±2.99 | 0.554 |
| 2 weeks | 33.03±4.73 | 5.65±0.68 | 14.35±3.29 | 0.011 |
| 4 weeks | 36.27±7.81 | 5.27±3.56 | 17.06±2.89 | 0.002 |
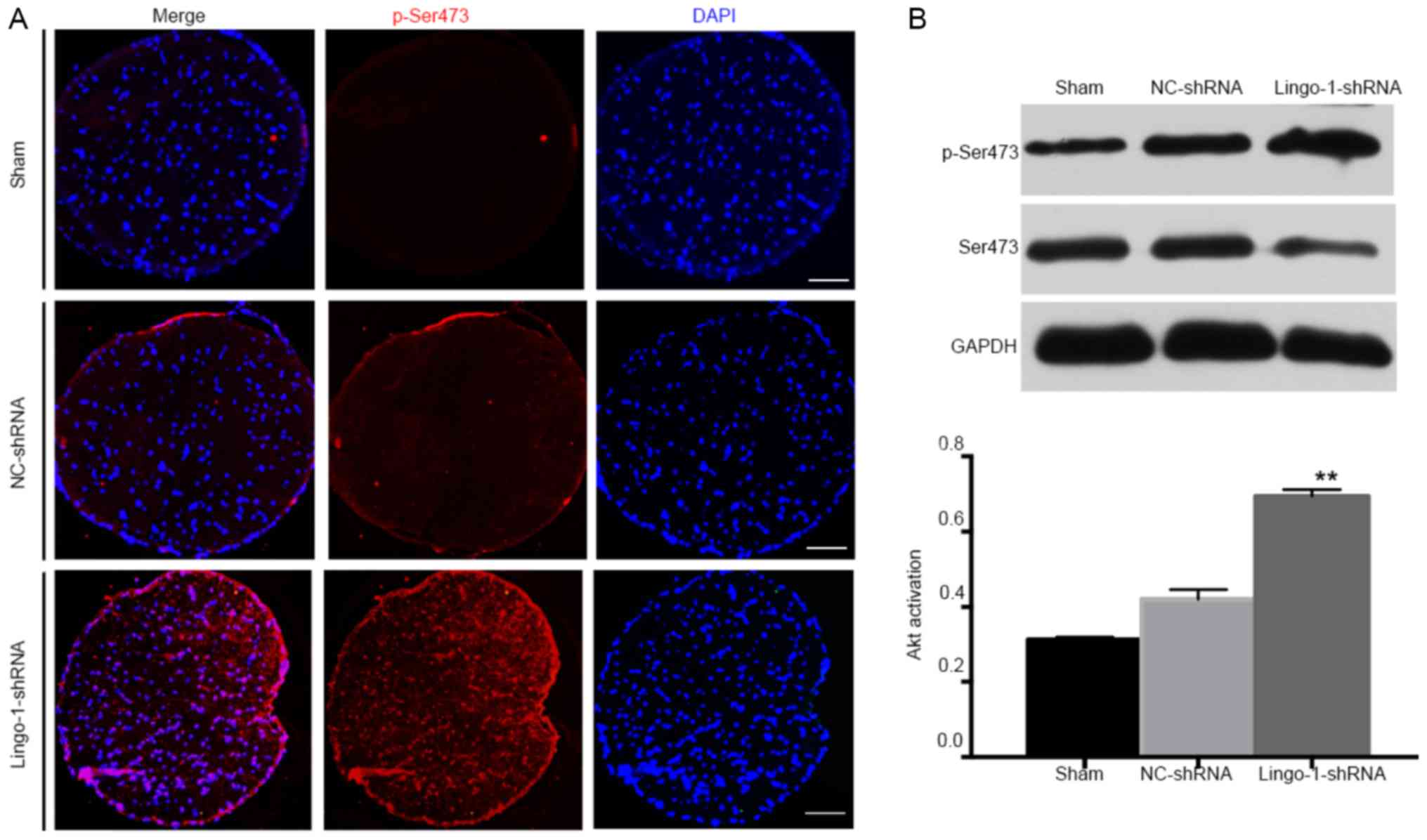
Knockdown of lingo-1 promotes Akt
activation in the ON after injury
To analyze the mechanisms underlying the
neuroprotective effects observed, the current study aimed to
determine whether the Akt signaling pathway was involved.
Phosphorylation of Akt is an important survival signal for neurons
and for RGCs after ON injuries (5,33,34).
Considering the neuroprotective role of Akt signaling (12,14,21), it
was hypothesized that inhibition of lingo-1 may promote Akt
activation. The effects of lingo-1-shRNA on Akt phosphorylation
were determined by measuring total Akt and pAkt (at Ser473) before
and after ONC.
pAkt expression in intact ON was very low and not
detectable by immunostaining (Fig.
7A). The difference of pAkt/Akt level between the 3 groups was
statistically insignificant before the injury and 2 weeks
post-operation (data not shown). However, 4 weeks after ONC,
western blotting revealed that there was a low level of Akt
phosphorylation in ON tissues in the sham and NC-shRNA groups
(Fig. 7B). p-Akt levels were very
low in sham ONs but increased 1.65-fold 4 weeks after ONC,
indicating that the neuroprotective activity may be mediated
through partial Akt phosphorylation at Ser473 in response to
lingo-1 silencing.
Discussion
The current study examined the strategy of
delivering lingo-1 shRNA vectors for ON injury repair. Knockdown of
lingo-1 significantly promoted functional recovery and increased
RGCs survival, providing neuroprotection through the activation of
Akt signaling in the lesioned ONs. The current study indicated that
intravitreal delivery of lingo-1 shRNA vectors may be an efficient
and effective approach for the treatment of optic neuropathy.
Delivering lingo-1 shRNA vectors into rats subjected
to ONC allowed for the evaluation of whether lingo-1 shRNA may
promote RGC survival after ONC in vivo. The ON is a part of
the CNS, injury of which is difficult to regenerate. The ON is
composed of RGC axons, injury of which may lead to permanent vision
loss. The anterograde ON damage causes the death of a large number
of RGCs (5). Furthermore, gradual
axonal degeneration following ON injury causes the death of RGCs,
which ultimately leads to irreversible loss of visual function
(4,22,27,28,34,35).
Therefore, promoting the survival of injured RGCs is crucial to the
treatment of optic neuropathy. In the current study, RGCs
transfected with lingo-1 shRNA vectors were administered
intravitreally to knock down lingo-1 expression in the ON. The
downregulation of lingo-1 protein expression confirmed successful
delivery of AAV in vivo.
RGC function in animal models of ONC was quantified
using the overall F-VEP response method, which allows for the
detection of changes in the anterograde degeneration in glaucoma
and optic neurotrauma. The N1 latency strongly reflects the
function of nerve impulse conduction and myelin sheath integrity
(28,34). The N1 amplitude demonstrates the
receptive function of RGCs and the number of synaptic contacts
between functional axons and their targets in V1 cortex (4,36).
Injury or degeneration of the ON leads to latency delay and
amplitude decrease of N1 waves to varying degrees (37). In the current study, following ONC,
F-VEP measurements revealed that rats treated with lingo-1 shRNA
exhibited a higher amplitude and a shorter latency of N1 waves
compared with the NC-shRNA group, indicating a protection on visual
function. There results indicate that lingo-1 negatively regulated
RGC survival and damage-resistance ability of axons, and postponed
the functional recovery of RGC after optic neuropathy.
In addition, as confirmed by HE staining, treatment
with lingo-1-shRNA significantly reduced the ON lesion volume,
which reflected the extent of tissue repair and contributed to
functional recovery of RGCs after ONC. The proximal ON damage
reduces the number of ON fibers in degenerative and traumatic
neuropathy (5,22,25,27,28). In
the current study, knockdown of lingo-1 decreased the extent of RGC
loss. Apart from the survival rate of RGCs, axon repair also serves
an important role in vision recovery following ON injury (12). In the current study, following ONC,
the injured area did not shrink around the lesion site. In NC shRNA
group, more axons survived were observed near the margin of
lesioned area, however, severe damage in the central area of the
ONs was observed 4 weeks post injury. In the current study,
lingo-1-shRNA application represented a meaningful approach to
enhance the capacity of RGC survival morphologically and
functionally, as evidenced by RGC quantification, assessment of the
cavity volume in ON and F-VEP measurement.
Lingo-1 is a CNS-specific membrane-associated
glycoprotein, which is known to be a potent inhibitor of neural
survival and axonal regeneration (15,20,38). In
animal models of Parkinson's disease, upregulation of lingo-1
coincided with decreased EGFR levels, suggesting that lingo-1 may
inhibit the EGFR/Akt signaling pathway in dopamine neurons
(12,14,15).
Anti-lingo-1 antibody underwent clinical trial in subjects with
relapsing remitting or secondary progressive multiple sclerosis
(clinicaltrials.gov; Identifier:
NCT01244139) (16). The role of
lingo-1 in neurodegeneration has been extensively studied, yet
caveats still remain in understanding the mechanism by which it
contributes to optic neuropathy.
Similar to other neurodegenerative diseases, in the
current study, lingo-1-shRNA protected against RGC death and axon
loss after ONC (15,39). These results indicated that lingo-1
inhibition may be efficient in promoting functional recovery of
axons after ON injury. Due to the tetramer structure burying a
large area into the cell membrane, it has been hypothesized that
lingo-1 could function at the sites of neuronal pathways to
terminate axon growth (15,39). In neurons, lingo-1 normally inhibits
the elongation and axonal growth (9,12).
Therefore, knocking down lingo-1 expression may maintain structural
and functional integrity of RGCs. It has been reported that
blocking lingo-1 function with lingo-1 antagonists may protect
cells from apoptosis via inhibition of RhoA activation, and enhance
neuronal survival through activation of the PI3K/Akt pathways in
chronic glaucoma and acute ON transaction models (5,14,40).
Furthermore, the neuroprotective activity through activation of the
Akt intracellular signals is independent of RhoA (33,41). The
phosphorylation of Akt at Ser473 and Thr-308 could be independently
regulated in different biological activities (33,38,42).
Thus, phosphorylation of Ser473 did not require concomitant
phosphorylation of Thr-308 by PI3K during neural injury (43). In the current study, the
phosphorylation of Akt at Ser473 was elevated after lingo-1-shRNA
treatment, and this result is consistent with a previous study
(15). Upregulation of p-Ser473 was
also observed in vivo in an animal model of Parkinson's
disease in lingo-1 knockout mice (15). Therefore, inhibition of lingo-1
appears to function through mechanisms similar to those of other
neuroprotective factors, such as BDNF (43) and erythropoietin (13) which rescue RGCs after axotomy by
activating Akt via Ser473 phosphorylation. In the current study,
the activation of Akt through Ser473 phosphorylation in response to
lingo-1 silencing not only enhanced RGC survival but also preserved
axon integrity. The present study had certain limitations and
whether the transportation of p-AKT from lesioned axons to RGC soma
remains to be determined.
In conclusion, the present study provided evidence
that lingo-1 is a negative regulator of the survival and integrity
maintenance of RGCs. The delivery of lingo-1-shRNA appears to be a
promising potential strategy for enhancing RGC survival for
individuals with traumatic or glaucomatous neurodegeneration. The
influence of lingo-1 on Akt signaling was confirmed, however, the
exact mechanism underlying these changes requires further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Guangdong
Innovative Research Team Program (grant no. 2015A030312016) and
National Natural Science Foundation of China (grant no.
81870655).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors approved the final version of the
manuscript. YQ, KW and MY conceived and designed the experiments.
YQ, YW, ZZ, XC, YY and KW performed the experiments. YQ and YW
analyzed the data. YQ and MY wrote the manuscript.
Ethics approval and consent to
participate
All experimental protocols and the ethical care of
the rats were reviewed and approved by the Institutional Animal
Care and Use Committee of the Zhongshan Ophthalmic Center, Sun
Yat-sen University (approval no. 2016187).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bourne RR, Stevens GA, White RA, Smith JL,
Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, et
al: Causes of vision loss worldwide, 1990–2010: A systematic
analysis. Lancet Glob Health. 1:e339–e349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonas JB, Aung T, Bourne RR, Bron AM,
Ritch R and Panda-Jonas S: Glaucoma. Lancet. 390:2183–2193. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stevens GA, White RA, Flaxman SR, Price H,
Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Resnikoff S,
et al: Global prevalence of vision impairment and blindness:
Magnitude and temporal trends, 1990–2010. Ophthalmology.
120:2377–2384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dhande OS, Stafford BK, Lim JA and
Huberman AD: Contributions of retinal ganglion cells to subcortical
visual processing and behaviors. Annu Rev Vis Sci. 1:291–328. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benowitz LI, He Z and Goldberg JL:
Reaching the brain: Advances in optic nerve regeneration. Exp
Neurol. 287:365–373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park KK, Liu K, Hu Y, Smith PD, Wang C,
Cai B, Xu B, Connolly L, Kramvis I, Sahin M and He Z: Promoting
axon regeneration in the adult CNS by modulation of the PTEN/mTOR
pathway. Science. 322:963–966. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim JH, Stafford BK, Nguyen PL, Lien BV,
Wang C, Zukor K, He Z and Huberman AD: Neural activity promotes
long-distance, target-specific regeneration of adult retinal axons.
Nat Neurosci. 19:1073–1084. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu K, Tedeschi A, Park KK and He Z:
Neuronal intrinsic mechanisms of axon regeneration. Annu Rev
Neurosci. 34:131–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andrews JL and Fernandez-Enright F: A
decade from discovery to therapy: Lingo-1, the dark horse in
neurological and psychiatric disorders. Neurosci Biobehav Rev.
56:97–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mi S, Lee X, Shao Z, Thill G, Ji B, Relton
J, Levesque M, Allaire N, Perrin S, Sands B, et al: LINGO-1 is a
component of the Nogo-66 receptor/p75 signaling complex. Nat
Neurosci. 7:221–228. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mi S, Miller RH, Lee X, Scott ML,
Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M,
et al: LINGO-1 negatively regulates myelination by
oligodendrocytes. Nat Neurosci. 8:745–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mi S, Pepinsky RB and Cadavid D: Blocking
LINGO-1 as a therapy to promote CNS repair: From concept to the
clinic. CNS Drugs. 27:493–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rex TS, Allocca M, Domenici L, Surace EM,
Maguire AM, Lyubarsky A, Cellerino A, Bennett J and Auricchio A:
Systemic but not intraocular Epo gene transfer protects the retina
from light-and genetic-induced degeneration. Mol Ther. 10:855–861.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu QL, Hu B, Wu W, Pepinsky RB, Mi S and
So KF: Blocking LINGO-1 function promotes retinal ganglion cell
survival following ocular hypertension and optic nerve transection.
Invest Ophthalmol Vis Sci. 49:975–985. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue H, Lin L, Lee X, Shao Z, Mendes S,
Snodgrass-Belt P, Sweigard H, Engber T, Pepinsky B, Yang L, et al:
Inhibition of the leucine-rich repeat protein LINGO-1 enhances
survival, structure, and function of dopaminergic neurons in
Parkinson's disease models. Proc Natl Acad Sci USA.
104:14430–14435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu QL, Hu B, Li X, Shao Z, Shi JB, Wu W,
So KF and Mi S: LINGO-1 negatively regulates TrkB phosphorylation
after ocular hypertension. Eur J Neurosci. 31:1091–1097. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu QL, Li X, Yip HK, Shao Z, Wu W, Mi S
and So KF: Combined effect of brain-derived neurotrophic factor and
LINGO-1 fusion protein on long-term survival of retinal ganglion
cells in chronic glaucoma. Neuroscience. 162:375–382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwon HS, Nakaya N, Abu-Asab M, Kim HS and
Tomarev SI: Myocilin is involved in NgR1/Lingo-1-mediated
oligodendrocyte differentiation and myelination of the optic nerve.
J Neurosci. 34:5539–5551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen N, Cen JS, Wang J, Qin G, Long L,
Wang L, Wei F, Xiang Q, Deng DY and Wan Y: Targeted inhibition of
leucine-rich repeat and immunoglobulin domain-containing protein 1
in transplanted neural stem cells promotes neuronal differentiation
and functional recovery in rats subjected to spinal cord injury.
Crit Care Med. 44:e146–e157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mi S, Hu B, Hahm K, Luo Y, Kam Hui ES,
Yuan Q, Wong WM, Wang L, Su H, Chu TH, et al: LINGO-1 antagonist
promotes spinal cord remyelination and axonal integrity in
MOG-induced experimental autoimmune encephalomyelitis. Nat Med.
13:1228–1233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Andereggen L, Yuki K, Omura K, Yin
Y, Gilbert HY, Erdogan B, Asdourian MS, Shrock C, de Lima S, et al:
Mobile zinc increases rapidly in the retina after optic nerve
injury and regulates ganglion cell survival and optic nerve
regeneration. Proc Natl Acad Sci USA. 114:E209–E218. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qu J, Wang D and Grosskreutz CL:
Mechanisms of retinal ganglion cell injury and defense in glaucoma.
Exp Eye Res. 91:48–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaw PX, Sang A, Wang Y, Ho D, Douglas C,
Dia L and Goldberg JL: Topical administration of a Rock/Net
inhibitor promotes retinal ganglion cell survival and axon
regeneration after optic nerve injury. Exp Eye Res. 158:33–42.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trost A, Bruckner D, Kaser-Eichberger A,
Motloch K, Bogner B, Runge C, Strohmaier C, Couillard-Despres S,
Reitsamer HA and Schroedl F: Lymphatic and vascular markers in an
optic nerve crush model in rat. Exp Eye Res. 159:30–39. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McKinnon SJ, Schlamp CL and Nickells RW:
Mouse models of retinal ganglion cell death and glaucoma. Exp Eye
Res. 88:816–824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu HF, Cen JS, Zhong Q, Chen L, Wang J,
Deng DY and Wan Y: The promotion of functional recovery and nerve
regeneration after spinal cord injury by lentiviral vectors
encoding Lingo-1 shRNA delivered by Pluronic F-127. Biomaterials.
34:1686–1700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huberman AD, Manu M, Koch SM, Susman MW,
Lutz AB, Ullian EM, Baccus SA and Barres BA: Architecture and
activity-mediated refinement of axonal projections from a mosaic of
genetically identified retinal ganglion cells. Neuron. 59:425–438.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pernet V and Schwab ME: Lost in the
jungle: New hurdles for optic nerve axon regeneration. Trends
Neurosci. 37:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cen LP, Liang JJ, Chen JH, Harvey AR, Ng
TK, Zhang M, Pang CP, Cui Q and Fan YM: AAV-mediated transfer of
RhoA shRNA and CNTF promotes retinal ganglion cell survival and
axon regeneration. Neuroscience. 343:472–482. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang R, Sun Q, Xia F, Chen Z, Wu J, Zhang
Y, Xu J and Liu L: Methane rescues retinal ganglion cells and
limits retinal mitochondrial dysfunction following optic nerve
crush. Exp Eye Res. 159:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rodriguez AR, de Sevilla Müller LP and
Brecha NC: The RNA binding protein RBPMS is a selective marker of
ganglion cells in the mammalian retina. J Comp Neurol.
522:1411–1443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahn JY: Neuroprotection signaling of
nuclear akt in neuronal cells. Exp Neurobiol. 23:200–206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crair MC and Mason CA: Reconnecting eye to
brain. J Neurosci. 36:10707–10722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kwon OJ, Lee ES and Jeon CJ: Density and
types of calretinin-containing retinal ganglion cells in rabbit.
Neuroscience. 278:343–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Groh A, Meyer HS, Schmidt EF, Heintz N,
Sakmann B and Krieger P: Cell-type specific properties of pyramidal
neurons in neocortex underlying a layout that is modifiable
depending on the cortical area. Cereb Cortex. 20:826–836. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gennarelli TA, Thibault LE, Tipperman R,
Tomei G, Sergot R, Brown M, Maxwell WL, Graham DI, Adams JH, Irvine
A, et al: Axonal injury in the optic nerve: A model simulating
diffuse axonal injury in the brain. J Neurosurg. 71:244–253. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Sapolsky RM and Steinberg GK:
Phosphoinositide- 3-kinase/akt survival signal pathways are
implicated in neuronal survival after stroke. Mol Neurobiol.
34:249–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mosyak L, Wood A, Dwyer B, Buddha M,
Johnson M, Aulabaugh A, Zhong X, Presman E, Benard S, Kelleher K,
et al: The structure of the Lingo-1 ectodomain, a module implicated
in central nervous system repair inhibition. J Biol Chem.
281:36378–36390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bei F, Lee HHC, Liu X, Gunner G, Jin H, Ma
L, Wang C, Hou L, Hensch TK, Frank E, et al: Restoration of visual
function by enhancing conduction in regenerated axons. Cell.
164:219–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wilson AM and Di Polo A: Gene therapy for
retinal ganglion cell neuroprotection in glaucoma. Gene Ther.
19:127–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pezet S, Spyropoulos A, Williams RJ and
McMahon SB: Activity-dependent phosphorylation of Akt/PKB in adult
DRG neurons. Eur J Neurosci. 21:1785–1797. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alessi DR, Andjelkovic M, Caudwell B, Cron
P, Morrice N, Cohen P and Hemmings BA: Mechanism of activation of
protein kinase B by insulin and IGF-1. EMBO J. 15:6541–6551. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martin KRG, Quigley HA, Zack DJ,
Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease
ME, Klein RL and Hauswirth WW: Gene therapy with brain-derived
neurotrophic factor as a protection: Retinal ganglion cells in a
rat glaucoma model. Invest Ophthalmol Vis Sci. 44:4357–4365. 2003.
View Article : Google Scholar : PubMed/NCBI
|