Introduction
Urinary tract infections (UTIs) are among the most
common bacterial infections acquired in the general population and
in hospitals (1). A variety of
pathogens, including bacteria, fungi, mycoplasma, chlamydia and
viruses, may cause UTIs, which are mainly characterized by painful,
frequent and urgent urination, as well as urethral burning
(2). In the USA, >7 million
outpatients and ~1 million in-patients with UTIs are encountered in
the clinic each year (3), while ~150
million patients are diagnosed with UTIs worldwide each year
(4). UTIs, which may cause septic
shock, rank third among all diseases that may lead to death as a
result of infection (5).
Gram-negative bacteria are the major cause of community-acquired
and hospital-acquired UTIs (4,6). At
present, antibiotics are the major treatment for UTIs, but not all
patients benefit from them. Abuse of antibiotics markedly increases
the drug resistance of bacteria, reduces the clinical efficacy of
antibiotics and increases the recurrence rate of bacterial
infection, which wastes medical resources and reduces the quality
of life of patients (7). Multiple
Chinese traditional medicines have been demonstrated to exert
bacteriostatic effects on pathogenic microorganisms, and may
therefore inhibit or destroy the formation of toxic substances
(8,9). A test of antibacterial properties in
mice revealed that Sanjin tablet (SJTs) has a marked bacteriostatic
effects (10).
SJT is composed of five types of Chinese herbal
medicines: Baqia (Chinaroot Greenbrier Rhizome), Jinyinggen (Root
of Cherokee Rose), Yangkaikou (Fruit of Fiverleaf Akebia),
Jinshateng (Lygodii Herba) and Jixuecao (Asiatic Pennywort Herb),
which were recorded in the Chinese Pharmacopoeia 2015 (11). According to the concepts of Chinese
Traditional Medicine, the herbal components have the following
properties: Jinyinggen is acerb and neutral in nature, and is able
to control nocturnal emission and astringent intestine (12). Baqia is bitter and neutral in nature;
it is able to alleviate rheumatism, promote blood circulation,
detoxicate, relieve convulsion and calm endogenous wind (13). Jinshateng is slightly sweet and
cold-natured, and is able to clear heat, detoxicate and remove
dampness (14). These three herbs
are monarch drugs (major components) in the prescription, which
have an enhancing effect on the functions of anti-inflammation,
dehumidification and detoxification (15). The above three drugs supplemented
with Yangkaikou and Jixuecao exhibit enhanced effects against UTIs
(16,17).
Previous clinical studies have demonstrated that
SJTs are able to reduce the symptoms of chronic UTIs, the number of
recurrences and the secretory level of urinary soluble interleukin
(IL)-2 receptor, IL-6 and IL-8 in patients with chronic
nephropyelitis (10). Electron
microscopy revealed that SJTs is able to make the flagella of
Escherichia coli drop (18).
Lower UTIs mainly comprise cystitis and urethritis, which have high
incidence and recurrence rates. In recent years, numerous clinical
studies have explored the efficacy and safety of SJTs combined with
antibiotics in the treatment of acute lower UTIs (ALUTIs) in China.
However, there is no meta-analysis on SJTs combined with
antibiotics for the treatment of ALUTIs. To the best of our
knowledge, the present study is the first comprehensive systematic
review that determined the efficacy and safety of SJTs combined
with antibiotics for the treatment of ALUTIs. The Grading of
Recommendations, Assessment, Development and Evaluations (GRADE)
system (19,20) was used to evaluate the quality of
evidence of the key outcomes of the present meta-analysis, which
provides a basis and serves as a reference for clinical practice
guidance.
Materials and methods
Search strategy
Electronic databases, including PubMed, EMBASE,
Cochrane Library, Web of Science, China National Knowledge
Infrastructure, Chinese BioMedical Database, WanFang Database and
VIP Database (VIP) were systematically searched for entries added
between inception and December 2018. The following search terms
were used separately or in combination: ‘Sanjin’ or ‘Sanjin tablet’
AND ‘acute lower urinary tract infection’ or ‘acute lower UTI’.
Selection criteria
Studies were selected according to the following
inclusion criteria: i) Participants were diagnosed with ALUTIs; ii)
the study was performed as a randomized controlled trial (RCT);
iii) efficacy of SJT combined with antibiotics vs. antibiotics,
including levofloxacin tablets (LTs), gatifloxacin tablets (GTs)
and ofloxacin tablets (OTs); iv) primary outcomes were the cure
rate (i.e. the symptoms disappeared and the leukocyte levels in the
urine returned to normal after treatment) and the recurrence rate
(i.e. the symptoms of the patients reappeared or their urine
leukocyte value increased again); and v) secondary outcomes
included the total effective rate (i.e. the symptoms partially
disappeared or the value of urine leukocytes was reduced but did
not return to normal after treatment), bacterial clearance rate
(the original infected part of the specimen did not regenerate
after treatment), incidence of adverse reactions (ADRs) and any
adverse events (ADEs), including headache, stomach ache, stomach
discomfort, mild nausea, skin rash and dizziness.
The exclusion criteria were as follows: i)
Insufficient data (miscalculation or missing data); ii) the full
text was not available; iii) duplicated data; and iv) the
intervention included other Chinese drugs, acupuncture and massage
(including a proprietary Chinese drug, Traditional Chinese Medicine
extract injection, decoction, auricular points and other
Traditional Chinese Medicine methods as auxiliary treatment).
Literature screening
EndNote (v. 8.1.11010; Clarivate Analytics) was used
to identify duplicates among the studies retrieved. After reading
the titles and abstracts of the studies obtained for preliminary
screening, those articles that did not meet the inclusion criteria
were excluded. The full text of the articles that potentially met
the inclusion criteria was further screened to determine whether
they should be included in the present study. The list of
references of the studies retrieved were also checked to identify
any further studies. The literature was independently screened by
two researchers (JL and MS) according to the inclusion/exclusion
criteria. Any disagreement between the reviewers was resolved by
consulting a third party (LW and YX).
Data extraction
Two independent researchers, namely JL and MS, were
responsible for data extraction and any disagreements were resolved
by a third author (YX). The number of events and the total number
of patients in each group were extracted from binary outcomes. The
mean, standard deviation and sample size for each group were
extracted or inputted from continuous outcomes. The data extracted
included the following: Name of the first author, year of
publication, method of randomization, number of patients, sex and
age in the comparison groups, as well as the total number of
patients, the drug dose and duration of treatment in the comparison
groups, primary and secondary outcomes, and any ADEs or ADRs. When
the study had ≥1 common intervention group, the method recommended
by the Cochrane Collaboration was followed, i.e. grouping and
merging, and conversion of the multi-arm trial into a 2-arm trial
(21).
Quality assessment
The Cochrane Handbook for Systematic Reviews of
Interventions 5.1 ‘bias risk assessment’ tool was used to assess
quality in seven domains: Random sequence generation; allocation
concealment; blinding of participants and personnel; blinding of
outcome assessment; incomplete outcome data; selective outcome
reporting; and other bias (21). The
risk of bias was classified as low, high or unclear. JL and CZ were
responsible for independent assessment of quality and any
disagreements were resolved by a third author (YX).
Statistical analysis
RevMan 5.3, provided by the Cochrane Collaboration
Network, was used for meta-analysis. The relative ratio (RR) and
95% CI were used for binary outcomes and the weighted mean
difference and 95% CIs were used for continuous outcomes. The
I2 statistic was used to assess heterogeneity; if
I2<50%, the statistical homogeneity was considered to
be acceptable and the fixed-effects model was used, and if
I2≥50%, a significant statistical heterogeneity was
assumed and the random-effects model was adopted. If the study was
not suitable for meta-analysis, a descriptive analysis was
performed. A funnel plot was used to detect publication bias.
Subgroup and sensitivity analyses
In order to address heterogeneity, subgroup analysis
was performed, which focused on the cure rate of the different
interventions. Interventions included SJTs combined with GTs vs.
GTs and SJTs combined with LTs vs. LTs. Sensitivity analysis was
performed to assess the influence of a single study on the overall
pooled estimate by removing one study at a time.
GRADE assessment
The GRADE system was used to classify the quality of
evidence of the cure rate, total effective rate, recurrence rate
and incidence of ADRs. According to the GRADE classification
method, the RCTs are initially classified as studies with the
highest quality of evidence and their quality was then decreased
based on 5 factors (risk of bias, inconsistency, indirectness,
imprecision and publication bias), and the quality of the final
evidence was classified as high, moderate, low and very low
(19,20).
Results
Literature search results
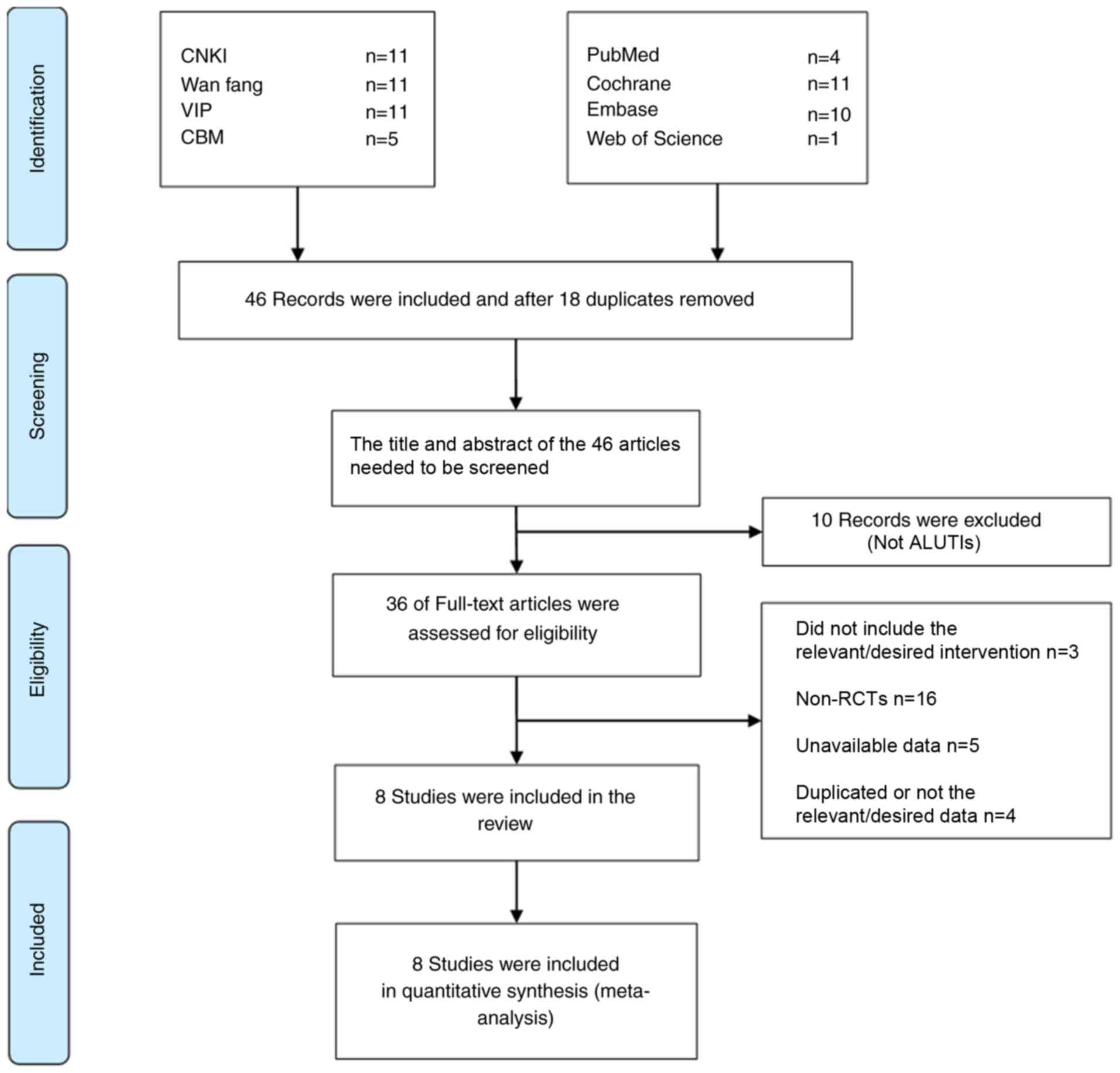
The initial literature search identified 64 studies.
After duplicates among different databases were removed by using
EndNote, the title and abstract of the studies were read, and the
inclusion and exclusion criteria were combined, and 36 articles
were selected for evaluation of their full text. A total of 28
articles were excluded for not being RCTs (n=16), not correctly
performing intervention measures (n=3), unavailability of data
(n=5) and containing duplicate or incorrect data (n=4). A total of
8 trials were eventually selected for inclusion in the present
meta-analysis, all of which were published in Chinese (22–29). The
selection process of the studies is presented in Fig. 1.
Characteristics of the studies
included
A total of 8 studies were included in the present
meta-analysis, all of which described that the baseline values of
the experimental and control groups were comparable. All patients
included in the present meta-analysis underwent a urine culture
test at the time-point of diagnosis. After treatment, a urine
culture test was performed to determine the curative effect. A
change in urine bacterial culture for the same strain from positive
to negative was considered to indicate cure. In total, 3 studies
were 3-arm trials (22,23,28), and
2 of them were double-blinded and double-simulated studies
(22,23). After grouping and combining these
3-arm trials, the interventions were as follows: SJTs vs.
antibiotics and SJTs combined with antibiotics vs. antibiotics. The
interventions of the other 5 studies were SJTs combined with
antibiotics vs. antibiotics. Other conventional and adjuvant
treatments were not mentioned in any of the studies. The total
sample size was 790 cases, including 405 cases in the experimental
group and 385 cases in the control group. The average daily dose of
SJTs was 12 pills (3.48 g). In 5 studies, patients received
treatment for 7 days, while in other studies, patients received
treatment for 3 (27), 5 (17) and 3–15 days (26). A total of 7 trials reported on
ADEs/ADRs. The characteristics of the 8 trials included are listed
in Table I.
 | Table I.Characteristics of trials
included. |
Table I.
Characteristics of trials
included.
|
| Sample size |
|
| Treatment group |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| First author
(year) | T | C | Total | Sex (M/F) | Age (years) | Dose of
SJTs/tablet | Combined
treatment | Control group | Duration
(days) | Outcomes | (Refs.) |
|---|
| Liu
(2017)a | 72 | 72 | 144 | T:28/42 | T:20 to 74
(39.70±14.37) | 12 | SJP + LT | SJP placebo + LT
0.4g | 7 | b–f | (22) |
|
|
|
|
| C:27/44 | C:18 to 73
(39.14±15.82) |
| placebo 0.4g |
|
|
|
|
|
| 72 | 72 | 144 | T:22/50 | T:18 to 75
(35.90±14.14) | 12 | SJT + LT 0.4g | SJP placebo + LT
0.4g |
|
|
|
|
|
|
|
| C:27/44 | C:18 to 73
(39.14±15.82) |
|
|
|
|
|
|
| Lyu
(2015)a | 71 | 72 | 143 | T:29/42 | T:(39.00) | 12 | SJP + LT | SJP placebo + LT
0.4g | 7 | b,f | (23) |
|
|
|
|
| C:27/45 | C:(39.00) |
| placebo 0.4g |
|
|
|
|
|
| 72 | 72 | 144 | T:22/50 | T:(35.00) | 12 | SJT + LT 0.4g | SJP placebo + LT
0.4g |
|
|
|
|
|
|
|
| C:27/45 | C:(39.00) |
|
|
|
|
|
|
| Hu (2014) | 40 | 40 | 80 | T:0/40 | 20 to 50
(35.40±2.70) | 15 | SJP + GT 0.4g | GT 0.4g | 7 | b,f | (24) |
|
|
|
|
| C:0/40 |
|
|
|
|
|
|
|
| Zheng (2013) | 38 | 38 | 76 | T:12/26 | T:20 to 58
(30.80±5.70) | 9 | SJP + GT 0.4g | GT 0.4g | 5 | b–g | (17) |
|
|
|
|
| C:14/24 | C:21 to 60
(31.30±4.90) |
|
|
|
|
|
|
| Wang (2011) | 28 | 28 | 56 | T:0/28 | T:20 to 49
(33.00±6.23) | 15 | SJP + GT 0.4g | GT 0.4g | 7 | b,f | (25) |
|
|
|
|
| C:0/28 | C:18 to 47
(32.00±5.46) |
|
|
|
|
|
|
| Tu (2011) | 40 | 40 | 80 | T:7/33 | T:16 to 50
(46.10) | 12 | SJP + OT 0.4g | OT 0.4g | 3–15 | b,c | (26) |
|
|
|
|
| C:7/33 | C:16 to 50
(48.10) |
|
|
|
|
|
|
| Qiu (2009) | 80 | 60 | 140 | T:0/80 | T:22 to 63
(33.70±15.90) | 12 | SJP + LT 0.4g | LT 0.4g | 3 | b,c,e,f | (27) |
|
|
|
|
| C:0/60 | C:- |
|
|
|
|
|
|
| aMei (2008) | 35 | 35 | 70 | T:13/22 | T:19 to 61
(47.00) | 12 | SJP | LT 0.4g | 7 | b,c,e,f,g | (28) |
|
|
|
|
| C:14/21 | C:20 to 59
(47.00) |
|
|
|
|
|
|
|
| 35 | 35 | 70 | T:15/20 | T:20 to 60
(43.00) | 12 | SJP + LT 0.4g | LT 0.4g |
|
|
|
|
|
|
|
| C:14/21 | C:20 to 59
(47.00) |
|
|
|
|
|
|
Methodological quality
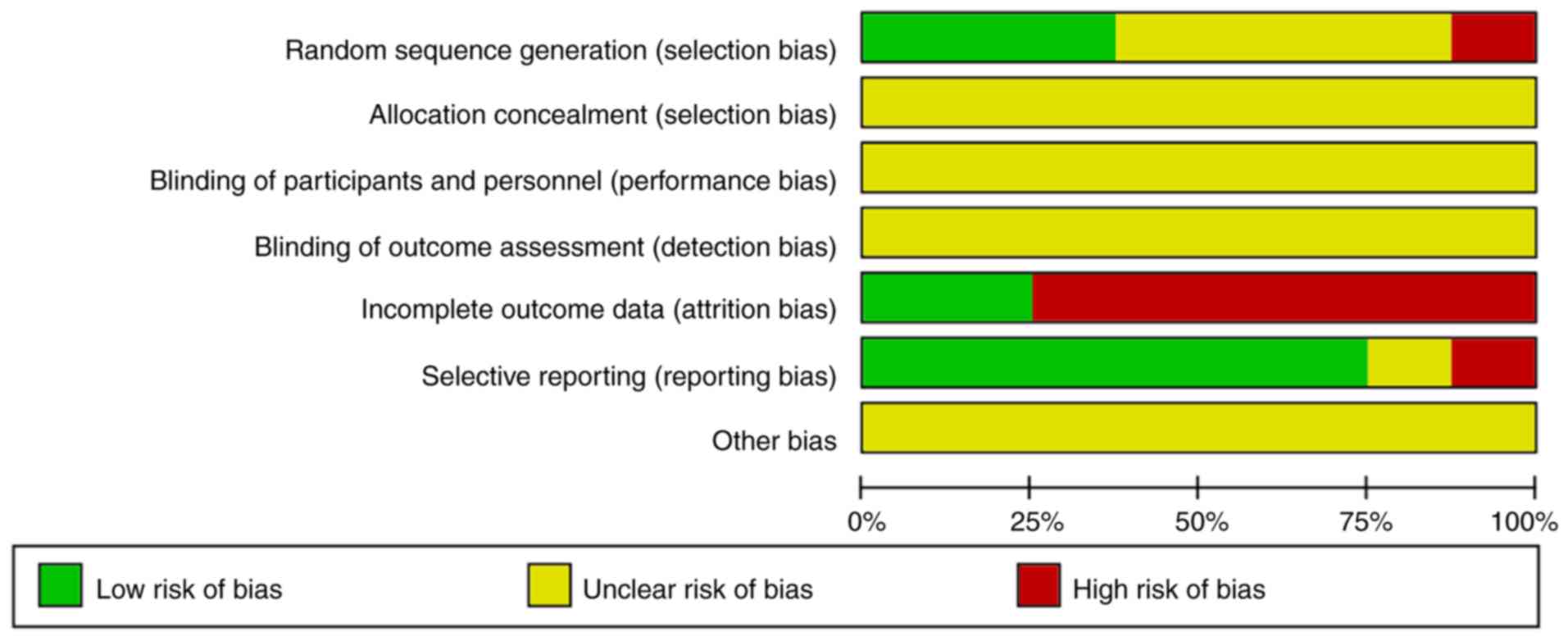
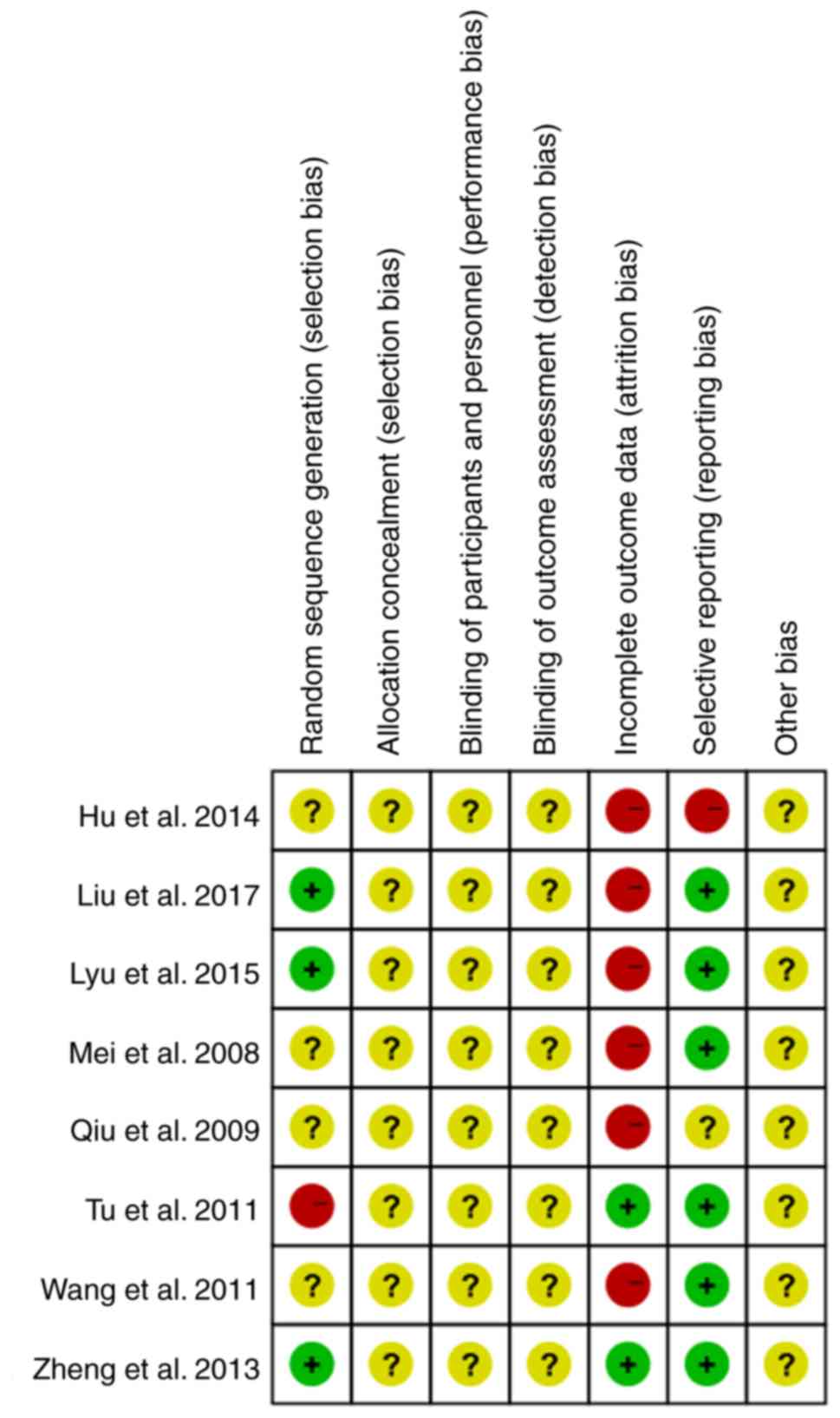
None of the 8 studies reported on the study
protocol, sample size estimates, randomization, blinding or
allocation concealment. A total of 6 studies mentioned that the
patients were followed up after treatment to evaluate recurrence,
but only 3 studies had followed up data (17,27,28). In
total, 6 studies reported on withdrawals and loss to follow-up, but
no intention analysis was performed (22–25,27,28). One
study did not fully report on pre-specified outcomes and exhibited
selective reporting of results (24). The results of the quality assessment
of the studies included are provided in Figs. 2 and 3.
Meta-analysis
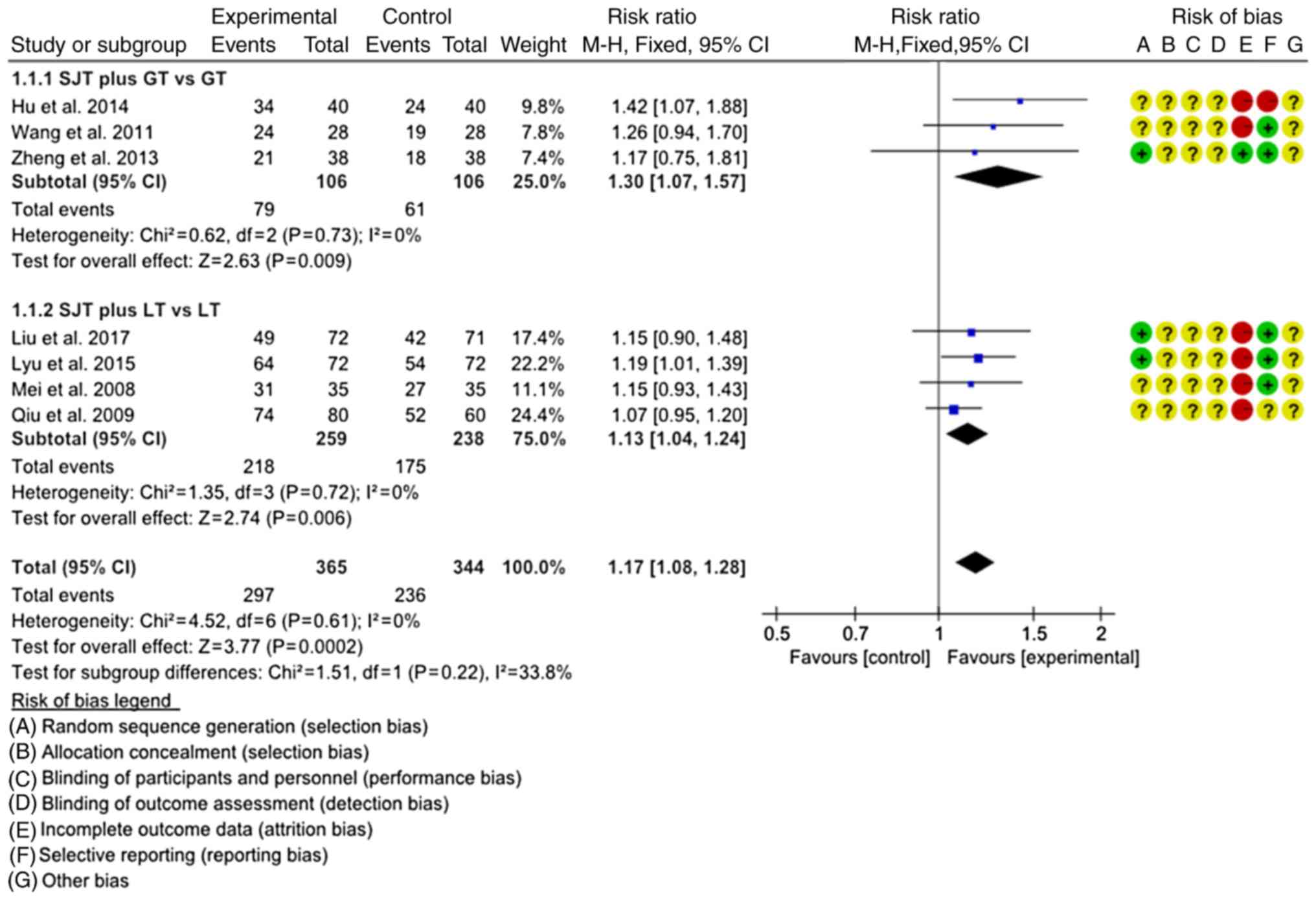
Cure rate
The cure rate was assessed for a total of 790
patients across all of the studies included. In total, 7 studies
(17,22–25,27,28) were
divided into 2 subgroups according to the different interventions
and the homogeneity was good in each subgroup (P=0.73/0.72,
I2=0). The fixed-effects model was used for the
meta-analysis. The results indicated that the cure rate of SJTs
combined with GTs was higher than that of GTs alone (RR=1.30, 95%
CI=1.07–1.57, P=0.009; Fig. 4), and
the cure rate of SJTs combined with LTs was higher than that of LTs
alone (RR=1.13, 95% CI=1.04–1.24, P=0.006; Fig. 4). One study (26) performed a descriptive analysis and
suggested that the cure rate of SJTs combined with OT was higher
than that of OT alone (RR=1.38, 95% CI=1.03–1.84, P<0.03).
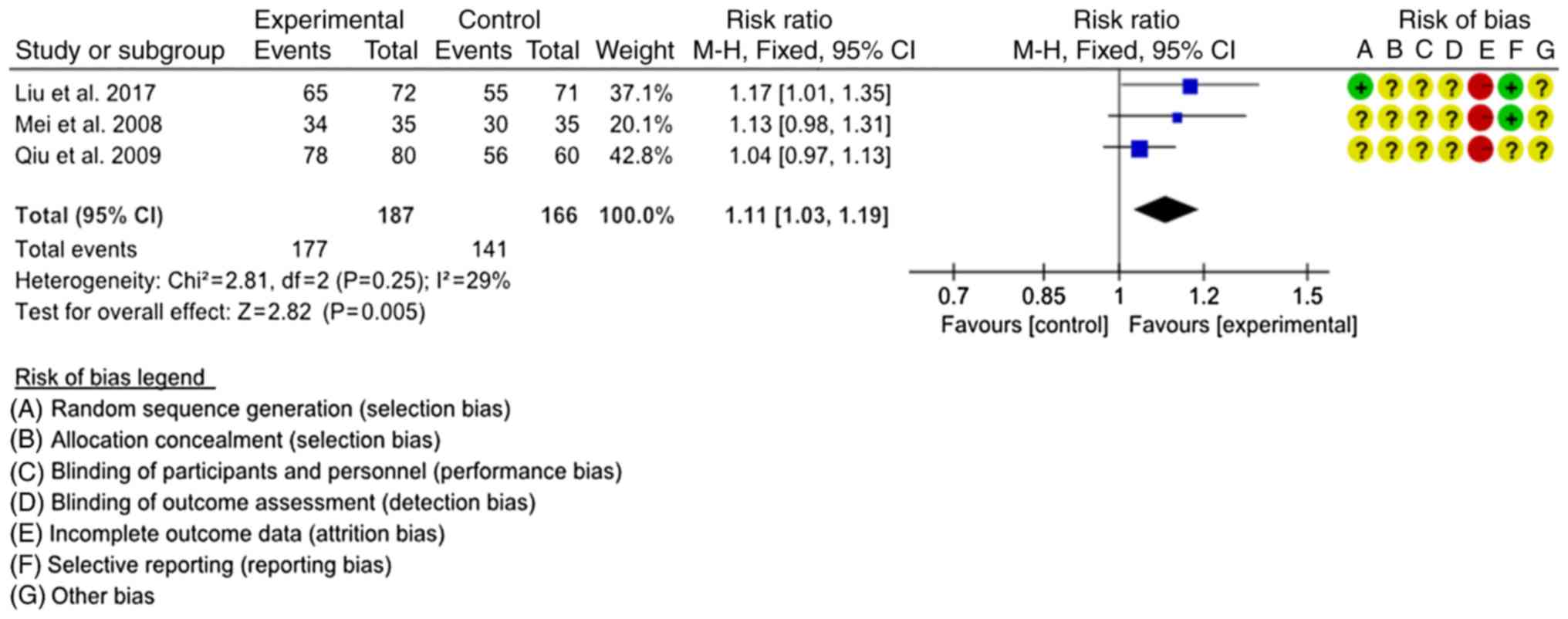
Total effective rate
A total of 510 patients from 5 studies were assessed
for the total effective rate. The intervention of 3 studies
consisted of SJTs combined with LTs vs. LTs alone (22,27,28). The
heterogeneity test indicated that the fixed-effects model was
appropriate for use (P=0.25, I2=29%). Meta-analysis
demonstrated that the total effective rate of SJTs combined with
LTs was higher than that of LTs alone (RR=1.11, 95% CI=1.03–1.19,
P=0.005; Fig. 5). Analysis of the
data of one study (17) suggested
that the effective rate of SJTs combined with GTs was higher than
that of GTs alone (RR=1.31, 95% CI=1.03–1.67, P=0.03). One study
(26) suggested that the total
effective rate of SJTs combined with OT and that of OTs alone was
not significantly different (RR=1.16, 95% CI=0.95–1.41;
P=0.14).
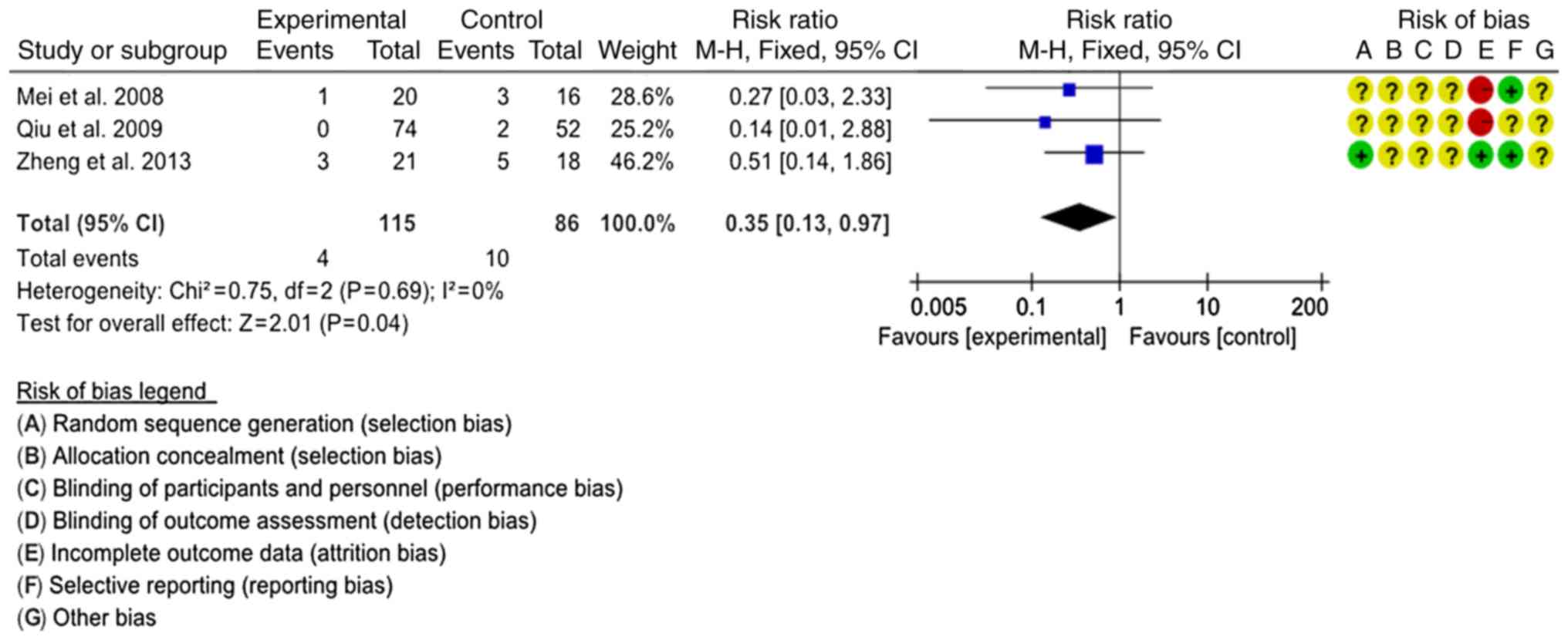
Recurrence rate
A total of 3 studies comprising 201 patients were
assessed regarding the recurrence rate (17,27,28). The
interventions were SJTs combined with GTs vs. GTs and SJTs combined
with LTs vs. LTs. In order to comprehensively evaluate the effect
of SJT combined with antibiotics, the 3 interventions were
classified as SJT combined with antibiotics vs. antibiotics alone
for combined analysis. The homogeneity of the 3 studies was good
(P=0.69, I2=0%) and the fixed-effects model was used for
the meta-analysis. Statistical analysis indicated that the
recurrence rate of SJT combined with antibiotics was lower than
that of antibiotics alone (RR=0.35, 95% CI=0.13–0.97, P=0.04;
Fig. 6).
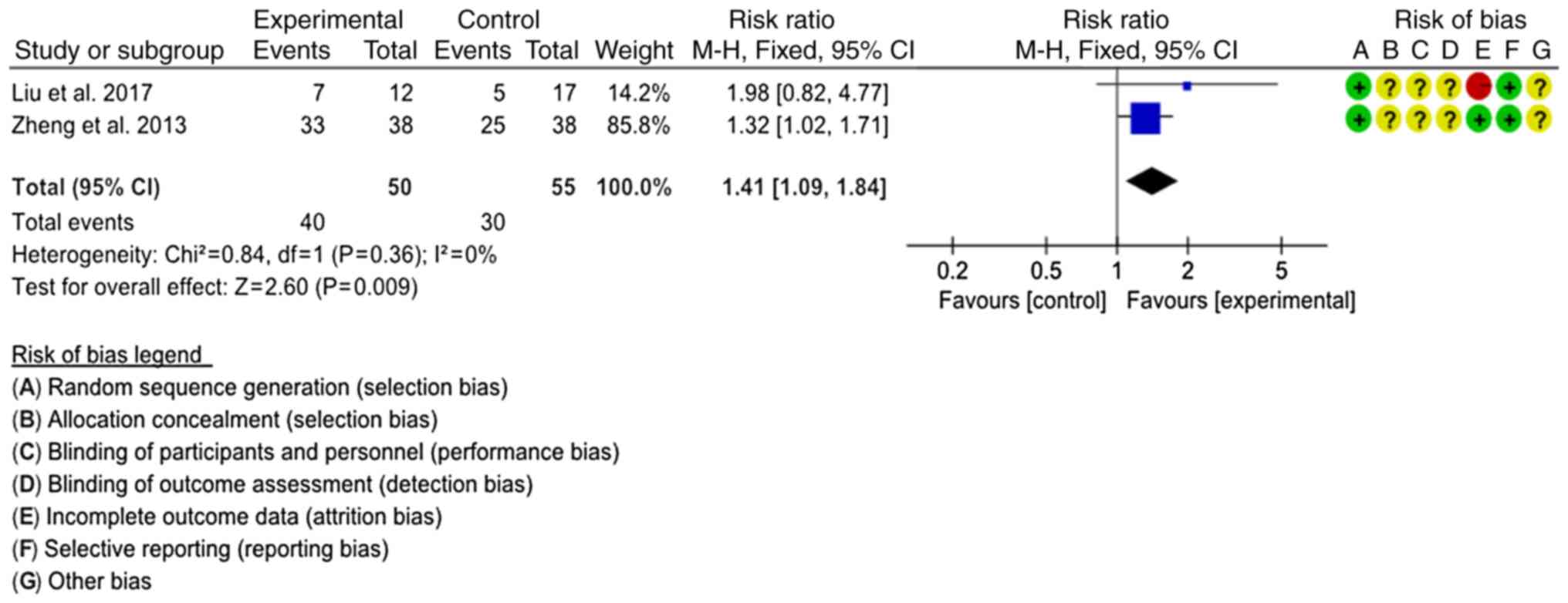
Bacterial clearance rate
Bacterial clearance rates were reported in two
studies (20,22). The interventions were SJTs combined
with LTs vs. LTs and SJTs combined with GTs vs. GTs, respectively.
The interventions were classified as SJT combined with antibiotics
vs. antibiotics to comprehensively evaluate the combined effect.
The fixed-effects model was used for analysis (P=0.36,
I2=0%), revealing that the bacterial clearance rate of
SJT combined with antibiotics was higher than that of antibiotics
alone (RR=1.41, 95% CI=1.09–1.84, P=0.009; Fig. 7).
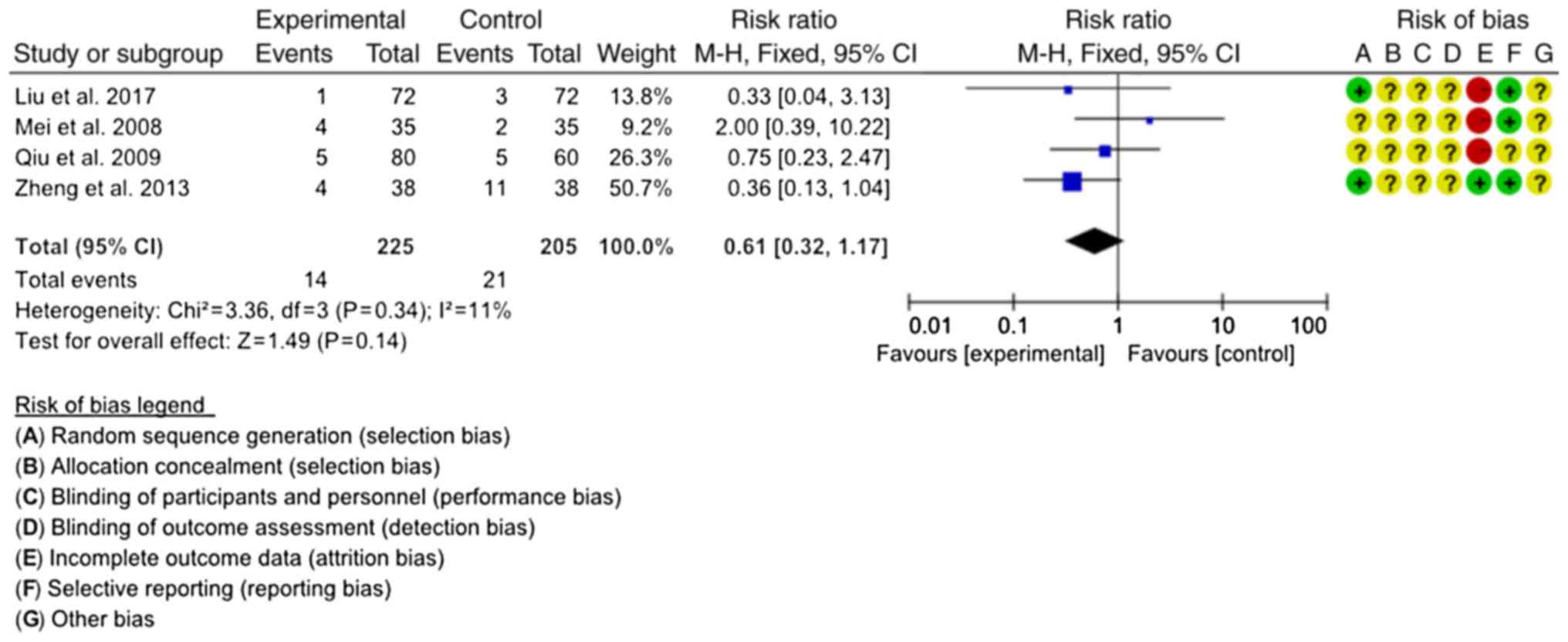
Incidence of ADRs
A total of four studies reported on the incidence of
ADRs (20,22,27,28). The
combined antibiotics in the four studies included LTs, GTs and OTs.
The homogeneity among the studies was good (P=0.34,
I2=11%), and the fixed-effects model was used for the
meta-analysis. The results demonstrated that there was no
significant difference in the incidence of ADRs between SJT
combined with antibiotics and antibiotics alone (RR=0.61, 95%
CI=0.32–1.17; P=0.14; Fig. 8).
ADRs/ADEs
In total, seven studies (710 cases) mentioned
observation regarding ADRs or ADEs and three studies (23–25)
reported positive results. A study (22) reported on one case of slight increase
in total bilirubin, one case of slight decrease in blood leukocytes
and one case of proneness to hunger in the SJT group, as well as
one case of headache, one case of stomach ache and one case of
elevated blood pressure in the LT group, and one case of thirst and
proneness to hunger in the combined group. Another study (17) reported two cases of nausea and
stomach discomfort, and two cases of mild diarrhea in the
experimental group, in addition to five cases of nausea and stomach
discomfort, three cases of loss of appetite and three cases of mild
dysuria in the control group. In addition, one study (27) reported five cases of mild nausea and
dizziness in the experimental and control groups, respectively.
Another study (28) reported three
cases of stomach discomfort and one case of skin rash in the
combined treatment group, one case of stomach discomfort in the SJT
group and two cases of stomach discomfort in the LT group.
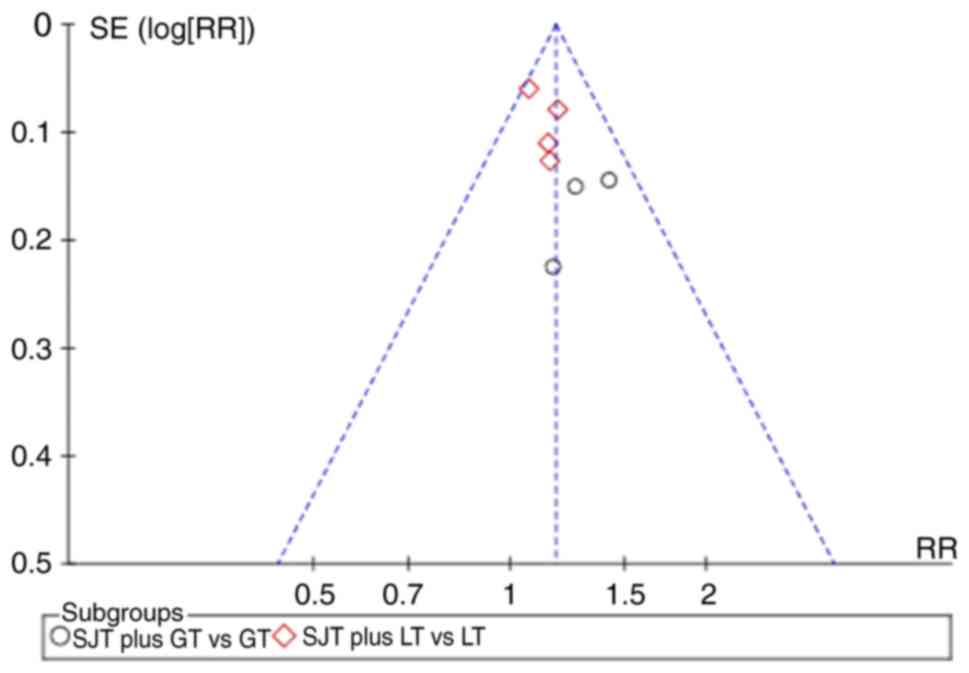
Publication bias
The funnel plot was asymmetric when pooling seven
trials on the cure rate (Fig. 9).
The potential publication bias may be due to the high proportion of
published positive results in China. All of the studies included in
the present meta-analysis are written in Chinese, which may cause
linguistic publication bias.
Sensitivity analysis
A sensitivity analysis of the six trials with
incomplete reporting was performed (22–25,27,28)
using Revman software. The P-value of the overall pooled estimate
changed significantly after removing one study at a time regarding
four outcomes (cure rate, total effective rate, recurrence rate,
incidence of adverse reactions). The results of the sensitivity
analysis indicated that the sensitivity was high and the results of
the meta-analysis were not stable and reliable. This suggests that
the present results require confirmation using high-quality RCTs
and larger samples. Clinicians should therefore exercise caution
when using the present results. The P-values obtained in the
sensitivity analysis for the six trials with incomplete reporting
are provided in Table II.
 | Table II.Sensitivity analysis of six trials
with incomplete reporting. |
Table II.
Sensitivity analysis of six trials
with incomplete reporting.
| Outcome | Study removed
[first author (year)] | P-value | RR (95% CI) |
|---|
| Cure rate (SJT + LT
vs. LT) | Liu (2017) | 0.008 | 1.13
(1.03–1.23) |
| Cure rate (SJT + LT
vs. LT) | Lyu (2015) | 0.060 | 1.11
(1.00–1.24) |
| Cure rate (SJT + LT
vs. LT) | Qiu (2009) | 0.010 | 1.17
(1.03–1.31) |
| Cure rate (SJT + LT
vs. LT) | Mei (2008) | 0.010 | 1.13
(1.02–1.25) |
| Cure rate (SJT + GT
vs. GT) | Hu (2014) | 0.140 | 1.22
(0.94–1.58) |
| Cure rate (SJT + GT
vs. GT) | Wang (2011) | 0.030 | 1.31
(1.02–1.67) |
| Total effective
rate (SJT + LT vs. LT) | Liu (2017) | 0.050 | 1.07
(1.00–1.15) |
| Total effective
rate (SJT + LT vs. LT) | Qiu (2009) | 0.010 | 1.15
(1.04–1.29) |
| Total effective
rate (SJT + LT vs. LT) | Mei (2008) | 0.020 | 1.10
(1.02–1.19) |
| Recurrence rate
(SJT + LT vs. LT) | Mei (2008) | 0.110 | 0.38
(0.12–1.22) |
| Bacterial clearance
rate (SJT + LT vs. LT) | Liu (2017) | 0.040 | 1.32
(1.02–1.71) |
| Incidence of
adverse reactions (SJT + antibiotics vs. antibiotics) | Liu (2017) | 0.220 | 0.66
(0.33–1.29) |
| Incidence of
adverse reactions (SJT + antibiotics vs. antibiotics) | Zheng (2013) | 0.740 | 0.87
(0.37–2.02) |
| Incidence of
adverse reactions (SJT + antibiotics vs. antibiotics) | Qiu (2009) | 0.140 | 0.56
(0.26–1.21) |
| Incidence of
adverse reactions (SJT + antibiotics vs. antibiotics) | Mei (2008) | 0.040 | 0.47
(0.23–0.98) |
GRADE evidence profile
The quality of evidence for the cure rate, total
effective rate, recurrence rate and incidence of ADRs was very low,
low, very low and low, respectively, due to the lack of
randomization, blinding and allocation concealment, small sample
size and publication bias, respectively. The GRADE evidence
profiles are provided in Table
III.
 | Table III.GRADE evidence profile: SJT plus
antibiotics for acute lower urinary tract infection. |
Table III.
GRADE evidence profile: SJT plus
antibiotics for acute lower urinary tract infection.
|
| Quality
assessment | No. of
patients | Effect |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Groups | No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other
considerations | SJT combined with
antibiotics | Antibiotics | RR (95% CI) | Absolute | Quality | Importance |
|---|
| Cure rate-SJTs
combined with GTs vs. GTs (7 days) | 3 | Randomized
trials |
Seriousa | No serious
inconsistency | No serious
indirectness |
Seriousb | Reporting
biasc,g | 79/106
(74.50%) | 61/106
(57.50%) | 1.3
(1.07–1.57) | 173 more per 1,000
(from 40 more to 328 more) | Very low | Critical |
|
|
|
|
|
|
|
|
|
| 60% |
| 180 more per 1,000
(from 42 more to 342 more) |
|
|
| Cure rate-SJT
combined with LT vs. LT (7 days) | 4 | Randomized
trials |
Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | Reporting
biasc,d,g | 218/259
(84.20%) | 175/238
(73.50%) | 1.13
(1.04–1.24) | 96 more per 1,000
(from 29 more to 176 more) | Low | Critical |
|
|
|
|
|
|
|
|
|
| 76.10% |
| 99 more per 1,000
(from 30 more to 183 more) |
|
|
| Total effective
rate-SJT combined with LT vs. LT (follow-up mean 7 days) | 3 | Randomized
trials |
Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | Reporting
biasc,d | 177/187
(94.70%) | 141/166
(84.90%) | 1.11
(1.03–1.19) | 93 more per 1,000
(from 25 more to 161 more) | Low | Critical |
|
|
|
|
|
|
|
|
|
| 85.70% |
| 94 more per 1,000
(from 26 more to 163 more) |
|
|
| Recurrence rate-SJT
combined with antibiotics vs. antibiotics (follow-up mean 7
days) | 3 | Randomized
trials |
Seriousa | No serious
inconsistency | No serious
indirectness |
Seriousb | Reporting
biasc–e | 4/115 (3.50%) | 10/86 (11.60%) | 0.35
(0.13–0.97) | 76 fewer per 1,000
(from 3 fewer to 101 fewer) | Very low | Critical |
|
|
|
|
|
|
|
|
|
| 18.80% |
| 122 fewer per 1,000
(from 6 fewer to 164 fewer) |
|
|
| Incidence of
adverse reactions-SJT combined with antibiotics vs. antibiotics
(follow-up mean 7 days) | 4 | Randomized
trials |
Seriousa | No serious
inconsistency | No serious
indirectness | No serious
imprecision | Reporting
biasc,d,f | 14/225 (6.20%) | 21/205
(10.20%) | 0.61
(0.32–1.17) | 40 fewer per 1,000
(from 70 fewer to 17 more) | Low | Critical |
|
|
|
|
|
|
|
|
|
| 7% |
| 27 fewer per 1,000
(from 48 fewer to 12 more) |
|
|
Discussion
The purpose of the present meta-analysis was to
evaluate the efficacy of SJT combined with antibiotics in the
treatment of ALUTIs. In order to provide accurate evidence for
clinical practice, the cure rate and total effective rate were
assessed. Under the same curative effect standard, combined
analysis of 3 studies revealed that the cure rate of SJT combined
with GT was higher than that of GT alone, while combined analysis
of 4 studies indicated that the cure rate of SJT combined with LT
was higher than that of LT alone. In addition, 1 study indicated
that the cure rate of SJT combined with OT was higher than that of
OT alone. Combined analysis of 3 studies suggested that the total
effective rate of SJT combined with LT was higher than that of LT
alone and 1 study indicated that the total effective rate of SJT
combined with GT was higher than that of GT alone. Combined
analysis of 3 studies revealed that the recurrence rate of SJT
combined with antibiotics was lower than that of antibiotics alone.
Combined analysis of 2 studies indicated that the bacterial
clearance rate of SJT combined with antibiotics was higher than
that of antibiotics alone. The present meta-analysis demonstrated
that SJT combined with antibiotics improved the clinical curative
effect in the treatment of ALUTIs. Addition of SJT to antibiotics
significantly improved the cure, total effective and bacterial
clearance rates, and decreased the recurrence rate. However, the
sensitivity analysis suggested that the stability and reliability
of the results were poor. More RCTs with better consistency and
fewer confounding factors are required to further verify the
results, so as to provide reliable evidence for clinical
practice.
The incidence of ADRs reported in the studies was
summarized. The results of 4 studies suggested that there was no
significant difference between SJT plus antibiotics and antibiotics
alone regarding the incidence of ADRs. Thus, the addition of SJT to
antibiotics may not increase the incidence of ADRs. No ADRs were
reported in 3 studies, while 4 studies reported a slight increase
in total bilirubin, a mild decrease in blood leukocytes, increased
hunger, headache, stomach ache, elevated blood pressure, thirst,
nausea, stomach discomfort, mild diarrhea and reduced appetite,
mild dysuria and skin rash in the control groups. All ADRs were
minor or tolerable and commonly disappeared naturally or after drug
withdrawal. There were no serious ADRs or ADEs reported in any of
the trials included. However, the methodological quality of the
studies included in the present analysis was poor and safety
requires to be further clarified by standard centralized monitoring
of hospital patients.
In terms of the cure rate, this was significantly
higher in the experimental group compared with that in the control
group. However, there was a marked risk of bias caused by blinding,
randomization, allocation concealment and publication bias.
Inaccuracy due to small sample size was also present. According to
the 5 degradation factors (risk of bias, inconsistency,
indirectness, imprecision and reporting bias) in the GRADE system,
the quality of evidence for the cure rate of SJT combined with
antibiotics for ALUTI was very low and low, respectively. The total
effective rate in the experimental group was higher than that in
the control group; however, due to the risk of bias caused by the
lack of blinding, randomization, allocation concealment and
publication bias due to the small sample size, the quality of
evidence for the total effective rate of SJT combined with LTs for
ALUTIs decreased from high to low. Meta-analysis demonstrated that
the recurrence rate of SJT combined with antibiotics was lower than
that of antibiotics alone. However, the quality of evidence
decreased from high to very low due to the risk of bias caused by
blinding, randomization, allocation concealment, imprecision and
publication bias caused by the small sample size. The quality of
evidence regarding the incidence of ADRs was low due to the risk of
bias caused by blinding, randomization, allocation concealment and
publication bias. The results of the evaluation of the quality of
evidence of the above outcomes should be combined with factors
including the patients' value intention and cost in order to
provide recommendations, and serve as a reference or basis for
clinical practice guidance.
The major limitations of the present study are as
follows: i) None of the studies included reported blinding,
randomization or allocation concealment, which may result in risk
of bias; ii) study protocol, informed consent or ethical statements
were not specified in any of the studies; iii) 6 studies reported
that follow-up was performed after treatment to evaluate
recurrence, but only 3 studies included follow-up data and it was
not possible to evaluate the long-term effect in the cohorts of the
other studies; iv) 6 studies reported on case shedding, all of
which failed to perform an intentionality analysis and had
incomplete reports; v) 1 study did not fully report on
pre-specified outcomes and featured selective reporting; vi) no
sample size estimation was reported in any of the studies included.
Specifically, 3 studies had a sample size ≥100 cases and 5 studies
had a sample size ≤80 cases. In various studies, the curative
effect index was unstable and the test efficiency was low due to
the small sample size. The overall methodological quality of the
studies included was low. It has been suggested that large-sample,
low-bias clinical RCTs should first refer to Consolidated Standards
of Reporting Trials (29).
The results of the present meta-analysis suggested
that, in clinical practice, addition of SJT to the use of
antibiotics may be considered in order to improve the curative
effect and reduce the recurrence rate in patients with ALUTIs. Due
to the poor methodological quality of the studies included, the
results of the present meta-analysis require to be further
confirmed. The level of evidence obtained in the present study is
low; thus, the expert consensus method was employed to confirm
whether it may be widely used in the clinic, including the nominal
group and Delphi methods. Clinicians should interpret the results
of the present study with caution with regard to the actual
situation and perform clinical treatments based on comprehensive
consideration of evidence, expert consensus, clinical experience
and the patients' preferences. In the present study, the patients
were not divided into those with complex and simple LUTIs. A
previous meta-analysis focused on simple LUTIs (30). In the future, the exact efficacy of
SJT for complex LUTIs may be further explored.
In conclusion, the present meta-analysis
demonstrated that, compared with antibiotics treatment, SJT
combined with antibiotics improved the cure rate, total effective
rate and bacterial clearance rate, and decreased the recurrence
rate without any serious ADRs in patients with ALUTIs. However, the
GRADE quality of evidence was low. Thus, additional large-sample,
high-quality RCTs with a rigorous design are required to improve
the quality of evidence.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Key
Research and Development Program of China (grant no.
2018YFC1707400).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL, YMX, MHS, CZ and LXW contributed to designing
the search strategy. JL and MHS conducted the searches. JL, MHS and
YMX performed the data extraction. JL, CZ and YMX contributed to
quality assessment. All authors contributed to drafting and
revising the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SJT
|
Sanjin tablet
|
|
ALUTI
|
acute lower urinary tract
infection
|
|
RCT
|
randomized controlled trial
|
|
RR
|
risk ratio
|
|
ADE
|
adverse event
|
|
ADR
|
adverse reaction
|
|
LT
|
levofloxacin tablet
|
|
GT
|
gatifloxacin tablet
|
|
OT
|
oxyfluoxacin tablet
|
|
GRADE
|
Grading of Recommendations,
Assessment, Development and Evaluations
|
References
|
1
|
Foxman B: The epidemiology of urinary
tract infection. Nat Rev Urol. 7:653–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li XS, Feng Y, Zhou X, Fan CL and Wu XY:
Study on clinical distribution and drug resistance of pathogenic
bacteria of urinary tract infection in inpatients in a hospital.
Chin J Disinfection. 36:279–281. 2019.
|
|
3
|
Foxman B: Epidemiology of urinary tract
infections: Incidence, morbidity, and economic costs. Dis Mon.
49:53–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khoshnood S, Heidary M, Mirnejad R,
Bahramian A, Sedighi M and Mirzaei H: Drug-resistant gram-negative
uropathogens: A review. Biomed Pharmacother. 94:982–994. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El Bcheraoui C, Mokdad AH, Dwyer-Lindgren
L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Shirude S, Naghavi M
and Murray CJL: Trends and patterns of differences in infectious
disease mortality among US counties 1980–2014. JAMA. 319:1248–1260.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bader MS, Loeb M and Brooks AA: An update
on the management of urinary tract infections in the era of
antimicrobial resistance. Postgrad Med. 129:242–458. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang SH, Gao YQ, Tan HG, et al:
Standardization study and curative effect Analysis of Clinical
treatment Scheme of Integrated traditional Chinese and Western
Medicine in the treatment of Lower urinary tract infection. J
Zhejiang Univ TCM. 37:1197–1200. 2013.
|
|
8
|
Peng YX, Liu XQ, Wen LL, et al:
Antibacterial Activities of Five Chinese Medicines of Rhei Radiset
Rhizoma and Their Chemical Constituents Against Multidrug-resistant
Clinical Bacteria Isolates. Chin J Exp Trad Med Formulae.
20:103–107. 2014.(In Chinese).
|
|
9
|
Li DY, Hou Y, Zhang KY, et al: Research
progress on mechanism of anti-drug resistance of traditional
Chinese medicine. Chin Med Engineering. 25:16–19. 2017.(In
Chinese).
|
|
10
|
Hou X and Wang LX: Research progress of
Sanjin tablets. Evaluation Ana Drug Use Chin Hospitals.
16:1148–1151. 2016.
|
|
11
|
National Pharmacopoeia Committee:
Pharmacopoeia of the People's Republic of China. China Medical
Science Press; 2015
|
|
12
|
Wei XY and Lu XL: Research progress on
pharmacological action of Jinyinggen. Trace Elements Health Res.
34:80812017.
|
|
13
|
Xie Y, Hu D, Zhong C, Liu KF, Fang E,
Zhang YJ, Zhou C and Tian LW: Anti-inflammatory furostanol saponins
from the rhizomes of Smilax china L. Steroids. 140:70–76.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou YL, Zhao X, Hua J, et al: Studies on
the Chemical constituents and Antioxidant activity of Jinshateng.
Chin J TCM. 28:1392–1396. 2013.(In Chinese).
|
|
15
|
Lyu J, Xie YM, Gao Z, Shen JW, Deng YY,
Xiang ST, Gao WX, Zeng WT, Zhang CH, Yi DH, et al: Sanjin tablets
for acute uncomplicated lower urinary tract infection (syndrome of
dampness-heat in the lower Jiao): Protocol for randomized,
double-blind, double-dummy, parallel control of positive drug,
multicenter clinical trial. Trials. 20:4462019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong XL: Clinical efficacy and safety of
Sanjin tablets combined with levofloxacin tablet in the treatment
of urinary tract infection. The World's Latest Med Information
Abstracts. 17:169–170. 2017.
|
|
17
|
Zheng HY and Hu JG: Efficacy of Sanjin
tablets combined with gatifloxacin in the treatment of acute simple
lower urinary tract infection. Zhejiang J Integrated Traditional
Chin Western Med. 23:724–726. 2013.
|
|
18
|
Mao X, Yao RM, Xu YH, et al: Establishment
of rat model of acute urinary tract infection and its application
in efficacy evaluation of traditional Chinese medicine. Pharmacol
Clinic TCM. 35:177–180. 2019.
|
|
19
|
Balshem H, Helfand M, Schünemann HJ, Oxman
AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S
and Guyatt GH: GRADE guidelines: 3. Rating the quality of evidence.
J Clin Epidemiol. 64:401–406. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guyatt GH, Oxman AD, Sultan S, Glasziou P,
Akl EA, Alonso-Coello P, Atkins D, Kunz R, Brozek J, Montori V, et
al: GRADE guidelines: 9. Rating up the quality of evidence. J Clin
Epidemiol. 64:1311–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higgins J and Green SE: Cochrane Handbook
for Systematic Reviews of Interventions. version 5.1.0. The
Cochrane Collaboration. 2011:https://handbook-5-1.cochrane.org/Updated.
March. 2011
|
|
22
|
Liu H, Xie JX and Xu Z: Clinical study on
treatment of Acute simple bacterial Lower urinary tract infection
with combination of traditional Chinese and Western Medicine. J
TCM. 32:2489–2492. 2017.
|
|
23
|
Lyu GR and Zhan YL: Clinical observation
of Sanjin tablet combined with levofloxacin in the treatment of
acute simple lower urinary tract infection. Chin Med J. 50:105–107.
2015.
|
|
24
|
Hu XL: Clinical analysis of Sanjin tablet
combined with gatifloxacin in the treatment of acute lower urinary
tract infection. Med Information. 27:659–660. 2014.
|
|
25
|
Wang DZ: Clinical analysis of Sanjin
tablet combined with gatifloxacin in the treatment of acute lower
urinary tract infection. J Med Forum. 32:164–165. 2011.
|
|
26
|
Tu Z and Wang T: Analysis of the efficacy
of Sanjin tablet combined with ofloxacin in the treatment of acute
lower urinary tract infection. China Grass-Roots Med. 18:2999–3000.
2011.
|
|
27
|
Qiu MS, Xu ZJ and Zhang CY: Analysis of 80
cases of female Acute Lower urinary tract infection treated by
combination of traditional Chinese and Western Medicine. China
Grass-Roots Med. 16:20762009.
|
|
28
|
Mei XF and Zhang CT: Clinical observation
of Sanjin tablet in the treatment of acute simple lower urinary
tract infection. J Modern Integration Traditional Chin Western Med.
26:4085–4086. 2008.
|
|
29
|
Moher D, Hopewell S, Schulz KF, Montori V,
Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M and Altman DG:
CONSORT: CONSORT 2010 explanation and elaboration: Updated
guidelines for reporting parallel group randomised trials. Int J
Surg. 10:28–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pu X, Zhang LY and Zhang JH: A systematic
review of Sanjin tablets in the treatment of simple urinary tract
infection: A randomized controlled trial. Lishizhen Med Mat Med
Res. 27:1012–1014. 2016.
|