Introduction
Congenital hypothyroidism (CH) is a form of general
endocrine disease caused by congenital thyroid hormone (TH)
deficiency (1). The incidence of CH
in newborn infants ranges between 1/3,000 and 1/4,000 (2). This condition is treatable if diagnosed
early, but later diagnoses can lead to physical and mental
abnormalities (3). Therefore, the
accurate timing of thyroid hormone replacement therapy in humans is
critical for optimizing neurocognitive recovery. CH results in
cognitive deficiency (4), motor
deficits (5) and behaviors
associated with anxiety (6). Timely
and effective screening of newborns would enable early diagnosis
and treatment, with the aim of improving the prognosis of children.
In addition, quality care can improve the completion rate of CH
screening in newborns more effectively. Therefore, early detection,
diagnosis, scientific and effective nursing and treatment are
crucial for improving the prognosis of children with congenital
hypothyroidism and improving the quality of the population.
TH is essential for the proper development of the
mammalian brain. TH deficiency results in serious structural and
functional damage to the central nervous system (7). In particular, CH leads to neutrophil
damage, abnormal cerebellar growth and differentiation (8–10). The
hippocampus is an important center responsible for cognitive
activities in humans. Indeed, children with CH who experience
neonatal thyroid hormone deficiency exhibit reduced hippocampal
volumes compared with healthy controls (11). A previous study has reported that
perinatal hypothyroidism may induce apoptosis in hippocampal
neurons in rats (12). Following
early diagnosis of CH, neurodevelopmental dysplasia could still
occur if treatment is not optimized within the first 2–3 years of
birth (13). Therefore, patients
with CH must undergo early treatment followed by close
follow-up.
MicroRNAs (miRNAs) are non-coding single stranded
small RNAs consisting of 18–25 nucleotides that do not encode
proteins (14). Mature miRNA
directly bind to mRNAs to regulate their expression (15). Recent research has suggested that
miRNAs participate widely in diseases involving the nervous system,
including congenital hypothyroidism (16). For example, a previous study has
demonstrated that miR-124 inhibits the progression of congenital
hypothyroidism (16). Previous
studies have revealed that miR-1236 can serve different roles in a
tissue- or physiological-dependent manners. miR-1236 attenuates
human lymphatic endothelial cell migration and tube formation
(in vitro angiogenesis detection) in addition to
angiogenesis in the lymphatic system in vivo (17). In contrast, miR-1236-3p has been
found to repress ovarian cancer metastasis (18). However, the role of miR-1236-3p in
congenital hypothyroidism remains unclear.
Translationally-controlled tumor protein 1 (TPT1) is
a highly conserved protein that has been reported to be strongly
expressed in a variety of malignant tumors, where it regulates cell
proliferation, invasion, cell cycle and apoptosis (19–21).
Indeed, TPT1 downregulation has been demonstrated to inhibit cell
proliferation and induce cell cycle arrest and apoptosis in
pancreatic cancer (22).
Furthermore, miR-489-3p has been revealed to inhibit glioblastoma
progression by acting through the downregulation of TPT1 (23).
The present study aimed to clarify the role of
miR-1236-3p in CH by investigating the function of this miRNA in
hippocampal neuron apoptosis in vivo and in vitro
using a rat model.
Materials and methods
Reagents
Propylthiouracil was obtained from Beyotime
Institute of Biotechnology. This protocol followed and dosage of
Propylthiouracil used was performed/selected according to a
previous study (24). The
miR-1236-3p inhibitor and its corresponding negative control
(inhibitor control), TPT1-siRNA (cat no. XWCRR2962; Zhejiang Huijia
Biotechnology Co., Ltd.) and control-siRNA (cat no. 9500C-1;
Zhejiang Huijia Biotechnology Co., Ltd.) were purchased from
Shanghai GenePharma Co., Ltd.
Experimental animals
A total of 50 female pregnant Sprague-Dawley rats
(weight, 200±10 g; age, 6 weeks) obtained from Vital River
Laboratories Co., Ltd. were used. All rats were maintained at room
temperature with a humidity of 55% and ad libitum access to
standard pellet feed and water under a 12-h light/dark cycle.
Propylthiouracil (50 mg/day) was injected intraperitoneally into
pregnant rats on day 15 of gestation and then carried out every day
thereafter until parturition to generate pups with congenital
hypothyroidism (24). For the
treatment of CH pups, animals were anesthetized with an
intraperitoneal injection of 2% sodium pentobarbital (40 mg/kg).
Newborn rats (12 days old) were subsequently fixed on a stereotaxic
apparatus and their skulls were opened at 1.0 mm from the former
fontanel and 1.7 mm from the mid-line (16). A micro syringe was then inserted
vertically into the left lateral ventricle (bregma: −0.58 mm;
dorsoventral: 2.1 mm; lateral: 1.2 mm) and pups were injected with
miR-1236-3p inhibitor control solution (5 µl; 1 nmol/l),
miR-1236-3p inhibitor (5 µl; 1 nmol/l) or miR-1236-3p inhibitor (5
µl; 1 nmol/l) + TPT1-siRNA solution (5 µl; 1 nmol/l) as previously
described (25). The newborn rats
(12 days old in all groups) were divided into five groups (n=5):
Control group (newborn rats from pregnant rat that was received
food and normal tap water ad libitum without
propylthiouracil treatment); congenital hypothyroidism (CH) group
[newborn pups from pregnant rats that were injected
intraperitoneally with Propylthiouracil (50 mg/d) on day 15 of
gestation each day until parturition]; miR-1236-3p inhibitor
control group [12 day old CH newborn rats injected miR-1236-3p
inhibitor control as previously described (25)]; miR-1236-3p inhibitor group [12 day
old CH newborn rats were injected with miR-1236-3p inhibitor as
previously described (25)]; and
miR-1236-3p inhibitor + TPT1-siRNA group [12 day old CH newborn
rats were injected with miR-1236-3p inhibitor + TPT1-siRNA as
previously described (25)].
This animal experiment lasted for 36 days in total,
and the health and behaviors (including diet, drinking, tail swing,
sucrose preference, swimming) of all rats were monitored every 2
days. No rats died for the duration the aforementioned experimental
procedures. The experiments were ended when the rats lost >15%
of their body weight (body weight prior to injection). On day 21
after birth, newborn rats (21 day old; body weight <200 g) were
anesthetized with pentobarbital (40 mg/kg) by intraperitoneal
injection and sacrificed by cervical dislocation (death defined as
the lack of heartbeat and breathing). The brain hippocampus tissue
was subsequently harvested following euthanasia. The mother rats
(24 h after the pups were born) from which the pups were obtained
were also anesthetized with pentobarbital (40 mg/kg) and then
sacrificed by cervical dislocation, with death defined as the lack
of heartbeat and breathing. The experimental procedure of the
present study regarding the establishment of the CH rat model and
treatment process is presented in Fig.
S1. All animal care and experimental procedures were performed
in accordance with the National Institutes of Health Guide for Care
and approved by the Animal Ethics Committee of the First Hospital
of Jilin University.
Cell culture and transfection
Hippocampal neurons were prepared from the
hippocampus of postnatal day 0 rat pups from the control group, as
previously described (26). Neurons
were cultured using Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) in poly-D lysine-coated
plates at a density of 1×106 cells/ml. After culturing
for 6 h at 37°C and 5% CO2, the cell culture medium was
replaced with neurobasal medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 2% B27 (cat. no. 17504-044; Life Technologies;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, 100 g/ml
streptomycin and 0.5 mM glutamine (Gibco; Thermo Fisher Scientific,
Inc.) and then incubated at 37°C in a humidified atmosphere under
5% CO2. The culture medium was replaced once every 3
days.
Neurons were seeded into 6-well plates
(5×104 cells/well) and transfected with miR-1236-3p
inhibitor, miR-1236-3p inhibitor control (inhibitor control),
TPT1-siRNA, control-siRNA, miR-1236-3p inhibitor + control-siRNA or
miR-1236-3p inhibitor + TPT1-siRNA using
Lipofectamine®2000 (Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol. Neurons were collected for
subsequent experiments after 48 h of transfection.
MTT assay
Cell viability of neurons was measured via an MTT
assay. Cells were first cultured in 96-well plates
(1×104 cells/well) at 37°C for 24 h and then transfected
with the miR-1236-3p inhibitor, inhibitor control, miR-1236-3p
inhibitor + control-siRNA or miR-1236-3p inhibitor + TPT1-siRNA for
48 h, as aforementioned. Following incubation, 10 µl MTT solution
was added into each well and incubated for a further 4 h at 37°C
under 5% CO2. A total of 150 µl dimethyl sulfoxide
(DMSO) was used to dissolve the formazan crystals. Optical density
at 490 nm was measured in each well using a microplate reader.
Flow cytometry
A BD FACSCalibur™ Flow cytometer (BD Biosciences)
was used to analyze neuronal cell apoptosis. Neurons
(1×105 cells/well) were first digested using 0.25%
trypsin, washed with PBS and fixed in 70% ice-cold ethanol
overnight at 4°C. The neurons were subsequently added with 5 µl
fluorescein isothiocyanate-labeled Annexin V and 5 µl propidium
iodide (PI; cat. no. 6592; Cell Signaling Technology, Inc.). Then,
the neurons were incubated at 4°C for 30 min in the dark. Cell
apoptosis was analyzed and calculated (right quadrants) using
FlowJo software (version 7.6.1; Tree Star Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the hippocampus tissues
and hippocampus neurons using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to manufacturer's protocol. RNA
was reverse transcribed into cDNA using the PrimeScript™ RT reagent
kit (Takara Biotechnology Co., Ltd.) according to manufacturer's
protocol. qPCR was performed using Maxima™ SYBR Green qPCR Master
Mix (2×) (Fermentas; Thermo Fisher Scientific, Inc.) according to
manufacturers' protocols. The primer sequences used for qPCR were
as follows: TPT1 forward, 5′-ATGATTATCTACCGGGACCTC-3′ and reverse,
5′-TACATTTTTCCATTTCTAAACCATCC-3′; GAPDH forward,
5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′; U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; miR-1236-3p forward,
5′-CCAATCAGCCTCT-TCCCCTT-3′ and reverse,
5′-TATGGTTGTTCACGACTCCT-TCAC-3′. The thermocycling conditions were
as follows: 5 min at 95°C and 40 cycles of 30 sec at 95°C, 30 sec
at 60°C and 30 sec at 72°C. U6 for (miRNA) and GAPDH (for mRNA)
were used as the internal control. Quantification was performed
using the 2−∆∆Cq method (27).
Western blot analysis
After cell transfection, the neuronal cells were
homogenized in cell lysis buffer (10×; Cell Signaling Technology,
Inc.) containing protease inhibitors. Proteins were quantified
using Bicinchoninic Acid Assay kit (Thermo Fisher Scientific, Inc.)
and subsequently separated (30 µg/lane) via 12% SDS-PAGE prior to
transferal onto PVDF membranes. The membranes were blocked with 5%
skim milk in TBS containing 0.1% Tween at room temperature for 2 h,
and then incubated with primary antibodies against TPT1 (cat. no.
5128; 1:1,000; Cell Signaling Technology, Inc.), Serine/Threonine
Kinase Pim-3 (Pim-3; cat. no. 4165; 1:1,000; Cell Signaling
Technology, Inc.), phosphorylated (p)-Bad (Ser112) (cat. no. 5284;
1:1,000; Cell Signaling Technology, Inc.), Bad (cat. no. 9268;
1:1,000; Cell Signaling Technology, Inc.), Bcl-xL (cat. no. 2764;
1:1,000; Cell Signaling Technology, Inc.) and β-actin (cat. no.
4970; 1:1,000; Cell Signaling Technology, Inc.) at 4°C overnight.
Subsequently, the membranes were then incubated with Anti-rabbit
IgG, HRP-linked Antibody (cat. no. 7074; 1:2,000; Cell Signaling
Technology, Inc.) at room temperature for 1 h. Protein bands were
visualized using enhanced chemiluminescence (Cell Signaling
Technology, Inc.). Densitometry analysis was performed using
Gel-Pro Analyzer densitometry software (Version 6.3; Media
Cybernetics, Inc.).
Dual luciferase reporter assay
Bioinformatics software 7.1 (http://www.targetscan.org/vert_71/) was used to
predict potential targets of miR-1236-3p. The binding sites between
the 3′-untranslated region (3′-UTR) of TPT1 and miR-1236-3p were
observed. A dual luciferase reporter assay was used to investigate
whether miR-1236-3p directly targets the 3′-UTR of TPT1. The
wild-type (WT) 3′UTR of TPT1 or the mutant (MUT) 3′UTR TPT1 were
cloned into the dual-luciferase reporter vector pmiR-RB-REPORT
(Guangzhou RiboBio Co., Ltd.) following the manufacturer's
instructions. Hippocampal neurons were subsequently prepared from
the hippocampus of postnatal day 0 rat pups in the control group
(5×104 cells per well) were first seeded into 24-well
plates and co-transfected with either TPT1-3′-UTR-wild type (WT) or
TPT1-3′-UTR-mutant (MUT) plasmids and miR-1236-3p mimics (forward
5′-CGCGGATCCCTGGCCCTCACTTACCTC-3′ and reverse
5′-CCGAATTCCCATCTACATTCCAACTTGGAG-3′ or mimic control (forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′) using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.). After 48 h, the relative
luciferase activities were measured using a dual luciferase
reporter assay kit (Promega Corporation) according to
manufacturer's protocol. All luciferase activities were normalized
to Renilla luciferase activity.
Statistical analysis
All experiments were repeated three times. Data were
presented as the mean ± standard deviation. The significance of
differences between groups was evaluated using Student's t-test or
analyzed via one-way ANOVA followed by a Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
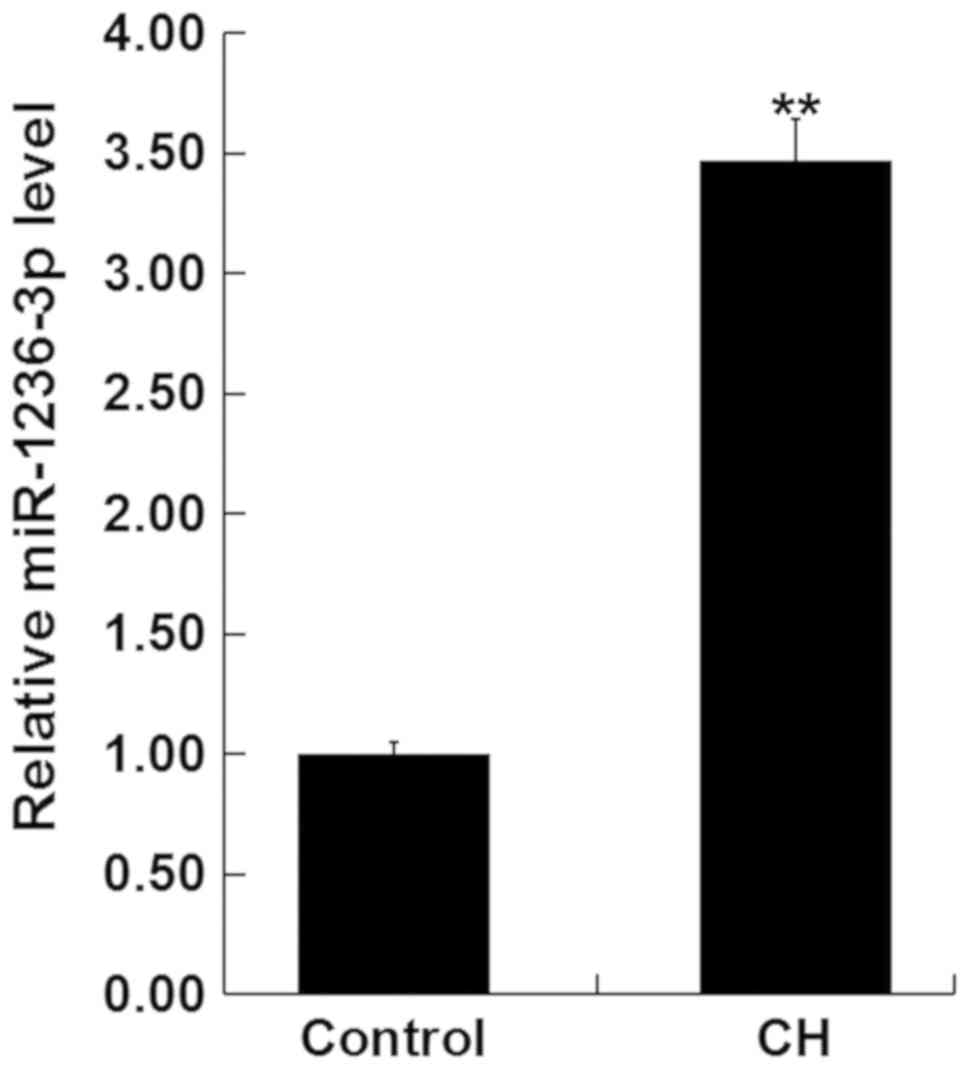
MiR-1236-3p is upregulated in rats
with CH
Changes in miR-1236-3p expression in the hippocampus
tissue of newborn pups after the establishment of CH were first
measured using RT-qPCR. Compared with the newborn pups of the
control group, the expression of miR-1236-3p was significantly
higher in the hippocampus tissue of newborn pups with CH (Fig. 1).
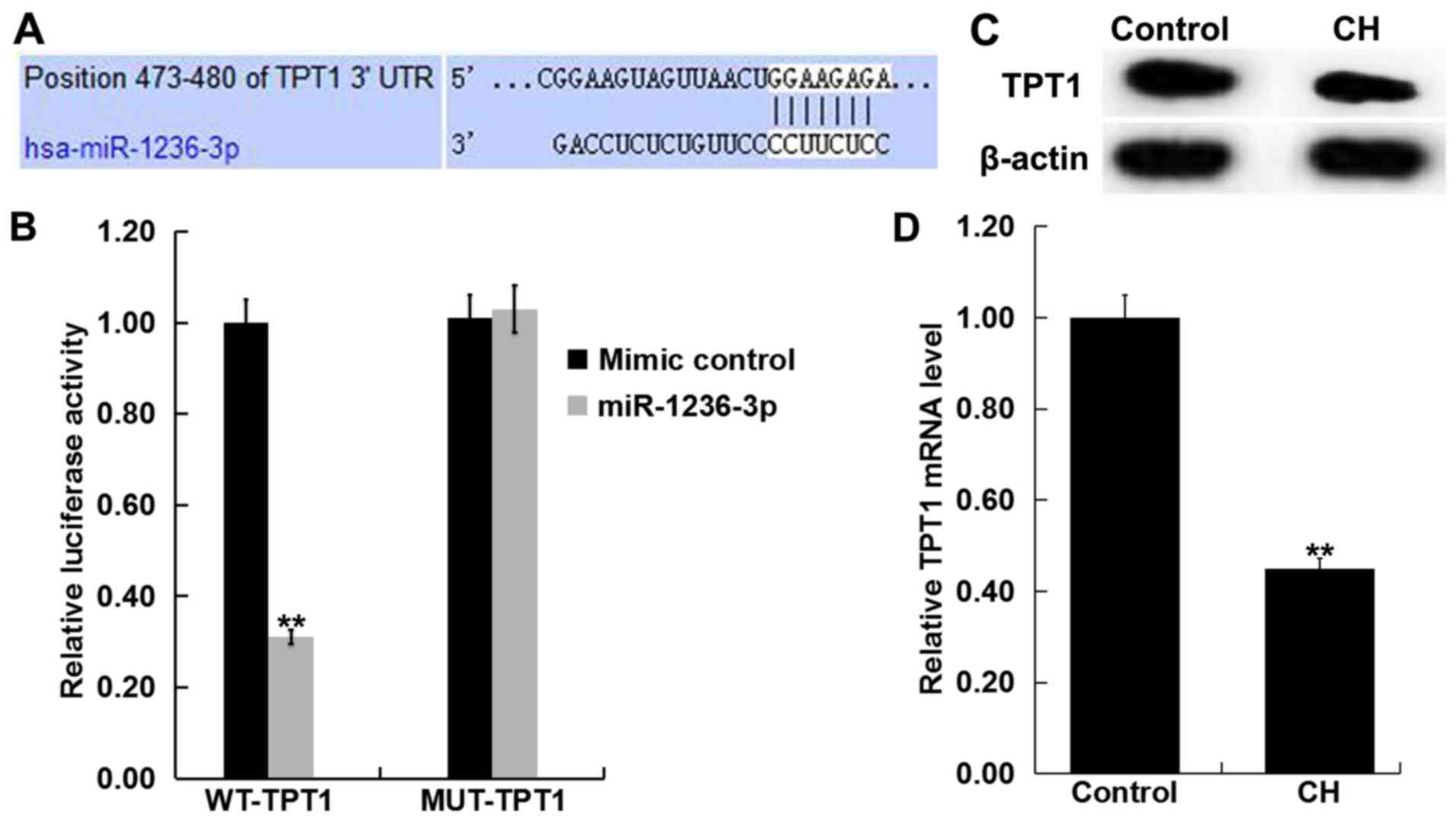
TPT1 is a target of miR-1236-3p
TargetScan revealed a potential miR-1236-3p binding
site on the 3′-UTR of TPT1 (Fig.
2A). Dual-luciferase reporter assay results demonstrated that
transfection with miR-1236-3p mimic significantly reduced
luciferase activity in neurons that were also co-transfected with
TPT1-WT group but not in those co-transfected with TPT1-MUT
(Fig. 2B). This observation
indicated that TPT1 is a direct target of miR-1236-3p. To support
this, levels of TPT1 expression in the hippocampus tissue of
newborn pups with or without CH was determined via RT-qPCR and
western blotting. Compared with the control group, TPT1 protein
(Fig. 2C) expression markedly
reduced in the hippocampus tissues of rats with CH. Compared with
the control group, TPT1 mRNA (Fig.
2D) expression was significantly reduced in the hippocampus
tissues of rats with CH.
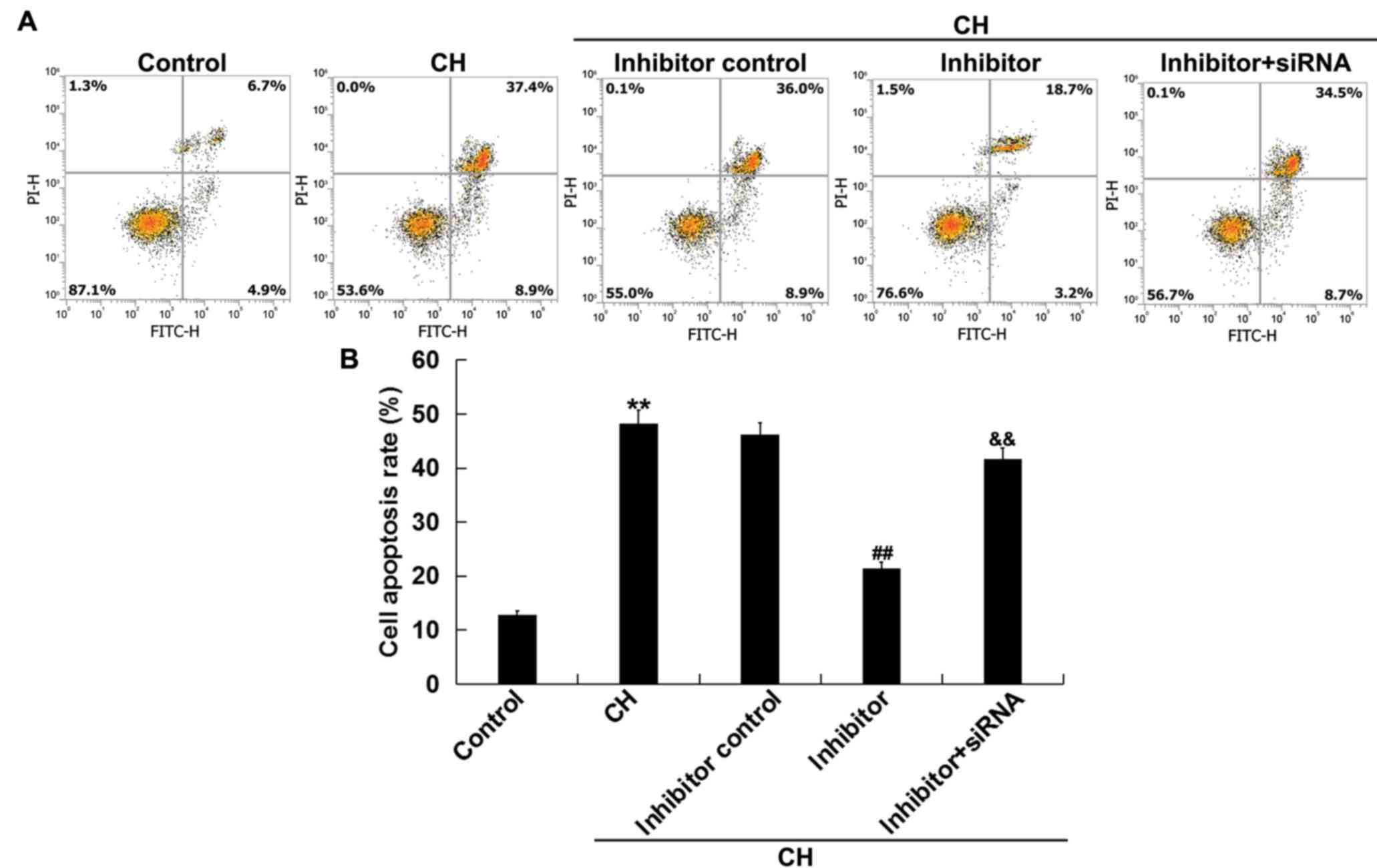
Inhibition of miR-1236-3p mitigates CH
newborn pups hippocampal neuronal cell apoptosis in vivo
The effects of miR-1236-3p on neuronal cell
apoptosis in rats with CH was assessed via flow cytometry. Compared
with the control group, neuronal cells isolated from rats in the CH
group exhibited significantly increased cell apoptosis (Fig. 3A and B). Injection with miR-1236-3p
inhibitor significantly reduced CH-induced neuronal cell apoptosis,
an effect that was reversed by co-injection with TPT1-siRNA
(Fig. 3A and B). Overall, these
results indicated that inhibition of miR-1236-3p prevented neuronal
cell apoptosis in rats with CH.
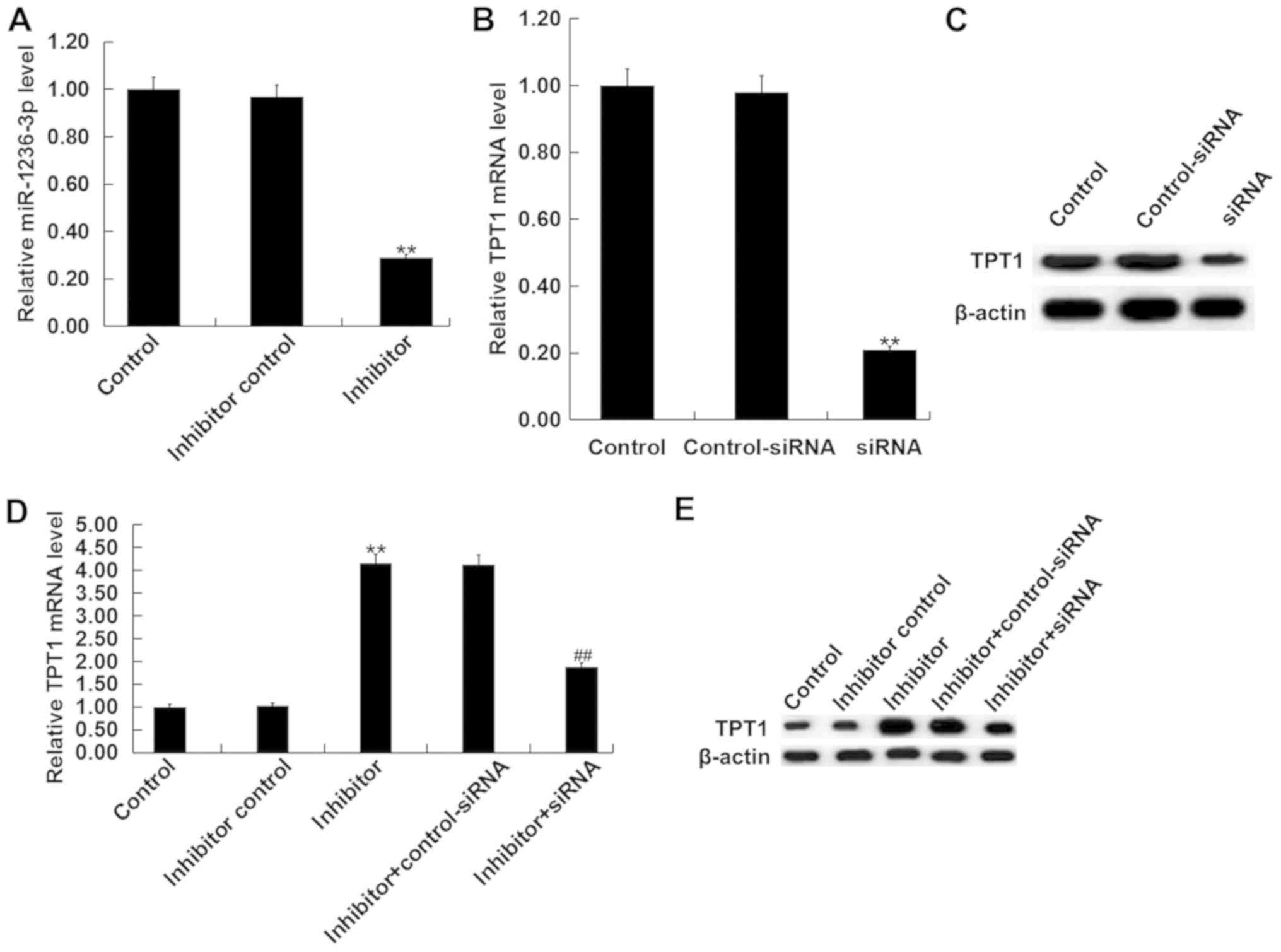
Inhibition of miR-1236-3p protects
cultured neurons from apoptosis in vitro
Neuronal cells were isolated from the hippocampus of
postnatal day 0 rat pups from the control group and subsequently
transfected with either miR-1236-3p inhibitor, inhibitor control or
miR-1236-3p inhibitor + TPT1-siRNA. Transfection efficiency was
first assessed via RT-qPCR. Transfection with the miR-1236-3p
inhibitor significantly reduced the levels of miR-1236-3p in
neurons (Fig. 4A), whilst TPT1-siRNA
transfection significantly reduced TPT1 mRNA level in cultured
neurons (Fig. 4B). TPT1-siRNA
transfection also markedly reduced TPT1 protein level in cultured
neurons (Fig. 4C). Compared with the
inhibitor control group, transfection with the miR-1236-3p
inhibitor significantly increased TPT1 mRNA expression, which was
reversed by co-transfection with TPT1-siRNA (Fig. 4D). Similar results were observed from
western blot assay (Fig. 4E).
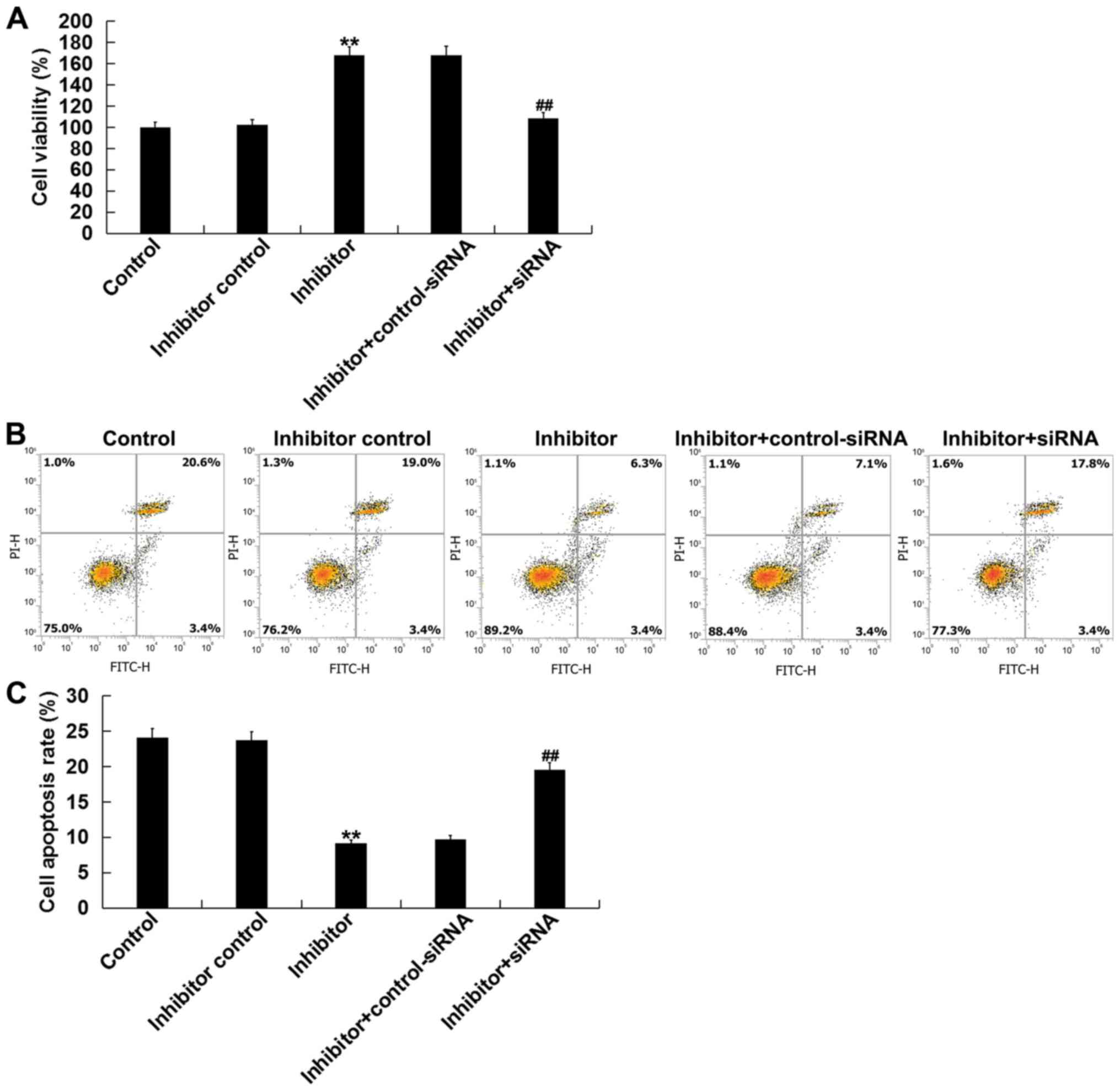
Next, the effects of miR-1236-3p on cultured
neuronal cell apoptosis in vitro was assesed. Compared with
the inhibitor control group, transfection with the miR-1236-3p
inhibitor significantly increased the neuronal cell viability after
48 h (Fig. 5A) whilst inhibiting
neuronal cell apoptosis (Fig. 5B and
C), effects that were significantly reversed by co-transfection
with TPTI-siRNA.
Inhibition of miR-1236-3p upregulates
Pim-3, p-Bad and Bcl-xL expression
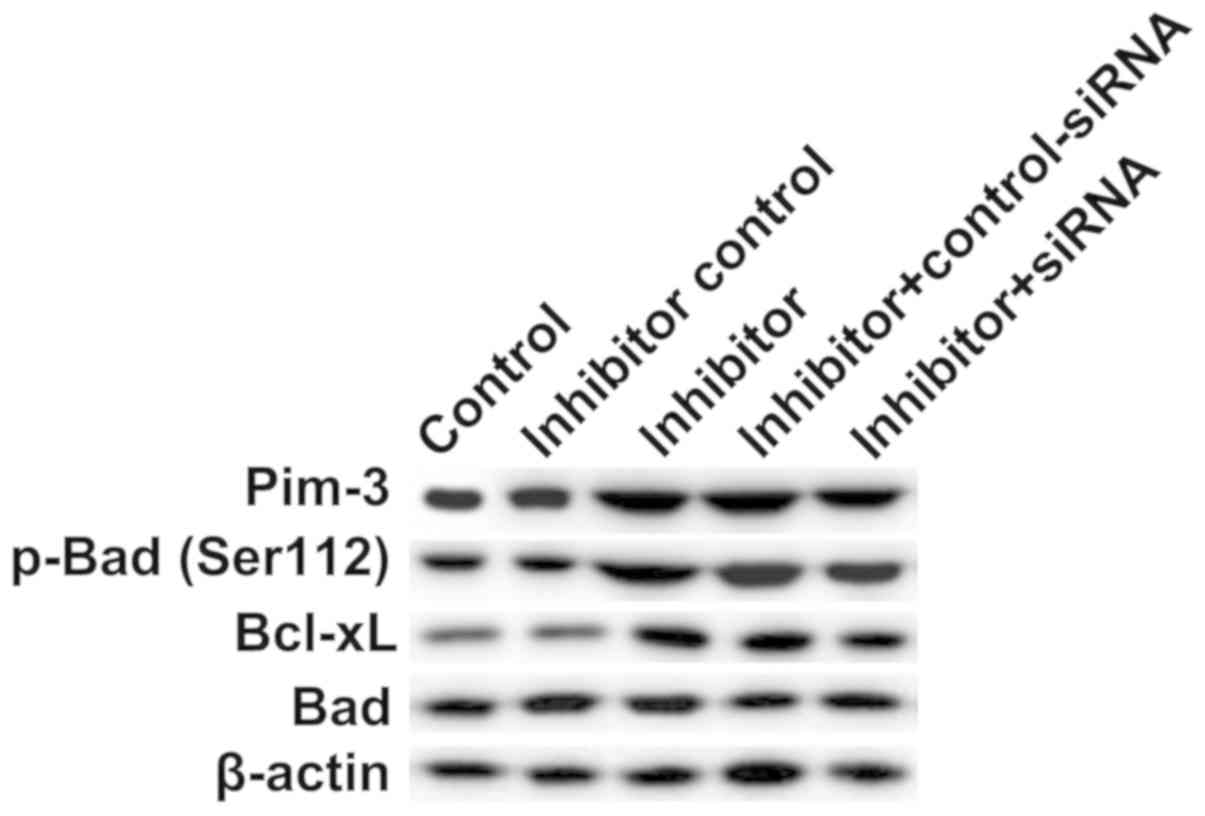
To explore the molecular mechanism by which
miR-1236-3p inhibition reduced neuronal apoptosis, the expressions
of Pim-3, p-Bad, Bad and Bcl-xL, proteins associated with
apoptosis, were analyzed via western blotting. Compared with the
inhibitor control group, transfection with miR-1236-3p inhibitor
significantly increased protein levels of Pim-3, p-Bad (Ser112) and
Bcl-xL in neuronal cells (Fig. 6),
which were in turn reversed by co-transfection with TPT1-siRNA
(Fig. 6).
Discussion
In the present study, it was demonstrated that
miR-1236-3p was upregulated in the hippocampus of newborn rats with
CH. TPT1 was a direct target of miR-1236-3p, which was revealed to
be downregulated in the hippocampus of newborn rats with CH.
Inhibition of miR-1236-3p expression protected hippocampal neurons
from apoptosis induced by CH in vivo. In addition, the
present study also revealed that miR-1236-3p inhibition prevented
neurons from apoptosis and upregulated Pim-3, p-Bad (Ser112) and
Bcl-xL protein expression in vitro, all of which were
reversed by TPT1 knockdown.
CH leads to decreased developmental quotient, mental
retardation and increased depression/anxiety scores in patients
(6,28–31).
Clinical phenomenon (including short stature and mental
retardation) suggested that thyroid hormone synthesis and secretion
reduction leads to developmental delay and neurological deficits
(32). In addition, TH deficiency
can lead to hippocampal neuronal cell injury and cell apoptosis
(33). miRNAs have been revealed to
serve a critical role in diseases associated with the nervous
system, including autoimmune neuroinflammation (34), Alzheimer's disease (35), Parkinson's disease (36) and CH (16). A previous study has reported that
miR-124 can protect neurons from apoptosis in rat models of
hypothyroidism (16). In the case of
miR-1236, it has been reported to induce cell apoptosis in bladder
cancer cells by inhibiting p21 expression (37). However, the expression and role of
miR-1236-3p in CH remain unclear. In the present study, it was
determined that miR-1236-3p was significantly upregulated in the
hippocampal tissue of newborn rats with CH, where TPT1 was
identified as one of its direct targets. Supporting this notion, it
was also identified that TPT1 was downregulated in the hippocampus
tissues of newborn rats with CH. TPT1 is a translationally
controlled oncogene, where it was found to be upregulated in a
variety of malignancies including glioma (20), pancreatic (21,22),
breast (38) and colorectal cancer
(39). It was also revealed to
enhance tumor cell proliferation whilst inhibiting cell apoptosis.
Hippocampal neuronal cell apoptosis is one of the main
characteristics of CH (11,12,16).
Therefore, the results from the present study suggested that
miR-1236-3p may affect CH progression by regulating neuronal cell
apoptosis by targeting TPT1. Subsequently, the effect of
miR-1236-3p on hippocampal neuronal cell apoptosis in rats with CH
was investigated. The results of the present study revealed that CH
induction increased apoptosis of neurons in the hippocampus of
rats, which was alleviated by the inhibition of miR-1236-3p
expression in vivo. The in vitro results also
indicated that miR-1236-3p inhibition enhanced neuronal cell
viability and prevented apoptosis.
Pim-3 is a member of the proto-oncogene Pim family
and is reported to regulate cell survival, proliferation and cell
cycle in during tumor development (40). A previous study demonstrated that
Pim-3 may inhibit cell apoptosis by upregulating the
phosphorylation of Bad and the expression of the anti-apoptotic
molecule Bcl-xL (41). In
particular, TPT1 can interact with Pim-3 to enhance Pim-3 protein
stability (22). A previous study
has reported that miR-216b-5p regulates pancreatic cancer
progression by regulating TPT1/Pim-3 signaling (42). miR-216b-5p upregulation suppressed
Pim-3, p-Bad and Bcl-xL protein expression in pancreatic cancer
cells, which was partly reversed by TPT1 upregulation (42). In the present study, the results
revealed that the inhibition of miR-1236-3p significantly increased
TPT1, Pim-3, p-Bad (Ser112) and Bcl-xL protein expression in
hippocampal neurons. In addition, it was demonstrated that
TPT1-siRNA transfection reversed the upregulation of Pim-3, p-Bad
(Ser112) and Bcl-xL protein expression caused by miR-1236-3p
inhibition. Therefore, miR-1236-3p could regulate neuronal cell
apoptosis in the hippocampus in a CH rat model by regulating
TPT1/Pim-3 signaling.
In conclusion, the present study indicated that
miR-1236-3p was upregulated in new born rats with CH. Inhibition of
miR-1236-3p protected neurons from apoptosis by upregulating the
TPT1/Pim-3 axis. These results indicated that miR-1236-3p may be a
new potential therapeutic target for the treatment of CH.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM and SS contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. CL and XL contributed to data collection
and statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal care and experimental procedures were
performed in accordance with the National Institutes of Health
Guide for Care and approved by the Animal Ethics Committee of the
First Hospital of Jilin University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pardo Campos ML, Musso M, Keselman A,
Gruñeiro L, Bergadá I and Chiesa A: Cognitive profiles of patients
with early detected and treated congenital hypothyroidism. Arch
Argent Pediatr. 115:12–17. 2017.PubMed/NCBI
|
|
2
|
Knowles RL, Oerton J, Cheetham T, Butler
G, Cavanagh C, Tetlow L and Dezateux C: Newborn screening for
primary congenital hypothyroidism: Estimating test performance at
different TSH thresholds. J Clin Endocrinol Metab. 103:3720–3728.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
LaFranchi S: Congenital hypothyroidism:
Etiologies, diagnosis, and management. Thyroid. 9:735–740. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akaike M, Kato N, Ohno H and Kobayashi T:
Hyperactivity and spatial maze learning impairment of adult rats
with temporary neonatal hypothyroidism. Neurotoxicol Teratol.
13:317–322. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hashimoto K, Curty FH, Borges PP, Lee CE,
Abel ED, Elmquist JK, Cohen RN and Wondisford FE: An unliganded
thyroid hormone receptor causes severe neurological dysfunction.
Proc Natl Acad Sci USA. 98:3998–4003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimokawa N, Yousefi B, Morioka S,
Yamaguchi S, Ohsawa A, Hayashi H, Azuma A, Mizuno H, Kasagi M,
Masuda H, et al: Altered cerebellum development and dopamine
distribution in a rat genetic model with congenital hypothyroidism.
J Neuroendocrinol. 26:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Porterfield SP and Hendrich CE: The role
of thyroid hormones in prenatal and neonatal neurological
development-current perspectives. Endocr Rev. 14:94–106. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mendes-de-Aguiar CB, Alchini R, Zucco JK,
Costa-Silva B, Decker H, Alvarez-Silva M, Tasca CI and Trentin AG:
Impaired astrocytic extracellular matrix distribution under
congenital hypothyroidism affects neuronal development in vitro. J
Neurosci Res. 88:3350–3360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koibuchi N: The role of thyroid hormone on
cerebellar development. Cerebellum. 7:530–533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koibuchi N: Animal models to study thyroid
hormone action in cerebellum. Cerebellum. 8:89–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zendel BR, Willoughby KA and Rovet JF:
Neuroplastic effects of music lessons on hippocampal volume in
children with congenital hypothyroidism. Neuroreport. 24:947–950.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang XW, Yin HM, Ji C, Qin YF, Yang RW
and Zhao ZY: Effects of perinatal hypothyroidism on rat behavior
and its relation with apoptosis of hippocampus neurons. J
Endocrinol Invest. 31:8–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bongers-Schokking JJ and de Muinck
Keizer-Schrama SM: Influence of timing and dose of thyroid hormone
replacement on mental, psychomotor, and behavioral development in
children with congenital hypothyroidism. J Pediatr. 147:768–774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaravinos A: The regulatory role of
MicroRNAs in EMT and cancer. J Oncol. 2015:8658162015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
16
|
Li W, Song D, Sun Y, Lv Y and Lv J:
microRNA-124-3p inhibits the progression of congenital
hypothyroidism via targeting programmed cell death protein 6. Exp
Ther Med. 15:5001–5006. 2018.PubMed/NCBI
|
|
17
|
Jones D, Li Y, He Y, Xu Z, Chen H and Min
W: Mirtron microRNA-1236 inhibits VEGFR-3 signaling during
inflammatory lymphangiogenesis. Arterioscler Thromb Vasc Biol.
32:633–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Yan S, Liu X, Zhang W, Li Y, Dong
R, Zhang Q, Yang Q, Yuan C, Shen K and Kong B: miR-1236-3p
represses the cell migration and invasion abilities by targeting
ZEB1 in high-grade serous ovarian carcinoma. Oncol Rep.
31:1905–1910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan TH, Chen L and Guan XY: Role of
translationally controlled tumor protein in cancer progression.
Biochem Res Int. 2012:369–384. 2012. View Article : Google Scholar
|
|
20
|
Gu X, Yao L, Ma G, Cui L, Li Y, Liang W,
Zhao B and Li K: TCTP promotes glioma cell proliferation in vitro
and in vivo via enhanced β-catenin/TCF-4 transcription. Neuro
Oncol. 16:217–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaarbo M, Storm ML, Qu S, Wæhre H, Risberg
B, Danielsen HE and Saatcioglu F: TCTP is an androgen-regulated
gene implicated in prostate cancer. PLoS One. 8:e693982013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang F, Liu B, Wang Z, Yu XJ, Ni QX, Yang
WT, Mukaida N and Li YY: A novel regulatory mechanism of Pim-3
kinase stability and its involvement in pancreatic cancer
progression. Mol Cancer Res. 11:1508–1520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Wang Q, Wang F, Zhang X, Zhang L,
Tang Y and Wang S: LncRNA LINC01446 promotes glioblastoma
progression by modulating miR-489-3p/TPT1 axis. Biochem Biophys Res
Commun. 503:1484–1490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fabian ID, Rosner M, Fabian I,
Vishnevskia-Dai V, Zloto O, Shinderman Maman E, Cohen K, Ellis M,
Lin HY, Hercbergs A, et al: Low thyroid hormone levels improve
survival in murine model for ocular melanoma. Oncotarget.
6:11038–11046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu LJ, Ouyang YB, Xiong X, Stary CM and
Giffard RG: Post-stroke treatment with miR-181 antagomir reduces
injury and improves long-term behavioral recovery in mice after
focal cerebral ischemia. Exp Neurol. 264:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang YN, Lai CC, Chiu CT, Lin JJ and Wang
JY: L-ascorbate attenuates the endotoxin-induced production of
inflammatory mediators by inhibiting MAPK activation and NF-κB
translocation in cortical neurons/glia Cocultures. PLoS One.
9:e972762014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Teng W, Gao Y, Fan C, Zhang H and
Shan Z: Early levothyroxine treatment on maternal subclinical
hypothyroidism improves spatial learning of offspring in rats. J
Neuroendocrinol. 24:841–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sartim AG, Brito BM, Gobira PH and Joca
SRL: Attenuation of glutamatergic and nitrergic system contributes
to the antidepressant-like effect induced by capsazepine in the
forced swimming test. Behav Pharmacol. 30:59–66. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berbel P, Mestre JL, Santamaria A, Palazón
I, Franco A, Graells M, González-Torga A and de Escobar GM: Delayed
neurobehavioral development in children born to pregnant women with
mild hypothyroxinemia during the first month of gestation: The
importance of early iodine supplementation. Thyroid. 19:511–519.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Velasco I, Carreira M, Santiago P, Muela
JA, García-Fuentes E, Sánchez-Muñoz B, Garriga MJ,
González-Fernández MC, Rodríguez A, Caballero FF, et al: Effect of
iodine prophylaxis during pregnancy on neurocognitive development
of children during the first two years of life. J Clin Endocrinol
Metab. 94:3234–3241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mastorakos G, Karoutsou EI, Mizamtsidi M
and Creatsas G: The menace of endocrine disruptors on thyroid
hormone physiology and their impact on intrauterine development.
Endocrine. 31:219–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo Y, Wan SY, Zhong X, Zhong MK and Pan
TR: Levothyroxine replacement therapy with vitamin E
supplementation prevents the oxidative stress and apoptosis in
hippocampus of hypothyroid rats. Neuro Endocrinol Lett. 35:684–690.
2014.PubMed/NCBI
|
|
34
|
Ghorbani S, Talebi F, Chan WF, Masoumi F,
Vojgani M, Power C and Noorbakhsh F: MicroRNA-181 variants regulate
T cell phenotype in the context of autoimmune neuroinflammation.
Front Immunol. 8:7582017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song J and Kim YK: Identification of the
role of miR-142-5p in Alzheimer's disease by comparative
bioinformatics and cellular analysis. Front Mol Neurosci.
10:2272017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grasso M, Piscopo P, Confaloni A and Denti
MA: Circulating miRNAs as biomarkers for neurodegenerative
disorders. Molecules. 19:6891–6910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang C, Chen Z, Ge Q, Hu J, Li F, Hu J, Xu
H, Ye Z and Li LC: Up-regulation of p21(WAF1/CIP1) by miRNAs and
its implications in bladder cancer cells. FEBS Lett. 588:4654–4664.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neuhäuser K, Küper L, Christiansen H and
Bogdanova N: Assessment of the role of translationally controlled
tumor protein 1 (TPT1/TCTP) in breast cancersusceptibility and ATM
signaling. Clin Transl Radiat Oncol. 15:99–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li R, Zhu H, Yang D, Xia J and Zheng Z:
Long noncoding RNA lncBRM promotes proliferation and invasion of
colorectal cancer by sponging miR-204-3p and upregulating TPT1.
Biochem Biophys Res Commun. 508:1259–1263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mukaida N, Wang YY and Li YY: Roles of
Pim-3, a novel survival kinase, in tumorigenesis. Cancer Sci.
102:1437–1442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li YY, Popivanova BK, Nagai Y, Ishikura H,
Fujii C and Mukaida N: Pim-3, a proto-oncogene with
serine/threonine kinase activity, is aberrantly expressed in human
pancreatic cancer and phosphorylates bad to block bad-mediated
apoptosis in human pancreatic cancer cell lines. Cancer Res.
66:6741–6747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
You Y, Tan J, Gong Y, Dai H, Chen H, Xu X,
Yang A, Zhang Y and Bie P: MicroRNA-216b-5p Functions as a
Tumor-suppressive RNA by targeting TPT1 in pancreatic cancer cells.
J Cancer. 8:2854–2865. 2017. View Article : Google Scholar : PubMed/NCBI
|