Introduction
Intervertebral disk degeneration (IVDD) that has
unclear specific pathogenesis is mainly manifested as lower back
pain and acute nerve root pain of lower extremity (1). Data show that the disease is extremely
common worldwide. More than 80% of adults may suffer from different
degrees of the degeneration (2), and
the degree greatly increases among the middle-aged and elderly
patients (3). As the population
ages, the risk of IVDD is increasing (4). The disease can easily cause a series of
lumbar diseases such as intervertebral disc herniation and
degenerative disc diseases, even impaired walking and lower limb
paralysis (5). According to
statistics, over 40% of the lumbar diseases are mainly caused by it
(6). Currently, the treatment of
IVDD is based on conservative treatment or surgical operation, but
both only relieve symptoms and cannot completely cure the disease
(7).
Worldwide researchers are exploring new therapeutic
methods for increasingly serious IVDD. With the deepening of
research, increasing number of reports point out that cytobiology
may be crucial to treat the disease in the future (8,9). Bone
morphogenetic protein (BMP) plays a pivotal role in affecting the
synthesis of intervertebral disc extracellular matrix. In addition
to rebuilding intervertebral disc function, its upregulation
promotes the expression of type II collagen and glycoprotein in
normal nucleus pulposus cells and in the extracellular matrix of
degenerated nucleus pulposus (10).
Statin simvastatin is a hydroxymethylglutaryl coenzyme A (HMG-CoA)
reductase. Its pharmacological mechanism has been proven to
upregulate BMP-2 in nucleus pulposus cells through the mevalonic
acid pathway (11), but its clinical
application is limited due to its water insolubility (12). As a drug sustained-release system
commonly used in clinical practice, polylactic-co-glycolic acid
(PLGA) can specifically administer drugs, improve drug stability,
and control drug release, so it has been applied in the treatment
of many diseases (13,14). It is speculated that
simvastatin-loaded PLGA sustained release microspheres can cure
IVDD, which remains uncertain due to lack of relevant research
support. Therefore, in this study, a rat model of IVDD was
established, and the treatment with simvastatin-loaded PLGA
microspheres was observed, and prostaglandin markers
6-keto-prostaglandin F1α (6-K-PGF1α) and hypoxia inducible
factor-1α (HIF-1α) were detected to analyze the therapeutic value
of simvastatin for the disease.
Materials and methods
Animal data
Eighty 3-month-old Srague-Dawley (SD) female healthy
rats, weighting 210±20 g, were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd., with a certificate number
of SCXK (Beijing) 2016-0011. They were normally fed and housed in
lighted cages (5 rats each), with the temperature of 29±2°C and the
humidity of 40–50%.
Methods
Preparation of simvastatin-loaded
PLGA
PLGA and simvastatin were dissolved in methylene
chloride at 10:3.3, shaken and well mixed, and then added with
distilled water (200 µl) for ultrasonic mixing. Next, the mixture
was slowly added with 5% polyvinyl alcohol aqueous solution (100
ml) for ultrasonic mixing. Then, the mixture was added with 0.3%
polyvinyl alcohol aqueous solution (50 ml), stirred, well mixed,
and then allowed to stand at room temperature for 12 h. After the
organic solution was removed, the mixture was frozen and dried for
48 h.
Detection of encapsulation efficiency
and drug loading of microspheres
Microspheres (5 mg) were selected and prepared into
an ethanol solution (100 ml), to measure the optical density (238
nm) of the solution with ethanol as a blank control. The content of
simvastatin in the microspheres was calculated according to the
standard curve. Encapsulation efficiency = the total amount of
simvastatin/the total amount of microspheres ×100%. Drug loading =
actual drug loading/theoretical drug loading ×100%. The release
curve of simvastatin was plotted according to the cumulative
release.
Modeling method
All rats were divided into four groups (n=20 each)
based on random number table. Rats in the model and treatment
groups were modeled for IVDD, with the method described by Chen
et al (15). After the rats
were intraperitoneally injected with 2% pentobarbital sodium (40
mg/kg) for anesthesia, the morphology was observed. They were
determined to enter deep anesthesia if they had disappearing
responses to skin pain, relaxing muscles, and disappearing
responses to eye and face irritation, as well as smooth breathing.
Next, the rat chest was incised to expose the lower segment of the
thoracic vertebra, and the upper segment of the lumbar vertebra and
the sacrum. All lumbar spinous processes, supraspinous ligaments,
lumbar segment of interspinous ligaments, and posterior outer 1/2
of lumbar facet joints on both sides were removed. Subsequently,
deep fascia and skin were sutured, while erector spinae muscles
were not sutured, and then bilateral ovaries were removed via
ventral approach. X-ray fluoroscopy was conducted to determine
whether the modeling was successful. Rats in the sham operation
group were anesthetized. After that, their chest was incised to
expose the lower segment of the thoracic vertebra, and the upper
segment of the lumbar vertebra and the sacrum. Finally, the
incision was sutured. Rats in the control group were not treated.
Rats in the treatment group were treated with simvastatin-loaded
PLGA microspheres, and injected with normal saline (2 µl) at 5
mg/ml. Rats in the model group were injected with the same dose of
normal saline.
Outcome measures
After modeling, 5 rats in each group were sacrificed
by cervical dislocation under anesthesia before simvastatin
injection (T0), at 2 weeks (T1) and 4 weeks (T2) after simvastatin
injection, respectively. Enzyme-linked immunosorbent assay (ELISA)
was used to detect the concentrations of 6-K-PGF1α (the kit was
purchased from Shanghai Hengfei Biotechnology Co., Ltd.;
CSB-E14411r-1) and HIF-1α (the kit was purchased from Shanghai
Qiaoyu Industrial Co., Ltd.; QY-Q11659) in the peripheral blood of
rats. The lumbar vertebrae L5-6 of the rats were
obtained, and the bone mineral density (BMD) of L5 and
L6 was detected by X-ray. After the injection, CT was
carried out to scan the vertebral body of the rats and reconstruct
the scanned area in three dimensions. The trabecular thickness,
number, and separation of the vertebral body were detected. Changes
in the sagittal T2-weighted signal were detected by MRI to detect
the intervertebral disc nucleus pulposus of the rats.
Evaluation program of humane
endpoint
Weight loss: The rats lost 15–20% of the original
body weight within 2 days, or they had no sustained weight gain in
growth period, or they had cachexia and sustained muscle
consumption without weight monitoring. Weakness: The rats were
unable to feed and drink on their own, and they were unable to
stand for 24 h or they could stand with extreme efforts. The
recovery period after anesthesia was excluded, and then whether
they were weak due to diseases, experiments, or other factors was
evaluated. Organ infection: The rats had abnormal physical indices
and blood test values, but no good response to drug treatment;
additionally, the disease progressed to a systemic disease.
Respiratory system: The rats had severe respiratory tract
infection, dyspnea, or cyanosis. Circulatory system: The rats had
severe anemia, uncontrollable hemorrhage (PVC <15%), or
jaundice. Nervous system: The rats had abnormal central nervous
system reactions (convulsion, trembling, paralysis, and
torticollis) or uncontrollable pain. Others: The rats had
persistent self-mutilation behavior, non-healing wounds, diseases
that seriously affected eating and drinking water, advanced
infectious diseases, persistent hypothermia, significant functional
damage to organs and facial features, or behaviors and
physiological phenomena when they suffered from distress and pain.
The study was approved by the Ethics Committee of Shandong
Provincial Hospital Affiliated to Shandong University.
Death determination
The rats were laid on one side and they were
determined to be dead if they could not turn over to a normal
posture within 30 sec.
Statistical analysis
In this study, SPSS 24.0 (Shanghai Yuchuang Network
Technology Co., Ltd.) was used to statistically analyze the
results. GraphPad 8 (Softhead Inc.) was used to plot figures.
Enumeration data were expressed by rate, and Chi-square test was
used for comparison between groups. Measurement data were expressed
as mean ± standard deviation, and t-test was used for comparison
between groups. Repeated measures analysis of variance and
Bonferroni post hoc test were used for comparison between multiple
time-points. P<0.050 indicated a statistically significant
difference.
Results
Simvastatin-loaded PLGA sustained
release microspheres
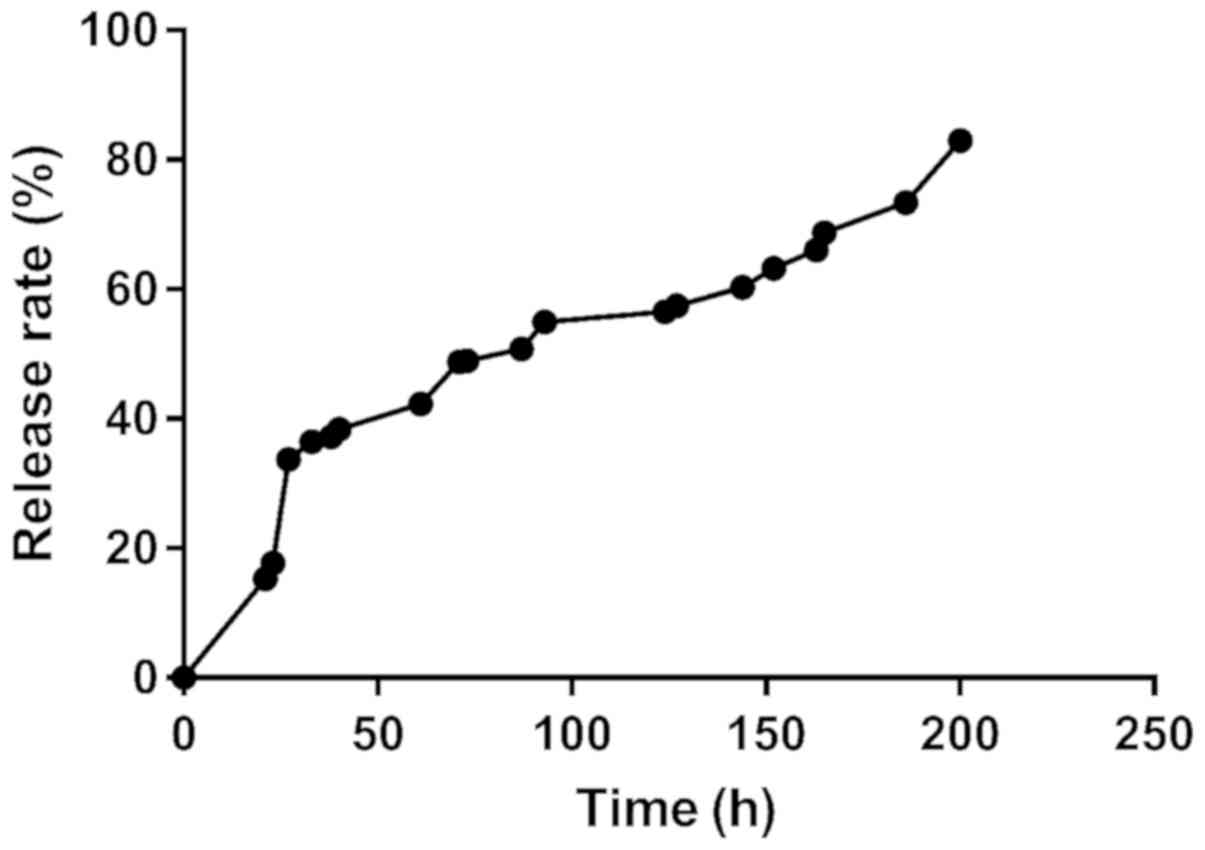
The drug loading of the microspheres was
22.85±1.85%, and the encapsulation efficiency was 91.12±2.78%, and
the cumulative release was >80%, which indicated successful
preparation (Fig. 1).
Modeling results
Of the 40 rats modeled, 20 in the treatment group
and 19 in the model group were successfully modeled, with a success
rate of 97.5%.
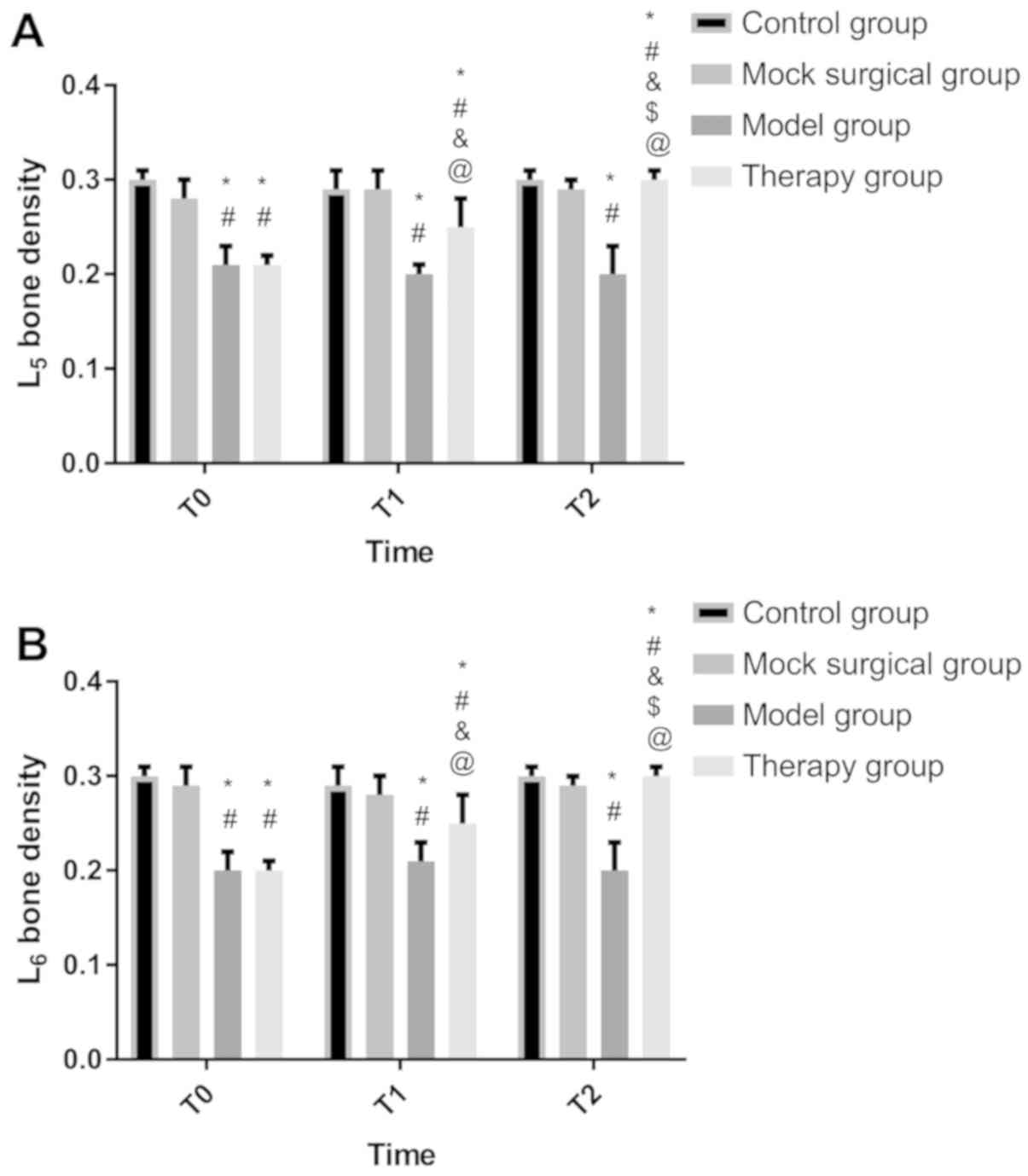
Detection results of BMD
At T0, there was no significant difference in BMD
between the treatment and model groups (P>0.050), and between
the sham operation and control groups (P>0.050). BMD in the
model and treatment groups was lower than that in the sham
operation and control groups (P<0.050). At T1, there was no
significant difference between the sham operation and control
groups (P>0.050). BMD in the two groups was higher than that in
the model and treatment groups, while BMD in the treatment group
was higher than that in the model group (P<0.050). At T2, there
was no significant difference between the sham operation, control,
and treatment groups (P>0.050), and BMD in the three groups was
higher than that in the model group (P<0.050). There was no
difference in BMD at T0, T1 and T2 between the sham operation,
control, and model groups (P>0.050). BMD in the treatment group
was the lowest at T0 and the highest at T2 (P<0.050) (Fig. 2).
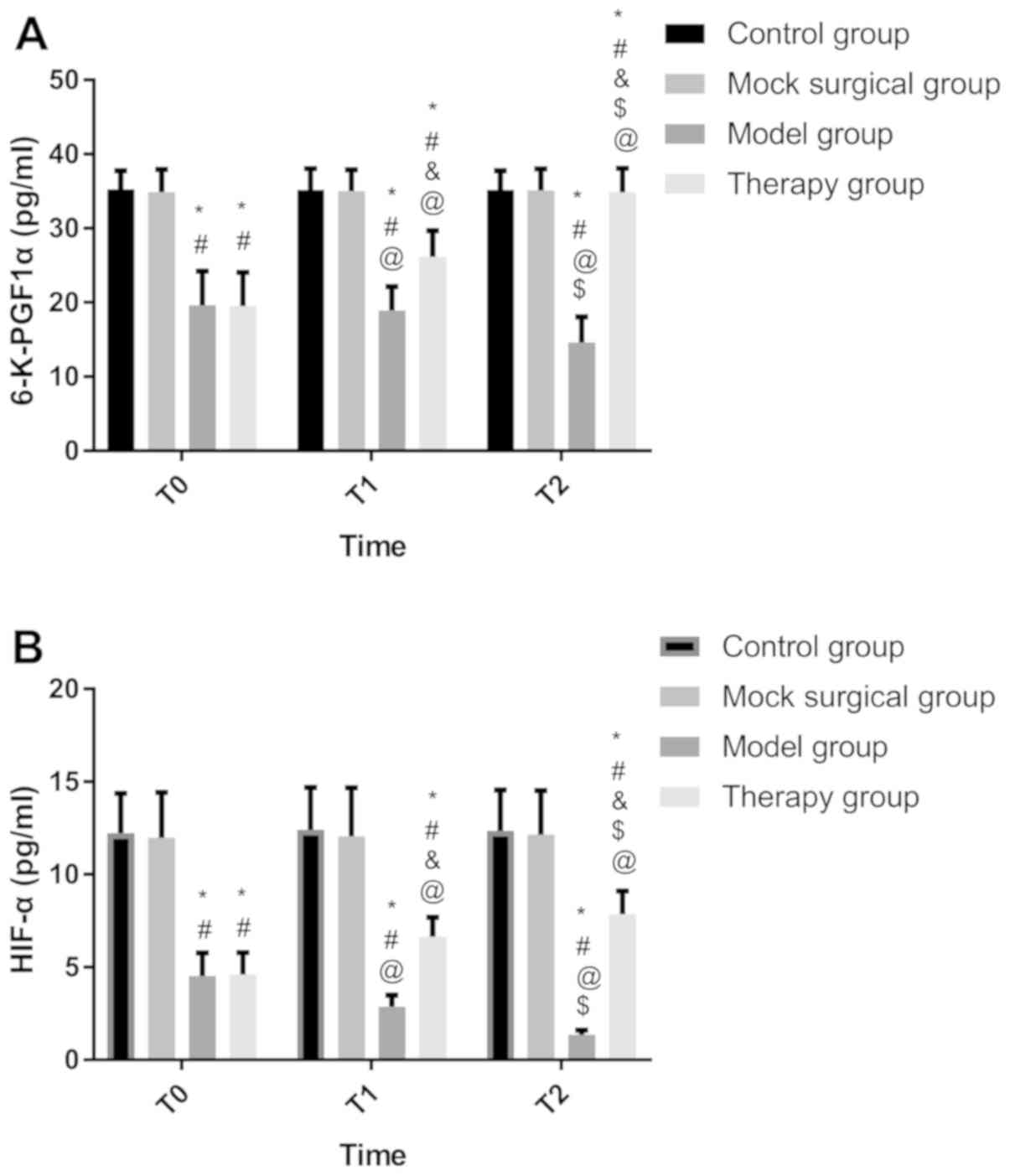
6-K-PGF1α and HIF-1α
At T0, there were no differences in 6-K-PGF1α and
HIF-1α between the control and sham operation groups (P>0.050),
but there were differences between the model and treatment groups
(P>0.050). The two indices in the control and sham operation
groups were higher than those in the model and treatment groups
(P<0.050). At T1 and T2, there were no differences between the
control and sham operation groups (P>0.050). The two indices in
the two groups were higher than those in the treatment and model
groups (P<0.050), while those in the treatment group were higher
than those in the model group (P<0.050). There were no
differences in the indices at T0, T1 and T2 between the control and
sham operation groups (P<0.050). The indices in the model group
were the highest at T0 and the lowest at T2 (P<0.050). The
indices in the treatment group were the lowest at T0 and the
highest at T2 (P<0.050) (Fig.
3).
CT results
There was no difference in the trabecular thickness
between the four groups (P>0.050), and in the trabecular number
and separation between the control and sham operation groups
(P>0.050). The trabecular number in the control and sham
operation groups was higher than that in the treatment and model
groups, and that in the model group was the lowest (P<0.050).
The trabecular separation in the two groups was lower than that in
the treatment and model groups, and that in the model group was the
highest (P<0.050) (Table I).
 | Table I.Comparison of CT results. |
Table I.
Comparison of CT results.
|
| Control | Sham operation
group | Model group | Treatment group | F-value | P-value |
|---|
| Trabecular thickness
(µm) | 105.52±4.28 | 101.52±3.15 | 103.62±3.42 | 103.57±4.16 | 0.933 | 0.448 |
| Trabecular number
(mm) | 2.34±0.42 | 2.28±0.39 |
1.42±0.24a,b |
1.83±0.25a–c | 8.249 | 0.002 |
| Trabecular separation
(µm) | 250.62±28.63 | 262.61±30.57 |
368.45±32.65a,b |
316.74±25.61a–c | 16.880 | <0.001 |
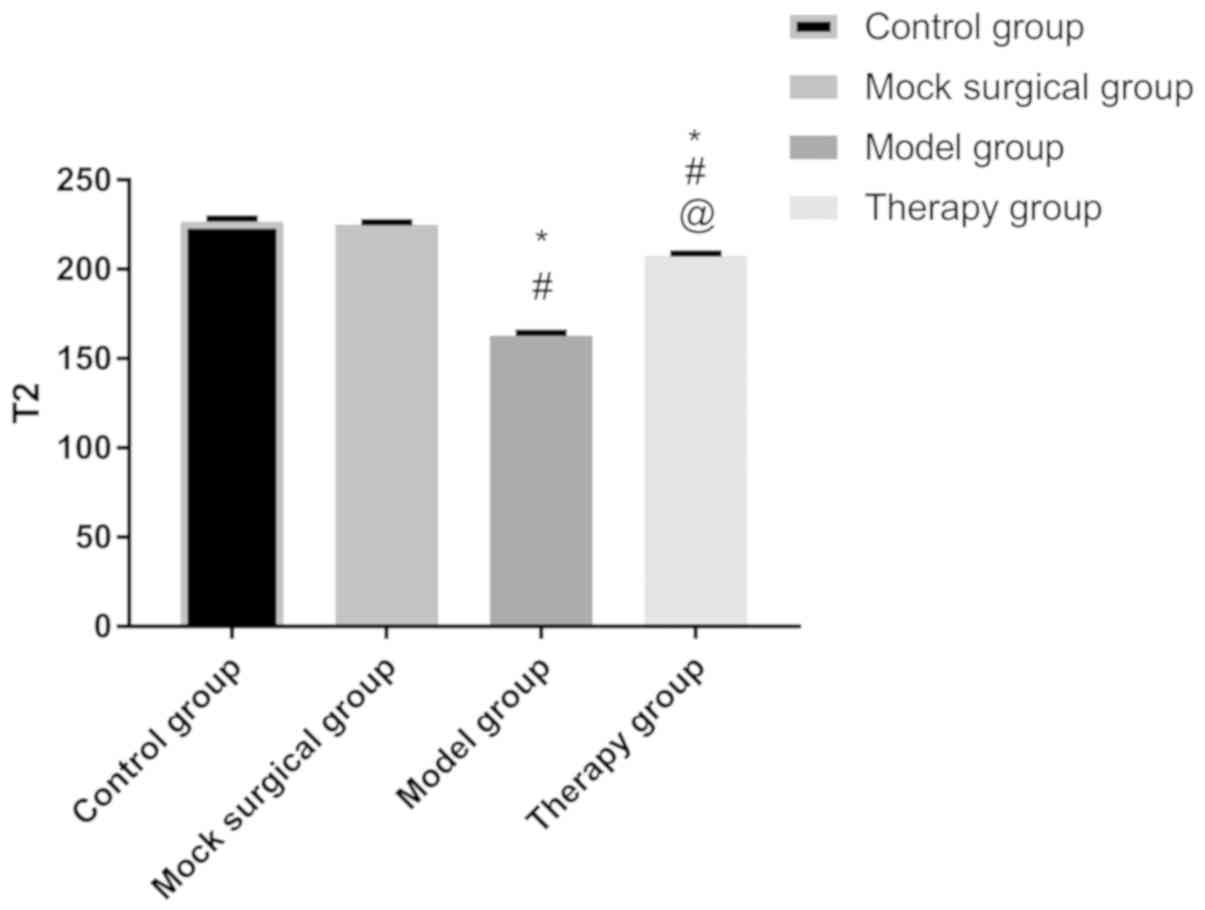
MRI results
After 8-week injection, there was no difference in
the sagittal T2-weighted MRI signal of the intervertebral disc
nucleus pulposus between the control and sham operation groups
(P>0.050). The signal in the two groups was higher than that in
the model and treatment groups (P<0.050), and that in the model
group was the lowest (Fig. 4).
Discussion
IVDD is a very common chronic disease around the
world with a high incidence and great harm, so it has long been a
major research project in clinical practice (16). The effect of simvastatin on IVDD has
been unanimously agreed, it improves bone quality by promoting the
aggregation of type II collagen. However, it has not yet been
widely used in clinical practice due to its extremely high
limitation in human body (17). In
this study, the effects of simvastatin-loaded PLGA microspheres on
treating rats with IVDD were observed, to analyze the effective
scheme of simvastatin in the treatment. In addition, 6-K-PGF1α and
HIF-1α in the rats were detected to preliminarily analyze the
influencing mechanism of simvastatin, which is of great
significance for the clinical treatment of the disease in the
future.
In this study, BMD in the treatment group was
significantly higher than that in the model group after treatment,
but partial BMD was not significantly different from that in the
control group at T2. This confirms the important role of
simvastatin in the bone tissue and the feasibility of treating IVDD
with simvastatin-loaded PLGA sustained release microspheres, which
was also proved by comparing the trabecular number, the trabecular
separation, and the sagittal T2-weighted MRI signal between the
four groups. Organic polymer material PLGA has good
biodegradability, compatibility, non-irritant, and
non-immunogenicity, so it has a great application prospect in
clinical practice (18). PLGA was
chosen as the sustained-release material of simvastatin, because
its excellent drug loading, encapsulation efficiency, and release
ensure its stable concentration for homeostasis during the action
of simvastatin on IVDD. Many studies have confirmed that
simvastatin mainly affects the intervertebral disc by upregulating
BMP-2 (19,20), which was therefore not repeated here.
According to the detection, 6-K-PGF1α and HIF-1α at T0 in the
treatment and model groups were lower than those in the control and
sham operation groups, while the two indices in the treatment group
significantly increased at T1 and T2, and were higher than those in
the control and sham operation groups at T2. These findings suggest
that simvastatin has a significant effect on 6-K-PGF1α and HIF-1α.
Prostaglandin I2 (PGI2), a substance
reflecting bioactivity and produced by arachidonic acid metabolism,
dilates blood vessels and controls platelet aggregation (21). In human body, it is quickly
hydrolyzed into inactive 6-K-PGF1α and maintains blood circulation
(22). Fan et al (23) investigated the role of 6-K-PGF1α in
hyperlipidemia and found that it is important for the formation of
thrombosis. Although the pathogenesis of IVDD is not yet clear,
previous studies have shown that the number of vascular buds in
cartilage endplate decreases during pathogenesis, so hemodynamic
abnormality may be a pathogenic factor for the disease (24). The significant effect of 6-K-PGF1α on
hemodynamics may also be a mechanism of simvastatin in the
treatment of IVDD. It is speculated that simvastatin may play an
antithrombotic role by increasing the content of 6-K-PGF1α, so as
to enhance the hemodynamics of patient tissues, and then protect
and improve the integrity of bone tissues. 6-K-PGF1α usually
functions in human body through coordinating with thromboxane
A2 (TXA2). In this study, TXA2 in
each group was not detected, but it is a research emphasis in the
future. As an important cytokine that makes tissues and cells adapt
rapidly to low oxygen environment, HIF-1α promotes the generation
of vascular endothelial growth factor (VEGF) and glucose receptor,
and regulates cell apoptosis and differentiation (25). Lumbar intervertebral disc cells have
been in a low oxygen microenvironment for a long time due to the
aging of the body. At this time, HIF-1α is activated, which
accelerates the synthesis of VEGF and protein (26). The increase in HIF-1α after
simvastatin injection is possibly because the upregulation of VEGF
expression promotes cardiovascular formation in the intervertebral
disc tissue, thus delaying the progression of IVDD. Additionally,
studies have confirmed the close correlations of 6-K-PGF1α and
HIF-1α with BMP-2 (27,28). However, it is also possible that
simvastatin regulates 6-K-PGF1α and HIF-1α through BMP-2 and then
affects IVDD, which needs further confirmation.
This study has deficiencies due to the limited
experimental conditions. For example, because of the differences
between animal models and human beings, the concentration of
simvastatin should be further confirmed, and the drug resistance of
humans should be considered.
In conclusion, simvastatin-loaded PLGA sustained
release microspheres can improve the BMD of the vertebral body and
increase the contents of 6-K-PGF1α and HIF-1α in the treatment of
rats with IVDD, so they are important for the clinical treatment of
the disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZK wrote the manuscript. ZK and ZF conceived and
designed the study. ZF and YY were responsible for the collection
and analysis of the experimental data. ZK and MW interpreted the
data and drafted the manuscript. ZK and YY revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shandong Provincial Hospital Affiliated to Shandong University,
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feng C, Liu H, Yang M, Zhang Y, Huang B
and Zhou Y: Disc cell senescence in intervertebral disc
degeneration: Causes and molecular pathways. Cell Cycle.
15:1674–1684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng Y, Egan B and Wang J: Genetic factors
in intervertebral disc degeneration. Genes Dis. 3:178–185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang F, Cai F, Shi R, Wang XH and Wu XT:
Aging and age related stresses: A senescence mechanism of
intervertebral disc degeneration. Osteoarthritis Cartilage.
24:398–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang F, Zhao X, Shen H and Zhang C:
Molecular mechanisms of cell death in intervertebral disc
degeneration (Review). Int J Mol Med. 37:1439–1448. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun D, Liu P, Cheng J, Ma Z, Liu J and Qin
T: Correlation between intervertebral disc degeneration, paraspinal
muscle atrophy, and lumbar facet joints degeneration in patients
with lumbar disc herniation. BMC Musculoskelet Disord. 18:1672017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Corniola MV, Stienen MN, Joswig H, Smoll
NR, Schaller K, Hildebrandt G and Gautschi OP: Correlation of pain,
functional impairment, and health-related quality of life with
radiological grading scales of lumbar degenerative disc disease.
Acta Neurochir (Wien). 158:499–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeckser J, Wolff M, Tucker J and Goodwin
J: Multipotent mesenchymal stem cell treatment for discogenic low
back pain and disc degeneration. Stem Cells Int. 2016:39083892016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakai D and Schol J: Cell therapy for
intervertebral disc repair: Clinical perspective. J Orthop
Translat. 9:8–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen K, Lv X, Li W, Yu F, Lin J, Ma J and
Xiao D: Autophagy is a protective response to the oxidative damage
to endplate chondrocytes in intervertebral disc: implications for
the treatment of degenerative lumbar disc. Oxid Med Cell Longev.
2017:40417682017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye S, Ju B, Wang H and Lee KB: Bone
morphogenetic protein-2 provokes interleukin-18-induced human
intervertebral disc degeneration. Bone Joint Res. 5:412–418. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai L, Xu M, Wu H, Xue L, Yuan D, Wang Y,
Shen Z, Zhao H and Hu M: The functional mechanism of simvastatin in
experimental osteoporosis. J Bone Miner Metab. 34:23–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elkadi S, Elsamaligy S, Al-Suwayeh S and
Mahmoud H: The development of self-nanoemulsifying liquisolid
tablets to improve the dissolution of simvastatin. AAPS
PharmSciTech. 18:2586–2597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steinbach JM, Seo YE and Saltzman WM: Cell
penetrating peptide-modified poly(lactic-co-glycolic acid)
nanoparticles with enhanced cell internalization. Acta Biomater.
30:49–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, An T, Wang D, Wan G, Zhang M,
Wang H, Zhang S, Li R, Yang X and Wang Y: Stepwise pH-responsive
nanoparticles containing charge-reversible pullulan-based shells
and poly(β-amino ester)/poly(lactic-co-glycolic acid) cores as
carriers of anticancer drugs for combination therapy on
hepatocellular carcinoma. J Control Release. 226:193–204. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Xuan J, Gu YT, Shi KS, Xie JJ,
Chen JX, Zheng ZM, Chen Y, Chen XB, Wu YS, et al: Celastrol reduces
IL-1β induced matrix catabolism, oxidative stress and inflammation
in human nucleus pulposus cells and attenuates rat intervertebral
disc degeneration in vivo. Biomed Pharmacother. 91:208–219. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Tao H, Wang H, Dong F, Zhang R, Li
J, Ge P, Song P, Zhang H, Xu P, et al: Biological behavior of human
nucleus pulposus mesenchymal stem cells in response to changes in
the acidic environment during intervertebral disc degeneration.
Stem Cells Dev. 26:901–911. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gentile P, Nandagiri VK, Daly J, Chiono V,
Mattu C, Tonda-Turo C, Ciardelli G and Ramtoola Z: Localised
controlled release of simvastatin from porous chitosan-gelatin
scaffolds engrafted with simvastatin loaded PLGA-microparticles for
bone tissue engineering application. Mater Sci Eng C. 59:249–257.
2016. View Article : Google Scholar
|
|
18
|
Ramazani F, Chen W, van Nostrum CF, Storm
G, Kiessling F, Lammers T, Hennink WE and Kok RJ: Strategies for
encapsulation of small hydrophilic and amphiphilic drugs in PLGA
microspheres: State-of-the-art and challenges. Int J Pharm.
499:358–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Terauchi M, Inada T, Tonegawa A, Tamura A,
Yamaguchi S, Harada K and Yui N: Supramolecular inclusion
complexation of simvastatin with methylated β-cyclodextrins for
promoting osteogenic differentiation. Int J Biol Macromol.
93:1492–1498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang P, Han F, Li Y, Chen J, Chen T, Zhi
Y, Jiang J, Lin C, Chen S and Zhao P: Local delivery of
controlled-release simvastatin to improve the biocompatibility of
polyethylene terephthalate artificial ligaments for reconstruction
of the anterior cruciate ligament. Int J Nanomedicine. 11:465–478.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Russell-Puleri S, Dela Paz NG, Adams D,
Chattopadhyay M, Cancel L, Ebong E, Orr AW, Frangos JA and Tarbell
JM: Fluid shear stress induces upregulation of COX-2 and
PGI2 release in endothelial cells via a pathway
involving PECAM-1, PI3K, FAK, and p38. Am J Physiol Heart Circ
Physiol. 312:H485–H500. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayoral-Andrade G, Pérez-Campos-Mayoral L,
Majluf-Cruz A, Perez-Campos Mayoral E, Perez Campos Mayoral C,
Rocha-Núñez A, Martinez M, Zenteno E, Hernandez-Gonzalez L, López
Juan MG, et al: Reduced platelet aggregation in women after
intercourse: A possible role for the cyclooxygenase pathway. Clin
Exp Pharmacol Physiol. 44:847–853. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan H, Li M, Yu L, Jin W, Yang J, Zhang Y
and Wan H: Effects of Danhong injection on platelet aggregation in
hyperlipidemia rats. J Ethnopharmacol. 212:67–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mok FPS, Samartzis D, Karppinen J, Fong
DY, Luk KD and Cheung KM: Modic changes of the lumbar spine:
Prevalence, risk factors, and association with disc degeneration
and low back pain in a large-scale population-based cohort. Spine
J. 16:32–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiang CH, Chu PY, Hou MF and Hung WC:
MiR-182 promotes proliferation and invasion and elevates the
HIF-1α-VEGF-A axis in breast cancer cells by targeting FBXW7. Am J
Cancer Res. 6:1785–1798. 2016.PubMed/NCBI
|
|
26
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang TH, Chung SY, Chua S, Chai HT, Sheu
JJ, Chen YL, Chen CH, Chang HW, Tong MS, Sung PH, et al: Effect of
early administration of lower dose versus high dose of fresh
mitochondria on reducing monocrotaline-induced pulmonary artery
hypertension in rat. Am J Transl Res. 8:5151–5168. 2016.PubMed/NCBI
|
|
28
|
Hong F, Wang X and Tu G: Underlying
mechanism of effect of Shenfu injection on Buerger's disease model
rats. 2011 International Conference on Human Health and Biomedical
Engineering IEEE. 1041–1044. 2011. View Article : Google Scholar
|