Introduction
Technological advancements in genetic engineering
and antigen purification technology have accelerated the production
of subunit antigens and peptides in recent years. These antigens
confer many advantages over traditional vaccines, including smaller
molecular weight, higher purity and generally improved safety
(1,2); however, following purification, they
exhibit lower reactivity and immunogenic potential compared with
conventional vaccines, especially when derived from recombinant
proteins and DNA (3). Therefore,
there is an urgent need to develop novel vaccination adjuvants to
improve antigen immunogenicity. Although various adjuvants have
been used in conjunction with experimental vaccines, most display
harmful effects, including poor immune efficacy and safety
problems, which limit their potential for use in vaccines (4,5).
Adjuvants have profound effects on the immune
response, which can skew the immune system toward either T helper
(Th)1- or Th2-type responses (6–8).
Elevated levels of the cytokines interleukin (IL)-2, tumor necrosis
factor-β (TNF-β) and interferon-γ (IFN-γ), coupled with the
enhanced production of immunoglobulin (IgG)2a, IgG2b and IgG3, are
characteristics of the Th1 immune response in mice (9). By contrast, the Th2 response is
characterized by elevated levels of the cytokines IL-4, IL-5 and
IL-10, alongside enhanced production of IgG1 and secretory IgA
(10). The adjuvants that are
currently used predominantly stimulate the Th2 rather than the Th1
immune responses. For instance, lipid A and its derivatives do not
stimulate cytotoxic T lymphocytes (CTLs) in animals immunized with
antigens, whilst water/oil emulsion adjuvants and aluminum
hydroxide gel (alum) adjuvants can only elicit Th2-based immunity
(11). It is of great interest to
improve the efficacy of adjuvants in activating both the Th1 and
Th2 responses as well as the safety.
In general, extracts from traditional Chinese
medicine enhance immune responses and do not exhibit toxicity to
normal, healthy cells, making them attractive candidates as vaccine
adjuvants (12). Over the past
decade, research on immune adjuvants has focused mainly on
polysaccharides and saponins (12,13).
However, they have disadvantages, including lack of activity,
toxicity, hemolysis and the potential to cause infection (4,5).
Isoflavones have been previously demonstrated to
exhibit a number of effects on the immune function of animals,
characterized by enhancement of the immune response and the
regulation of excessive inflammatory cytokine expression (14). Rasouli and Jahanian (15) demonstrated that the isoflavone
genistein not only increased the weight and growth of broiler
chicks, but also exerted beneficial effects on the immunological
response and increased the proportion of lymphocytes to
heterophils. In addition, isoflavones significantly improved serum
antibody levels against swine fever and enhance the reactivity of T
lymphocytes to phytohemagglutinin (16). Importantly, a previous study found
that diet supplementation with red clover isoflavone extract (RCIE)
significantly increased the levels of antibodies in the serum of
piglets, eased the inhibition of piglet growth induced by immune
stress, and significantly decreased the secretion and expression of
inflammatory cytokines including TNF-γ and IL-6 (17). The aforementioned studies suggest
that RCIE has immunoregulatory properties, highlighting its promise
as a potential vaccine adjuvant. Although previous studies of RCIE
have focused on its ability to improve immune function in humans
(18), the possible use of RCIE as
an immune adjuvant has not been previously reported. Therefore, in
the present study, the role of RCIE as possible vaccine adjuvant
was explored.
Materials and methods
Materials
Ovalbumin (OVA), concanavalin A (ConA) and
lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (Merck
KGaA). Goat anti-mouse IgG (cat. no. 1036-05), IgG1 (cat. no.
1070-05) and IgG2a (cat. no. 1080-05) horseradish peroxidase
conjugates were purchased from SouthernBiotech. The RPMI-1640
medium was purchased from Hyclone (GE Healthcare Life Sciences).
Fetal bovine serum (FBS) was supplied by Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd. Aluminum hydroxide gel
(alum) was supplied by Zhejiang Wanma Pharmaceutical Co., Ltd. Cell
Counting kit-8 (CCK-8) was purchased from Vazyme. All other
chemicals were of analytical reagent grade. The ELISA kits (IFN-γ,
cat. no. F10660; TNF-α cat. no. F11630; IL-2 cat. no. F10780; IL-4
cat. no. F10810; and IL-5 cat. no. F10820) were from Shanghai
Westang Biotechnology Co., Ltd. FITC/phycoerythrin (PE) rat
anti-mouse CD4/CD8 antibodies (cat. no. FMD001-050) were purchased
from Beijing 4A Biotech Co., Ltd. RNAiso plus® (cat. no.
9108), First Strand cDNA Synthesis kit (cat. no. RR036A) and
SYBR® Premix Ex Taq™ were purchased from Takara
Biotechnology Co., Ltd. Pathogenic porcine Escherichia coli
(E. coli) (19) was kindly
provided by Dr Jingxuan Ni (Longyan University). RCIE (cat. no.
NAT-177) was purchased from Naturalin Bio-Resources Co., Ltd and
standardized to obtain a concentration of 10% isoflavone
(consisting of 10.2% formononetin, 9.6% biochanin A, 0.32%
genistein and 0.08% daidzein). The solubility of RCIE was improved
by treating with NaOH at pH 12 and 60°C for 6 h as previously
reported (20,21).
Animal experiments
A total of 100 female ICR (CD-1) mice (age, 6 weeks,
grade II; weight range, 18–22 g) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (certificate no. SCXK2016-0003). All
mice were maintained under the conventional housing conditions for
one week prior to experiments. They were housed under controlled
conditions, specifically at 24±2°C, with 50±10%, humidity with a 12
h light/dark cycle, and were provided free access to food and
drinking water.
Induction of the immune response
following OVA immunization with adjuvants
The experimental protocol for testing the immune
adjuvant response induced by RCIE combined with OVA was as
described in previous studies (3,22).
Briefly, the mice were divided into 6 groups (n=10 mice/group):
Saline group was defined as blank control; other groups of mice
were subcutaneously immunized with 100 µg OVA alone or 100 µg OVA +
adjuvant (200 µg alum or 50, 100 or 200 µg RCIE) on day 1. Mice
received a booster injection after 2 weeks. Splenocytes, serum and
peripheral blood were collected 2 weeks following the booster
injection. After immunization, the mental state (23), diet and activity of the mice was
monitored every day.
Vaccine challenge using E. coli with
adjuvants
A challenge test using RCIE combined with E.
coli vaccine was conducted in mice as previously described
(19,21). Pathogenic E. coli was
inactivated using 0.2% formaldehyde at 65°C for 8 h. Following a
sterility test, the live E. coli vaccine was prepared to a
final concentration of 1×108 CFU/ml.
A total of 40 mice were randomly divided into the
following four groups (n=10/group): i) Vehicle group, which was
subcutaneously injected with 0.2 ml saline; ii) saline + E
coli group, which was subcutaneously injected with 0.1 ml E.
coli vaccine (containing 1×107 CFU) and 0.1 ml
saline; iii) alum + E. coli group, which was subcutaneously
injected with 0.1 ml E. coli vaccine (containing
1×107 CFU) and 0.1 ml alum (200 µg/0.1 ml); and iv) RCIE
+ E. coli group, which was subcutaneously injected with 0.1
ml E. coli vaccine (containing 1×107 CFU) and 0.1
ml RCIE (100 µg/0.1 ml). Each mouse was subsequently injected with
0.2 ml (2×107 CFU) pathogenic E. coli 3 days
following immunization. Mouse mortality was recorded 2 h following
bacterial challenge, and every 6 h thereafter. The survival rate
(%) was calculated as follows: (The number of surviving mice/total
number of mice) ×100.
Humane and experimental endpoints
For the present study, humane endpoints were
established and applied at the earliest experimental timepoint
without adversely affecting scientific objectives. The endpoints
for the animal experiments were 2 weeks following the booster
injection and 48 h after challenge with pathogenic E. coli
bacteria, following which all animals were humanely euthanized.
Firstly, each mouse was anesthetized with 2–5% isoflurane by
inhalation and anesthesia was subsequently confirmed by blink
reflex examination. Blood samples were then extracted by orbital
sinus puncture following anesthesia, after which the mice were
sacrificed by cervical dislocation. To minimize animal suffering,
the experimental design was optimized such that alternatives were
considered, pain and the number of animals used were kept to a
minimum, and only qualified personnel were permitted to perform the
experiments. All experiments were performed in accordance with the
guidelines of the Animal Ethics Committee of Fujian province
(Fujian, China) and were approved by the Institutional Animal Care
and Use Committee of Longyan University (Longyan, China).
Splenocyte proliferation assay
Splenic tissues were collected from the mice under
sterile conditions. The spleens were transferred to a 200-mesh cell
strainer and ground to obtain cell suspensions in PBS. Following
erythrocyte lysis, the splenocytes were cultured in RPMI-1640
medium supplemented with 10% FBS in an incubator with 37°C and 5%
CO2. Next, the splenocytes (100 µl/well) were seeded
into 96-well plates at a density of 1×107 cell/ml before
ConA (5 µg/ml), LPS (5 µg/ml), OVA (10 µg/ml) or media were added
to the wells to a final volume of 200 µl. After 72 h incubation,
cell viability was measured using the CCK-8 assay, according to the
manufacturer's protocols. The stimulation index (SI) was calculated
using the following formula: SI = absorbance value at OD 450 nm for
mitogen-activated cultures/absorbance value for non-stimulated
cultures.
Measurement of OVA-specific
antibodies
The levels of OVA-specific IgG, IgG1 and IgG2a
antibodies in mouse serum were measured using an indirect ELISA
method as previously described (5).
Briefly, microtiter plate wells were first coated with 100 µl OVA
solution (50 µg/ml dissolved in 50 mM carbonate-bicarbonate buffer,
pH 9.6) for 24 h at 4°C. The wells were then washed three times
with PBS containing 0.05% Tween-20 and then blocked with 5% bovine
serum albumin (cat. no. ST023; Beyotime Institute of Biotechnology)
at 37°C for 2 h. The serum was diluted 1:10 with 0.5% BSA/PBS.
Following a further three washing steps, 100 µl diluted serum
sample or 0.5% BSA/PBS (control) was added (in triplicate) to the
wells and incubated for 2 h at 37°C. Next, after three washing
steps, 100 µl horseradish peroxidase (HRP)-conjugated antibody
(IgG, 1:10,000; IgG1, 1:4,000; IgG2a, 1:4,000) or 0.5% BSA/PBS
(control) was added to each well. The plates were further incubated
for 1 h at 37°C. After washing, the wells were incubated with TMB
chromogenic substrate in 37°C for 30 min. The substrate reaction
was stopped by the addition of 50 µl of 2 M sulfuric acid. The
absorbance value at 490 nm was measured for each well using a
microplate reader.
Serum cytokine measurements by
ELISA
Serum levels of IL-2, IFN-γ, TNF-α, IL-4 and IL-5
were quantified using commercial ELISA kits according to the
manufacturers' protocols.
Flow cytometry
Blood was collected from the mice by retroorbital
exsanguination into tubes containing the anticoagulant heparin. The
number of peripheral blood cells in serum isolated from the mice
immunized with OVA was detected using flow cytometry. A total of 10
µl FITC-conjugated CD4 (0.25 µg) and 5 µl PE-conjugated CD8 (0.25
µg) monoclonal antibodies were added to 50 µl anticoagulant blood.
This mixture was mixed and incubated gently at room temperature for
30 min in the dark. 1.8 ml hemolysin solution was added to each
tube, and incubated at 37°C for 10 min. Following centrifugation at
1,500 × g, the pelleted cells were rinsed using
fluorescence-activated cell sorting (FACS) buffer (2% FBS in PBS)
before resuspension in 0.5 ml FACS buffer. FACS data acquisition
was performed on the BD FACS Calibur™ system (BD Biosciences), and
the data was analyzed on Novo express 1.3 software (ACEA
Biosciences, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR) for cytokine gene expression
Splenocytes were seeded (5×106 cells/ml,
1 µl/well) into 24-well plates, and ConA (5 µg/ml) was then
immediately added. After 12 h treatment, cells were harvested and
total RNA was extracted using TRIzol® according to the
manufacturer's protocol. The RNA was reverse-transcribed to cDNA
using a First Strand cDNA Synthesis kit according to the
manufacturer's protocol. The cDNA samples were stored at −20°C
until further use. qPCR was subsequently performed in a 20 µl total
reaction volume consisting of 2X SYBR® Premix Ex Taq™
(10 µl), 0.5 µl each primer (10 µM), 2 µl cDNA and 7 µl
ddH2O with a concomitant reaction condition (10 min at
95°C, then followed by 35 cycles of 30 sec at 94°C, 30 sec at 55°C
and 60 sec at 72°C). The sequences of the primers used are shown in
Table I. Relative expression levels
of mRNAs using β-actin as an internal control were analyzed using
the 2−ΔΔCq method (24).
 | Table I.Sequences of primers used for
PCR. |
Table I.
Sequences of primers used for
PCR.
| Gene | Primer
sequence |
|---|
| β-actin |
5′-AGCGGTTCCGATGCCCT-3′ |
|
|
5′-AGAGGTCTTTCGGACGGATGTCAACG-3′ |
| IL-2 |
5′-GCACCCACTTCAAGCTCCA-3′ |
|
|
5′-AAATTTGAAGGTGAGCATCCTG-3′ |
| IFN-γ |
5′-GCTTTGCAGCTCTTCCTCATG-3′ |
|
|
5′-CTTCCACATCTATGCCACTTGAG-3′ |
| IL-4 |
5′-GAGACTCTTTCGGGCTTTTCG-3′ |
|
|
5′-CAGGAAGTCTTTCAGTGATGTGG-3′ |
| IL-10 |
5′-CCAGTTTTACCTGGTAGAAGTGATG-3′ |
|
|
5′-CTTGCTCTTATTTTCACAGGGGAG-3′ |
| T-bet |
5′-ATTGCCCGCGGGGTTG-3′ |
|
|
5′-GACAGGAATGGGAACATTCGC-3′ |
| GATA-3 |
5′-GGTCAAGGCAACCACGTC-3′ |
|
|
5′-CATCCAGCCAGGGCAGAG-3′ |
Statistical analysis
Values are expressed as the mean ± SEM. Differences
between the experimental and control groups were analyzed using
SPSS 20.0 software (IBM Corp.). One-way ANOVA followed by
Student-Newman-Keuls multiple comparison test was used for
multigroup analyses. Survival rates were analyzed using the
log-rank test in GraphPad Prism software (version 6; GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical observations
No notable stress responses, including visible
changes in daily appetite, mental state or motor ability were
observed in the mice following the first immunization and booster
immunization 2 weeks later. In addition, no differences in body
weight were observed after immunization between the adjuvant groups
(OVA + RCIE, OVA + alum) and control groups (saline and OVA alone)
(data not shown).
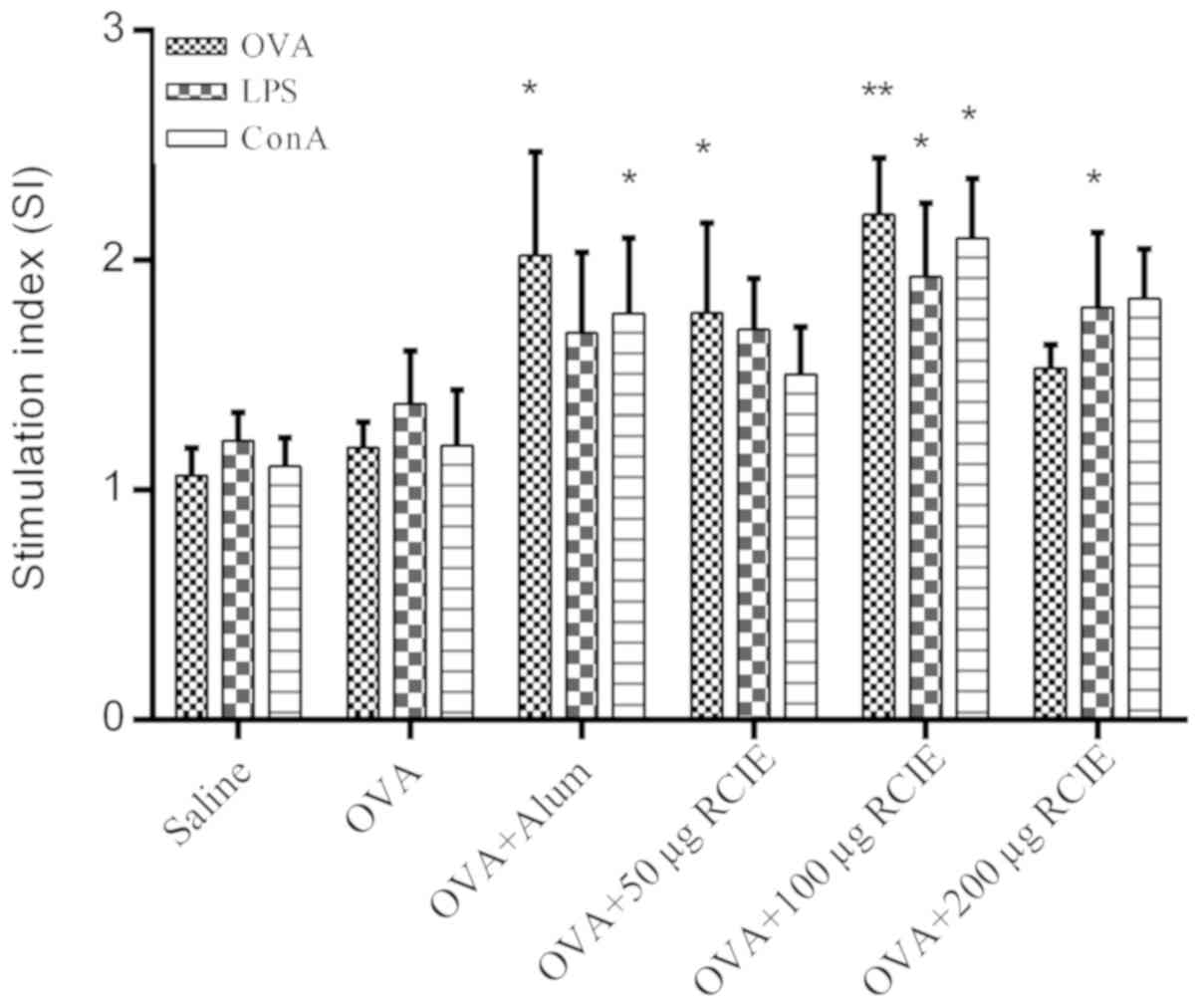
Effect of OVA/RCIE immunization on
splenocyte proliferation
The effects of RCIE-OVA immunization on splenocyte
proliferation in mice are shown in Fig.
1. Splenocyte viability in the OVA + RCIE (50 or 100 µg) and
OVA + alum groups was significantly higher compared with that of
the OVA control group following OVA stimulation (P<0.05 or
P<0.01). Splenocytes isolated from mice immunized with OVA +
RCIE (100 and 200 µg) exhibited significantly higher cell viability
following treatment with LPS compared with those from mice
immunized with OVA alone (P<0.05). Splenocytes isolated from OVA
+ RCIE (100 µg)- and OVA + alum-immunized mice stimulated with ConA
displayed significantly increased cell viability compared with
those from the OVA control group (P<0.05). However, no
significant differences were observed between the OVA and OVA +
RCIE (50 or 200 µg) groups following ConA stimulation. The results
demonstrated the effect of RCIE on improving the cellular immune
response.
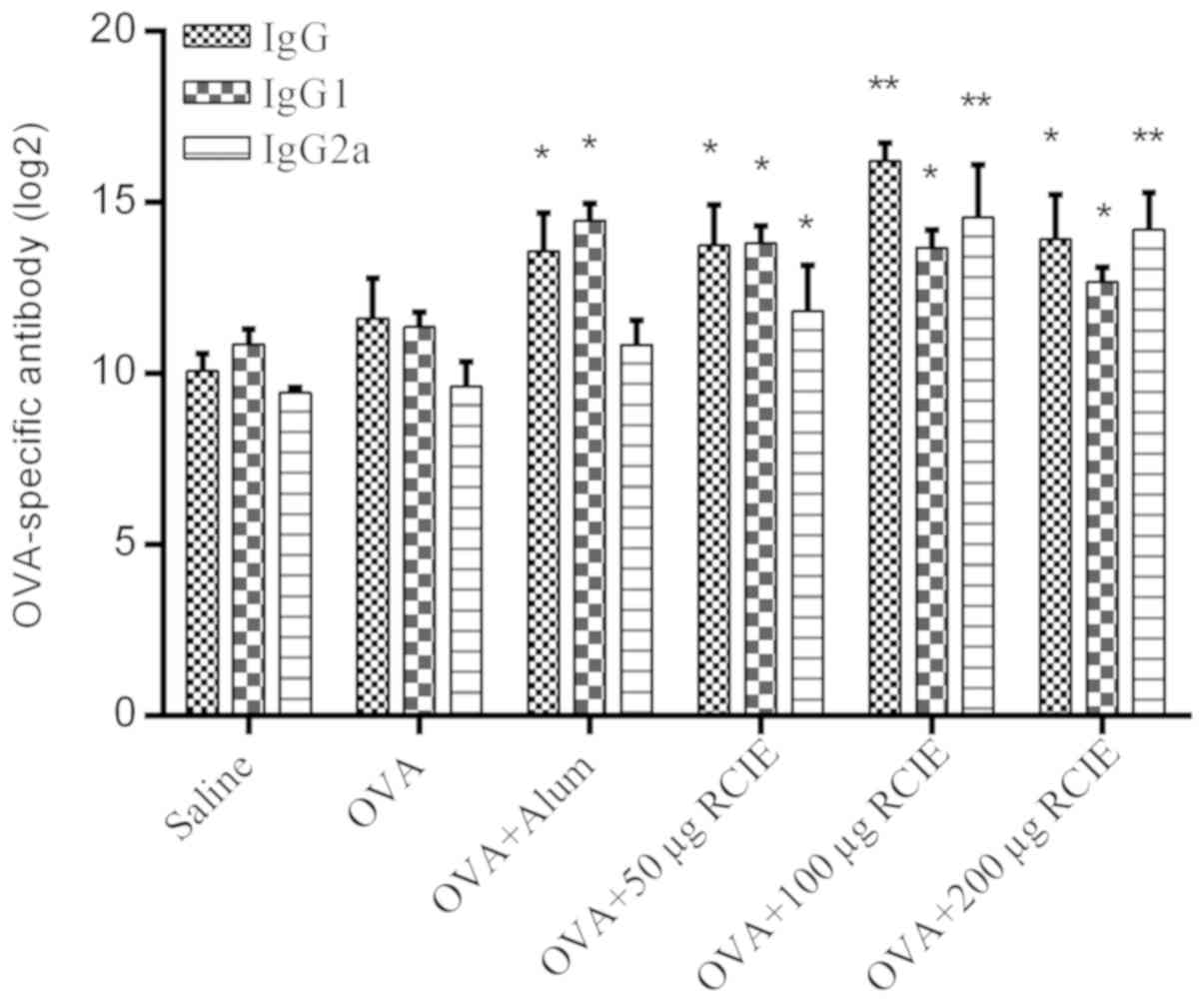
Effect of OVA/RCIE immunization on the
serum antibody response
The levels of OVA-specific IgG, IgG1 and IgG2a
antibodies are shown in Fig. 2.
Serum IgG levels were significantly higher in the OVA + alum and
OVA + RCIE (50, 100 and 200 µg) groups compared with the OVA group
(P<0.05 or P<0.01). In addition, serum IgG1 levels were
significantly higher in all RCIE-immunized groups of mice compared
with the OVA group. No significant differences were observed in the
total serum IgG1 levels between the OVA + alum group and the mouse
groups immunized with OVA + RCIE. RCIE (50 µg) and RCIE (100 and
200 µg) also significantly increased total serum IgG2a levels in
OVA-immunized mice (P<0.05 or P<0.01, respectively), but no
significant differences in serum IgG2a levels were identified
between the OVA + alum and OVA alone groups. Therefore, these
observations suggest that alum only significantly enhanced the
levels of Th2-type antibodies, whereas the levels of Th1-type
antibodies were significantly increased by RCIE compared with alum
at appropriate levels.
Levels of cytokines in OVA-immunized
mice
The levels of the cytokines IFN-γ, TNF-α, IL-2, IL-4
and IL-5 were next measured using ELISA kits and the results are
shown in Fig. 3. The serum levels of
IFN-γ, IL-2 and IL-4 in mice immunized with OVA + RCIE (100 µg)
were significantly higher compared with those in the OVA group
(P<0.05). Although increases were observed in the levels of
TNF-α and IL-5 in the OVA + RCIE group compared with the OVA group,
no statistically significant differences were detected. This
suggests that RCIE can significantly enhance the levels of Th1 and
Th2 cytokines in OVA-immunized mice.
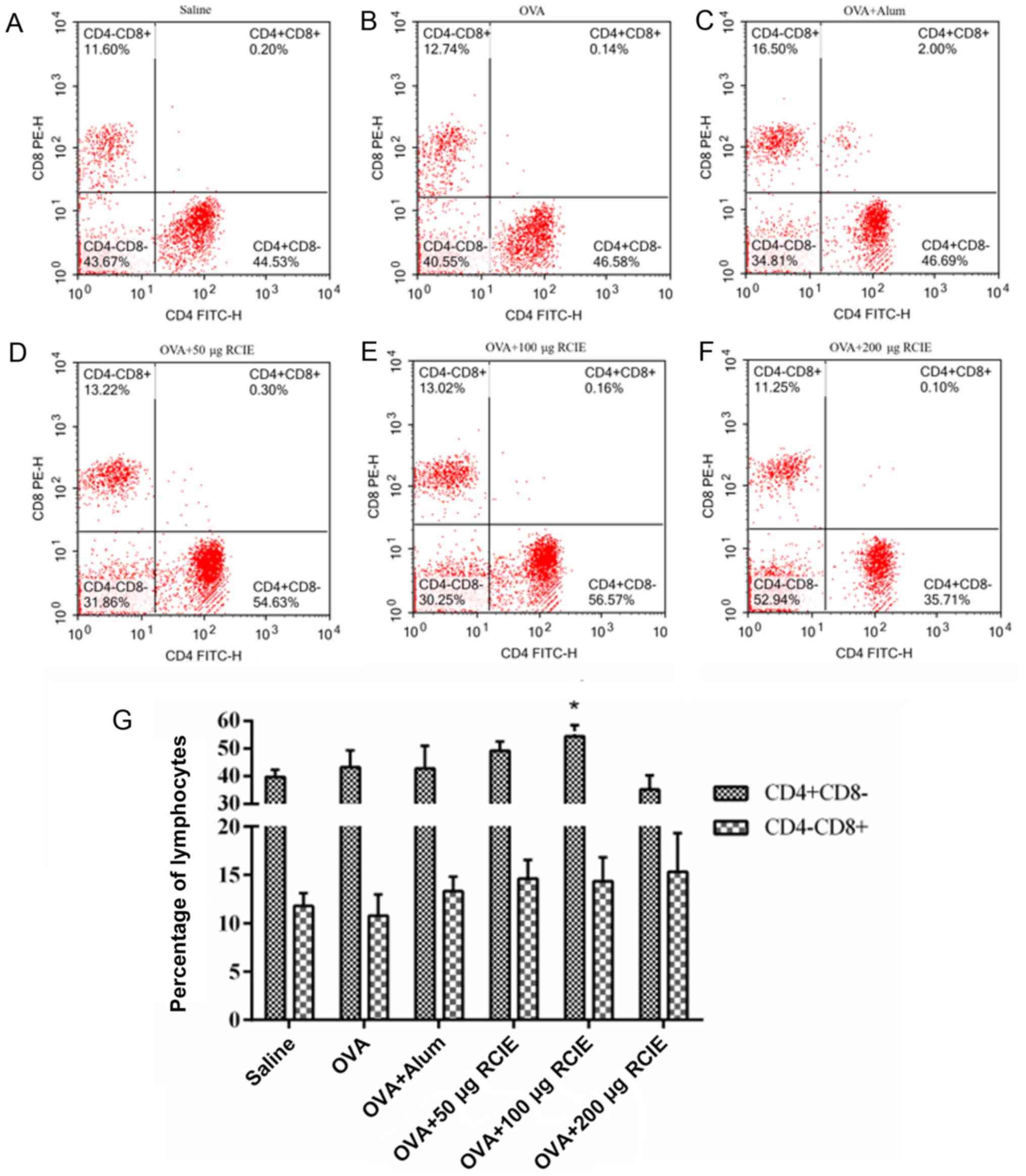
Effect of RCIE on T-lymphocyte subsets
in peripheral blood
The effects of RCIE on the levels of CD4+
and CD8+ T cells in the blood from OVA-immunized mice
are summarized in Fig. 4. Compared
with the OVA group, the proportion of CD4+ T cells in
mice immunized with OVA + RCIE (100 µg) was significantly increased
(P<0.05). No significant differences were detected in the
proportion of CD4+ T cells between the OVA + RCIE (50
µg) and OVA + alum groups. Additionally, the proportion of
CD8+ T cells in the OVA-immunized mice was not
significantly affected by RCIE.
Effect on cytokine mRNA levels in
splenocytes
The levels of IL-2, IFN-γ, T-bet, IL-10, GATA-3 and
IL-4 mRNA expression in ConA-stimulated mouse splenocytes are shown
in Table II. Following ConA
treatment, the mRNA expression levels of the Th2-associated
cytokines IL-4, IL-10 and transcription factor GATA-3, as well as
those of Th1-associated cytokines IL-2, IFN-γ and transcription
factor T-bet were significantly increased in splenocytes from the
OVA + RCIE groups at certain concentrations compared with those in
the OVA group (P<0.05 or P<0.01). However, only IL-4, IL-10
and GATA-3 mRNA expression levels were observed to be significantly
higher in the OVA + alum group compared with the OVA group
(P<0.05 or P<0.01). These results suggest that the inclusion
of RCIE during immunization induced the gene expression of Th1/Th2
cytokines and transcription factors in splenocytes following
stimulation with ConA.
 | Table II.mRNA expression levels of cytokines
and transcription factors in splenocytes isolated from
OVA-immunized mice. |
Table II.
mRNA expression levels of cytokines
and transcription factors in splenocytes isolated from
OVA-immunized mice.
| Gene | OVA | OVA + alum | OVA + 50 µg
RCIE | OVA + 100 µg
RCIE | OVA + 200 µg
RCIE |
|---|
| IL-2 | 1.01±0.12 | 1.27±0.34 | 1.07±0.32 |
1.99±0.39a |
1.65±0.29a |
| IFN-γ | 1.01±0.13 | 1.08±0.32 |
2.25±0.37b |
2.32±0.39b |
1.63±0.63a |
| T-bet | 1.00±0.08 | 1.19±0.12 |
2.13±0.52a |
2.28±0.63a | 1.26±0.32 |
| IL-4 | 1.02±0.22 |
4.07±1.32b |
2.33±0.60a |
2.49±0.18a | 1.51±0.12 |
| IL-10 | 1.01±0.17 |
3.07±1.02a | 1.70±0.77 |
4.93±1.49b |
4.53±1.88b |
| GATA-3 | 1.02±0.21 |
5.32±1.16b | 1.24±0.44 |
2.19±0.62a | 1.40±0.26 |
RCIE promotes anti-infection effects
in immunized mice
Three days following inoculation with E. coli
vaccine, the mice were challenged with pathogenic porcine E.
coli. In the vehicle group, the mice began to exhibit symptoms
associated with infection 4 h following bacterial challenge
including curling, diminished movement, increased depression,
ruffled fur, shortness of breath and death within 6 h, with no mice
surviving beyond 24 h. In the RCIE + E. coli group, deaths
were observed commencing at 18 h following bacterial challenge with
no additional deaths observed 24 h after challenge. Since
statistical significance could already be observed in the mortality
rates between the four experimental groups at 48 h after bacterial
challenge, all experiments were terminated at 48 h post-challenge.
The survival rates of immunized mice were found to be 80% (8/10)
for the RCIE group, 60% (6/10) for the alum + E. coli group
and 30% (3/10) for the saline + E. coli group (Fig. 5). Compared with the vehicle and
saline + E. coli groups, the survival rate in the RCIE +
E. coli group was significantly higher (P<0.05). These
results suggest that RCIE can significantly enhance the efficacy of
E. coli vaccine.
Discussion
An ideal adjuvant should exhibit the following
characteristics: i) Promotion of humoral and cell-mediated immunity
with no toxic side effects; ii) be able to be delivered to the body
via different routes and with different antigens; iii) leave no
long-term residue as well as maintain stability when delivered via
the oral route (25,26). Although a number of adjuvants have
been applied in vaccines, a majority of the materials used only
elicit humoral immunity or exert undesirable side effects, limiting
their potential use in vaccines (27). In the present study, no adverse
clinical responses, including immune stress, growth inhibition and
adverse reactions, were observed when RCIE was applied as the
adjuvant. In addition, it was found that RCIE acted as an adjuvant
to enhance the cellular immune responses in OVA-immunized mice.
Compared with the control group, the levels of IgG and IgG-subclass
antibodies were significantly increased, whereas splenocyte
viability and cytokine expression were also significantly enhanced
in the OVA + RCIE groups.
The type of adjuvant determines the type of elicited
immune response, which in turn has a significant effect on the
immunoprotective properties of the vaccine (28). The Th1-type immune response, mainly
mediated by Th1 helper cells, is associated with increased levels
of IL-2, IFN-γ, IgG2a, IgG2b and IgG3. These immune responses are
necessary for the activation of CTLs (24). By contrast, the Th2-type immune
response is mainly associated with increased levels of IL-4, IL-5,
IL-10, IgG1 and IgA (24).
Currently, the most commonly used adjuvants, including water/oil
emulsions and alum, only activate Th2-type responses (29). Although, other adjuvants such as
liposomes and their derivatives have been found to give rise to
Th-type responses characterized by the production of cytokines and
IgG antibody subclasses, they are incapable of stimulating the
production of CTLs against soluble or exogenous antigens (e.g.,
viral particles and soluble MHC molecules) (30).
To the best of our knowledge, there have not been
any studies investigating the adjuvant activity of RCIE. In the
present study, the effect of RCIE as an adjuvant for inducing Th1
or Th2 immune responses in OVA-immunized mice was evaluated. ConA
and LPS are able to induce the proliferation of T cells and B
cells, respectively (31). In the
RCIE immunization groups in the present study, ConA and LPS were
demonstrated to significantly enhance splenocyte viability in
vitro. However, no significant differences were detected
between the OVA and OVA + alum groups. Since the majority of spleen
cells are lymphocytes (3), these
results suggest that RCIE could significantly increase the
activation potential of lymphocytes in OVA-immunized mice. IgG is
the most abundant immunoglobulin isoform in serum, which can
provide protection against most blood-borne diseases. IgG is
divided into four subtypes: IgG1, IgG2a, IgG2b and IgG3, with their
distribution regulated by the cytokine profile (32). The levels of IgG and IgG1 in the
OVA-immunized mice were enhanced by 50, 100 or 200 µg RCIE compared
with those in the OVA group. Although alum significantly enhanced
the levels of IgG1 in the OVA-immunized mice, the levels of IgG2a
remained unchanged compared with those in the mice immunized with
OVA alone. As GATA-3 is a Th2 cytokine transcription factor
(33), this may be related to the
induction of the GATA-3 gene expression. Therefore, RCIE
application may be effective in activating the Th1- and Th2-type
immune response at appropriate levels, which is associated with
increased levels of IgGl and IgG2a.
The possible effects of RCIE on the Th1/Th2 immune
response were also examined by flow cytometry and ELISA. The
proportion of CD4+ T cells in the blood of mice
immunized with OVA + RCIE (100 µg) was higher compared with that in
the OVA group. In addition, the levels of IL-4 and IL-5 as well as
the levels of IL-2, IFN-γ and TNF-α were increased by RCIE in the
OVA-immunized mice. These results suggest that RCIE elicited both
Th1 and Th2 immune responses to OVA in mice. Subsequently, the mice
were immunized with RCIE and E. coli vaccine, following
which a lethal dose of pathogenic E. coli was given after 3
days. Pathogenic E. coli produces exotoxins that can cause
diarrhea, bleeding and even death in animals (34). Compared with the vehicle group, in
which all mice died following bacterial challenge, RCIE
significantly improved the survival rate of mice, which may be
associated with the induction of Th1- and Th2-type immune responses
and the rapid promotion of antibody production.
To examine the mechanism of Th1/Th2 cytokines
further, RT-qPCR was utilized to analyze the mRNA expression levels
of IL-2, IFN-γ, IL-4 and IL-10, all of which were found to be
enhanced by RCIE. The original decision of T cells to differentiate
into Th1 or Th2 subtypes is regulated by transcription factors
T-bet or GATA-3 (33). The present
study found that the mRNA levels of T-bet and GATA-3 were increased
in splenocytes isolated from the immunized mice in the RCIE group.
These results suggest that RCIE has the potential to induce Th1 and
Th2 immune responses.
There was a notable change in the type of Th1 or Th2
immune response that was induced with varying concentrations of
RCIE, in agreement with other studies (35,36). The
type of immune response activated is dependent upon the type of
infection involved. The Th1-type immune response can neutralize
intracellular microorganisms, primarily via the involvement of
IgG2a, IFN-γ, CTLs and IL-12, whereas the clearance of
extracellular pathogens is dependent on the humoral immune
response, primarily involving IgG1, IL-4, IL-5 and IL-10 (37). However, a key challenge in the field
of vaccine adjuvants remains the activation of an aberrant immune
response and disease deterioration induced by an adjuvant. The
selection of the correct adjuvant may be an effective strategy for
producing different types of immune responses. Indeed, CpG-DNA has
been demonstrated to stimulate local susceptibility, causing potent
CTL responses and Th1-polarized immune responses to subsequent
infections or antigen challenge (38). Alum adjuvants are generally
recognized as safe and effective stimulators of Th2 immunity,
resulting in the production of high levels of IgG1 (39). Additionally, total ginseng saponins
have been reported to potentiate natural killer cell activity,
increase IFN production and stimulate the activity of CTLs and the
Th1 immune response. In particular, Rivera et al reported
that ginsenoside Rb1 stimulated higher antibody titers compared
with alum adjuvant vaccines (40).
It has also been reported that adjuvants such as oil/ginseng
saponin or alum/Rg1 regulate Th1 or Th2 immune responses (38). These previous reports emphasize the
importance of the type of adjuvant applied to the immune activation
that is induced.
Based on the present study, it may be concluded that
the use of RCIE as an adjuvant increased specific antibody and
cellular responses against OVA in mice by regulating the gene
expression of Th1/Th2 cytokines and transcription factors. In
addition, the subsequent potentiation of both Th1 and Th2 immune
responses by RCIE significantly improved the efficacy of the E.
coli vaccine.
Acknowledgements
The authors would like to thank Dr Jingxun Ni
(Longyan University, Longyan, Fujian, P.R. China) for kindly
providing the Pathogenic porcine Escherichia coli.
Funding
This work was supported by the Fujian Science and
Technology Plan Guiding Project of China (grant no. 2015N0029), the
Longyan Qimai Science and Technology Innovation Fund (grant no.
2018LYQM0201) and special projects for local science and technology
development guided by the central government (grant no.
2019L3011).
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HC and LQ designed the experiments. HC performed the
experiments. HC, XZ, MC and LL collected the data. HC and ZG
analyzed the data. HC prepared the manuscript and LQ revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the guidelines of the Animal Ethics Committee of Fujian province
(Fujian, China) and were approved by the Institutional Animal Care
and Use Committee of Longyan University (Fujian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martins KAO, Cooper CL, Stronsky SM,
Norris SLW, Kwilas SA, Steffens JT, Benko JG, van Tongeren SA and
Bavari S: Adjuvant-enhanced CD4 T Cell responses are critical to
durable vaccine immunity. EbioMedicine. 3:67–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tandrup Schmidt S, Foged C, Korsholm KS,
Rades T and Christensen D: Liposome-based adjuvants for subunit
vaccines: Formulation strategies for subunit antigens and
immunostimulators. Pharmaceutics. 8:E72016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun HX, Ye YP, Pan HJ and Pan YJ: Adjuvant
effect of panax notoginseng saponins on the immune responses to
ovalbumin in mice. Vaccine. 29:3882–3889. 2004. View Article : Google Scholar
|
|
4
|
Jiang W, Zhu T, Wang YX, Zhang ZX and Shan
JJ: Application of polysaccharide adjuvants in vaccines. Chin J N
Drugs. 21:1470–1478. 2012.
|
|
5
|
Xie Y, Pan H, Sun H and Li D: A promising
balanced Th1 and Th2 directing immunological adjuvant, saponins
from the root of platycodon grandiflorum. Vaccine. 26:3937–3945.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eshaghkhani Y, Sanati MH, Nakhjavani M,
Safari R, Khajavi A, Ataei M and Jadali Z: Disturbed Th1 and Th2
balance in patients with graves' disease. Minerva Endocrinologica.
1:28–36. 2016.
|
|
7
|
Hussein MM and Ahmed MM: The Th1/Th2
paradigm in lambda cyhalothrin-induced spleen toxicity: The role of
thymoquinone. Environ Toxicol Pharmacol. 41:14–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haghshenas MR, Khademi B, Ashraf MJ,
Ghaderi A and Erfani N: Helper and cytotoxic T-cell subsets (Th1,
Th2, Tc1, and Tc2) in benign and malignant salivary gland tumors.
Oral Dis. 22:566–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Germann T, Bongartz M, Dlugonska H, Hess
H, Schmitt E, Kolbe L, Kölsch E, Podlaski FJ, Gately MK and Rüde E:
Interleukin-12 profoundly up-regulates the synthesis of
antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody
subclasses in vivo. Eur J Immunol. 25:823–829. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rostamian M, Sohrabi S, Kavosifard H and
Niknam HM: Lower levels of IgG1 in comparison with IgG2a are
associated with protective immunity against Leishmania tropica
infection in BALB/c mice. J Microbiol Immunol Infect. 50:160–166.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mochizuki S, Morishita H, Kobiyama K,
Aoshi T, Ishii KJ and Sakurai K: Immunization with antigenic
peptides complexed with β-glucan induces potent cytotoxic
T-lymphocyte activity in combination with CpG-ODNs. J Control
Release. 220:495–502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu L, Yu C, Wang C, Shao M, Yan Z, Jiang
X, Chi S, Wang Z, Wei K and Zhu R: Immuno-enhancement of taishan
pinus massoniana pollen polysaccharides on recombinant Bordetella
avium ompA expressed in pichia pastoris. Microb Pathog. 95:54–61.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song X and Hu S: Adjuvant activities of
saponins from traditional Chinese medicinal herbs. Vaccine.
27:4883–4890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu YJ, Tian SY, Zhang Y, Ren HL and Bing
LI: Effect of red clover isoflavones on immune function and
antioxidant activity in broilers. J Shenyang Agricultural
University. 39:699–703. 2008.
|
|
15
|
Rasouli E and Jahanian R: Improved
performance and immunological responses as the result of dietary
genistein supplementation of broiler chicks. Animal. 9:1473–1480.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang BP, Liu YB and Liu YH: Effects of
astragalus polysaccharides on production performance of weanling
piglets and immune effect of classical swine fever vaccine. J Anhui
Agricultural Sci. 17:5493–5496. 2014.
|
|
17
|
Cao P, Jeyabalan J, Aqil F, Ravoori S,
Gupta RC and Vadhanam MV: Polymeric implants for the delivery of
green tea polyphenols. J Pharm Sci. 103:945–951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryanborchers TA, Park JS, Chew BP, Mcguire
MK, Fournier LR and Beerman KA: Soy isoflavones modulate immune
function in healthy postmenopausal women. Am J Clin Nutr.
83:1118–1125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni J, Huang H, Chen T, Shen J and Lin W:
Improved early humoral immune response to antigens by Protamine
protein. Chin J Prev Veterinary Med. 4:342–346. 2018.
|
|
20
|
Chen HQ and Jin ZY: Study on extraction
technology of trifolium pratense isoflavones. Food Sci. 26:156–159.
2005.
|
|
21
|
Feng YL, Yuan YM and Xia Y: Hydrolysis of
soybean isoflavone by alkali. China Oils Fats. 34:56–58. 2009.
|
|
22
|
Yang ZG, Sun HX and Fang WH: Haemolytic
activities and adjuvant effect of Astragalus membranaceus saponins
(AMS) on the immune responses to ovalbumin in mice. Vaccine.
44:5196–5203. 2005. View Article : Google Scholar
|
|
23
|
Cao ZM, Wang MZ, Wang L, Zhang JY, Wang
HR, Li JX and Wang XZ: Establishment and evaluation of mouse model
of ulcerative colitis. China Animal Husbandry Veterinary Med.
1:171–175. 2016.
|
|
24
|
Ni J, Bi S, Xu W, Zhang C, Lu Y, Zhai L
and Hu S: Improved immune response to an attenuated pseudorabies
virus vaccine by ginseng stem-leaf saponins (GSLS) in combination
with thimerosal (TS). Antiviral Res. 132:92–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aucouturier J, Dupuis L and Ganne V:
Adjuvants designed for veterinary and human vaccines. Vaccine.
19:2666–2672. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Hagan DT, Mackichan ML and Singh M:
Recent developments in adjuvants for vaccines against infectious
diseases. Biomol Eng. 18:69–85. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hunter RL: Overview of vaccine adjuvants:
Present and future. Vaccine. 3 (Suppl 20):S7–S12. 2002. View Article : Google Scholar
|
|
28
|
Longhi MP, Trumpfheller C, Idoyaga J,
Caskey M, Matos I, Kluger C, Salazar AM, Colonna M and Steinman RM:
Dendritic cells require a systemic type I interferon response to
mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp
Med. 206:1589–1602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie Y, Sun HX and Li D: Platycodin D is a
potent adjuvant of specific cellular and humoral immune responses
against recombinant hepatitis B antigen. Vaccine. 27:757–764. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao W, Gang YU, Hao PL, Han XX, Huang XY
and Yang XM: Effect from cationic liposomes DOTAP as adjuvant on
H5N1 influenza split vaccine. Prog Microbiol Immunol. 44:1–9.
2016.
|
|
31
|
Huong PT, Lee CH, Li MH, Lee MY, Kim JK,
Lee SM, Seon JH, Lee DC and Jeon YJ: Characterization and
immunopotentiating effects of the glycoprotein isolated from
dioscorea batatas. Korean J Physiol Pharmacol. 15:101–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adame-Gallegos JR, Shi J, Mcintosh RS and
Pleass RJ: The generation and evaluation of two panels of
epitope-matched mouse IgG1, IgG2a, IgG2b and IgG3 antibodies
specific for plasmodium falciparum and Plasmodium yoelii merozoite
surface protein 1–19 (MSP1(19)). Exp Parasitol. 130:384–393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chakir H, Wang H, Lefebvre DE, Webb J and
Scott FW: T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine
profile in mixed cell populations: Predominant role of GATA-3. J
Immunol Methods. 278:157–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Watson VE, Jacob ME, Flowers JR, Strong
SJ, Debroy C and Gookin JL: Association of atypical
enteropathogenic Escherichia coli with diarrhea and related
mortality in kittens. J Clin Microbiol. 9:2719–2735. 2017.
View Article : Google Scholar
|
|
35
|
Sun H, He S and Shi M: Adjuvant-active
fraction from Albizia julibrissin saponins improves immune
responses by inducing cytokine and chemokine at the site of
injection. Int Immunopharmacol. 22:346–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouyang K, Chen L, Sun H, Du J and Shi M:
Screening and appraisal for immunological adjuvant-active fractions
from platycodon grandiflorum total saponins. Immunopharmacol
Immunotoxicol. 34:126–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Z, Chen A, Sun H, Ye Y and Fang W:
Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in
mice. Vaccine. 25:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marinaro M, Fasano A and De Magistris MT:
Zonula occludens toxin acts as an adjuvant through different
mucosal routes and induces protective immune responses. Infect
Immun. 71:1897–1902. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jafari M, Moghaddam Pour M, Taghizadeh M,
Masoudi S and Bayat Z: Comparative assessment of humoral immune
responses of aluminum hydroxide and oil-emulsion adjuvants in
influenza (H9N2) and Newcastle inactive vaccines to chickens. Artif
Cells Nanomed Biotechnol. 45:84–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rivera E, Hu S and Concha C: Ginseng and
aluminium hydroxide act synergistically as vaccine adjuvants.
Vaccine. 21:1149–1157. 2003. View Article : Google Scholar : PubMed/NCBI
|