Introduction
Spinal anesthesia is a widely used anesthetic method
for cesarean delivery (CD), and is associated with a high incidence
of maternal hypotension, at 70–80% (1). Persistent hypotension can lead to
maternal nausea and vomiting, as well as fetal acidosis owing to
reduced uterine blood flow (2).
Therefore, common vasopressors such as ephedrine, phenylephrine and
norepinephrine are recommended to decrease the occurrence of
hypotension (3). However,
controversies exist regarding the choice and use of vasopressors
(3). Compared with ephedrine,
phenylephrine is associated with improved fetal acid-base status
(4) and is considered a better
choice for hypotension prevention during cesarean delivery
(5,6). However, phenylephrine has a potential
risk of maternal bradycardia, with significant effects on maternal
heart rate (HR) and cardiac output (CO) (7). Norepinephrine has pharmacologic
characteristics that make it a potential alternative to
phenylephrine for blood pressure stabilization during spinal
anesthesia (8). In comparison with
phenylephrine, norepinephrine is a potent α-adrenergic receptor
agonist that can also excite β-adrenergic receptors (3). A recent study demonstrated that
norepinephrine may be a useful vasopressor for stabilizing blood
pressure with fewer side effects, such as reducing HR and CO
(9). However, there are limited
studies on the use of norepinephrine for treating hypotension
during spinal anesthesia, and few studies have reported its
application in obstetric patients. Norepinephrine infusion has been
used to prevent hypotension during spinal anesthesia (10) but the use of this drug as a bolus has
not been fully investigated.
Although the ED90 of norepinephrine that
prevents hypotension during spinal anesthesia has been assessed
(11), the use of ED50
and ED95 for treating hypotension has not been
completely defined. The objective of the present study was to
determine the intravenous bolus dose of norepinephrine in treating
maternal hypotension in 50% (ED50) and 95%
(ED95) of puerperas undergoing spinal anesthesia. The
secondary outcomes of the present study included maternal
anesthetic block level, hemodynamic changes, bradycardia, adverse
effects, umbilical arterial blood gases, Apgar scores and the ratio
of hypotensive patients to hypertensive patients following
treatment.
Patients and methods
Patient characteristics
A total of 42 patients were enrolled into the
present study between 3rd November 2018 and 31st December 2018. The
present study was conducted in Jiaxing Maternity and Child
Healthcare Hospital (Jiaxing, China), and informed consent was
provided by each participating patient. Inclusion criteria were
elective cesarean delivery under spinal anesthesia, healthy
singleton full-term pregnancy beyond 37 weeks of gestation,
American Society of Anesthesiologists physical status I or II
(12), weight of 50–100 kg, height
of 150–180 cm, and fasting for >6 h. Exclusion criteria were
hypertension, cardiovascular diseases, preeclampsia,
anisorrhythmia, diabetes mellitus, spinal cord malformation,
abnormal fetus and patient refusal. The included puerperas received
different doses of norepinephrine (0.025, 0.050, 0.075, 0.100 and
0.125 µg/kg) to stabilize blood pressure. The present prospective,
double-blinded study was approved by the Ethical Committee of
Jiaxing Maternity and Child Healthcare Hospital, and was registered
in the Chinese Clinical Registry Center (registration no.
ChiCTR1800018474).
Patient monitoring
No patients received premedication. Non-invasive
systolic blood pressure (SBP), diastolic blood pressure (DBP) and
HR were recorded in the supine position on the morning of the
surgery. Baseline systolic arterial BP was the average of three
continuous measurements at 1 min intervals using an automated
device for non-invasive BP assessment on the morning of surgery. In
the operating room, patients underwent continuous standard
monitoring throughout the whole surgery, including
electrocardiography, non-invasive blood pressure measurement and
pulse oximetry on a patient monitor system (B650; GE Healthcare
Finland Oy). A 18G intravenous catheter cannula was inserted into a
right forearm vein and infused with Ringer's lactate solution at 10
ml/kg within 20 min of infusion, followed by a maintained dose of
20 ml/min. The patients were placed in the left lateral position.
Spinal anesthesia was performed using a 16G epidural puncture
needle, with a 25G Whitacre needle at the Lumbar 3–4 interspace.
Upon entry into the subarachnoid space and noticing the
cerebrospinal fluid flowing out, 0.5% bupivacaine, which was
obtained by mixing 2 ml 0.75% bupivacaine (Shanghai Hefeng
Pharmaceutical Co., Ltd.) with 1 ml sterile saline, and was
administered intrathecally at the rate of 0.1 ml/sec. The dose of
bupivacaine is referred to in the Harten dose table, and was
adjusted according to the height and weight of a patient (13). Then, the patient was placed in the
supine position with left uterine displacement by tilting the bed
to the left by 15 (14). Oxygen was
administered via a mask at 5 l/min. The spinal sensory block level
was evaluated by pinprick and confirmed to be within Thoracic (T)
4–6. If not, the case was excluded from the current study.
Treatment preparation
Prior to treatment, the anesthesiologist assistant,
who was not involved in patient management or data acquisition,
prepared the study bolus dose by adding a measured volume of 2 mg
norepinephrine (Changzhou Yuanda Pharmaceutical Chemical Co. Ltd.)
to 500 ml of physiological saline. The patient and the attending
anesthetic manager were blinded to group allocation. Based on
patient weight, the corresponding dose of the drug was determined
by the assistant anesthesiologist. The anesthetic manager who
collected the data was blinded to the drug and its dose. After
delivery, 5U oxytocin (Nanjing Xinbai Pharmaceutical Co., Ltd.) was
administered slowly by intravenous infusion, and an additional 5U
oxytocin was injected into the uterine muscle. Meanwhile, the
pediatric nurse, who was unaware of the study, assessed Apgar
scores (15) at 1 and 5 min,
including activity, pulse, grimace, appearance and respiration.
Arterial blood specimens were collected from the clamped umbilical
cord for immediate blood gas analysis using a blood gas analyzer
(COBAS B123; Roche Diagnostics).
Treatment regime
Previous studies have suggested that a 100 µg dose
of phenylephrine approximates an 8 µg dose of norepinephrine
(16), though a dose 100 µg of
phenylephrine is a suitable dosage (17,18), and
the use of a 6 µg bolus norepinephrine appears sufficient to
prevent hypotension (11). In the
present trial, the dosage of norepinephrine was decided by the
up-and-down sequential allocation method with an initial dose of
0.075 µg/kg and a 0.025 µg/kg gradient. For the dose finding study
by the up-and-down method, the data distribution was unknown and
non-independent (19–21). As required by the sequential
approach, the dose level for the next patient was determined by the
response of the preceding patient. In the present study, the first
patient received a dose of 0.075 µg/kg, which was thought to be
closest to the estimated dose. If the first patient showed a
positive response, the second patient would be exposed to a lower
dose (0.05 µg/kg). In case of a negative response by the first
patient at 0.075 µg/kg, the second patient would be exposed to a
next higher dose (0.1 µg/kg). Successive patients were assigned
respective doses similarly based on the responses of the preceding
participants.
In the present study the norepinephrine regimen was
started immediately after intrathecal injection. According to our
standard practice, SBP was assessed every min, beginning
immediately after intrathecal injection until delivery. The study
drug was administered manually by the attending anesthetic manager
whenever SBP was <80% of baseline, to maintain SBP within the
95% baseline value. Hypotension was defined as SBP <80% of
baseline, and hypertension as a 20% increase from baseline. In case
SBP returned to within the 95% baseline value within 1 min after
administration, treatment was considered to be successful. If SBP
was still <80% of baseline, 6 mg ephedrine was administered.
Bradycardia, which is a heart rate below 50 beats per minute, was
treated with 0.5 mg atropine.
SBP, DBP and HR were recorded at baseline and 1 min
after administration. The times of norepinephrine injection before
delivery and throughout the surgery were recorded. Maternal
infusion volume, blood loss, urine output and adverse effects such
as nausea, vomiting, bradycardia, hypertension and hypotension were
also recorded. In addition, fetal heart rates before and after
spinal anesthesia, Apgar scores at 1 and 5 min, and umbilical
arterial blood gases were assessed.
Statistical analysis
In the present UDM study, data distribution was
non-independent and unknown, which prevents the development of
theoretical rules for determining the necessary sample size from an
accurate estimation of the ED50 (21). Simulation studies suggested that a
stopping rule with 20–40 patients would provide stable estimates of
the study dose for most realistic cases (21). The present study required a sample
size of 42 patients for the stopping rule. The data were used to
calculate the ED50 with 95% CI, using the up-and-down
method. Data were further analyzed using the probit regression
model to calculate the ED50 and ED95. The
sequential method was performed according to the following formula:
ED50=lg−1ΣrlgC/ΣrC, where C is the dose, r is
the number of injections. The standard error was calculated as
SlgED50=d {Σ[p(1-p)/(r-1)]}1/2, where p is
the effective rate. The 95%CI was determined for the
ED50 obtained by the sequential method as
lg−1(lgED50±1.96SlgED50) (22). Data analysis was performed using the
SPSS 20.0 software (IBM Corp.).
Results
Patient recruitment
A total of 60 patients undergoing elective cesarean
delivery under spinal anesthesia were recruited in the present
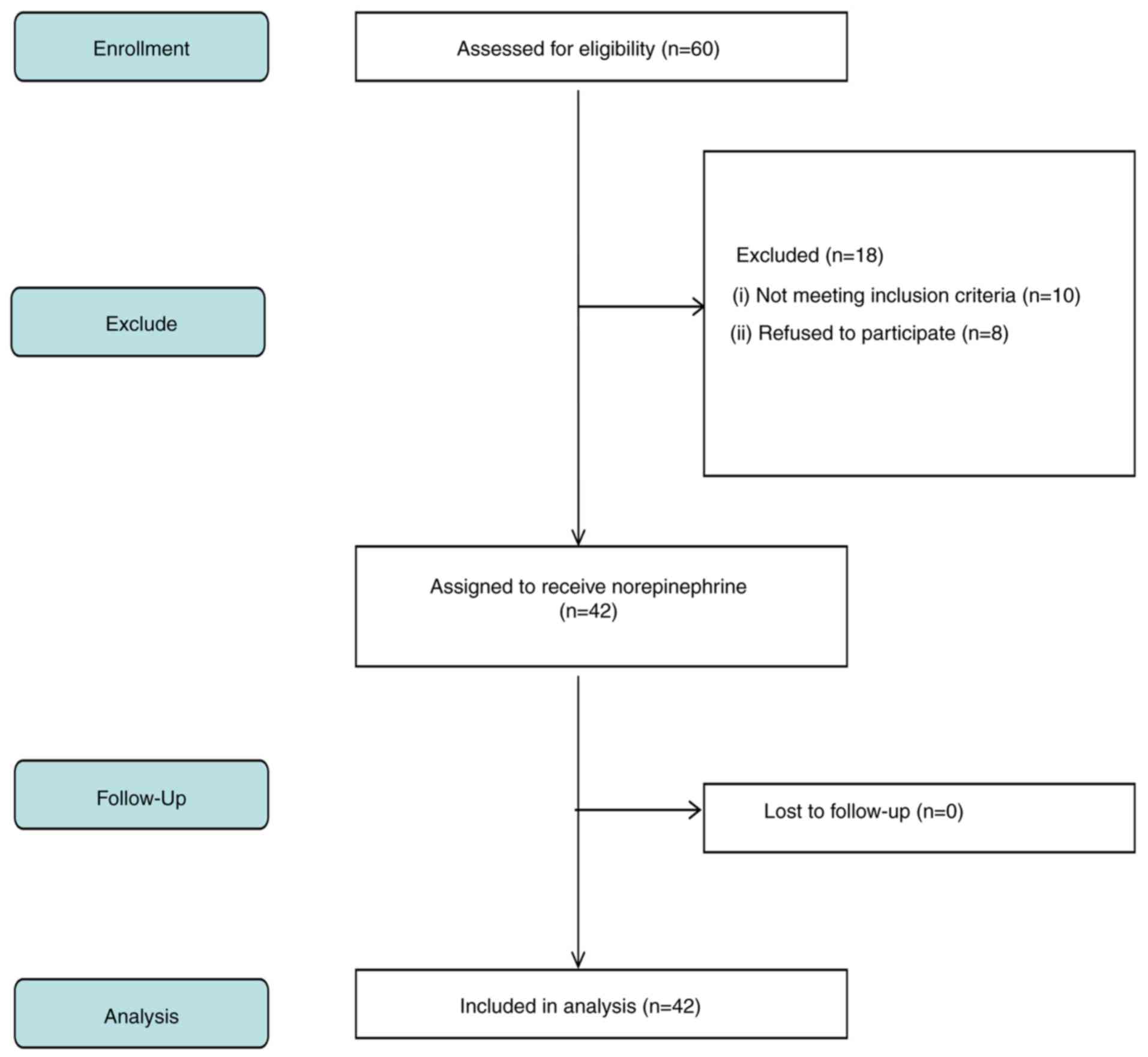
study, and 42 were included in the final analysis. Fig. 1 indicates a flow diagram detailing
patient enrolment. The demographics of the mothers are presented in
Table I, with parameters presented
as mean ± SD or number. The mean age of the participants was
30.4±4.3 years, the mean body weight was 70.5±8.4 kg. The
anesthesia block level was between T4 and T6.
 | Table I.Demographic data and surgical
characteristics. Data are presented as mean ± SD. |
Table I.
Demographic data and surgical
characteristics. Data are presented as mean ± SD.
| Characteristic | Index |
|---|
| Age, years | 30.4±4.3 |
| Weight, kg | 70.5±8.4 |
| Height, cm | 159.2±6.3 |
| Gestation,
weeks | 38.0±1.4 |
| SBP at baseline,
mmHg | 120.4±11.4 |
| DBP at baseline,
mmHg | 71.5±6.1 |
| HR at baseline,
beats/min | 77.8±11.2 |
| Block level
(T) | T5(T4 to T6) |
Neonatal measurements
Umbilical arterial blood gas data after delivery are
presented as mean ± SD (Table II).
Umbilical artery base excess values ranged from −7.5 to −0.4 mEq/l.
The pH values ranged from 7.23–7.34. All pH values were within the
normal range, and no neonate experienced fetal acidosis defined as
pH <7.2, which is the lower limit of normal (data not shown)
(23). Values in blood gas analysis
of the umbilical artery fluctuated within the normal range without
obvious abnormality. Apgar scores at 1 and 5 min were eight or
above for all cases. No neonates required intubation or
ventilation.
 | Table II.Neonatal umbilical artery
outcomes. |
Table II.
Neonatal umbilical artery
outcomes.
| Parameters | Index |
|---|
| PO2, mm
Hg | 21.4±4.8 |
| PCO2, mm
Hg | 46.4±4.1 |
| pH | 7.3±0.1 |
|
HCO3−, mmol/l | 24.6±2.5 |
| Base excess,
mEq/l | −3.2±2.0 |
| Apgar score at 1
min | 8.7±0.5 |
| Apgar score at 5
min | 8.8±0.4 |
Maternal outcomes
Side effects observed after treatment included
nausea, vomiting, bradycardia, hypertension and hypotension
(Table III). Of the eight patients
(19.1%) who developed nausea, six received a dose <0.1 µg/kg,
and one case (2.4%) had vomiting symptoms. Of the 19 patients
(45.2%) who developed hypotension, 12 received bolus norepinephrine
doses <0.075 µg/kg (data not shown). Table IV indicates the observed response
rates for various norepinephrine dose levels. A total of 21
participants reported the norepinephrine dosage to be effective,
and 21 considered their received dosage to be ineffective.
 | Table III.Maternal outcomes. |
Table III.
Maternal outcomes.
| Parameter | Index |
|---|
| Nausea | 8 (19.1%) |
| Vomiting | 1 (2.4%) |
| Bradycardia | 2 (4.8%) |
| Hypertension | 2 (4.8%) |
| Hypotension | 19 (45.2%) |
| Transfusion volume,
ml | 871.4±74.2 |
| Bleeding volume,
ml | 260.7±59.0 |
| Urine volume,
ml | 104.3±15.3 |
 | Table IV.Observed response rate. |
Table IV.
Observed response rate.
| Groups | Assigned dose,
µg/kg | Effective | Total number | Effective rate |
|---|
| NE 1 | 0.025 | 0 | 3 | 0.00 |
| NE 2 | 0.050 | 3 | 12 | 0.3 |
| NE 3 | 0.075 | 9 | 16 | 0.6 |
| NE 4 | 0.100 | 7 | 9 | 0.8 |
| NE 5 | 0.125 | 2 | 2 | 1.00 |
Norepinephrine dose response
Effective and ineffective responses were evaluated
at various norepinephrine dose levels, including 0.025, 0.05,
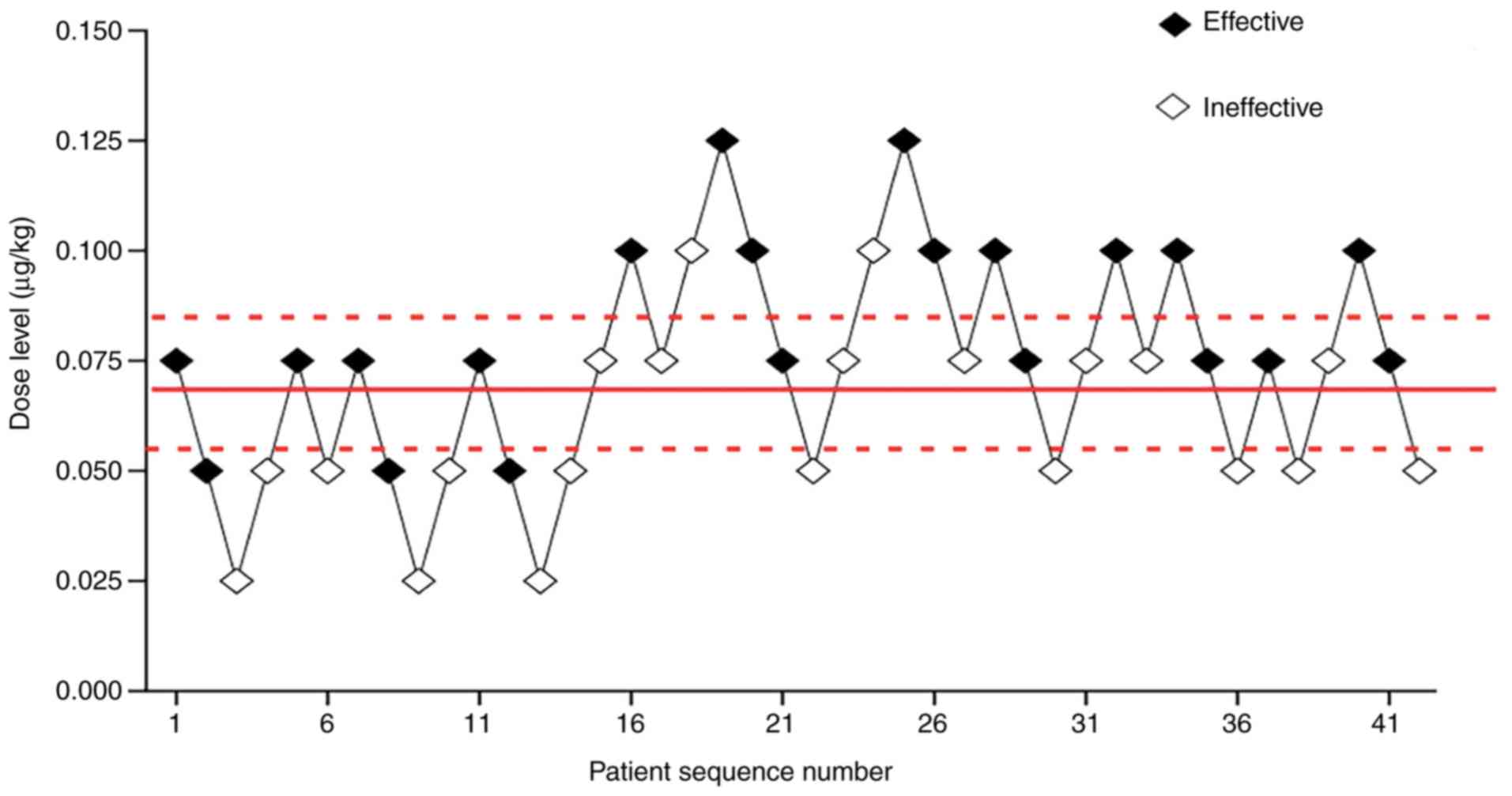
0.075, 0.1 and 0.125 µg/kg. Fig. 2
showed each dose, anesthetic result and subsequent dosing. The
ED50 of norepinephrine determined by the up-and-down
sequential allocation method was 0.067 µg/kg (Fig. 2; 95% CI, 0.056–0.081). In addition,
effective and ineffective responses to norepinephrine were
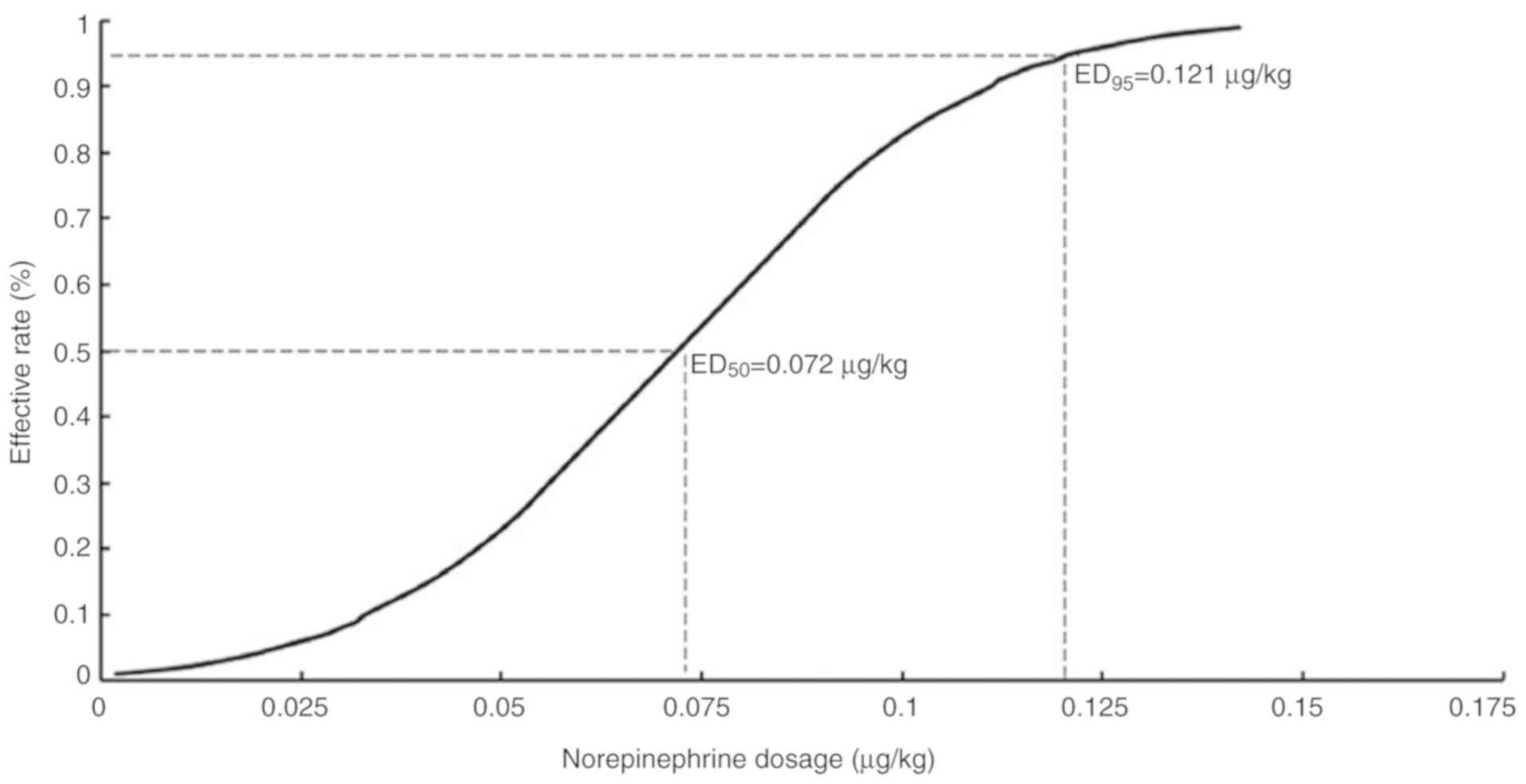
evaluated by the probit regression model. The ED50 of
norepinephrine was 0.072 µg/kg (95% CI, 0.056–0.088) and the
ED95 identified by the probit regression model was 0.121
µg/kg (Fig. 3; 95% CI,
0.1–0.207).
Discussion
The present study determined the weight-based dose
of norepinephrine given as a bolus to treat the first episode of
hypotension in patients undergoing spinal anesthesia for elective
CD. The present results suggested that ED50 values for
norepinephrine were 0.067 µg/kg (95% CI, 0.056–0.081) and 0.072
µg/kg (95% CI, 0.056–0.088) as assessed by the up-and-down method
and the probit regression model, respectively. The ED95
calculated by the probit regression model was 0.121 µg/kg (95% CI,
0.1–0.207). The ED50 measured by the up-and-down method
was close to that obtained by the probit regression model. No
significant adverse effects were identified in the present study;
however, it is possible that the low incidence of side effects was
due to the small sample size. For the dosage determination study by
the up-and-down method, data distribution was unknown and
non-independent. As required by the sequential approach, the dose
for the subsequent patient was determined by the response of the
preceding patient (21). Using the
sequential approach to calculate median effective dose, once six
pairs of reversal of sequence was achieved, it was possible to
consider the sample size as adequate (20,24). The
present study had >6 pairs of reversal of sequence, so the
sample size was sufficient. In addition, previous simulation
studies suggest that including ≥20–40 patients will provide stable
estimates of the target dose for most realistic scenarios (19). The present study required a sample
size of 42 patients for the stopping rule.
A previous study reported an ED90
preventing hypotension for norepinephrine of 6 µg (11), however the weight based
ED50 and ED95 of norepinephrine have not been
widely investigated in patients. Therefore, the present study
assessed the ED50 and ED95 of norepinephrine.
Understanding the ED50 of a drug is important as it is
located in the most sensitive portion of the dose-response curve,
and small adjustments are expected to have significant increases in
therapeutic response (25). In
addition, determining the ED50 can potentially limit the
total number of patients enrolled in a clinical trial, which is
important when there is limited published information related to
the side effects of a drug. The present study used the up-and-down
method to determine the effective dose of norepinephrine, when the
starting dose approximates the therapeutic scope for the drug,
which resulted in the identification of the ED50 in a
limited number of patients. A limitation of the up-and-down method
is the inability to accurately determine the ED95
(26); therefore, the probit
regression model was used for this purpose.
Ngan Kee et al (16) conducted a random-allocation, graded
dose-response study of norepinephrine and phenylephrine and the
authors estimated the ED50 (dose yielding a 50%
response) of norepinephrine at 10 µg (95% CI, 6 to 17 µg), which
was different from the results of the present study. With
dose-response analysis, the value represents the dose that results
in responses of 50% magnitude, which differs from the same term in
the more traditional quantal dose-response methodology (27). In the present study, the intrathecal
anesthetic dose was 11 mg hyperbaric bupivacaine 0.5% w/v and 15 µg
fentanyl, while the dose of bupivacaine was 10 mg bupivacaine (2 ml
0.75% bupivacaine). The magnitude of response is measured as the
percentage of full restoration of SBP to baseline (16), which is different to the present
study where SBP returning to within 95% of the baseline value was
satisfactory. The ED50 calculated was much higher in the
study by Ngan Kee et al (16)
than the ED50 values for norepinephrine in the present
study. This variation could be attributed to the differing study
designs. Compared with the random-allocation graded dose-response
study, the present study used the sequential method to collect
cases, with the advantage of obtaining the effective dose with a
fewer number of patients. Differences in patient populations could
have also affected the study results.
A previous study has suggested that the incidence of
maternal hypotension is 50–80% depending upon the position of the
patient, the rate of spinal anesthetic agent injected, intravenous
fluid loading and whether the women is laboring or has associated
morbidity, including pregnancy induced hypertension (28). Anesthesiologists are required to be
careful since hypotension is common during cesarean delivery under
spinal anesthesia (28). Commonly
used vasopressors include ephedrine, phenylephrine and
norepinephrine (3). Ephedrine has
been previously considered the most appropriate vasopressor during
spinal anesthesia (28). However,
ephedrine is associated with decreased umbilical artery pH compared
with other vasopressors such as phenylephrine (29). In addition, ephedrine does not
completely prevent hypotension, nausea and vomiting and fetal
acidosis (9). On the contrary, it
may cause reactive hypertension in some patients (30). Phenylephrine is more effective for
venoconstriction than arterial constriction, and predictably
increases blood pressure by elevating both systemic vascular
resistance and preload (28). In
addition, due to its minimal β-2 receptor activity, phenylephrine
does not cause tachycardia, and instead induces reflex bradycardia
with increasing blood pressure (28).
Ali Elnabtity and Selim (3) compared ephedrine and norepinephrine,
and found that the latter is associated with reduced numbers of
hypotension and hypertension episodes as well as decreased
frequencies of bradycardia and tachycardia, while maintaining
maternal blood pressure and uterine artery blood flow during
cesarean delivery. Furthermore, the study also found that the
number of norepinephrine boluses used during spinal anesthesia is
lower compared with that of ephedrine. Norepinephrine does not
readily cross the placental barrier, because of the ability of the
placenta to degrade catecholamines (31). However, it is a mild β-adrenergic and
a potent α-adrenergic receptor agonist (7,8). Hence,
norepinephrine may be a more appropriate choice for stabilizing
maternal blood pressure with fewer adverse effects on HR and
cardiac output in the setting of elective cesarean delivery under
spinal anesthesia.
Although prophylactic infusion is the recommended
method for treating spinal hypotension to reduce hemodynamic
fluctuation and maternal side effects, it may be associated with a
higher incidence of reactive hypertension (32). Doherty et al (33) reported that no significant difference
could be found in maintaining baseline maternal CO and providing BP
stability between both options of an infusion regimen and a bolus
dose of phenylephrine in elective cesarean delivery under spinal
anesthesia. Compared with the bolus regimen, the infusion regimen
required a higher total dose of phenylephrine to maintain maternal
arterial blood pressure at baseline during the pre-delivery period
(33). Administration of the bolus
regimen resulted in a smaller reduction in baseline SBP in the
initial minutes after intrathecal injection. The bolus regimen
allows faster delivery of an effective dose of phenylephrine
recovering maternal vascular resistance rapidly during the
establishment of spinal blockade. Of note, many anesthesiologists
may favor bolus doses of vasopressors, while being prepared for
repeated doses rather than selecting infusion initially during
spinal anesthesia (34). Therefore,
the present study selected a bolus dose of norepinephrine, which
may be familiar to the majority of anesthesiologists. To the best
of our knowledge, no previous study has precisely determined the
ED50 and ED95 of weight-based norepinephrine
as a bolus in the setting of elective cesarean delivery under
spinal anesthesia. Furthermore, using norepinephrine as a bolus to
treat hypotension during cesarean delivery is not well
understood.
Chen et al (10) performed a randomized double-blinded
controlled study of 120 patients for elective section delivery
under spinal anesthesia; the patients treated by infusion were
assigned to four groups, and administered saline or norepinephrine
at 5, 10 and 15 µg/kg/h, respectively. Onwochei et al
(11) carried out a prospective,
double-blind sequential allocation dose-finding study, using the
biased coin up-and-down design. In the latter trial, 40 pregnant
women received a set intermittent norepinephrine bolus of 3, 4, 5,
6, 7 or 8 µg, when systolic blood pressure fell below 100% of
baseline. Vallejo et al (14)
conducted an open-label randomized controlled clinical trial
including 85 patients undergoing spinal anesthesia for elective
cesarean delivery, who were randomized to the phenylephrine (0.1
µg/kg/min) and norepinephrine (0.05 µg/kg/min) fixed-rate infusion
groups. The present results suggested the ED50 and
ED95 of norepinephrine were 0.067 µg/kg (95% CI,
0.056–0.081) and 0.121 µg/kg (95% CI, 0.1–0.207), respectively,
during cesarean delivery, corroborating the previous studies.
The issue of tissue injury caused by bolus
norepinephrine through peripheral venous catheters has become the
main concern in the field (35).
Previous studies have reported complications associated with
norepinephrine administered through peripheral venous catheters,
e.g. skin necrosis, with a 3.6% complication rate in 55 patients
administered the vasopressor (36).
However, a recent retrospective trial in neonates using
vasopressors via peripheral venous catheters found no complications
(37). In another prospective study,
55 patients received vasopressors via peripheral venous catheters,
and the rate of complications (5.45%) was very low, with no
significant morbidity (38). In the
present study, the highest single bolus dose of norepinephrine was
0.125 µg/kg, and was associated with no complications.
However, the present study does have limitations
that need to be mentioned. First, the present study, which was
based on treatment for the first episode of hypotension following
spinal anesthesia, was not extended until the end of surgery;
responses to subsequent episodes of hypotension could be different.
Secondly, the individual sensitivity to vasoactive drugs differs,
which may impact the results. Thirdly, all participants were from
the same geographic area. Finally, the present study had no control
group.
In conclusion, the present results indicated that
the ED50 values of a single bolus of norepinephrine for
preventing hypotension in elective CD were 0.067 µg/kg (95% CI,
0.056–0.081) and 0.072 µg/kg (95% CI, 0.056–0.088) by the
up-and-down method and the probit regression model, respectively.
The ED95 obtained by the probit regression model was
0.121 µg/kg (95% CI, 0.1–0.207).
Acknowledgements
Not applicable.
Funding
The current study was supported, in part, by grants
from the Natural Science Foundation of Zhejiang Province (grant no.
LY17H090019), the Science and Technology Project of Jiaxing City
(grant no. 2018AY32012), Medical Scientific Research Foundation of
Zhejiang Province, China (grant no. 2020358554) the Medical and
Health General Research Program of Zhejiang Province (grant no.
2019KY687), the Construction Project of Anesthesiology Discipline
Special Disease Center in Zhejiang North Region (grant no. 201524),
the Key Medical Subjects Established by Zhejiang Province and
Jiaxing City Jointly, Pain Medicine (grant no. 2019-ss-ttyx), and
the Construction Project of Key Laboratory of Nerve and Pain
Medicine in Jiaxing City.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW and QH conducted the majority of the experiments
and wrote the manuscript. WZ and JZ conceived and designed the
study. HN and RY performed the data analysis. QL and LX collected
the data and MY coordinated and supervised the experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Jiaxing Maternity and Child Healthcare Hospital
(Zhejiang, China) and registered in the Chinese Clinical Registry
Center (registration no. ChiCTR1800018474). All of the participants
who participated in the study provided written informed consent at
the time of enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mercier FJ, Augè M, Hoffmann C, Fischer C
and Gouez AL: Maternal hypotension during spinal anesthesia for
caesarean delivery. Minerva Anestesiol. 79:62–73. 2013.PubMed/NCBI
|
|
2
|
Antoine C and Young BK: Fetal lactic
acidosis with epidural anesthesia. Am J Obstet Gynecol. 142:55–59.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ali Elnabtity AM and Selim MF:
Norepinephrine versus ephedrine to maintain arterial blood pressure
during spinal anesthesia for cesarean delivery: A prospective
double-blinded trial. Anesth Essays Res. 12:92–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee A, Kee WD and Gin T: A quantitative,
systematic review of randomized controlled trials of ephedrine
versus phenylephrine for the management of hypotension during
spinal anesthesia for cesarean delivery. Anesth Analg. 94:920–926.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ngan Kee WD, Lee A, Khaw KS, Ng FF,
Karmakar MK and Gin T: A randomized double-blinded comparison of
phenylephrine and ephedrine infusion combinations to maintain blood
pressure during spinal anesthesia for cesarean delivery: The
effects on fetal acid-base status and hemodynamic control. Anesth
Analg. 107:1295–1302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ngan Kee WD, Khaw KS, Tam YH, Ng FF and
Lee SW: Performance of a closed-loop feedback computer-controlled
infusion system for maintaining blood pressure during spinal
anaesthesia for caesarean section: A randomized controlled
comparison of norepinephrine versus phenylephrine. J Clin Monit
Comput. 31:617–623. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart A, Fernando R, McDonald S, Hignett
R, Jones T and Columb M: The dose-dependent effects of
phenylephrine for elective cesarean delivery under spinal
anesthesia. Anesth Analg. 111:1230–1237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ngan Kee WD, Lee SW, Ng FF, Tan PE and
Khaw KS: Randomized double-blinded comparison of norepinephrine and
phenylephrine for maintenance of blood pressure during spinal
anesthesia for cesarean delivery. Anesthesiology. 122:736–745.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chooi C, Cox JJ, Lumb RS, Middleton P,
Chemali M, Emmett RS, Simmons SW and Cyna AM: Techniques for
preventing hypotension during spinal anaesthesia for caesarean
section. Cochrane Database Syst Rev. 8:CD0022512017.PubMed/NCBI
|
|
10
|
Chen D, Qi X, Huang X, Xu Y, Qiu F, Yan Y
and Li Y: Efficacy and safety of different norepinephrine regimens
for prevention of spinal hypotension in cesarean section: A
randomized trial. Biomed Res Int. 2018:27081752018.PubMed/NCBI
|
|
11
|
Onwochei DN, Ngan Kee WD, Fung L, Downey
K, Ye XY and Carvalho JC: Norepinephrine intermittent intravenous
boluses to prevent hypotension during spinal anesthesia for
cesarean delivery: A sequential allocation dose-finding study.
Anesth Analg. 125:212–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doyle DJ and Garmon EH: American Society
of Anesthesiologists Classification (ASA Class). StatPearls
Publishing LLC; Treasure Island, FL: 2019
|
|
13
|
Harten JM, Boyne I, Hannah P, Varveris D
and Brown A: Effects of a height and weight adjusted dose of local
anaesthetic for spinal anaesthesia for elective Caesarean section.
Anaesthesia. 60:348–353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vallejo MC, Attaallah AF, Elzamzamy OM,
Cifarelli DT, Phelps AL, Hobbs GR, Shapiro RE and Ranganathan P: An
open-label randomized controlled clinical trial for comparison of
continuous phenylephrine versus norepinephrine infusion in
prevention of spinal hypotension during cesarean delivery. Int J
Obstet Anesth. 29:18–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Apgar V: A proposal for a new method of
evaluation of the newborn infant. Anesthesia Analgesia. 32:250–259.
1953. View Article : Google Scholar
|
|
16
|
Ngan Kee WD: A Random-allocation graded
dose-response study of norepinephrine and phenylephrine for
treating hypotension during spinal anesthesia for cesarean
delivery. Anesthesiology. 127:934–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ngan Kee WD, Khaw KS and Ng FF: Comparison
of phenylephrine infusion regimens for maintaining maternal blood
pressure during spinal anaesthesia for Caesarean section. Br J
Anaesth. 92:469–474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Huang Y, Diao M, Li H, Ma Y, Lin X
and Zhou J: Determination of the 90% effective dose (ED90) of
phenylephrine for hypotension during elective cesarean delivery
using a continual reassessment method. Eur J Obstet Gynecol Reprod
Biol. 194:136–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stylianou M and Flournoy N: Dose finding
using the biased coin up-and-down design and isotonic regression.
Biometracs. 58:171–177. 2002. View Article : Google Scholar
|
|
20
|
Dixon WJ: Staircase bioassay: The
up-and-down method. Neurosci Biobehav Rev. 15:47–50. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pace NL and Stylianou MP: Advances in and
limitations of up-and-down methodology: A précis of clinical use,
study design, and dose estimation in anesthesia research.
Anesthesiology. 107:144–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dixon WJ: The up-and-down method from
small samples. J Am Stat Assoc. 60:967–978. 1965. View Article : Google Scholar
|
|
23
|
Miller JM Jr, Bernard M, Brown HL, St
Pierre JJ and Gaber HA: Umbilical cord blood gases for term healthy
newborns. Am J Perinatol. 7:157–159. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sell A, Olkkola KT, Jalonen J and Aantaa
R: Minimum effective local anaesthetic dose of isobaric
levobupivacaine and ropivacaine administered via a spinal catheter
for hip replacement surgery. Br J Anaesth. 94:239–242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lynde GC: Determination of ED50
of hydromorphone for postoperative analgesia following cesarean
delivery. Int J Obstet Anesth. 28:17–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnston DF, Sondekoppam RV, Giffin R,
Litchfield R and Ganapathy S: Determination of ED50 and
ED95 of 0.5% Ropivacaine in adductor canal block to produce
Quadriceps weakness: A dose-finding study. Reg Anesth Pain Med.
42:731–736. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tallarida RJ: Drug Synergism and
Dose-Effect Data Analysis. Chapman and Hall/CRC; New York, NY: pp.
21–39. 2000
|
|
28
|
Macarthur A and Riley ET: Obstetric
anesthesia controversies Vasopressor choice for postspinal
hypotension during cesarean delivery. Int Anesthesiol Clin.
45:115–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomas D, Robson S, Redfern N, Hughes D
and Boys R: Randomized trial of bolus phenylephrine or ephedrine
for maintenance of arterial pressure during spinal anaesthesia for
Caesarean section. Br J Anaesth. 76:61–65. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ngan Kee WD, Khaw KS, Lee BB, Lau TK and
Gin T: A dose-response study of prophylactic intravenous ephedrine
for the prevention of hypotension during spinal anesthesia for
cesarean delivery. Anesth Analg. 90:1390–1395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Puolakka J, Kauppila A, Tuimala R,
Jouppila R and Vuori J: The effect of parturition on umbilical
blood plasma levels of norepinephrine. Obstet Gynecol. 61:19–21.
1983.PubMed/NCBI
|
|
32
|
Kinsella SM, Carvalho B, Dyer RA, Fernando
R, McDonnell N, Mercier FJ, Palanisamy A, Sia AT, Van de Velde M
and Vercueil A; Consensus Statement Collaborators, : International
consensus statement on the management of hypotension with
vasopressors during caesarean section under spinal anaesthesia.
Anaesthesia. 73:71–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Doherty A, Ohashi Y, Downey K and Carvalho
JC: Phenylephrine infusion versus bolus regimens during cesarean
delivery under spinal anesthesia: A double-blind randomized
clinical trial to assess hemodynamic changes. Anesth Analg.
115:1343–1350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Langesæter E, Rosseland LA and Stubhaug A:
A randomized, double-blind comparison of low-dose versus high-dose
spinal anesthesia with intravenous phenylephrine or placebo
infusion. Anesthesiology. 109:856–863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smiley RM: More perfect? Int J Obstet
Anesth. 29:1–4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Greenwald HP, Gootnick A, Luger NM and
King JA: Tissue necrosis following subcutaneous infiltration with
nor-epinephrine; Report of two cases. N Engl J Med. 246:252–253.
1952. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Turner DA and Kleinman ME: The use of
vasoactive agents via peripheral intravenous access during
transport of critically III infants and children. Pediatr Emerg
Care. 26:563–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Medlej K, Kazzi AA, El Hajj Chehade A,
Saad Eldine M, Chami A, Bachir R, Zebian D and Abou Dagher G:
Complications from administration of vasopressors through
peripheral venous catheters: An observational study. J Emerg Med.
54:47–53. 2018. View Article : Google Scholar : PubMed/NCBI
|