Introduction
Endometriosis is defined as the presence of
endometrial tissue (glands and stroma) outside of the uterine
cavity, and is one of the most common benign gynecological diseases
in women of reproductive age worldwide (1). The most common site of endometriosis is
the pelvic peritoneum and ovaries (1). Clinical symptoms of endometriosis are
non-specific and include chronic pelvic pain, dysmenorrhea, deep
pain during or after the sexual intercourse, infertility and
irregular menstruation (1).
Treatment choices vary significantly according to the symptoms of
each patient, and include analgesics, hormonal treatment and
surgical management (1,2). All of the current treatments aim to
only alleviate pain, regulate the menstrual cycle and promote
fertility, but not to cure the disease completely. Recurrence of
endometriosis is very high, ranging from 21.5% within 2 years to
40–50% within 5 years (3). Although
endometriosis appears to be widely recognized and well-treated, the
etiology and pathophysiology of this disease is still largely
unknown. One of the well-documented and accepted theories is the
retrograde menstruation theory, which states that viable menstrual
endometrial cells retrograde back through the fallopian tubes into
the peritoneal cavity and implant onto the peritoneal surface or
ovaries (4). Based on this theory, a
number of in-vitro and in-vivo models have been
established to investigate the pathology behind endometriosis and
its pathology. In animal models, the rhesus monkeys can develop
endometriosis spontaneously, and endometriosis can be induced by
transplanting endometrial tissues or cells from the same species or
from humans into the peritoneum of primate or rodents, which has
been reviewed in detail previously (5,6). Animal
models are able to recapitulate the potential pathogenesis of human
endometriosis in vivo. However, they use different species
other than humans, lack cellular and molecular investigations and
require a large amount of capital and time. In-vitro models
are easier to establish, can aid in understanding the process of
endometriosis and can be used to investigate cellular and molecular
mechanisms in detail. In the current review, the majority of
in-vitro models to date are summarized and discussed, and
further insights are obtained for the study of endometriosis.
In-vitro models
Cell lines
There are several endometriotic cell lines that are
commercially available, including epithelial type 12-Z, 49-Z, 108-Z
and 11-Z, and stromal type 22-B (7),
which are immortalized endometriotic cells. By comparing these
cells with primary human normal endometrial cells, it has been
indicated that they exhibit a higher potency of cell migration and
invasion, have a higher expression of genes regulating steroid
metabolism, especially the hormone receptors (estrogen receptor β
and progesterone receptor), and produce a larger amount of
prostaglandin (PG) E2, invasion related molecules [including the
activity of matrix metalloproteinase (MMP)2 and MMP9], vascular
endothelial growth factor (VEGF) and epidermal growth factor and
cytokines [interleukin (IL)-1β and tumor necrosis factor (TNF)α]
(7). Similar to the immortalized
normal endometrial cells, endometriotic cell lines also express
genes related to hormone biosynthesis and signaling, cell cycle
regulation, cell growth/survival and angiogenesis (7). These cell lines are widely used in
vitro to investigate the mechanisms of action behind
endometriosis due to them being a stable resource and the fact they
are easy to handle.
In a cell monolayer culture model, the association
between the function of endometriotic cells and hormonal related
molecules has been studied. In this aforementioned study, cell
proliferation and viability of 12-Z and 22-B cells was
significantly decreased following transfection of siRNA designed to
knock down the expression levels of EP2 and EP4 (receptors of PGE2;
Fig. 1A) (8). In an endometriosis mouse model, an
antagonist of growth hormone-releasing hormone, MIA-602, was
demonstrated to significantly reduce the size of the endometriotic
lesion (9). To investigate the
underlying mechanisms of action, cell lines (12-Z and 49-Z) were
exposed to MIA-602 in vitro (Fig.
1A). It was demonstrated that MIA-602 inhibited cell
proliferation, reduced the expression of epithelial growth factor
receptors and inhibited the activation of the MAP-kinases, ERK-1/2
(9). MicroRNA (miR)-145 was abundant
in the ectopic endometrium, and the overexpression of miR-145 in
12-Z cells inhibited cell proliferation and invasion of the
Matrigel®-coated invasion chamber (Fig. 1B). miR-145 was also indicated to
reduce the expression of a number of cytoskeletal elements and
stemness-associated factors in 12-Z cells (10). These results demonstrated that cell
line models are beneficial tools to verify the results from
clinical discoveries and murine models, as well as to explore the
underlying mechanisms of action. They may provide information on
novel biomarkers and treatment strategies for use in
endometriosis.
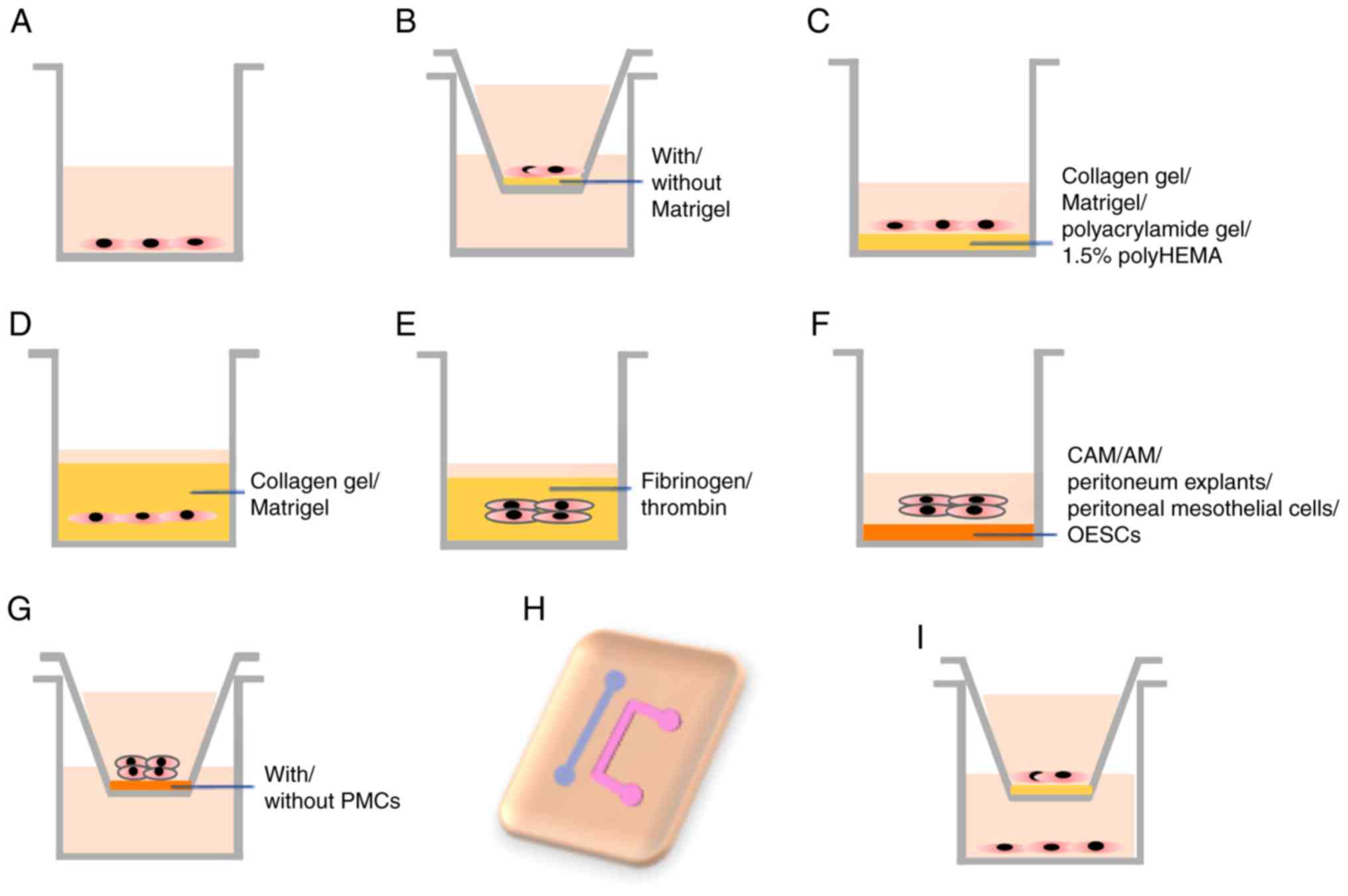 | Figure 1.In vitro models of
endometriosis. (A) Endometriotic cell lines, primary EECs and ESCs,
EN-MSCs, OESCs and PMCs are cultured in plates. (B) The migration
and invasion of the endometriotic cell lines, ESCs, and mixed
population with ESCs and PMCs in a chamber with/without Matrigel.
(C) Endometriotic cell lines, EEC and ESCs are grown on top of
plates coated with various matrices. (D) 3D model of endometriotic
cell lines, ESCs, EN-MSCs and OSE cells in collagen gel or Matrigel
solution. (E) 3D model of endometrial explants in fibrinogen or
thrombin solution. (F) Endometrial explants and cell lines are
grown on top of CAM, AM, peritoneum explants, PMCs or OESCs. (G)
Endometrial explants or ESCs grown in inserts coated with/without
PMCs. (H) The microfluidic channels model with ESCs and PMCs. (I)
EECs, ESCs, EN-MSCs, PMCs, immune cells and human umbilical cord
endothelial cells are cultured in inserts or in the lower well in
various combinations. 3D, three dimensional; AM, amniotic membrane;
CAM, chicken chorioallantotic membrane; EEC, endometrial epithelial
cells; EN-MSC, endometrial mesenchymal stem cells; OESC, ovarian
endometrioma stromal cells; ESC, endometrioma stromal cells; PMC,
peritoneal mesothelial cells. |
Since endometriosis is recognized as a chronic
inflammatory disease, the immune response of endometriotic cells
have been investigated in vitro. Although endometriotic cell
lines are not immune cells, they can respond to many immune
regulators by producing cytokines or regulating cell functions,
which indicate the importance of the immune response in the process
of endometriosis and the close involvement of endometriotic cells
in this inflammatory reaction (11).
For example, IL-33 stimulates the production of pro-inflammatory
cytokines in 12-Z cells, including chemokine ligand (CXCL)1, IL-6,
granulocyte-macrophage colony-stimulating factor and IL-15
(12) (Fig. 1A). CXCL12 and its specific receptor,
chemokine receptor (CXCR)4 are highly expressed in patients with
endometriosis, and the invasive and migratory ability of 12-Z cells
was increased by CXCL12 treatments, while this could be reversed by
AMD3100, a CXCR4-specific inhibitor (13) (Fig.
1B).
In addition to the traditional 2D monolayer
cultures, 3D models have also been established to mimic
endometriosis using the endometriotic cell lines, EEC15 and 12-Z
(Fig. 1C). In a previous study,
cells were cultured in 1.5% polyHEMA-coated culture plates, and
began to aggregate and form symmetrical spheroid structures within
7 days, which were visualized and measured under the microscope
(14). The histological and
molecular features of these 3D spheroids were similar to primary
human endometriotic lesions. In these 3D models, molecules related
to the immune response (including IL6, IL8, CXCL12 and CXCR4),
micro-environmental interactions [(including MMP2 and hepatocyte
growth factor (HGF)) and hormonal signaling [including
prostaglandin-endoperoxide synthase 2 (PTGS2) and cytochrome P450
family 19 subfamily A member 1 (CYP19A1)] were significantly
upregulated compared with the 2D models (14). Therefore, careful interpretation is
required of the results from the 2D and 3D models using these cell
lines, and 3D models using primary endometriotic cells and in
vivo models are required to support the results gained.
Human primary endometrial epithelial
cells (EECs) and endometrial stromal cells (ESCs)
Compared with cell lines, human primary cells are a
better model to use, with greater homology to the real in
vivo situation. Human EECs and ESCs can be isolated from the
eutopic endometrium and the ectopic endometriotic tissues, which
allows for the maintenance of their individual in vivo
phenotypic and functional markers (15). Isolated EECs and ESCs from deep
endometriotic tissue have previously been immortalized using
retroviral transfection of the human telomerase reverse
transcriptase to develop individual cell lines, which maintain the
characteristics of the primary cells, including the morphology and
expression of hormone receptors (16). Estradiol induces the proliferation
and invasion of ESCs from the eutopic endometrium of endometriosis
through the phosphorylation of cofilin1 mediated by LIM domain
kinase (LIMK)1, which indicated that the LIMK1/cofilin1 pathway may
be a therapeutic target for this disease (17). Celecoxib is a selective
cyclooxygenase (COX)-2 inhibitor. In EECs isolated from the eutopic
endometrium of endometriosis patients, Celecoxib inhibited the
proliferation, induced the apoptosis and reduced the secretion of
PGE2 and VEGF (Fig. 1A) (18). To investigate the association between
the extracellular matrix stiffness and the behavior of the
endometriotic stromal cells, ESCs are grown on top of
polyacrylamide gels with a variety matrix stiffness (Fig. 1C). The increased matrix stiffness
induces the formation of F-actin stress fibers and the expression
of procollagen I in cells from healthy patients and patients with
endometriosis, while the production of alpha smooth muscle actin
(αSMA)-containing stress fibers was only induced in the
endometriotic stromal cells. The increased matrix stiffness also
regulated the expression of molecules related to collagen synthesis
and degradation (such as type I collagen, cyclin D1, MMP-1 and
MMP-14), which suggests that an aberrant extracellular matrix is
involved in the process of endometriosis (19).
Similar to cell lines, primary endometrial cells can
be used to generate 3D models (Fig.
1D). The 3D polymerized collagen matrices contain the
endometriotic or ESCs mixed with the type I collagen solution.
After polymerization, the proliferation of the ESCs in the 2D
culture were significantly less inhibited compared with the ESCs in
the 3D polymerized collagen, and the activation of AKT and ERK
pathway was more intense in the endometriotic stromal cells than
that in the ESCs. The inhibition of the AKT and ERK signaling
pathway decreases the cell proliferation but does not increase the
caspase 3/7 activity in the 3D system of the endometriotic stromal
cells (20). These results
demonstrated that differences exist between the endometrial cells
from patients with endometriosis and that from women without
endometriosis. By further comparing these two cell types, it is
likely that more knowledge can be obtained about the pathology
behind endometriosis. Similar to cell lines, primary cells still
perform differently in 2D and 3D culture systems, which researchers
should take account of when designing experiments and interpreting
results.
Previous results have indicated that the peritoneal
fluid (PF) may be associated with the process of the endometriosis.
PF collected from patients with endometriosis or women without
endometriosis exhibit a similar effect on the cell proliferation of
the eutopic EECs and ESCs in vitro (Fig. 1A) (21), and induce the expression of VEGF-A,
urokinase plasminogen activator and plasminogen activator
inhibitor-1 in the eutopic ESCs. PF from patients with
endometriosis reduces the expression of a number of miRs in the
eutopic ESCs compared with the PF from women without endometriosis,
including miR-16-5p, miR-29c-3p and miR-424-5p (22,23).
Overexpression of these miRs reduces the secretion of VEGF-A in
eutopic ESCs (22,23). These results demonstrated that PF
contributes to the endometriosis process, especially during
angiogenesis, via the regulation of miRs.
Using the primary cell model, factors regulating
endometriosis have been previously investigated, including
platelet-derived growth factor (24), which is a macrophage secretory
product, and miR-200c and miR-145 (10,25),
which regulate the proliferation of eutopic ESCs. Similarly, the
anti-fibrotic factor epigallocatechin-3-gallate (EGCG), has been
revealed to inhibit the proliferation, migration and invasion of
eutopic and ectopic ESCs (26). EGCG
also significantly decreases the expression levels of αSMA,
Collagen-I (Col-I), connective tissue growth factor (CTGF) and
fibronectin (FN) in both cell types (26). Di-(2-ethylhexyl)-phthalate, which is
an environmental contaminant, induces the invasion of the eutopic
ESCs through the activation of MMP-2 and 9 and the ERK signaling
pathway (27). Inhibition of the
Wnt-β-catenin pathway significantly decreases the expression of the
fibrotic marker genes αSMA, Col-I, CTGF and FN in endometriotic and
ESCs, and activation of the Wnt-β-catenin pathway not only induces
the expression of these four markers, but also increases the cell
proliferation and migration of ESCs (28). By analyzing the secretion in the
culture medium of ESCs and EECs, a number of factors associated
with angiogenesis have been detected (29,30),
including platelet-derived endothelial cell growth factor, VEGF,
macrophage migration inhibitory factor and IL-8.
The aforementioned in-vitro models were based
on monocultures of EECs or ESCs. Co-culturing ESCs and ECCs may
represent the whole endometrium (Fig.
1A). In this model, IL-8 increases cell survival with no
differences observed between cells from women with or without
endometriosis, while IL-12 inhibits cell survival from women
without endometriosis, but not in cells obtained from women with
endometriosis (31). When these
endometrial cells are grown on top of the solidified Matrigel
solution (Fig. 1C), the
micro-tubular structures are visualized under the microscope as a
simulation of angiogenesis. Overexpression of protein tyrosine
phosphatase (PTEN) increases cell apoptosis, inhibits the cell
cycle and inhibits the formation of the micro-tubular structures,
with the reciprocal observations indicated when PTEN is inhibited
(32). However, in this model, it
was impossible to differentiate the effect of ILs and PTEN on EECs
or ESCs. The ratio of EECs and ESCs from individual patients was
not consistent throughout the aforementioned study, and the
reproducibility of the results is a concern.
Endometrial stem cells
The endometrial stem cell implantation theory is an
expansion of the retrograde menstruation theory (33). Endometrial epithelial progenitor
cells and mesenchymal stem-cell-like cells, together with the
menstruation are shed into the peritoneum, where they implant and
establish the ectopic lesions. Cells isolated from the ectopic
endometrium (endometriosis lesions) express markers of multipotent
mesenchymal stem cells (MMSCs), including CD90, CD73 and CD105, and
these cells are capable of adipogenic and osteogenic
differentiation. Although differences are indicated between the
MMSCs from the eutopic endometrium and from the ectopic
endometrium, the similarities suggest that the ectopic MMSCs can be
used as a novel model for investigating endometriosis (34,35).
Endometrial mesenchymal stem cells (EN-MSCs) are isolated from
uterine and ovarian endometriotic tissues from the same patient as
eutopic EN-MSCs and ectopic EN-MSCs. The two cells have different
gene profiles, especially IL-1b and COX-2, which are more highly
expressed in the ectopic EN-MSCs, and enhance the migratory and
invasive ability of ectopic EN-MSCs (36). The EN-MSCs collected from the eutopic
and ectopic endometrial tissues represent a heterogenic population
of mesenchymal stem cells and stromal fibroblast cells. When the
EN-MSCs are directly co-cultured with the endothelial cell line,
human umbilical vein endothelial cells (HUVECs), EN-MSCs
differentiate into endothelial cells and the co-cultures form
tube-like structures in Matrigel (Fig.
1C) (37), which can be used as
a model for studying the angiogenesis of endometriosis in
vitro.
Recently, Hapangama et al (38) identified endometrial basalis-like
(SSEA1+/SOX9+) cells contributing to the pathogenesis of
endometriosis. The basalis-like cells (SSEA1+, SOX9+) were abundant
in the functional layer during the secretory phase, from the
eutopic endometrium of patients with endometriosis. In-vitro
differentiation experiments demonstrated that SSEA1 + EECs are not
pluripotent as they are unable to differentiate down the same
mesodermal lineages as EN-MSCs. However, the cells from patients
with endometriosis are able to generate ectopic endometriotic
lesion-like structures in a 3D model with Matrigel (Fig. 1D), and these structures are
morphologically similar to the ectopic endometrial epithelium. This
3D model expresses cytokeratin18, MUC-1, β-catenin and the hormone
receptors (ERα, ERβ and PR). The evidence indicated the importance
of SSEA1+/SOX9+ cells in the development of endometriosis, which
may be a potential therapeutic target for endometriosis.
Endometrial explants
Along with isolated endometrial cells, endometrial
explants could also be utilized for the establishment of models to
investigate endometriosis. Small endometrial fragments could
outgrow, form glands/vessels, proliferate and invade the 3D culture
models with a fibrinogen/thrombin solution, and could be used to
study the early events of endometriosis (39,40)
(Fig. 1E). The tubular structures in
3D models have been examined using transmission electron
microscopy, and these cells are polarized, with microvilli on the
apical surface and present as desmosome-like structures on the
basement membrane, with features that are consistent with the
glandular epithelial cells observed in vivo (41). In this model, treatment with
dienogest, which is a current treatment for endometriosis, inhibits
the outgrowth of endometrial explants and induces apoptosis
(41). A total of two angiogenic
factors, glycodelin and COX-2 have been detected in >80% of 3D
cultures, which indicates that angiogenesis occurs in this model
(42). Statin inhibits cell
proliferation and impairs angiogenesis in a concentration-dependent
manner, which implies that statin may be a potential treatment
strategy for the early stages of endometriosis (43). In this 3D model, only the normal
endometrium was used, but endometriotic endometrium should be used
to investigate the pathogenesis of the disorder and for drug
screening. The endometrial explants took a number of weeks to
generate a fully developed model, and an obvious disadvantage to
this model is that it is time-consuming compared to cell lines or
primary endometrial cells. However, the glandular formation and
angiogenesis are great merits of this 3D model.
Chicken chorioallantoic membranes
(CAMs) and amniotic membranes culture model
In the retrograde menstruation theory, endometrium
encounters the peritoneum, implants, invades and forms the ectopic
endometrium and endometriotic lesions (33). Therefore, the peritoneum represents
the other side of the endometriosis pathogenesis. In vitro,
selected biological membranes have successfully replaced the
peritoneum, such as the CAM, which can be attached to and be
invaded by the endometrium (Fig.
1F). The CAM assay is widely used to investigate the angiogenic
properties of the endometrium (44).
Only endometrial fragments, instead of single endometrial cells,
have been demonstrated to induce the formation of
endometriosis-like lesions within 72 h after implanting onto the
CAM (45). A previous study
demonstrated that the endometrium collected from women, who were
using oral contraceptives (OCs), presented fewer endometriosis-like
lesions in the CAM compared with the endometrium collected from the
menstrual endometrium from women experiencing normal cycles
(46), which suggested that OCs
affected the characteristics of the endometrium and aided in the
regulation of endometriosis. In this model, the angiostatic
compounds, such as the anti-hVEGF antibody, TNP-470, endostatin and
anginex, impair the formation of endometriosis-like lesions and
decrease vessel densities. This suggests that the angiostatic
compounds may provide an alternative therapy for endometriosis
(47).
Similar to the CAM, the human amniotic membranes
(AM) can also be used as a surrogate for the peritoneum (48,49).
Endometrial fragments are unable to adhere to the intact epithelial
side of the AM; however, they can adhere to the damaged and
non-epithelial side. Endometrial carcinoma cell lines have been
indicated to attach to both sides of the AM, which may suggest that
primary endometrial fragments are superior to endometrial cell
lines in this model. This also indicates that the integrity of the
epithelia on the peritoneum may prevent the adhesion and growth of
the retrograde endometrium, prohibiting the initial stages of
endometriosis (50,51) (Fig.
1F). When AMs are stripped of the epithelial lining, the
endometrial fragments, but not the isolated primary endometrial
cells, can adhere to both sides of the membrane (48,49). The
isolated endometrial cells are associated with lower expression
levels of β1 integrins as well as E- and P-cadherin.
The CAMs and AMs are good models to use in the study
of the endometrium implantation, the formation of endometriotic
lesion and the angiogenesis of endometrium in vitro. These
models have stable resources, a simplified procedure and a standard
analysis of results (44,49).
Co-culture models with peritoneal
cells and explants
In addition to CAMs and AMs, human primary
peritoneum tissues are also used to create in vitro
co-cultures with endometrium (Fig.
1F). The peritoneum explants collected from the anterior
abdominal wall are cut into small pieces and cultured in plates or
inserts, and endometrium biopsies are added to the top of
peritoneum explants. Within 2 days, endometrial cells attach and
invade the tissue (48,52,53). The
majority of endometrial fragments attach to a peritoneum denuded of
mesothelial cells (48,54). However, when mesothelial cells are
isolated from the PF from normal women and cultured to form a
monolayer in the collagen-coated plates, the endometrial fragments
can also grow on top of the monolayer, demonstrating the
characteristic morphology of endometriosis in vivo (Fig. 1F) (55). ESCs are also able to attach to the
peritoneum mesothelial cell (PMC) monolayer (56). In adhesion assays, the labelled
endometrial epithelial carcinoma cell line, CRL-1671, attaches to
the human mesothelial cell line, CRL-9444, and pretreatment of
mesothelial cells with the cytokines TNFα, IL-6 and IL-8, inhibits
the number of attached EECs (57).
In the same model, ESCs attach and invade through the LP-9 PMC
monolayer, and the treatment of ESCs with an active tyrosine kinase
inhibitor, Imatinib, does not affect the attachment of ESCs onto
the LP-9 monolayer, but does inhibit the invasion of the ESCs
(58). In order to investigate the
role of PMCs in the invasion of ESCs, Nair et al (59) developed a model where ESCs invaded
through the Matrigel-coated chamber with or without the presence of
PMCs growing on top of the Matrigel (Fig. 1G). In this aforementioned study, it
was indicated that with the presence of the PMCs monolayer, the
invasive ability of the ESCs was increased, and the transcription
of extracellular signal-related kinase, colony stimulating
factor-1, c-fms and c-Met were also increased in ESCs and PMCs
after the endometrial-PMC attachment (59). This demonstrated that PMCs
contributed to endometrial invasion.
Chen et al (60) established a novel model using
microfluidic channels system to observe the interaction of PMCs and
ESCs in real time (Fig. 1H). In this
model, two microfluidic channels were designed in a glass slide,
and PMCs and ESCs were incubated in the two channels, respectively.
After removal of the channel mold, these two cell types migrate and
interact with each other. On day 3 of culture, the two cell types
contact, and the invasion and interaction of these cells can be
visualized on day 7. PMCs from control patients were indicated to
resist the invasion of ESCs, irrespective of whether the cells were
from patients with or without endometriosis, but PMCs from patients
with endometriosis were able to be invaded by normal and
endometriotic ESCs (60). The
endometriotic PMCs lose their adhesion and undergo apoptosis when
invaded by ESCs. These results indicated the critical role of
intact PMCs in the pathogenesis of endometriosis.
Using peritoneal cells or explants together with
endometrium tissue, instead of surrogates, to establish in
vitro models is a step closer to mimicking the real in
vivo environment. This approach reconstructs the relationship
between the ectopic endometrium and the peritoneum. The
disadvantage of these models is the diversity of cell types, the
unstable cell or tissue resources and the complexity to its
formation, which makes it difficult to reproduce these experiments
in respective labs.
Co-culture models with immune
cells
It is well-known that the immune system is
associated with the development of endometriosis, including
macrophages and a number of cytokines (11). In addition to the culture system with
secretory immune-factors, endometrium co-cultures with immune cells
can be used to study the immune reaction observed in endometriosis.
A previous study used U937 (human immune cells monocyte cell line),
HMrSV5 (human PMC cell line) and eutopic ESCs from patients with
endometriosis to generate a number of co-culture models with a
variety of combinations of these three types of cells, including as
a direct co-culture of any two combinations of the cells with or
without the third cell type, cultured in the transwell chamber
inserts and a directly co-culture of all three cell types (Fig. 1I). It was indicated that co-cultures
of the three cell types promoted the secretion of RANTES, a
chemoattractant for both monocytes and activated T-cells, as well
as macrophage-inflammatory protein-1 α (MIP-1α) compared to
individual cell cultures of these three cell types and co-culturing
of any two types of cells (61). The
combination of 17b-estradiol and dioxin
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; an environmental
contaminant) increases the secretion of RANTES and MIP-1α in these
models, promotes the invasiveness of ESCs and increases the
expression of MMP-2 and MMP-9 in ESC, which is mediated by CC-motif
chemokines (61). These observations
may indicate that 17b-estradiol and TCDD promote the onset of
endometriosis (61). In another
model, macrophages from the PF of patients with endometriosis or
control women have been cultured indirectly with EECs or ESCs in
the lower well of transwell chambers (Fig. 1I). PF macrophages from endometriosis
increase the proliferation of EECs and ESCs compared with control
women (62), which suggests that
macrophages from patients with endometriosis have developed
differences compared with those from women without endometriosis.
Further investigation is required to identify these differences and
to elicit the mechanism of action in endometriosis.
Ovarian endometriosis model
As well as the peritoneum, ovaries are another
common ectopic site that the retrograde endometrium is able to
adhere to and grow (33). Ovarian
endometrioma stromal cells (OESCs) can be used to establish in
vitro endometriosis models (Fig.
1A). Daidzein-rich isoflavone aglycones, which is a dietary
supplement, inhibits the proliferation of OESCs and reduces the
expression of IL-6, IL-8, COX-2 and aromatase in OESCs (63). To study the association of miR-214
and fibrosis in endometriosis, the overexpression of miR-214 can be
used, and in OESCs, significantly decreases the mRNA expression of
αSMA, Col-I and CTGF (64). EEC16
cells are an ovarian endometriosis epithelial cell line that are
isolated from superficial endometriosis lesions on the surface of
the ovaries, which express a number of biomarkers at different
levels when compared to normal ovarian epithelial cells, including
the lack of N-Cadherin expression (14). EEC16 can form smooth spheroids in
1.5% polyHEMA-coated culture plates to generate a 3D model which
mimics endometriotic lesions (Fig.
1C). In this model, a number of genes associated with immune
responses, micro-environmental interactions and hormonal signaling
were upregulated, including IL6, IL8, MMP2, HGF, PTGS2 and CYP19A1
(14). EEC16 cells can be
immortalized using TERT, but EEC16-TERT cells differentially
express epithelial and mesenchymal markers, specifically, they do
not express cytokeratin nor other epithelial markers, including
BerEP4, but do express vimentin. EEC16 cells express keratin and
vimentin but lack E-cadherin expression (65). The colony formation ability of the
EEC16-TERT cell line has been indicated to be inhibited by the Src
and Wnt signaling pathways, respectively, which may provide a novel
strategy to manage endometriosis (65).
Human ovarian surface epithelial (OSE) cells from
healthy ovaries together with ESCs and 17β-estradiol (E2) form a
lumen structure in the collagen gel (Fig. 1D), while OSEs alone can only form
circular arrangements. Without E2, no lumen structure is detected.
In this 3D model, OSE cells form a lumen structure surrounded by
ESCs. Cytokeratin and epithelial membrane antigen were detected in
glandular cells, and cilia were observed on the cell surface
(66), which revealed that there was
an interaction between ESCs and ovarian cells, and an importance of
E2 in the process of endometriosis. The disadvantage of this model
with the collagen gel solution is that the gel shrinks within 3
days, is released from the culture well and floats in the medium.
The gel divides into multiple small pieces after 9 to 12 days.
Therefore, it is important to define the exact time point to
observe the lumen structure, making observations difficult and
meaning experienced researchers are required.
Discussion
Endometriosis is a complex disease involving a
number of processes, including implantation, proliferation,
invasion, angiogenesis and apoptosis resistance (33). A variety of signaling pathways are
involved in the development and progression of this disease.
Various in vitro models have been established to study the
pathogenesis of endometriosis in the past decades, and the
molecular mechanisms of action and potential therapies for this
disease have also been widely investigated (7,15,21,33,38–40,44,50,60,61).
Each model exhibits unique characteristics and functions, and can
represent one or a number of aspects of endometriosis as summarized
in Table I, including cell
proliferation, invasion, angiogenesis and formation of
endometriotic lesions. Cell lines and primary cells can be used to
study the majority of aspects of the endometriosis process, except
the formation of endometriotic lesions, which requires a 3D matrix
to provide sufficient solid space. For angiogenesis, co-culturing
with CAM/AM or HUVECs is preferable than culturing primary cells or
tissues only. It should be noted that cell lines and primary cells
in different situations, including in 2D or 3D models, demonstrate
distinct potencies in regards to proliferation and the production
of factors related to the immune response and hormonal signaling
(14,20). Based on the purpose of the study and
the available resources of cells or tissues, investigators should
select appropriate models or establish a novel model of their
own.
 | Table I.Functions of in vitro models
of endometriosis. |
Table I.
Functions of in vitro models
of endometriosis.
| A,
Proliferation |
|---|
|
|---|
| Models | (Refs) |
|---|
| Cell lines | 8–10 |
| Primary EECs,
ESCs | 10,18,21,25,26 |
| OESCs | 63 |
| Primary EECs, ESCs
in 3D | 20 |
| Stem cells | 36 |
| Immune cell
cultured with ESCs | 62 |
|
| B, Migration,
invasion |
|
| Models | (Refs) |
|
| Migration, invasion
Cell lines | 10,13 |
| Primary EECs,
ESCs | 16,26,28 |
| Stem cells | 36 |
| Endometrial
fragments and peritoneum | 48,52,53 |
| ESCs and peritoneal
cell monolayer | 57,58 |
| Microfluidic
channels system with PMCs and ESCs | 60 |
| Immune cell
cultured with ESCs | 61 |
|
| C, Inflammatory
response |
|
| Models | (Refs) |
|
| Cell lines | 12,13 |
| OESCs | 63 |
| Cell lines in
3D | 14 |
| Immune cell
cultured with ESCs | 11,61 |
|
| D, Formation of
endometriotic lesions or glands |
|
| Models | (Refs) |
|
| Cell lines in
3D | 14 |
| Stem cell in
3D | 38 |
| Endometrial
explants in 3D | 39,40 |
| Endometrial
explants in CAM | 45,46 |
| Endometrial
explants and mesothelial cells | 55 |
| OSEs and ESCs in
3D | 66 |
|
| E, Extracellular
matrix regulation and fibrosis |
|
| Models | (Refs) |
|
| Cell lines | 10 |
| Primary EECs,
ESCs |
19,22,23,26,27,28 |
| OESCs | 64,68 |
|
| F,
Angiogenesis |
|
| Models | (Refs) |
|
| Primary EECs,
ESCs | 29,30 |
| EECs and ESCs in
matrigel | 32 |
| EN-MSCs and
HUVECs | 37 |
| Endometrial
explants in 3D | 42,43 |
| Endometrial
explants in CAM | 47 |
|
| G,
Implantation |
|
| Models | (Refs) |
|
| Endometrial
explants in CAM | 45 |
| Endometrial
explants in AM | 48–51 |
| ESCs and PMCs | 56 |
When using cell lines to study the process of
endometriosis, results should be carefully interpreted due to their
carcinomic origins varying from that of human primary endometriotic
cells. Alternatively, human primary EECc and ESCs are ideal cells
to generate models, especially in 3D models with peritoneum cells
and explants, which provide a reliable situation to investigate
cell-cell interactions, cross-talks and angiogenesis. These models
emulate the in vivo processes, aid in the clarification of
the potential mechanisms of action and can be used to identify
therapeutic advances (20,31). By co-culturing endometriotic cells
with immune cells, the mechanisms of action behind the inflammatory
process in endometriosis can be further investigated. Each model
has only demonstrated one or several aspects of endometriosis, but
not the whole picture. Currently, a number of representative models
of endometriosis have been generated in various laboratories, with
no consensus of opinions on the standardization of models being
achieved. More comprehensive and reliable in vitro models
are required to establish increasingly detailed studies.
Vigano et al (67) proposed a new concept of the
definition of endometriosis in 2017, according to its pro-fibrotic
nature. It has been demonstrated that the endometrial stroma and
glands are only present as a minor component of endometriotic
lesions, and are often absent in some forms of the disease,
including rectovaginal nodules and ovarian endometrioma.
Additionally, alterations in smooth muscle and fibrosis are
consistent features of all forms of the disease (67). Therefore, the definition of
endometriosis needs to be reconsidered and reworded as ‘a fibrotic
condition in which endometrial stroma and epithelium can be
identified’. The fibrosis is a condition caused by the accumulation
and contraction of the collagenous extracellular matrix, which is
produced by activated myofibroblasts (67). Therefore, the differentiation and
activation of myofibroblasts in endometriotic lesions should be a
focus of future research. Currently, the model for studying
myofibroblasts is the differentiation of ESCs. For example,
endometriotic stromal cells isolated from the ovarian endometrioma
are driven into the epithelial-mesenchymal transition and the
fibroblast-to-myofibroblast trans-differentiation by activated
platelets through the TGF-β/Smad signaling pathway, which results
in increased cell contractility, collagen production and ultimately
to the fibrosis (68). In the
future, 3D culture or co-cultures with peritoneal explants and
immune cells could also be used to investigate the fibrosis and its
dynamic changes, as well as its interaction with the peritoneum and
the immune system. Except for understanding the development and
mechanisms of action behind fibrosis in endometriosis, in
vitro models are a promising tool to investigate therapeutic
advances for managing endometriosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HF designed and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declare that they have no competing
interests.
References
|
1
|
Kuznetsov L, Dworzynski K, Davies M and
Overton C; Guideline Committee, : Diagnosis and management of
endometriosis: Summary of NICE guidance. BMJ. 358:j39352017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dunselman GA, Vermeulen N, Becker C,
Calhaz-Jorge C, D'Hooghe T, De Bie B, Heikinheimo O, Horne AW,
Kiesel L, Nap A, et al: ESHRE guideline: Management of women with
endometriosis. Hum Reprod. 29:400–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo SW: Recurrence of endometriosis and
its control. Hum Reprod Update. 15:441–461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halme J, Hammond MG, Hulka JF, Raj SG and
Talbert LM: Retrograde menstruation in healthy women and in
patients with endometriosis. Obstet Gynecol. 64:151–154.
1984.PubMed/NCBI
|
|
5
|
Bruner-Tran KL, Mokshagundam S, Herington
JL, Ding T and Osteen KG: Rodent models of experimental
endometriosis: Identifying mechanisms of disease and therapeutic
targets. Curr Womens Health Rev. 14:173–188. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
King CM, Barbara C, Prentice A, Brenton JD
and Charnock-Jones DS: Models of endometriosis and their utility in
studying progression to ovarian clear cell carcinoma. J Pathol.
238:185–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banu SK, Lee J, Starzinski-Powitz A and
Arosh JA: Gene expression profiles and functional characterization
of human immortalized endometriotic epithelial and stromal cells.
Fertil Steril. 90:972–987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee J, Banu SK, Rodriguez R,
Starzinski-Powitz A and Arosh JA: Selective blockade of
prostaglandin E2 receptors EP2 and EP4 signaling inhibits
proliferation of human endometriotic epithelial cells and stromal
cells through distinct cell cycle arrest. Fertil Steril.
93:2498–2506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Köster F, Jin L, Shen Y, Schally AV, Cai
RZ, Block NL, Hornung D, Marschner G, Rody A, Engel JB and Finas D:
Effects of an antagonistic analog of growth hormone-releasing
hormone on endometriosis in a mouse model and in vitro. Reprod Sci.
24:1503–1511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adammek M, Greve B, Kassens N, Schneider
C, Brüggemann K, Schüring AN, Starzinski-Powitz A, Kiesel L and
Götte M: MicroRNA miR-145 inhibits proliferation, invasiveness, and
stem cell phenotype of an in vitro endometriosis model by targeting
multiple cytoskeletal elements and pluripotency factors. Fertil
Steril. 99:1346–1355.e5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Králíčková M, Fiala L, Losan P, Tomes P
and Vetvicka V: Altered immunity in endometriosis: What came first?
Immunol Invest. 47:569–582. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller JE, Monsanto SP, Ahn SH, Khalaj K,
Fazleabas AT, Young SL, Lessey BA, Koti M and Tayade C:
Interleukin-33 modulates inflammation in endometriosis. Sci Rep.
7:179032017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruiz A, Ruiz L, Colòn-Caraballo M,
Torres-Collazo BJ, Monteiro JB, Bayona M, Fazleabas AT and Flores
I: Pharmacological blockage of the CXCR4-CXCL12 axis in
endometriosis leads to contrasting effects in proliferation,
migration, and invasion. Biol Reprod. 98:4–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brueggmann D, Templeman C,
Starzinski-Powitz A, Rao NP, Gayther SA and Lawrenson K: Novel
three-dimensional in vitro models of ovarian endometriosis. J
Ovarian Res. 7:172014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryan IP, Schriock ED and Taylor RN:
Isolation, characterization, and comparison of human endometrial
and endometriosis cells in vitro. J Clin Endocrinol Metab.
78:642–649. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boccellino M, Quagliuolo L, Verde A, La
Porta R, Crispi S, Piccolo MT, Vitiello A, Baldi A and Signorile
PG: In vitro model of stromal and epithelial immortalized
endometriotic cells. J Cell Biochem. 113:1292–1301. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Zhang Z, Liu J and Wang D: LIM
Kinase 1 mediates estradiol effects on the phosphorylation of
Cofilin1 in eutopic endometrial stromal cells during the invasion
and proliferation of endometriosis. Reprod Sci. 26:1499–1505. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olivares C, Bilotas M, Buquet R, Borghi M,
Sueldo C, Tesone M and Meresman G: Effects of a selective
cyclooxygenase-2 inhibitor on endometrial epithelial cells from
patients with endometriosis. Hum Reprod. 23:2701–2708. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuzaki S, Canis M, Pouly JL and Darcha
C: Soft matrices inhibit cell proliferation and inactivate the
fibrotic phenotype of deep endometriotic stromal cells in vitro.
Hum Reprod. 31:541–553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuzaki S and Darcha C: Co-operation
between the AKT and ERK signaling pathways may support growth of
deep endometriosis in a fibrotic microenvironment in vitro. Hum
Reprod. 30:1606–1616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Overton CE, Fernandez-Shaw S, Hicks B,
Barlow DH and Starkey P: In vitro culture of endometrial stromal
and gland cells as a model for endometriosis: The effect of
peritoneal fluid on proliferation. Fertil Steril. 67:51–56. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Braza-Boïls A, Salloum-Asfar S,
Marí-Alexandre J, Arroyo AB, González-Conejero R, Barcelo-Mólina M,
García-Oms J, Vicente V, Estelles A, Gilabert-Estelles J and
Martínez C: Peritoneal fluid modifies the microRNA expression
profile in endometrial and endometriotic cells from women with
endometriosis. Hum Reprod. 30:2292–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Braza-Boïls A, Gilabert-Estélles J, Ramon
LA, Gilabert J, Marí-Alexandre J, Chirivella M, Espana F and
Estelles A: Peritoneal fluid reduces angiogenesis-related microRNA
expression in cell cultures of endometrial and endometriotic
tissues from women with endometriosis. PLoS One. 8:e623702013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Surrey ES and Halme J: Effect of
platelet-derived growth factor on endometrial stromal cell
proliferation in vitro: A model for endometriosis? Fertil Steril.
56:672–679. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Z, Chen Y, Zhao Y, Xu C, Zhang A,
Zhang Q, Wang D, He J, Hua W and Duan P: miR-200c suppresses
endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell
Res Ther. 8:2512017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuzaki S and Darcha C: Antifibrotic
properties of epigallocatechin-3-gallate in endometriosis. Hum
Reprod. 29:1677–1687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SH, Cho S, Ihm HJ, Oh YS, Heo SH, Chun
S, Im H, Chae HD, Kim CH and Kang BM: Possible role of phthalate in
the pathogenesis of endometriosis: In vitro, animal, and human
data. J Clin Endocrinol Metab. 100:E1502–E1511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsuzaki S and Darcha C: Involvement of
the Wnt/β-catenin signaling pathway in the cellular and molecular
mechanisms of fibrosis in endometriosis. PLoS One. 8:e768082013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Laschke MW and Menger MD: In vitro and in
vivo approaches to study angiogenesis in the pathophysiology and
therapy of endometriosis. Hum Reprod Update. 13:331–342. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujimoto J, Sakaguchi H, Hirose R and
Tamaya T: Expression of platelet-derived endothelial cell growth
factor (PD-ECGF) related to angiogenesis in ovarian endometriosis.
J Clin Endocrinol Metab. 84:359–362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gazvani R, Smith L and Fowler PA: Effect
of interleukin-8 (IL-8), anti-IL-8, and IL-12 on endometrial cell
survival in combined endometrial gland and stromal cell cultures
derived from women with and without endometriosis. Fertil Steril.
77:62–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv J, Zhu Q, Jia X, Yu N and Li Q: In
vitro and in vivo effects of tumor suppressor gene PTEN on
endometriosis: An experimental study. Med Sci Monit. 22:3727–3736.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Nicholes K and Shih IM: The origin
and pathogenesis of endometriosis. Annu Rev Pathol. Sep
3–2019.(Epub ahead of print). PubMed/NCBI
|
|
34
|
Savilova AM, Yushina MN, Rudimova YV,
Khabas GN, Chuprynin VD and Sukhikh GT: Characteristics of
multipotent mesenchymal stromal cells isolated from human
endometrium and endometriosis lesions. Bull Exp Biol Med.
161:610–615. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Savilova AM, Farkhat KN, Yushina MN,
Rudimova YV, Makiyan ZN and Adamyan LV: Characteristics of
multipotent mesenchymal stromal cells isolated from the endometrium
and endometriosis lesions of women with malformations of the
internal reproductive organs. Bull Exp Biol Med. 162:539–544. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kao AP, Wang KH, Long CY, Chai CY, Tsai
CF, Hsieh TH, Hsu CY, Chang CC, Lee JN and Tsai EM: Interleukin-1β
induces cyclooxygenase-2 expression and promotes the invasive
ability of human mesenchymal stem cells derived from ovarian
endometrioma. Fertil Steril. 96:678–684 e671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Canosa S, Moggio A, Brossa A, Pittatore G,
Marchino GL, Leoncini S, Benedetto C, Revelli A and Bussolati B:
Angiogenic properties of endometrial mesenchymal stromal cells in
endothelial co-culture: An in vitro model of endometriosis. Mol Hum
Reprod. 23:187–198. 2017.PubMed/NCBI
|
|
38
|
Hapangama DK, Drury J, Da Silva L,
Al-Lamee H, Earp A, Valentijn AJ, Edirisinghe DP, Murray PA,
Fazleabas AT and Gargett CE: Abnormally located SSEA1+/SOX9+
endometrial epithelial cells with a basalis-like phenotype in the
eutopic functionalis layer may play a role in the pathogenesis of
endometriosis. Hum Reprod. 34:56–68. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Esfandiari N, Nazemian Z and Casper RF:
Three-dimensional culture of endometrial cells: An in vitro model
of endometriosis. Am J Reprod Immunol. 60:283–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fasciani A, Bocci G, Xu J, Bielecki R,
Greenblatt E, Leyland N and Casper RF: Three-dimensional in vitro
culture of endometrial explants mimics the early stages of
endometriosis. Fertil Steril. 80:1137–1143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prechapanich J, Kajihara T, Fujita K, Sato
K, Uchino S, Tanaka K, Matsumoto S, Akita M, Nagashima M, Brosens
JJ and Ishihara O: Effect of a dienogest for an experimental
three-dimensional endometrial culture model for endometriosis. Med
Mol Morphol. 47:189–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Esfandiari N, Ai J, Nazemian Z, Javed MH,
Gotlieb L and Casper RF: Expression of glycodelin and
cyclooxygenase-2 in human endometrial tissue following
three-dimensional culture. Am J Reprod Immunol. 57:49–54. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Esfandiari N, Khazaei M, Ai J, Bielecki R,
Gotlieb L, Ryan E and Casper RF: Effect of a statin on an in vitro
model of endometriosis. Fertil Steril. 87:257–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maas JW, Le Noble FA, Dunselman GA, de
Goeij AF, Struyker Boudier HA and Evers JL: The chick embryo
chorioallantoic membrane as a model to investigate the angiogenic
properties of human endometrium. Gynecol Obstet Invest. 48:108–112.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nap AW, Groothuis PG, Demir AY, Maas JW,
Dunselman GA, de Goeij AF and Evers JL: Tissue integrity is
essential for ectopic implantation of human endometrium in the
chicken chorioallantoic membrane. Hum Reprod. 18:30–34. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nap AW, Groothuis PG, Punyadeera C,
Klein-Hitpass L, Kamps R, Delvoux B and Dunselman GA: Oral
contraceptives prevent the development of endometriosis in the
chicken chorioallantoic membrane model. Contraception. 78:257–265.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nap AW, Dunselman GA, Griffioen AW, Mayo
KH, Evers JL and Groothuis PG: Angiostatic agents prevent the
development of endometriosis-like lesions in the chicken
chorioallantoic membrane. Fertil Steril. 83:793–795. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Koks CA, Groothuis PG, Dunselman GA, de
Goeij AF and Evers JL: Adhesion of shed menstrual tissue in an
in-vitro model using amnion and peritoneum: A light and electron
microscopic study. Hum Reprod. 14:816–1822. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
van der Linden PJ, de Goeij AF, Dunselman
GA, Erkens HW and Evers JL: Amniotic membrane as an in vitro model
for endometrium-extracellular matrix interactions. Gynecol Obstet
Invest. 45:7–11. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Groothuis PG, Koks CA, de Goeij AF,
Dunselman GA, Arends JW and Evers JL: Adhesion of human endometrium
to the epithelial lining and extracellular matrix of amnion in
vitro: An electron microscopic study. Hum Reprod. 13:2275–2281.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
van der Linden PJ, de Goeij AF, Dunselman
GA, Erkens HW and Evers JL: Endometrial cell adhesion in an in
vitro model using intact amniotic membranes. Fertil Steril.
65:76–80. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Witz CA, Dechaud H, Montoya-Rodriguez IA,
Thomas MR, Nair AS, Centonze VE and Schenken RS: An in vitro model
to study the pathogenesis of the early endometriosis lesion. Ann N
Y Acad Sci. 955:296–307, 340–342, 396–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Witz CA, Monotoya-Rodriguez IA and
Schenken RS: Whole explants of peritoneum and endometrium: A novel
model of the early endometriosis lesion. Fertil Steril. 71:56–60.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Groothuis PG, Koks CA, de Goeij AF,
Dunselman GA, Arends JW and Evers JL: Adhesion of human endometrial
fragments to peritoneum in vitro. Fertil Steril. 71:1119–1124.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wild RA, Zhang RJ and Medders D: Whole
endometrial fragments form characteristics of in vivo endometriosis
in a mesothelial cell co-culture system: An in vitro model for the
study of the histogenesis of endometriosis. J Soc Gynecol Investig.
1:65–68. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lucidi RS, Witz CA, Chrisco M, Binkley PA,
Shain SA and Schenken RS: A novel in vitro model of the early
endometriotic lesion demonstrates that attachment of endometrial
cells to mesothelial cells is dependent on the source of
endometrial cells. Fertil Steril. 84:16–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Debrock S, De Strooper B, Vander Perre S,
Hill JA and D'Hooghe TM: Tumour necrosis factor-alpha,
interleukin-6 and interleukin-8 do not promote adhesion of human
endometrial epithelial cells to mesothelial cells in a quantitative
in vitro model. Hum Reprod. 21:605–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Griffith JS, Binkley PA, Kirma NB,
Schenken RS, Witz CA and Tekmal RR: Imatinib decreases endometrial
stromal cell transmesothial migration and proliferation in the
extracellular matrix of modeled peritoneum. Fertil Steril.
94:2531–2535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nair AS, Nair HB, Lucidi RS, Kirchner AJ,
Schenken RS, Tekmal RR and Witz CA: Modeling the early
endometriotic lesion: Mesothelium-endometrial cell co-culture
increases endometrial invasion and alters mesothelial and
endometrial gene transcription. Fertil Steril. 90:1487–1495. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen Z, Dai Y, Dong Z, Li M, Mu X, Zhang
R, Wang Z, Zhang W, Lang J, Leng J and Jiang X: Co-cultured
endometrial stromal cells and peritoneal mesothelial cells for an
in vitro model of endometriosis. Integr Biol (Camb). 4:1090–1095.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu J, Wang Y, Zhou WH, Wang L, He YY and
Li DJ: Combination of estrogen and dioxin is involved in the
pathogenesis of endometriosis by promoting chemokine secretion and
invasion of endometrial stromal cells. Hum Reprod. 23:1614–1626.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Loh FH, Bongso A, Fong CY, Koh DR, Lee SH
and Zhao HQ: Effects of peritoneal macrophages from women with
endometriosis on endometrial cellular proliferation in an in vitro
coculture model. Fertil Steril. 72:533–538. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Takaoka O, Mori T, Ito F, Okimura H,
Kataoka H, Tanaka Y, Koshiba A, Kusuki I, Shigehiro S, Amami T and
Kitawaki J: Daidzein-rich isoflavone aglycones inhibit cell growth
and inflammation in endometriosis. J Steroid Biochem Mol Biol.
181:125–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wu D, Lu P, Mi X and Miao J: Exosomal
miR-214 from endometrial stromal cells inhibits endometriosis
fibrosis. Mol Hum Reprod. 24:357–365. 2018.PubMed/NCBI
|
|
65
|
Lawrenson K, Lee N, Torres HA, Lee JM,
Brueggmann D, Rao PN, Noushmehr H and Gayther SA: Src as a novel
therapeutic target for endometriosis. Gynecol Oncol. 135:100–107.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ohtake H, Katabuchi H, Matsuura K and
Okamura H: A novel in vitro experimental model for ovarian
endometriosis: The three-dimensional culture of human ovarian
surface epithelial cells in collagen gels. Fertil Steril. 71:50–55.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vigano P, Candiani M, Monno A, Giacomini
E, Vercellini P and Somigliana E: Time to redefine endometriosis
including its pro-fibrotic nature. Hum Reprod. 33:347–352. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang Q, Duan J, Liu X and Guo SW:
Platelets drive smooth muscle metaplasia and fibrogenesis in
endometriosis through epithelial-mesenchymal transition and
fibroblast-to-myofibroblast transdifferentiation. Mol Cell
Endocrinol. 428:1–16. 2016. View Article : Google Scholar : PubMed/NCBI
|















