Introduction
Aged garlic extract (AGE) is produced by extracting
and aging garlic slices in an aqueous ethanol solution for >10
months and has been shown to modulate several immune functions,
such as decreasing inflammatory cytokines and chemokines in animal
models (1–4). In addition, the supplementation of AGE
was previously shown to increase the numbers of γδ-T and natural
killer (NK) cells, and to reduce the levels of inflammatory
cytokines, such as interleukin (IL)-6 and tumor necrosis factor
(TNF)-α, in a clinical study (5).
AGE has been shown to exert immuno-enhancing and anti-inflammatory
effects (1–5). S−1-propenylcysteine (S1PC), a
major characteristic sulfur compound in AGE, which exhibits good
oral bioavailability in rats and canines (6), has been shown to exert several
beneficial effects, such as immunoregulatory, anti-hypertensive and
blood flow-promoting effects (7–10).
Moreover, in our previous recent studies, it was indicated that
S1PC promoted intestinal immunoglobulin A (IgA) production and
inhibited lipopolysaccharide (LPS)-induced IL-6 production
(7,10).
Autophagy is a major degradation system of cellular
components, including abnormal proteins, protein aggregates and
damaged organelles (11–13). In addition, autophagy maintains
cellular homeostasis and regulates various cellular events, such as
signal transduction, cell growth, apoptosis and differentiation.
Autophagy has been shown to regulate the immune response and immune
cell differentiation (14–18). The inhibition of autophagy prevents
monocyte-to-macrophage differentiation as it contributes to the
transition from apoptosis to differentiation (19). In addition, the regulatory T (Treg)
cell-specific deletion of autophagy-related gene (Atg) 7 triggers
the loss of Treg cells by inducing apoptosis and promotes the
development of inflammatory disorders (20). Thus, autophagy plays an important
role in the regulation of immune developments and functions. The
aim of the present review is to provide a summary and discussion of
the mechanisms responsible for the immunoregulatory effects of S1PC
which are mediated via the activation of autophagy.
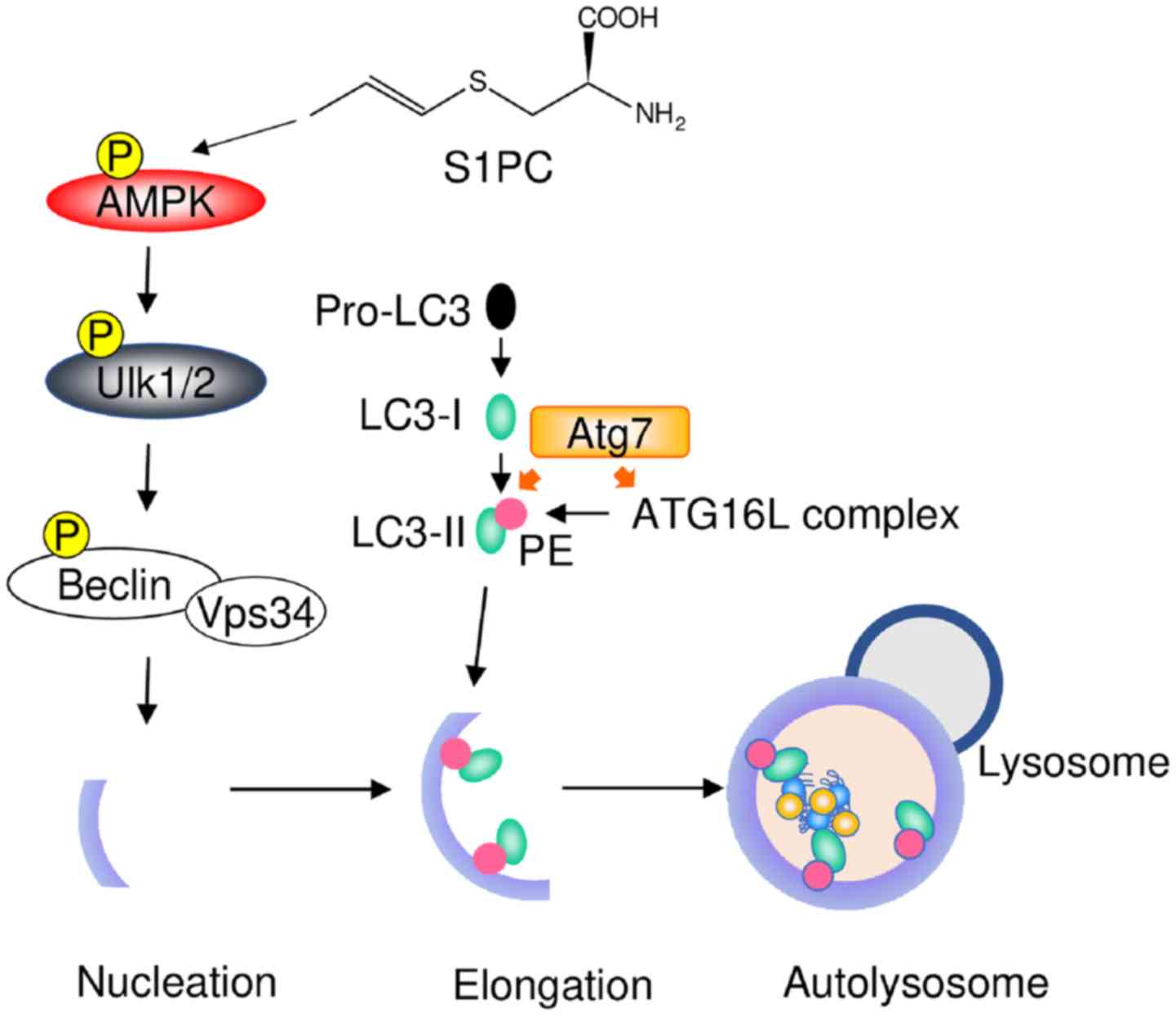
S1PC induces the activation of
autophagy
Autophagy is activated by several stress conditions,
such as nutrient starvation, unfolded proteins and infection
(11–13). Autophagy-mediated proteolysis occurs
through different steps, which include the elongation of the
phagophore and delivery to lysosomes. These processes are regulated
by several signaling molecules (21,22).
S1PC has been shown to promote the phosphorylation of AMP-activated
protein kinase (AMPK), which is a cellular energy sensor and
regulates the initial steps of autophagy activation (10). AMPK triggers the phosphorylation of
unc-51-like kinase 1/2 (Ulk1/2) and inhibits the phosphorylation of
mammalian target of rapamycin (mTOR), a repressor of autophagy.
These steps initiate the elongation of the phagophore by
phosphorylating the complex of Beclin1/vesicular sorting protein 34
(VPS34) (21–23). Following the formation of the
autophagosome membrane, microtubule-associated protein 1 light
chain 3 (LC3-I) conjugates with phosphatidylethanolamine by
ubiquitin-like enzymes, such as Atg7, Atg3 and the
Atg16L:Atg5-Atg12 complex, and is converted to lipidated LC3
(LC3-II). LC3-II interacts with target proteins via adaptor protein
p62 on the autophagosome membrane. The LC3-II/LC3-I ratio usually
increases upon the activation of autophagy (24,25),
whereas S1PC has been shown to increase the levels of both LC3-I
and LC3-II. Accordingly, S1PC can not only promote the conversion
of LC3-I to LC3-II, but can also increase the production of LC3-I.
Subsequently, the autophagosome fuses with the lysosome and then
target proteins are degraded with LC3-II and p62 (10). S1PC has been shown to induce the
degradation of target proteins and p62 (10). In addition, both 3-methyladenine
(3-MA), an autophagy inhibitor and compound C, an AMPK inhibitor,
have been shown to block the S1PC-induced activation of autophagy
(10). A schematic diagram of the
mechanisms through which S1PC induces autophagy is presented in
Fig. 1. It is thus suggested that
S1PC triggers the activation of autophagy by inducing AMPK
phosphorylation.
Anti-inflammatory effects of S1PC
Chronic inflammation is associated with the onset of
several human conditions and diseases, including aging, allergies,
autoimmune diseases, atherosclerosis, cancer, chronic wounds,
cystic fibrosis, metabolic syndrome and obesity (26,27). The
pattern recognition receptors (PRRs) play an important role in
innate immunity and host defense by recognizing pathogen-associated
molecular patterns (PAMPs). However, PRRs trigger chronic
inflammation by consecutively interacting with danger-associated
molecular patterns (DAMPs) released from dying cells (28–30).
Toll-like receptors (TLRs), which are important members of the PRR
family, recognize microbial components and cellular debris
(28–30). Therefore, PRRs recognize not only
pathogens, but also cellular components. The activation of TLRs
recruits myeloid differentiation response protein 88 (MyD88), a
common adaptor protein of TLRs, apart from TLR3, and IL-1
receptor-associated kinase 4 (IRAK4) to the plasma membrane
(30–32). TLR signaling induces the production
of the inflammatory cytokines, IL-6 and TNF-α, and the chemokines,
C-C motif chemokine ligand 2 (CCL2) and C-X-C motif chemokine
ligand 8 (CXCL8) via the activation of nuclear factor (NF)-κB
(34,35). S1PC has been shown to inhibit IL-6
production by suppressing the TLR signaling pathway via the
degradation of MyD88 (10). In
addition, S1PC blocks the mRNA expression of Ccl2 in the
livers of spontaneously hypertensive rats (SHRs) (10). A schematic diagram of the mechanisms
through which S1PC induces the degradation of MyD88 and paired box
protein 5 (Pax5) by activating autophagy is presented in Fig. 2. The constituents of fresh garlic and
AGE have been reported to inhibit the TLR signaling pathway.
Alliin, a constituent of fresh garlic, decreases the LPS-induced
phosphorylation of extracellular signal-regulated kinase (ERK)1/2
in adipocytes (36).
S-allylcysteine (SAC), a constituent of AGE, has been shown
to reduce the production of inflammatory cytokines by inhibiting
NF-κB phosphorylation (37). The
inhibitory effects of S1PC could be considered to be different from
those of other garlic constituents. S1PC has been shown to degrade
MyD88 by activating autophagy (see schematic diagram in Fig. 2) (10). However, the activation of autophagy
alone cannot degrade MyD88 due to the inability of SAC to induce
the degradation of MyD88, although SAC also activates autophagy
(10). S1PC has been shown to have
another distinct feature that directly denatures and aggregates
MyD88, whereas SAC is unable to denature MyD88 (10). Aggregated MyD88 is modified with both
acetylation and ubiquitination, and forms the histone deacetylase 6
(HDAC6)-dependent aggresome. Subsequently, ubiquitin of the
aggresome interacts with p62 and is degraded by the
autophagy-lysosome system (10).
Thus, as discussed above, it has been suggested by both in
vitro and in vivo studies that the anti-inflammatory
mechanisms of S1PC involve the degradation of MyD88 by triggering
the denaturation of MyD88 and the activation of autophagy.
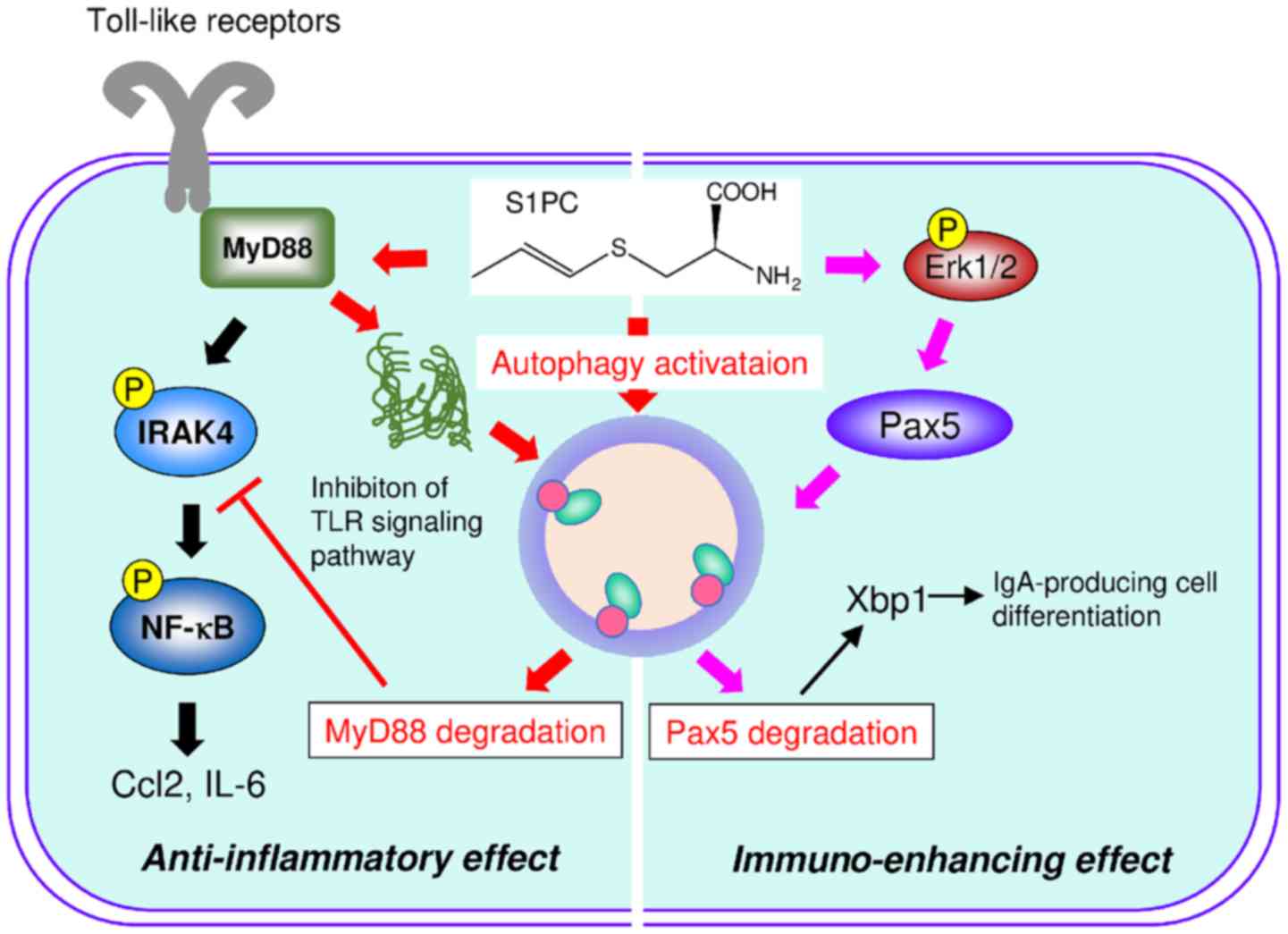 | Figure 2.S1PC induces the degradation of MyD88
and Pax5 by activating autophagy. S1PC directly denatures MyD88 and
then induces the formation of protein aggregates by the lysine
acetylation and ubiquitination. S1PC triggers the degradation of
MyD88 by inducing AMPK-mediated autophagy activation. Consequently,
the degradation of MyD88 inhibits TLR signaling pathway (left part
of diagram). S1PC induces the phosphorylation of ERK1/2 and
triggers AMPK-induced autophagy activation. Pax5 is phosphorylated
by ERK1/2 and then is degraded by autophagy. Therefore, the
degradation of Pax5 induces the expression of Xbp1 mRNA and
triggered the differentiation of B cells into IgA-producing cells
(right part of diagram). S1PC, S−1-propenylcysteine; MyD88,
myeloid differentiation response protein 88; AMPK, AMP-activated
protein kinase; TLR, Toll-like receptor; ERK1/2,
extracellular-regulated kinase 1/2; Pax5, paired box protein 5;
Xbp1, X-box binding protein 1; IRAK4, interleukin 1 receptor
associated kinase 4. |
Immuno-enhancing effects of S1PC
The intestine is the largest tissue of the immune
system and is the first defense line of the body against foreign
antigens, such as infectious pathogens, toxins and food allergens
(38,39). IgA is the most abundant secreted
antibody involved in protecting intestinal epithelial cells
(40). Immunoglobulin class
switching from IgM to IgA is induced by the action of both
cell-cell contact and cytokines in Peyer's patches (PPs) and
becomes rapidly plasmablasts. The oral administration of S1PC has
been shown to increase the intestinal IgA level and IgA-producing
cells in PPs (7). In addition, S1PC
has been found to act on B cells and increase IgA production by
promoting the differentiation of B cells into IgA-producing B cells
in vitro. Therefore, S1PC is more likely to promote the
expression of transcription factors related to immunoglobulin class
switching. Several transcription factors, including X-box binding
protein 1 (Xbp1), and B cell-induced maturation protein-1 (Blimp1)
regulate immunoglobulin class switching (41). S1PC increases the expression of
Xbp1 mRNA in vitro and in vivo, whereas the
mRNA expression of Blimp1 is not affected. Xbp1 requires the
formation of pre-plasmablasts, the early process of plasma cell
differentiation that is independent of Blimp1 function. It is
possible that S1PC induces the early process of plasma cell
differentiation. The mRNA expression of Xbp1 is repressed by
Pax5. It is known that ERK1/2 triggers the degradation of Pax5 by
inducing its phosphorylation (41).
S1PC has been found to induce the degradation of Pax5 by enhancing
ERK1/2 phosphorylation (see schematic diagram in Fig. 2) (7).
Therefore, on the whole, it is suggested that S1PC induces the
degradation of Pax5 by activating both ERK1/2 and autophagy, and
then triggers the differentiation of B cells into IgA-producing
cells by increasing the mRNA expression of Xbp1.
Conclusions and future perspectives
S1PC, a major characteristic sulfur compound in AGE,
induces the activation of autophagy via the phosphorylation of
AMPK. The activation of autophagy regulates the immune response
through the degradation of key molecules. S1PC has been shown not
only to induce the activation of autophagy, but also to trigger the
post-translational modification of target proteins. Thus, it is
suggested that S1PC selectively induces the degradation of
proteins. In addition, it is suggested that S1PC exerts
immuno-enhancing and anti-inflammatory effects, and may contribute
to the maintenance of immune homeostasis by regulating
autophagy.
Acknowledgements
The authors would like to thank Dr Takami Oka,
Wakunaga Pharmaceutical Co., Ltd., for providing many helpful
discussions and useful advice for this manuscript.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JIS and YK conceived this review. JIS, SM and MU
analyzed the relevant literature. JIS wrote the first draft of the
manuscript and produced the figures. JIS, SM, MU and YK critically
revised the manuscript. All authors have reviewed and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr.
131:1075S–1079S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morihara N, Hino A, Miki S, Takashima M
and Suzuki JI: Aged garlic extract suppresses inflammation in
apolipoprotein E-knockout mice. Mol Nutr Food Res. 61:17003082017.
View Article : Google Scholar
|
|
3
|
Morihara N, Hino A, Yamaguchi T and Suzuki
J: Aged Garlic Extract Suppresses the Development of
Atherosclerosis in Apolipoprotein E-Knockout Mice. J Nutr.
146:460S–463S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miki S, Inokuma K-I, Takashima M, Nishida
M, Sasaki Y, Ushijima M, Suzuki JI and Morihara N: Aged garlic
extract suppresses the increase of plasma glycated albumin level
and enhances the AMP-activated protein kinase in adipose tissue in
TSOD mice. Mol Nutr Food Res. 61:612017. View Article : Google Scholar
|
|
5
|
Nantz MP, Rowe CA, Muller CE, Creasy RA,
Stanilka JM and Percival SS: Supplementation with aged garlic
extract improves both NK and γδ-T cell function and reduces the
severity of cold and flu symptoms: A randomized, double-blind,
placebo-controlled nutrition intervention. Clin Nutr. 31:337–344.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amano H, Kazamori D and Itoh K:
Pharmacokinetics and N-acetylation metabolism of
S-methyl-l-cysteine and trans-S−1-propenyl-l-cysteine
in rats and dogs. Xenobiotica. 46:1017–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki J, Yamaguchi T, Matsutomo T, Amano
H, Morihara N and Kodera Y: S-1-Propenylcysteine promotes the
differentiation of B cells into IgA-producing cells by the
induction of Erk1/2-dependent Xbp1 expression in Peyer's patches.
Nutrition. 32:884–889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsutomo T, Ushijima M, Kodera Y,
Nakamoto M, Takashima M, Morihara N and Tamura K: Metabolomic study
on the antihypertensive effect of S-1-propenylcysteine in
spontaneously hypertensive rats using liquid chromatography coupled
with quadrupole-Orbitrap mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1046:147–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsutomo T, Ushijima M, Kunimura K and
Ohtani M: Metabolomic study reveals the acute hypotensive effect of
S-1-propenylcysteine accompanied by alteration of the plasma
histidine level in spontaneously hypertensive rats. J Pharm Biomed
Anal. 168:148–154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki JI, Kodera Y, Miki S, Ushijima M,
Takashima M, Matsutomo T and Morihara N: Anti-inflammatory action
of cysteine derivative S-1-propenylcysteine by inducing MyD88
degradation. Sci Rep. 8:141482018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N, Yoshimori T and Ohsumi Y: The
role of Atg proteins in autophagosome formation. Annu Rev Cell Dev
Biol. 27:107–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eskelinen E-L and Saftig P: Autophagy: A
lysosomal degradation pathway with a central role in health and
disease. Biochim Biophys Acta. 1793:664–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saha S, Panigrahi DP, Patil S and Bhutia
SK: Autophagy in health and disease: A comprehensive review. Biomed
Pharmacother. 104:485–495. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cuervo AM and Macian F: Autophagy,
nutrition and immunology. Mol Aspects Med. 33:2–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arroyo DS, Gaviglio EA, Peralta Ramos JM,
Bussi C, Rodriguez-Galan MC and Iribarren P: Autophagy in
inflammation, infection, neurodegeneration and cancer. Int
Immunopharmacol. 18:55–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deretic V and Levine B: Autophagy balances
inflammation in innate immunity. Autophagy. 14:243–251. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Into T, Horie T, Inomata M, Gohda J, Inoue
JI, Murakami Y and Niida S: Basal autophagy prevents autoactivation
or enhancement of inflammatory signals by targeting monomeric
MyD88. Sci Rep. 7:10092017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riffelmacher T, Richter FC and Simon AK:
Autophagy dictates metabolism and differentiation of inflammatory
immune cells. Autophagy. 14:199–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Morgan MJ, Chen K, Choksi S and
Liu ZG: Induction of autophagy is essential for monocyte-macrophage
differentiation. Blood. 119:2895–2905. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei J, Long L, Yang K, Guy C, Shrestha S,
Chen Z, Wu C, Vogel P, Neale G, Green DR, et al: Autophagy enforces
functional integrity of regulatory T cells by coupling
environmental cues and metabolic homeostasis. Nat Immunol.
17:277–285. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abounit K, Scarabelli TM and McCauley RB:
Autophagy in mammalian cells. World J Biol Chem. 3:1–6. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J and Guan KL: AMPK connects energy
stress to PIK3C3/VPS34 regulation. Autophagy. 9:1110–1111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanida I, Ueno T and Kominami E: LC3 and
Autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cherra SJ III, Kulich SM, Uechi G,
Balasubramani M, Mountzouris J, Day BW and Chu CT: Regulation of
the autophagy protein LC3 by phosphorylation. J Cell Biol.
190:533–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung HY, Kim DH, Lee EK, Chung KW, Chung
S, Lee B, Seo AY, Chung JH, Jung YS, Im E, et al: Redefining
Chronic Inflammation in Aging and Age-Related Diseases: Proposal of
the Senoinflammation Concept. Aging Dis. 10:367–382. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017.PubMed/NCBI
|
|
28
|
Falck-Hansen M, Kassiteridi C and Monaco
C: Toll-like receptors in atherosclerosis. Int J Mol Sci.
14:14008–14023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piccinini AM and Midwood KS: DAMPening
inflammation by modulating TLR signalling. Mediators Inflamm.
2010(pii): 6723952010.PubMed/NCBI
|
|
30
|
Drexler SK and Foxwell BM: The role of
toll-like receptors in chronic inflammation. Int J Biochem Cell
Biol. 42:506–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Troutman TD, Bazan JF and Pasare C:
Toll-like receptors, signaling adapters and regulation of the
pro-inflammatory response by PI3K. Cell Cycle. 11:3559–3567. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deguine J and Barton GM: MyD88: A central
player in innate immune signaling. F1000Prime Rep. 6:972014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boyd JH, Divangahi M, Yahiaoui L, Gvozdic
D, Qureshi S and Petrof BJ: Toll-like receptors differentially
regulate CC and CXC chemokines in skeletal muscle via NF-kappaB and
calcineurin. Infect Immun. 74:6829–6838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bassett SA and Barnett MP: The role of
dietary histone deacetylases (HDACs) inhibitors in health and
disease. Nutrients. 6:4273–4301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Quintero-Fabián S, Ortuño-Sahagún D,
Vázquez-Carrera M and López-Roa RI: Alliin, a garlic (Allium
sativum) compound, prevents LPS-induced inflammation in 3T3-L1
adipocytes. Mediators Inflamm. 2013:3818152013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arreola R, Quintero-Fabián S, López-Roa
RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Quintanar L and
Ortuño-Sahagún D: Immunomodulation and anti-inflammatory effects of
garlic compounds. J Immunol Res. 2015:4016302015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ley RE, Peterson DA and Gordon JI:
Ecological and evolutionary forces shaping microbial diversity in
the human intestine. Cell. 124:837–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qin J, Li R, Raes J, Arumugam M, Burgdorf
KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al
MetaHIT Consortium, : A human gut microbial gene catalogue
established by metagenomic sequencing. Nature. 464:59–65. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fagarasan S and Honjo T: Intestinal IgA
synthesis: Regulation of front-line body defences. Nat Rev Immunol.
3:63–72. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yasuda T, Hayakawa F, Kurahashi S,
Sugimoto K, Minami Y, Tomita A and Naoe T: B cell receptor-ERK1/2
signal cancels PAX5-dependent repression of BLIMP1 through PAX5
phosphorylation: A mechanism of antigen-triggering plasma cell
differentiation. J Immunol. 188:6127–6134. 2012. View Article : Google Scholar : PubMed/NCBI
|