Introduction
Pancreatic cancer is a highly aggressive malignancy
with an unacceptably high mortality rate. The overall 5-year
survival rate of patients with pancreatic cancer is generally
<5% in China and is even <1% in certain regions of the world
(1). It has been reported that
pancreatic cancer causes more deaths than breast cancer in the
European Union, in spite of having a much lower incidence rate
(2). The 5-year survival rate of
patients after proper surgical resection may be up to 25% (3). However, the application of surgical
operation is limited by the high prevalence of tumor metastasis by
the time of diagnosis (4).
Therefore, early diagnosis and treatment still has pivotal roles in
the survival of patients with pancreatic cancer.
Rho-associated protein kinase 1 (ROCK1) is a
serine/threonine kinase protein that widely participates in
numerous aspects of cancer biology (5,6). ROCK
kinases regulate associated gene expression to participate in cell
proliferation, differentiation and apoptosis, so as to affect
oncogenic transformation (7). A
growing body of evidence has indicated that inhibition of ROCK1 may
serve as a potential therapeutic target for cancer treatment
(5–8). ROCK1 participates in cancer biology
through interaction with various functional molecules, including
long non-coding RNAs (lncRNAs) (9–11). Long
intergenic non-coding RNA for kinase activation (LINK-A) is an
lncRNA with characterized functionality only in triple-negative
breast cancer (12) and ovarian
carcinoma (13). The interaction
between LINK-A and ROCK1 is unknown. Preliminary microarray data
(unpublished; 24 pancreatic adenocarcinoma tissues, 24 control
tissues) revealed the close correlation between them, indicating a
possible interaction. The present study indicated that LINK-A may
have a role in pancreatic cancer by upregulating ROCK1.
Materials and methods
Patients and specimens
A total of 42 patients with pancreatic
adenocarcinoma were enrolled in the present study (Table I). All of these patients were
diagnosed and treated at the First Affiliated Hospital of Nanjing
Medical University (Nanjing, China) between March 2016 and March
2018. The inclusion criteria were as follows: i) Pancreatic
adenocarcinoma patients confirmed by pathological examination; ii)
patients at stage IA-IIA prior to development of lymph node
metastasis; iii) patients received surgical resection. The
exclusion criteria were as follows: i) Any treatments within 3
months prior to admission; ii) complication with other
malignancies. During the same time period, 36 healthy controls were
also enrolled from a population of healthy people undergoing
physical examination. One day after admission, fasting blood was
extracted from the patients and controls in the morning to prepare
plasma. The patient group was composed of 24 males and 18 females
with an age range of 24–66 years and a mean age of 45.4±7.1 years.
The control group was composed of 19 males and 17 females with an
age range of 26–67 years and a mean age of 46.9±6.4 years. The two
groups had similar age and gender distributions. The ethics
committee of the First Affiliated Hospital of Nanjing Medical
University (Nanjing, China) approved the present study. All
participants provided written informed consent. All specimens were
stored in liquid nitrogen prior to use.
 | Table I.Clinicopathological characteristics of
the 42 patients. |
Table I.
Clinicopathological characteristics of
the 42 patients.
| Pathology | Number of cases, n
(%) | Stagea |
|---|
| Pancreatic ductal
adenocarcinoma | 37 (88.1) | I A (3), I B (6), II
A (14), II B (10), III (4) |
| Adenosquamous
carcinoma | 3 (7.1) | I A (1), I B (2) |
| Mucinous
cystadenoma | 1 (2.4) | II A (1) |
| Serous
cystadenoma | 1 (2.4) | II B (1) |
Reverse transcription-quantitative
(RT-q)PCR
Plasma samples were obtained by venipuncture of the
patients (n=42) and controls (n=36). Venous blood (3–5 ml) was
collected into tubes containing EDTA, and a 2 step centrifugation
protocol was followed: samples were first centrifuged at 1,500 × g
for 15 min at 4°C, before the supernatant was centrifuged again at
14,000 × g for 15 min at 4°C. The supernatant plasma was removed,
split into aliquots and frozen at −80°C until use. Total RNA was
extracted from plasma samples of the patients and controls using an
RNeasy Mini Spin kit (Qiagen China Co., Ltd.) and cDNA synthesis
was carried out from 1 µl of RNA, using 5X PrimeScript RT Master
Mix (Takara Biotechnology Co., Ltd.; 4 µl, in total volume of 20 µl
reaction mixture). PCR reactions were prepared using the
SuperScript III Platinum One-Step qRT-PCR Kit (Thermo Fisher
Scientific, Inc.). The PCR conditions were as follows: 95°C for 56
sec, followed by 40 cycles of 95°C for 14 sec and 58.5°C for 30
sec. The primers used for PCR had the following sequences: Human
LINK-A forward, 5′-TTCCCCCATTTTTCCTTTTC-3′ and reverse,
5′-CTCTGGTTGGGTGACTGGTT-3′; β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
Data were normalized using the 2−ΔΔCq method (14).
ELISA
Plasma levels of ROCK1 were measured by ELISA using
a ROCK1 ELISA Kit (human; cat. no. OKEH06554; AVIVA Systems
Biology). All operations were performed following the
manufacturer's protocol.
Cell lines, cell culture and
transfection
The HPAF-II [American Type Culture Collection
(ATCC)® CRL-1997™] and BxPC-3 (ATCC®
CRL-1687™) human pancreatic adenocarcinoma cell lines were
purchased from ATCC. Cells were cultured under conditions described
in the manufacturer's protocol. LINK-A small interfering (si)RNA
(5′-UGUCUAAGGUGGAGAUUAC-3′) and negative control siRNA
(5′-GGAATGCAGCTGAAAGATTCC-3′) were purchased from GenePharma.
LINK-A and ROCK1 expression pIRSE2 vectors and empty vectors were
purchased from GeneCopoeia. Lipofectamine™ 2000 (11668-019; Thermo
Fisher Scientific, Inc.) was used to transfect 15 nM vectors or 50
nM siRNA into cancer cells, and was further incubated for 15 min
following the manufacturer's protocol. Empty vector or negative
control siRNA transfection was performed in the negative control
(NC) group. Cells without any transfections were used as control
cells (C). An overexpression rate of >180% (180–245%) and a rate
of silencing to <50% (30–50%) were confirmed by RT-qPCR prior to
subsequent experiments.
Cell proliferation assay
Cell suspensions (3×104/ml) were
prepared. A total of 100 µl cell suspension was added to each well
of a 96-well plate. Cells were cultured and CCK-8 solution (10 µl)
was added 24, 48, 72 and 96 h later. Cells were cultured for an
additional 4 h, and optical density values at 450 nm were measured
using a Fisherbrand™ accuSkan™ GO ultraviolet/visible light
Microplate Spectrophotometer (Thermo Fisher Scientific, Inc.).
Transwell® migration and
invasion assay
Serum-free cell suspensions (3×104/ml)
were prepared. Migration and invasion assays were performed using
the same protocol except that Matrigel® (10 µg/ml, cat.
no. 356234; EMD Millipore) was used to coat the upper chamber at
37°C overnight prior to the invasion assay. The upper chamber was
of the Transwell® plate (pore size, 0.45 µm; Corning,
Inc.) was filled with 100 µl cell suspension, while the lower
chamber was filled with medium containing 20% fetal calf serum
(Sigma-Aldrich; Merck KGaA). Subsequently, the plates were
incubated for 12 h, followed by membrane staining using 0.5%
crystal violet (Sigma-Aldrich; Merck KGaA) at room temperature for
20 min. Cells that had migrated or invaded to the lower side of the
membrane were counted under an optical microscope (Olympus BX-51;
Olympus Corporation).
Western blot analysis
Total protein was extracted using RIPA buffer and
protein concentration was estimated by the BCA method. A total of
40 µg of protein per lane was loaded and separated using 10%
SDS-PAGE. Following transfer to polyvinylidene difluoride membranes
(EMD Millipore), blocking was performed in 5% skimmed milk at room
temperature for 2 h. Subsequently, the membranes were incubated
with rabbit anti-human primary antibodies to ROCK1 (1:1,200
dilution; cat. no. ab97582; Abcam) and GAPDH (1:1,200 dilution;
cat. no. ab8245; Abcam) overnight at 4°C. The next day,
IgG-horseradish peroxidase secondary antibody (goat anti-rabbit;
1:1,000 dilution; cat. no. MBS435036; MyBioSource) was used to
further incubate the membranes at room temperature for 1 h. Signals
were developed using enhanced chemiluminescence (Sigma-Aldrich;
Merck KGaA) and scanned with the MYECL™ Imager (Thermo Fisher
Scientific, Inc.). Data normalization was performed with ImageJ
v1.6 software (National Institutes of Health).
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc.) was used
for statistical analysis. Values are expressed as the mean ±
standard deviation. Comparisons between two groups were performed
by using Student's t-test. Comparisons among multiple groups were
performed using one-way analysis of variance and Tukey's test.
Correlation analysis was performed by determining Pearson's
correlation coefficient. Receiver operating characteristics (ROC)
curve analysis was performed to evaluate the diagnostic value.
P<0.05 was considered to indicate statistical significance.
Results
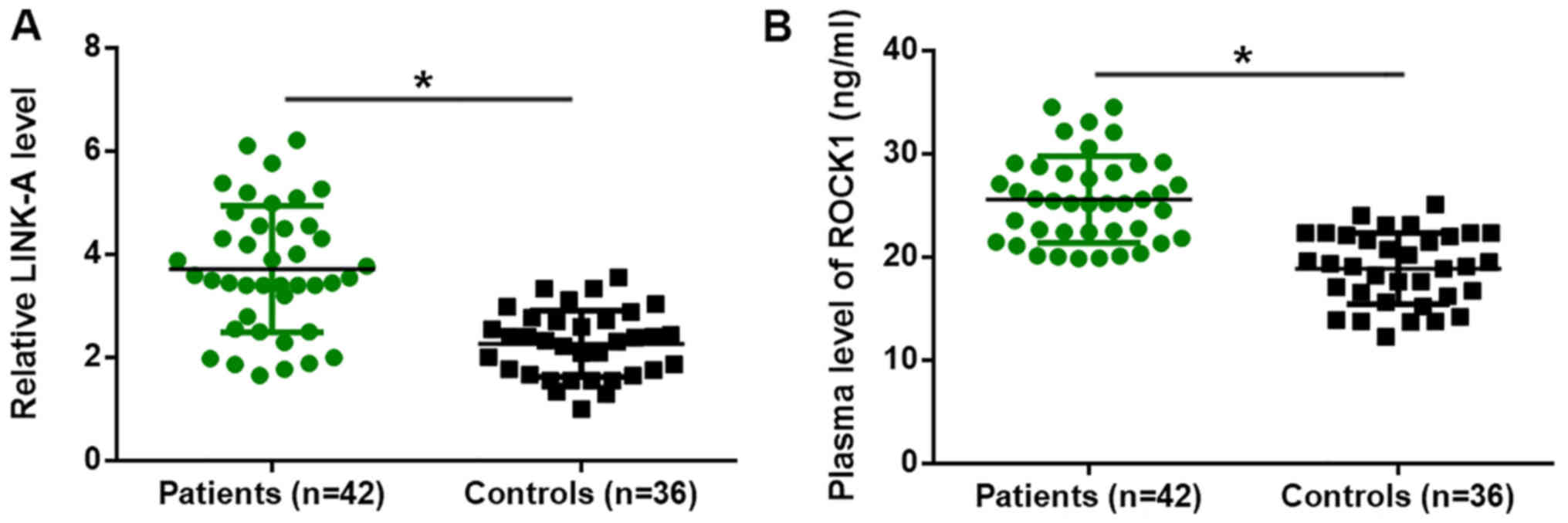
Altered plasma levels of LINK-A lncRNA
and ROCK1 in patients with pancreatic adenocarcinoma
Differential expression of genes in patients vs.
healthy controls indicates the involvement of the respective genes
in diseases. In the present study, RT-qPCR analysis revealed that,
compared with those in healthy controls, the plasma levels of
LINK-A lncRNA were significantly increased in patients with
pancreatic adenocarcinoma (P<0.05; Fig. 1A). In addition, ELISA demonstrated
that the plasma levels of ROCK1 were also significantly higher in
patients with pancreatic adenocarcinoma compared with those in
healthy controls (P<0.05; Fig.
1B).
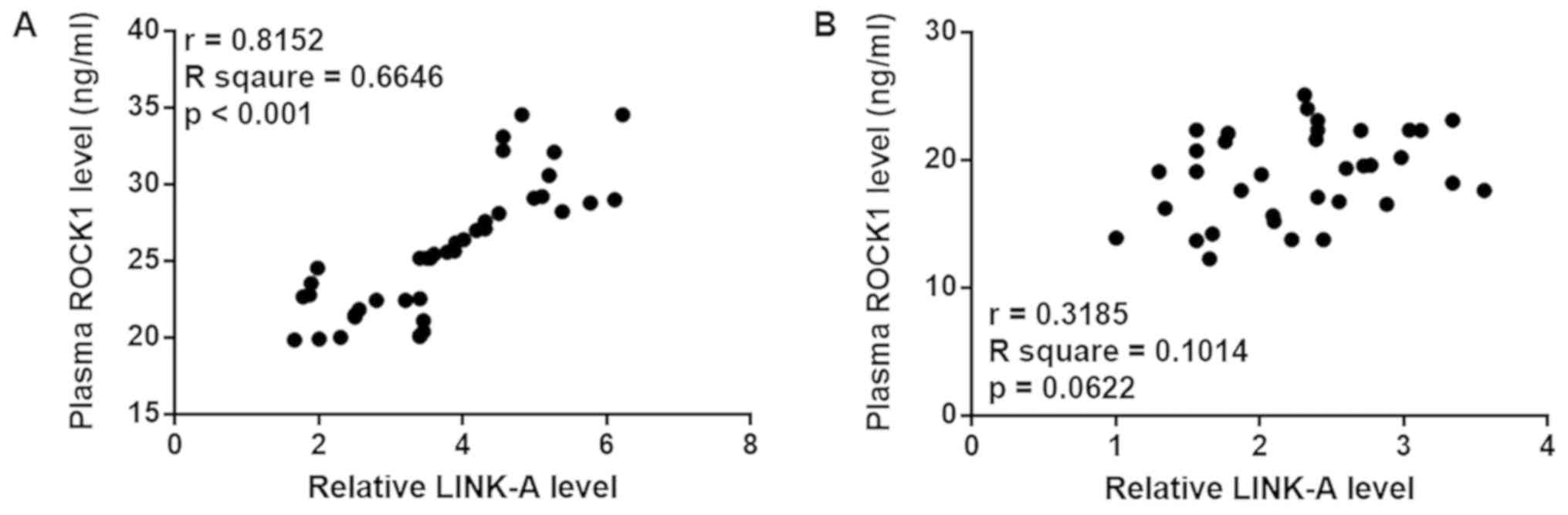
Plasma levels of LINK-A lncRNA and
ROCK1 are positively correlated in patients with pancreatic
adenocarcinoma
Pearson's correlation coefficient analysis revealed
a significant positive correlation between the plasma levels of
LINK-A lncRNA and ROCK1 in patients with pancreatic adenocarcinoma
(Fig. 2A). However, the correlation
between LINK-A lncRNA and ROCK1 was not significant in healthy
controls (Fig. 2B).
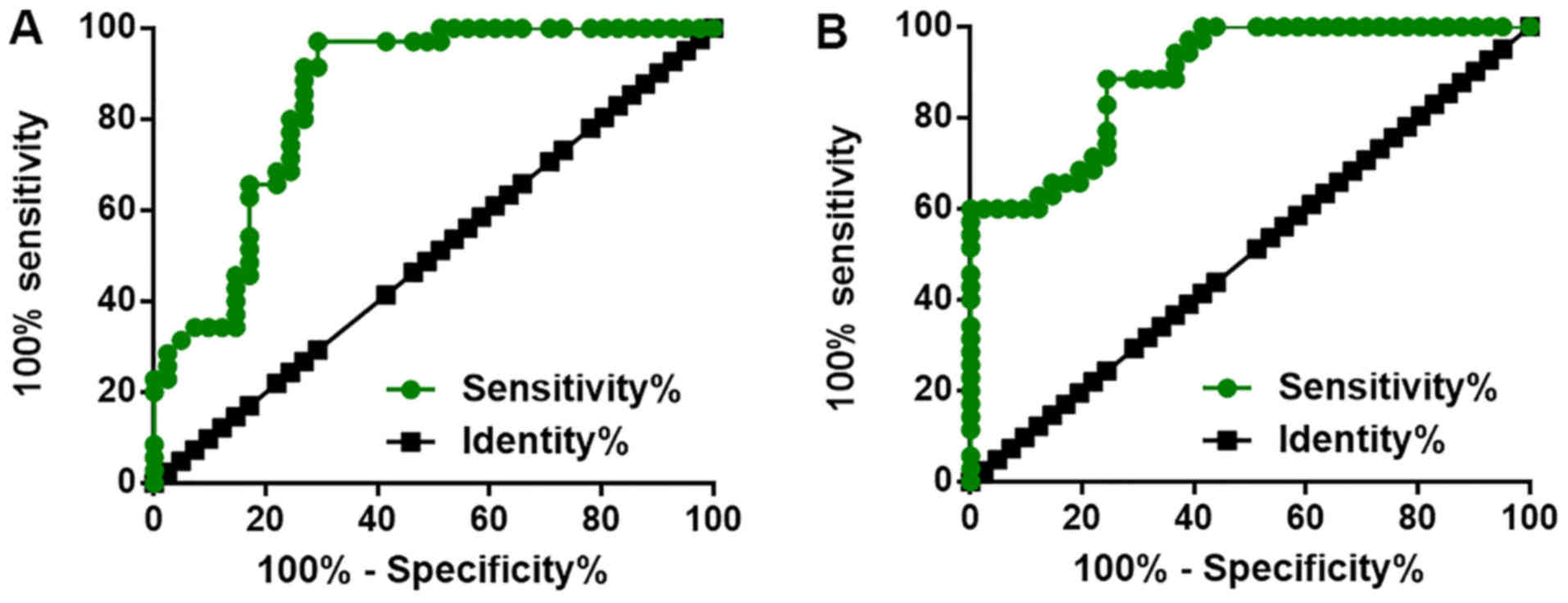
Altered plasma levels of LINK-A lncRNA
and ROCK1 distinguish early-stage pancreatic adenocarcinoma
patients from healthy controls
ROC curve analysis was performed with pancreatic
adenocarcinoma patients used as true-positive subjects and healthy
controls as true-negative samples. As displayed in Fig. 3, the average area under the curve
(AUC) for plasma LINK-A lncRNA was 0.8488 (sensitivity, 0.83;
specificity, 0.85; standard error, 0.04449; 95% confidence
interval, 0.7616–0.9360; P<0.0001), the cut-off value was
determined from the point closest to the upper left-hand corner of
the graph, the sensitivity was 94.7%. For plasma ROCK1, the average
AUC was 0.8948 (sensitivity, 0.84; specificity, 0.90; standard
error, 0.03421; 95% confidence interval, 0.8277–0.9618;
P<0.0001), the cut-off value was determined from the point
closest to the upper left-hand corner of the graph, the sensitivity
was 90.1%.
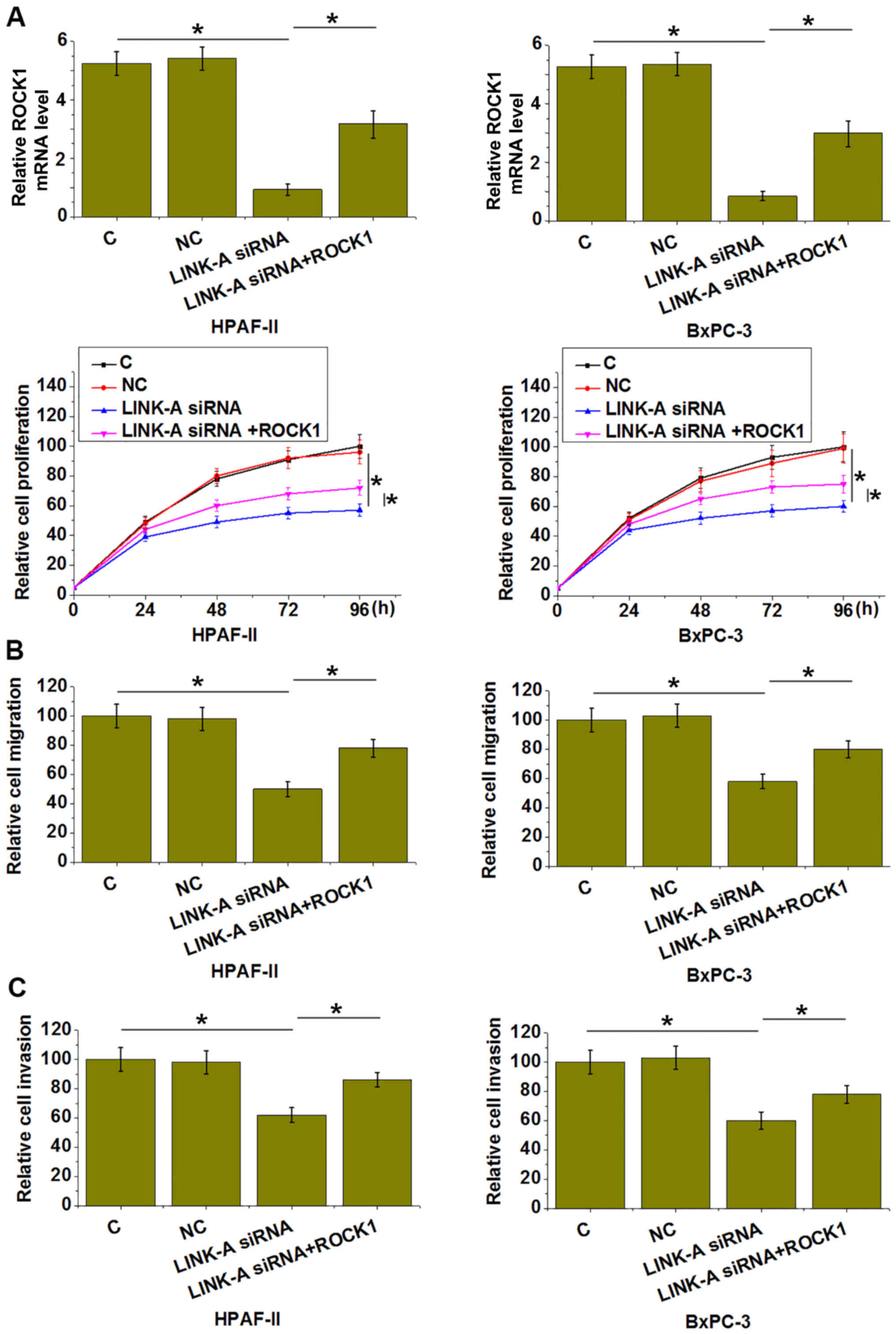
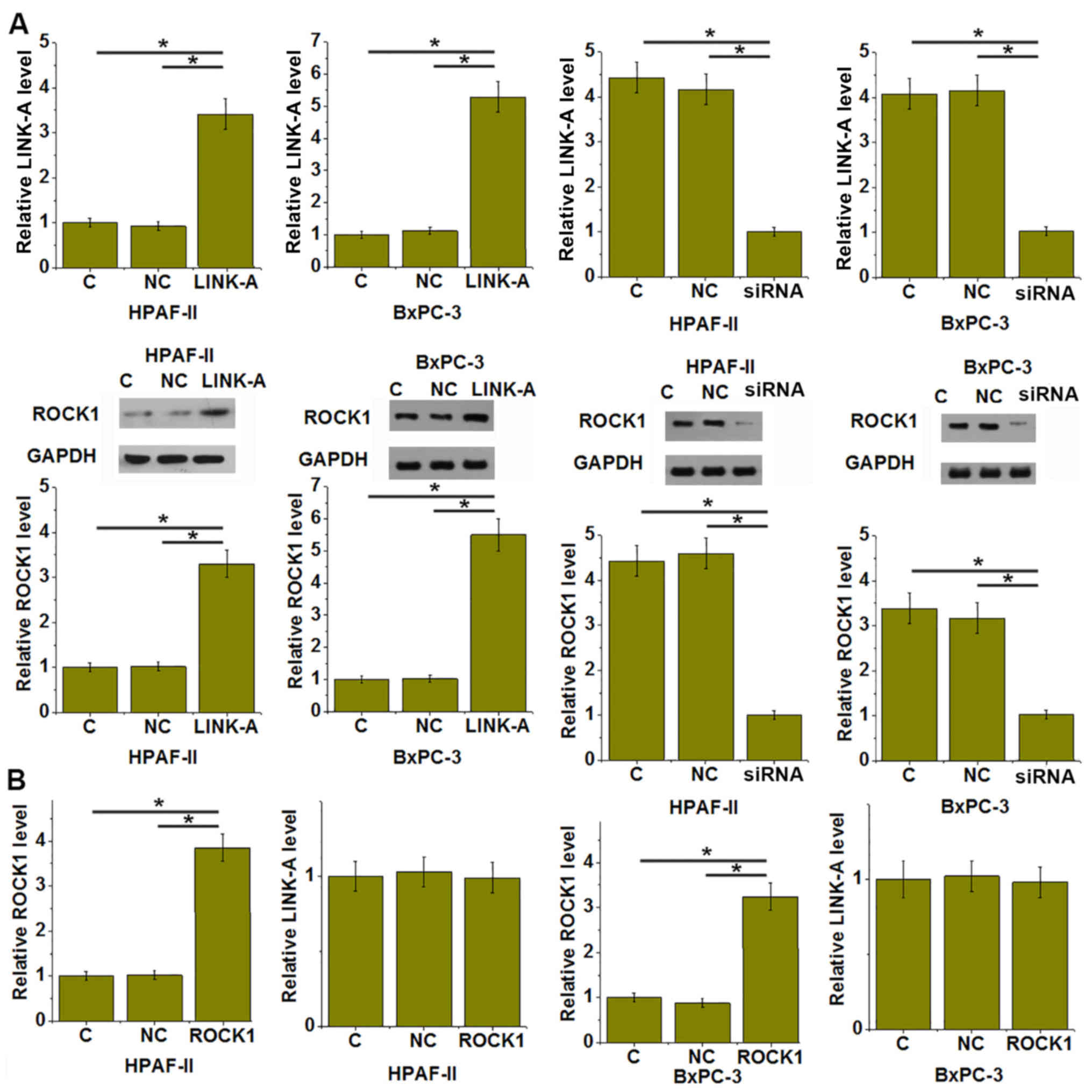
LINK-A and ROCK1 participate in the
proliferation, migration and invasion of pancreatic adenocarcinoma
cells
Compared to the C and NC groups, LINK-A silencing
led to significantly reduced proliferation (Fig. 4A), migration (Fig. 4B) and invasion (Fig. 4C) of HPAF-II and BxPC-3 human
pancreatic adenocarcinoma cells (P<0.05). However, simultaneous
overexpression ROCK1 significantly attenuated those inhibitory
effects of LINK-A siRNA silencing on cancer cell proliferation
(Fig. 4A), migration (Fig. 4B) and invasion (Fig. 4C; P<0.05).
LINK-A overexpression leads to
upregulation of ROCK1 in pancreatic adenocarcinoma cells
Compared with that in the C and NC groups, LINK-A
overexpression led to significantly increased expression of ROCK1,
while siRNA-mediated silencing of LINK-A significantly inhibited
the expression of ROCK1 in the HPAF-II and BxPC-3 cell lines
(P<0.05; Fig. 5A). By contrast,
ROCK1 vector transfection did not significantly affect the
expression of LINK-A in those cells (Fig
5B).
Discussion
The present study was the first, to the best of our
knowledge, to report on the involvement of LINK-A lncRNA in
pancreatic adenocarcinoma, which is the major type of pancreatic
cancer (15). The results revealed
that LINK-A lncRNA influences the proliferation, migration and
invasion of pancreatic adenocarcinoma cells, at least partially
through affecting the expression of ROCK1, which is a
well-characterized oncogenic serine/threonine kinase protein in
various types of cancer.
To simplify the experimental design, only patients
with pancreatic adenocarcinoma at stages IA-IIA, which represents
the early stage of this disease in the absence of cancer
metastasis, were enrolled in the present study. This design is
based on the fact that tumor metastasis globally affects gene
expression (16), which may bring
uncertainties to the conclusions. Another purpose of only including
patients at early stages was to investigate the early diagnostic
value of LINK-A lncRNA for pancreatic adenocarcinoma. To date,
altered expression of LINK-A has only been observed in
triple-negative breast cancer (12)
and ovarian carcinoma (13). The
present study revealed that the plasma levels of LINK-A were
significantly increased in pancreatic adenocarcinoma patients vs.
healthy controls. It was demonstrated that elevated plasma levels
of LINK-A were able to effectively distinguish patients with
pancreatic adenocarcinoma from healthy controls. Therefore, plasma
LINK-A may have a potential application for the early diagnosis of
pancreatic adenocarcinoma. However, the expression of LINK-A may
also be changed in other human diseases, such as ovarian carcinoma
(13), diabetic nephropathy
(17) and osteosarcoma (18). The diagnostic specificity may be
improved by combined use of multiple biomarkers.
ROCK1 exerts oncogenic functions and activated ROCK1
signaling has been observed in various types of human malignancy
(19,20). Consistent with previous studies, the
present study also observed significantly increased plasma levels
of ROCK1 in pancreatic adenocarcinoma patients compared with those
in healthy controls. The present study focused on ROCK1 due to the
observations from preliminary microarray data (unpublished; 24
patient samples, 24 healthy controls), which suggested that ROCK1
expression levels are likely positively correlated with the
expression levels of LINK-A in pancreatic adenocarcinoma patients.
In the present study, the existence of a significant positive
correlation between ROCK1 and LINK-A in pancreatic adenocarcinoma
was further proved. The regulation of ROCK1 by lncRNAs has been
reported by a previous study (21).
The present study proved that LINK-A is likely an upstream
activator of ROCK1 to regulate the proliferation, migration and
invasion of pancreatic adenocarcinoma cells. This conclusion is
based on following observations: i) LINK-A overexpression led to
significantly upregulated ROCK1 expression; ii) ROCK1
overexpression did not significantly affect LINK-A expression; iii)
ROCK1 overexpression attenuated the inhibitory effects of LINK-A
overexpression on the proliferation, migration and invasion of
pancreatic adenocarcinoma cells.
It can be hypothesized that the interaction between
LINK-A and ROCK1 is indirect. This is based on the observation that
plasma levels of LINK-A and ROCK1 are not significantly correlated
in healthy controls, indicating the existence of disease-associated
mediators that mediate interaction between LINK-A and ROCK1 in
pancreatic adenocarcinoma cells. LINK-A may also interact with
other pathways to participate in the proliferation, migration and
invasion of pancreatic adenocarcinoma cells due to the fact that
ROCK1 overexpression only attenuated, but not totally reversed the
inhibitory effects of LINK-A overexpression on the proliferation,
migration and invasion of pancreatic adenocarcinoma cells. Further
studies are required to elucidate the signaling pathways that
mediate the interaction between LINK-A and ROCK1.
In conclusion, LINK-A and ROCK1 were upregulated in
pancreatic adenocarcinoma. LINK-A may upregulate ROCK1 to
participate in the proliferation, migration and invasion of
pancreatic adenocarcinoma cells.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Jiangsu Provincial Department of Education (grant no.
17KJB320007) and CSCO-Hausoh Cancer Research Foundation (grant no.
Y-HS2017-032).
Availability of data and materials
The datasets generated and analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW designed the experiments. MZ performed the
experiments. MZ, RW, XZ and LL analyzed the data. XZ performed the
statistical analysis. TW drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Nanjing Medical
University (Nanjing, China). All patients and healthy volunteers
provided written informed consent prior to their inclusion in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Partensky C and Bray F: More
deaths from pancreatic cancer than breast cancer in the EU by 2017.
Acta Oncol. 55:1158–1160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sohal DP, Mangu PB, Khorana AA, Shah MA,
Philip PA, O'Reilly EM, Uronis HE, Ramanathan RK, Crane CH,
Engebretson A, et al: Metastatic pancreatic cancer: American
Society of Clinical Oncology clinical practice guideline. J Clin
Oncol. 34:2784–2796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rath N and Olson MF: Rho-associated
kinases in tumorigenesis: Re-considering ROCK inhibition for cancer
therapy. EMBO Rep. 13:900–908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chin VT, Nagrial AM, Chou A, Biankin AV,
Gill AJ, Timpson P and Pajic M: Rho-associated kinase signalling
and the cancer microenvironment: Novel biological implications and
therapeutic opportunities. Expert Rev Mol Med. 17:e172015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng B, Liang L, Wang C, Huang S, Cao X,
Zha R, Liu L, Jia D, Tian Q, Wu J, et al: MicroRNA-148a suppresses
tumor cell invasion and metastasis by downregulating ROCK1 in
gastric cancer. Clin Cancer Res. 17:7574–7583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui M, Wang J, Li Q, Zhang J, Jia J and
Zhan X: Long non-coding RNA HOXA11-AS functions as a competing
endogenous RNA to regulate ROCK1 expression by sponging miR-124-3p
in osteosarcoma. Biomed Pharmacother. 92:437–444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dimartino D, Colantoni A, Ballarino M,
Martone J, Mariani D, Danner J, Bruckmann A, Meister G, Morlando M,
Bozzoni I, et al: The long non-coding RNA lnc-31 interacts with
rock1 mRNA and mediates its YB-1-dependent translation. Cell Rep.
23:733–740. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Wang Y, Fu X and Lu Z: Long
non-coding RNA NEAT1 promoted ovarian cancer cells' metastasis
through regulation of miR-382-3p/ROCK1 axial. Cancer Sci.
109:2188–2198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin A, Li C, Xing Z, Hu Q, Liang K, Han L,
Wang C, Hawke DH, Wang S, Zhang Y, et al: The LINK-A lncRNA
activates normoxic HIF1α signalling in triple-negative breast
cancer. Nat Cell Biol. 18:213–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J and Xue M: LINK-A lncRNA promotes
migration and invasion of ovarian carcinoma cells by activating
TGF-β pathway. Biosci Rep. (pii): BSR201809362018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ting DT, Wittner BS, Ligorio M, Vincent
Jordan N, Shah AM, Miyamoto DT, Aceto N, Bersani F, Brannigan BW,
Xega K, et al: Single-cell RNA sequencing identifies extracellular
matrix gene expression by pancreatic circulating tumor cells. Cell
Rep. 8:1905–1918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Li L, Hong S, Zhou Z and Fan W:
LINK-A lncRNA activates HIF1α signaling and inhibits podocyte cell
apoptosis in diabetic nephropathy. Exp Ther Med. 18:119–124.
2019.PubMed/NCBI
|
|
18
|
Zhao B, Liu K and Cai L: LINK-A lncRNA
functions in the metastasis of osteosarcoma by upregulating HIF1α.
Oncol Lett. 17:5005–5011. 2019.PubMed/NCBI
|
|
19
|
Luo D, Chen H, Li X, Lu P, Long M, Peng X,
Lin S, Tan L, Zhu Y, Ouyang N and Li H: Activation of the
ROCK1/MMP-9 pathway is associated with the invasion and poor
prognosis in papillary thyroid carcinoma. Int J Oncol.
51:1209–1218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akagi EM, Lavorato-Rocha AM, Maia Bde M,
Rodrigues IS, Carvalho KC, Stiepcich MM, Baiocchi G, Sato-Kuwabara
Y, Rogatto SR, Soares FA and Rocha RM: ROCK1 as a novel prognostic
marker in vulvar cancer. BMC Cancer. 14:8222014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu X, Wang X, Geng C, Nie X and Bai C:
Long-chain non-coding RNA DAPK1 targeting miR-182 regulates
pancreatic cancer invasion and metastasis through ROCK-1/rhoa
signaling pathway. Int J Clin Exp Pathol. 10:9273–9283. 2017.
|