Introduction
Cerebral microbleeds (CMB) are subclinical lesions
caused by deposition of hemosiderin and other substances in the
brain parenchyma as a result of leakage of intravascular components
into the surrounding space following damage of the cerebral small
vessel wall (1). The prevalence rate
of CMB in healthy adults is ~5% (2).
CMB is rarely detected in young people (aged <40 years)
(3), but the prevalence rate and
case number increase with age. In a Rotterdam Scan study, the
prevalence rate of CMB increased from 18% in people aged 60–69
years to 38% in people aged ≥80 years (4). CMB occurred to 34% of patients with
ischemic stroke and 60% of patients with intracerebral hemorrhage
(2), indicating that CMB is
associated with the severity of underlying vascular diseases.
Association of CMB with stroke was reported in many studies
(5,6). Among patients with dementia, the
prevalence rate of CMB is ~14% in patients with mild cognitive
impairment and 23% in patients with Alzheimer's disease (7,8). The
increased prevalence rate of CMB in the elderly indicates its
association with conditions of deep perforating vessels and
cerebral amyloid angiopathy. It was reported that CMB is spatially
associated with amyloid deposition areas in the brain (9,10), and
thus it was speculated that CMB may pose a risk of developing
dementia (4–11). Two hypotheses were proposed for
possible mechanism. Firstly, CMB may have an impact on the complex
cortical connections, leading to disruption of the neural network
(12,13). Secondly, it may induce conditions of
deep perforating vessels and cerebral amyloid angiopathy (14). As far as we know, there are few
reports on specific molecular mechanisms.
Cystatin C (CysC) is a member of the type 2 cystatin
superfamily of cysteine protease inhibitors. It is a small protein
(13 kDa) produced and secreted by almost all karyocytes in the body
(15,16). Cystatin C is a potent competitive
cysteine protease inhibitor that primarily modulates extracellular
protease activity. Secreted extracellular cystatin C (CysC) can
also regulate target tissue homeostasis after in vivo
cellular uptake (17,18). CysC is a negative regulator of
angiogenesis and endothelial cell homeostasis both in vitro
and in vivo (16).
Clinically, the levels of CysC in serum of patients with CMB and
patients with stroke were found significantly higher than the
normal level in healthy people (19–22).
Elevated expression of CysC is a common response of the body to
injury. However, researchers have not yet reached a consensus about
its implication and mechanism. It was reported that CysC played a
neuroprotective role in preclinical disease models (23,24). It
was also reported that CysC was negatively correlated with
cognitive function (25,26), and expression of CysC in the elderly
was higher than that in young people (27,28). As
far as we know, there has been no report on the role of CysC in
cognitive development in CMB patients.
In this study, the difference in expression of CysC
in serum of patients with CMB and healthy subjects was confirmed.
CMB model mice were treated with CysC drug, followed by
investigation of the effect of CysC on the cognitive function of
CMB mice and the molecular mechanism.
Materials and methods
Materials
Subjects
Serum samples of 60 patients with cerebral
microbleeds, including 32 males and 28 females, were collected.
Furthermore, serum samples of 60 healthy subjects of similar age
were collected, including 29 males and 31 females. Fasting blood
samples were drawn in the morning from all subjects after an 8-h
overnight fast. All subjects signed informed consent. Patients who
met the following criteria (29)
were eligible for the study: i) patients whose age was ≥18 years
but <65 years; ii) patients who were diagnosed with brain
microbleeds (CMB) by MRI in accordance with the diagnostic criteria
of CMB; and iii) patients' family agreed to participate in the
study and signed an informed consent form. Patients who met the
following criteria were excluded from this study: i) patients who
did not take an MRI exam; ii) patients who had intracerebral
hemorrhage due to abnormal structures in the brain; iii) patients
who were experiencing parenchymal hemorrhage due to intracranial
aneurysm rupture; iv) patients who had cerebral bleeding due to
traumatic brain injury; v) patients who had circulatory system
diseases; vi) patients who had moyamoya disease; vii) patients who
were pre-treated with anticoagulant therapy; and viii) patients who
had severe respiratory diseases, advanced cancers, severe liver and
kidney dysfunction, severe heart dysfunction, hyperthyroidism or
severe endocrine system diseases. The study was approved by the
Ethics Committee of The Third Affiliated Hospital of Qiqihar
Medical University (Qiqihar, China).
Animals
Spontaneously hypertensive rats (SHR) were purchased
from Shanghai Experimental Animal Center affiliated with Chinese
Academy of Sciences. The animals were given a batch number of SYXK
Black 2008004 and were raised and reproduced in our institution's
laboratory animal center.
Reagents
Materials and reagents used in this study were
purchased from commercial sources: Human cystatin C kit (item no.
ab179883) from Abcam; CysC drug from Enzo Life Sciences; RIPA lysis
buffer from Beyotime Biotechnology Co., Ltd.; sucrose, glucose,
KCl, NaHCO3, NaH2PO4,
CaCl2 and MgCl2 from Sigma-Aldrich; Merck
KGaA; rat brain stereotaxic device from Anhui Zhenghua Biological
Instrument Co., Ltd.; primary antibodies to cystatin C (item no.
ab109508), p-extracellular signal-regulated kinase 1/2 (ERK1/2)
(item no. ab223500), synapsin I (item no. ab8), and
p-synapsinI-S549 (item no. ab119370) from Abcam; and β-actin
primary antibody (item no. 66009-1-Ig) from Proteintech.
Methods
Blood sample collection and
processing
Venous blood was drawn from subjects into a 15 ml
tube after more than 8 h fast. Immediately after collection, the
blood was centrifuged at 1,006.2 × g at room temperature (25–28°C)
for 20 min. The supernatant was carefully collected, aliquoted into
200 µl, and stored at −80°C. Multiple freeze-thaw cycles were
avoided. In accordance with the CysC kit manual, absorbance of the
sample was measured at 450 nm using a microplate reader, and the
expression level of CysC in the serum was calculated.
Animal housing and handling
Following conditions were provided in SHR housing.
All the SHRs were housed in a specific pathogen-free room
maintained at 18–26°C with a daily temperature difference of ≤3°C.
The housing was kept at a relative humidity of 40–70%, a noise
level of ≤60 dB, and a 12 h light (150–300 Lux)/12 h dark cycle.
The animals were maintained with free access to food and water. All
experimental procedures were consistent with experimental animal
welfare and ethical principles.
The animals were randomly divided into 4 groups:
sham surgery control group (sham), model group (CMB), model + empty
vector control group (CMB + vehicle), and model + cystatin C
overexpression group (CMB + CysC). Of each group, 12 animals were
used for behavioral experiment, 6 for electrophysiological
experiment, and the remaining 6 for molecular biology
experiments.
Following protocol was for establishing the animal
model. Forty-eight specific pathogen-free SHRs at 10 weeks of age
were selected, of which 36 were randomly chosen for the rat model
with brain microbleeds. A published protocol was followed (30). Rats were first restricted from food
and water for 8 h prior to the experiment. The rats were
anesthetized with 1% sodium pentobarbital via intraperitoneal
injection at 0.6 ml/10 g. After induction of deep anesthesia, the
rats were fixed on a stereotactic instrument. The skin area in the
head was prepared and disinfected for surgery. Two holes were
drilled 5 mm behind the anterior fontanelle, one at 2 mm left and
the other at 2 mm right of the midline. To elicit microbleed
formation, the rat brain was pierced perpendicularly to a depth of
4 mm from the dura mater using stainless steel needles of 474 and
159 µm in diameter, respectively, at both sides of the midline.
Bone wax was used to patch the holes, and the skin over it was
closed with sutures. The other 12 rats were used for sham surgery
which was the same as the procedure described above except that
there was no piercing of stainless-steel needles into the
brain.
To elicit cystatin C overexpression, following steps
were performed. The drug CysC was dissolved in physiological saline
to a final concentration of 50 µg/ml (31). The lateral ventricle was injected
with 10 µl of the drug solution. The same amount of saline was
injected into the brain of rats in the negative control group. The
lateral ventricle injection was at 0.8 mm behind the anterior
fontanelle, 1.5 mm distal to the midline, and 4.5 mm deep.
Y-maze test
Behavioral experiment was conducted 5 days after rat
model creation. Y-maze test was performed using a Y-maze apparatus.
The Y-maze as a labyrinth was composed of three arms of equal
length, i.e. arm I, arm II and arm III, as well as their junction
area. The bottom of the box was covered with an electric grid (0.2
cm in wire diameter, 14 cm in length and 1 cm in grid spacing).
There were multiple buttons on the Y-maze control panel such as I,
II, III and 0, as well as a voltage adjusting knob and a time
control knob. When button I, II or III was pressed, a signal light
corresponding to that arm became on, indicating the arm was not
energized and therefore was a safe zone, while the other two arms
and the junction area were dark and energized and therefore were
non-safe zones. When the button 0 was pressed, the junction area
was energized while the three arms were not. Five seconds after the
signal light was on, electricity (60 v) was on in the grid. Each
rat was tested 20 times a day for 2 days. Day 1 test was for study,
and day 2 test was for memory retention. Safe zones were changed in
a random and alternating way. In the beginning of the test, two
arms including the one where the rat hid were energized. The animal
would escape to the light area after receiving an electric shock. A
test was completed when the rat stayed in the safe zone for 30 sec.
Then next test started from where the rat was. It would be a
correct reaction if the rat fled directly to the safe zone after an
electric shock or ran to the safe zone within 10 sec after the
shock. It would be an incorrect reaction if the rat escaped to any
other arm without lights.
Testing indicators are listed below: i) Error number
(EN) was defined as the number of icorrect reactions in all
reactions. ii) Total reaction time (TRT) was defined as sum of the
time required to complete correct reactions and incorrect
reactions. Reaction time referred to the time taken from when the
signal light was on to when the rat escaped to the safe zone. iii)
Active avoidance rate (AAR) was defined as the percentage of times
a rat completed its escape reaction within 5 sec after the light
was on but the arm was not energized.
Electrophysiology
Preparation of acute hippocampal
slices
Artificial cerebrospinal fluid (ACSF) for anatomical
use was made containing 210 mM sucrose, 12 mM glucose, 2 mM KCl, 24
mM NaHCO3, 1 mM NaH2PO4, 0.5 mM
CaCl2 and 7 mM MgCl2. A mixed gas of 5%
CO2/95% O2 was bubbled through the ACSF for
at least half an hour at an osmotic pressure of 310–320 Osm in
order to saturate it with oxygen. The pH was adjusted to 7.4. A rat
was rapidly decapitated, and the head was placed in ice-cold
artificial cerebrospinal fluid where the brain tissue was dissected
out. The well-extracted brain tissue was transferred to fresh
ice-cold and oxygen-saturated artificial cerebrospinal fluid. The
brain tissue was trimmed with a scalpel while it was completely
submerged in ACSF. The trimmed brain tissue was fixed on a sample
tray, and a 2% agar block was used to hold the ventral side of the
brain to prevent the slice from tilting. Ice-cold artificial
cerebrospinal fluid for anatomical use was poured into the sample
tray to completely submerge the brain tissue. The sample tray
together with the brain tissue was mounted on a microtome. A mixed
gas of 5% CO2/95% O2 was bubbled through the
ACSF. After a blade was installed and parameters such as slicing
speed and oscillation frequency were set, the brain tissue was cut
into coronal slices of 400 µm thickness.
MED64 planar microelectrode array recording system
was purchased from Alpha Med Science, Japan and used for
electrophysiological recordings (32). ACSF for recording use was prepared
containing 120 mM NaCl, 27 mM NaHCO3, 20 mM glucose, 1
mM NaH2PO4, 3 mM KCl, 2.6 mM CaCl2
and 1 mM MgCl2. A mixed gas of 5% CO2/95%
O2 was bubbled through the ACSF for half an hour at an
osmotic pressure of 310–320 Osm, followed by pH adjustment to 7.4.
The well-cut brain slices were incubated in the ACSF for recording
use at room temperature for at least one hour to restore brain
slice activity. A slice was selected, and after it was placed on
the MED64 probe, the slice was infused continuously. At this stage
it was ready for recording. A stimulation site in the hippocampal
CA3 region was selected. All the sites in the CA1 region were for
recording. An input-output (I-O) curve was constructed. The current
corresponding to 30–50% of the maximum excitatory post-synaptic
potential amplitude was chosen as the subsequent stimulation
intensity. High-frequency stimulation was delivered to the brain
slice after baseline recording. The change in amplitude was
recorded during the 60 min period after stimulation.
Western blot analysis
Cells were lysed with ultrasound in RIPA lysis
buffer. Protein concentration was measured using the BCA kit.
Samples were subjected to electrophoresis, membrane transfer,
blocking, incubation with antibodies and image development.
Incubation with primary antibodies, i.e. anti-cystatin C (dilution
factor, 1:20,000), p-ERK1/2 (dilution factor, 1:400), synapsin I
(dilution factor, 1:1,000), p-synapsinI-S549 (dilution factor,
1:1,000), and β-actin (dilution factor, 1:10,000), was maintained
at 4°C overnight. After washing, the membrane was incubated with
corresponding secondary antibody (dilution factor, 1:4,000),
followed by exposure and image development. Quantity One software
was used to analyze the image in Grayscale format.
Statistical analysis
Experimental data were expressed as mean ± standard
deviation (mean ± SEM). Statistical analysis was performed using
SPSS 16.6 statistical software. The results were analyzed using
one-way ANOVA. A Bonferroni test was used for comparison between
groups. A difference was statistically significant at P<0.05,
P<0.01 and P<0.001. GraphPad Prism 5 software was used for
graph drawing.
Results
Measurement of CysC level in serum of
patients with cerebral microbleeds (CMB)
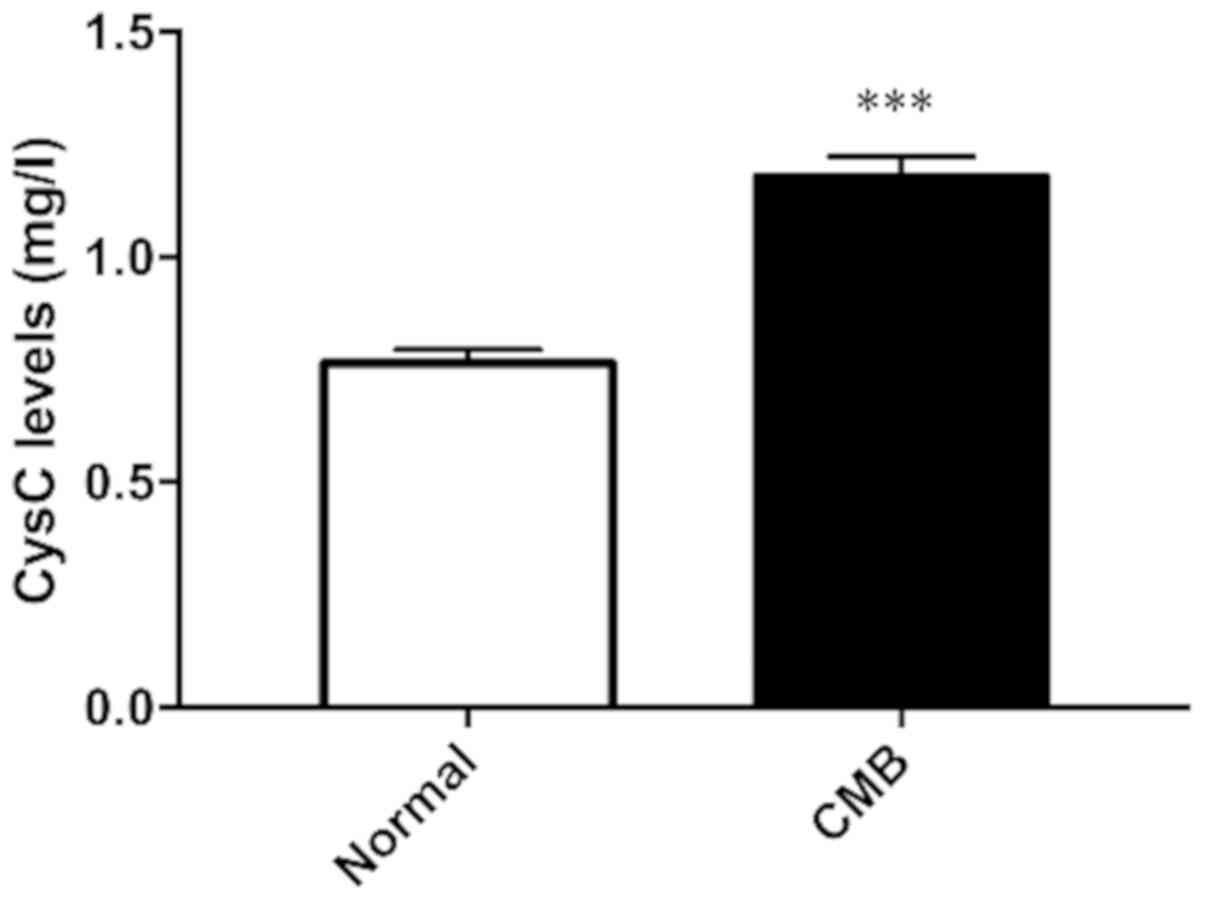
CysC expression levels in serum of clinically
healthy subjects and patients with cerebral microbleeds were
measured. The results in Fig. 1 show
that compared with the control group, expression level of CysC in
serum of patients with cerebral microbleeds increased
significantly. The difference was statistically significant.
CysC expression in rat
hippocampus
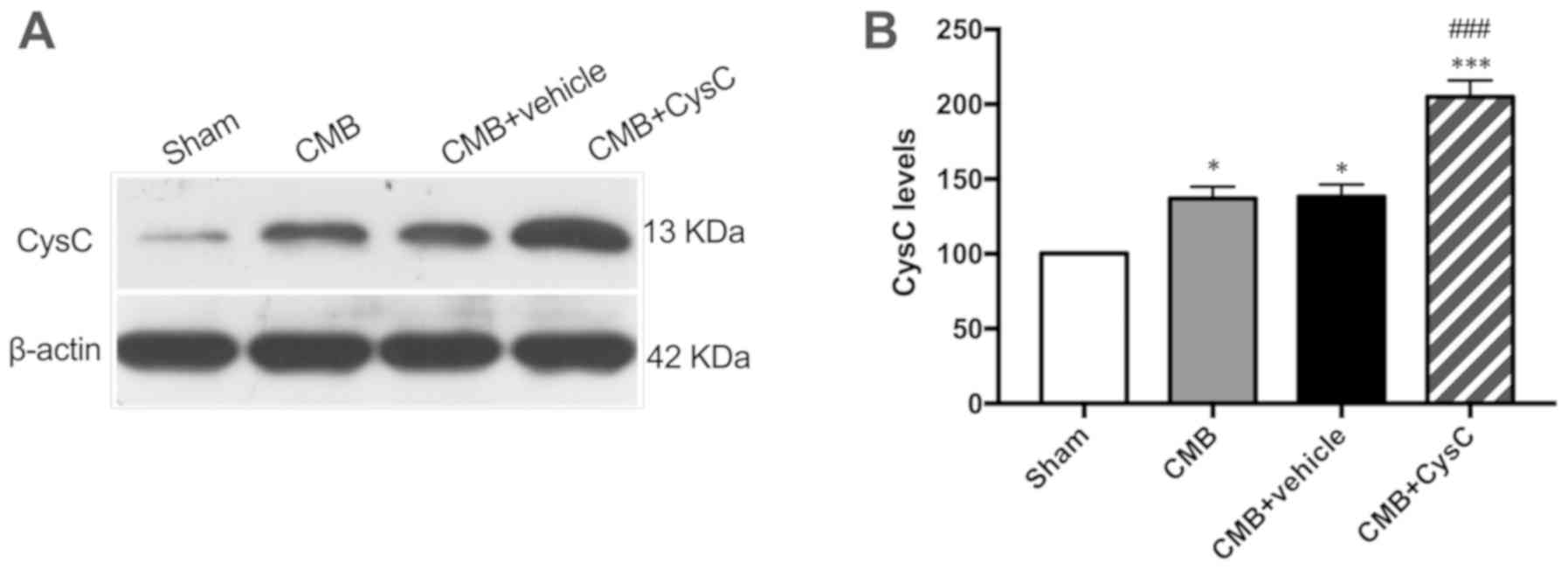
Expression levels of CysC in hippocampus of rats in
each group were measured. As shown in Fig. 2, expression level of CysC in the CMB
model group increased slightly after model creation. When rats in
the CMB model group were administered with CysC, the expression
level of CysC in hippocampus increased significantly.
Cognitive function evaluation of rats
in each group
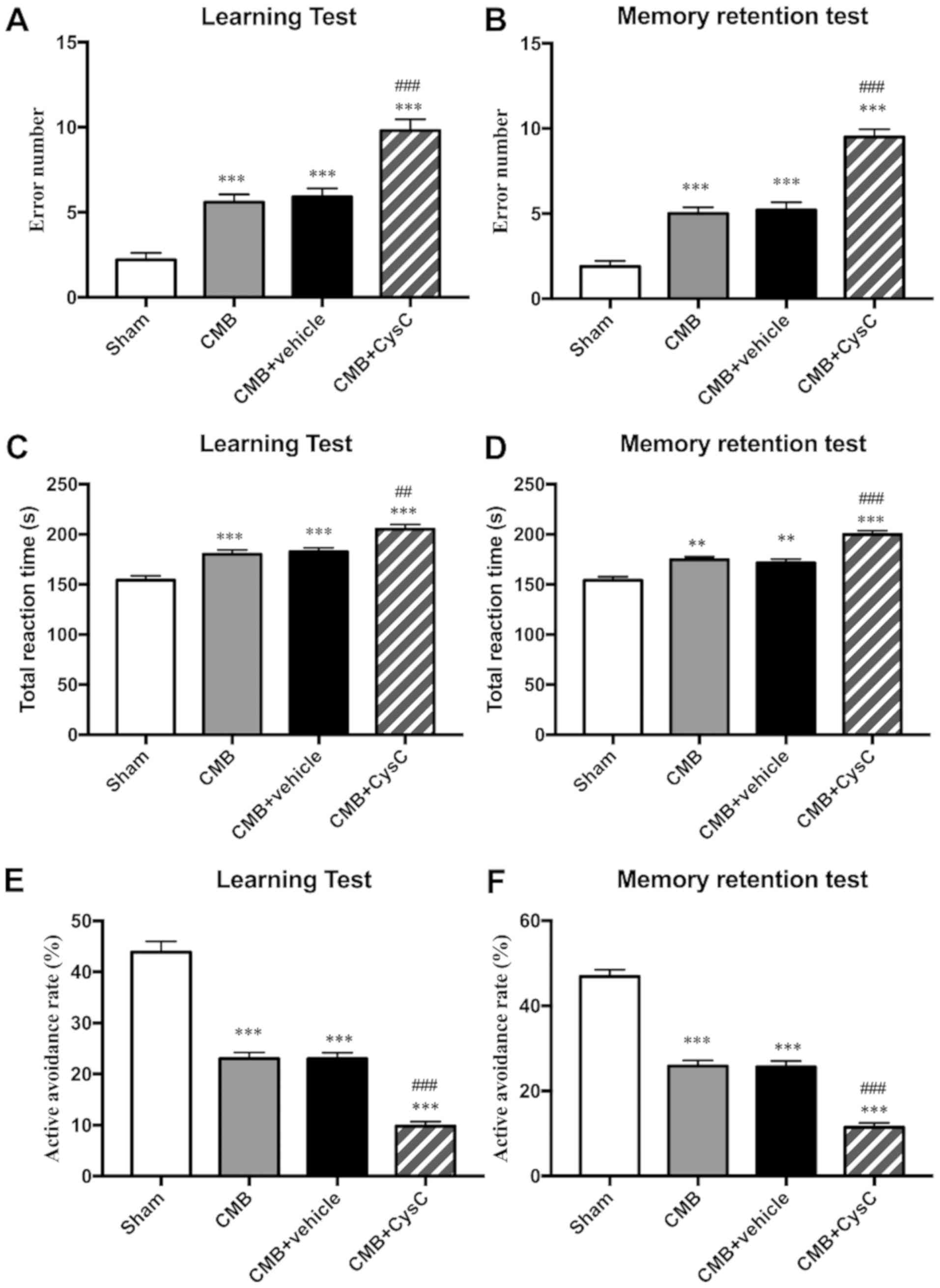
Cognitive functions, including learning and memory,
were evaluated in each group using the Y-maze test. Results showed
that compared with the sham group, the number of incorrect
reactions (Fig. 3A and B) and the
total reaction time (Fig. 3C and D)
increased, while the active avoidance rate (Fig. 3E and F) decreased in learning and
memory test in the CMB model group, indicating impairment of rat
learning and memory function. After administration of drug CysC,
the number of incorrect reactions and the total reaction time
(Fig. 3A–3D) further increased, while the active
avoidance rate (Fig. 3E and F)
further decreased, indicating that CysC aggravated impairment of
learning and memory function in the model rats.
Recording rat hippocampal LTP
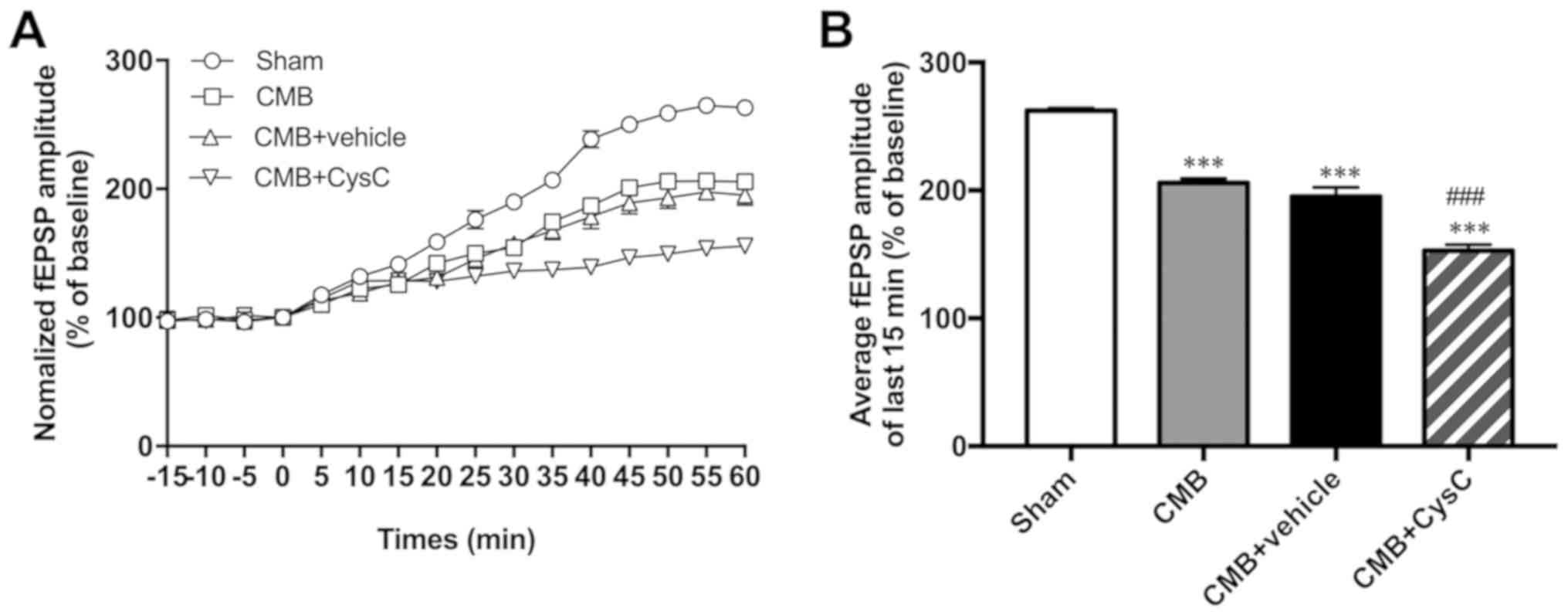
LTP is widely considered one of the major cellular
mechanisms that underlies learning and memory. In this study,
changes in LTP were recorded in each group using slice
electrophysiological techniques. As shown in Fig. 4, compared with the sham group, LTP of
the CMB model group was significantly reduced. Compared with the
CMB model group, LTP of the cystatin C overexpression group showed
further reduction as well.
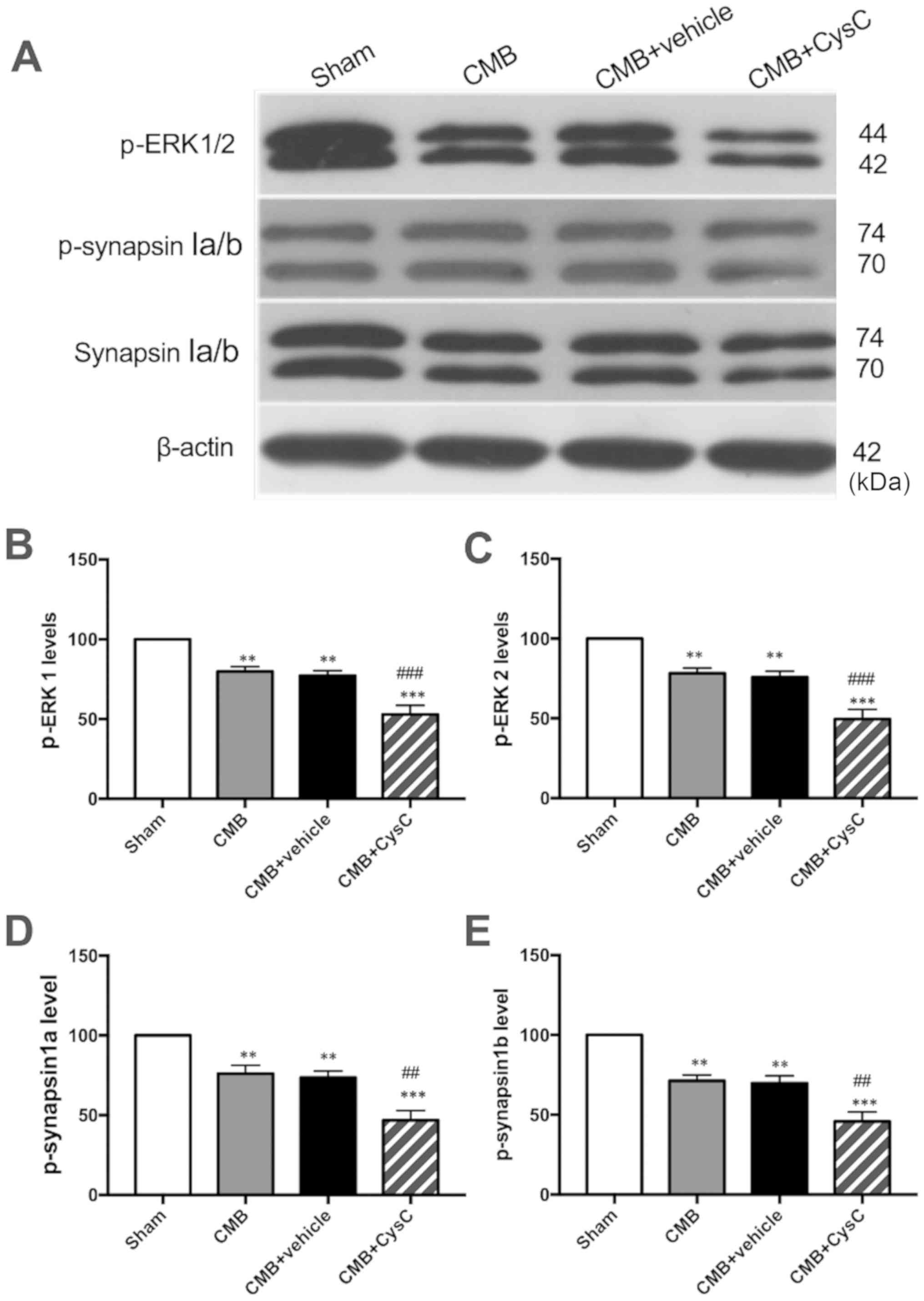
ERK/synapsinIa/b pathway
expression
Western blot analysis was performed to observe
changes in expression of p-ERK and p-synapsinIa/b (Ser549) which
are associated with cognitive functions. As shown in Fig. 5, compared with the sham group,
expression levels of p-ERK and p-synapsinIa/b (Ser549) were
significantly reduced in the CMB model group. Compared with the CMB
model group, CysC overexpression led to a further reduction in
expression levels of p-ERK and p-synapsinIa/b (Ser549).
Discussion
In clinic, expression level of cystatin C (CysC) was
found significantly higher in serum of patients with cerebral
microbleeds (CMB) than in healthy subjects (Fig. 1). In this study, a rat CMB model was
created, and was injected with CysC in the hippocampus aimed at
studying the impact of CysC on rat cognitive functions (Fig. 2). In behavioral study, cognitive
dysfunction was observed in the CMB model rats. After
administration of CysC, the learning and memory functions were
further deteriorated in the rats (Fig.
3). In electrophysiological study, a decrease in hippocampal
LTP was found in the CMB model rats. The hippocampal LTP was
further decreased after administration of CysC in the model rats
(Fig. 4). Among biomarkers
associated with learning and memory functions, it was found that
the expression levels of phosphorylated ERK1/2 and phosphorylated
synapsin Ia/b (Ser549) were reduced in hippocampus of the CMB model
rats. After administration of CysC, these levels were further
reduced (Fig. 5). These findings
suggested that overexpression of CysC can promote cognitive
dysfunction in rats with cerebral microbleeds by inhibiting the
ERK/synapsin Ia/b pathway.
CMB is the main cause of mild vascular cognitive
impairment and vascular dementia (33). The underlying mechanism may be that
CMB affects specific cognitive domain functions such as executive
functions and attention, and CMB may also damage the cerebral white
matter association fiber bundles, cholinergic fiber bundles, and
frontal subcortical circuitry (34).
In addition, CMB is often associated with neurodegenerative
diseases such as Alzheimer's disease, aggravating cortical atrophy
and increasing the risk of cognitive impairment (35). The present study demonstrated
learning and memory dysfunction in CMB model rats through Y-maze
learning and memory tests, indicating association of CMB with rat
cognitive functions. Our findings were consistent with related
literature reports. For example, CMB was reported to cause reduced
cognitive ability and increased risk of dementia (36,37). CMB
may play a role in development of cerebrovascular diseases and
neurodegenerative diseases. Its detection rate in Alzheimer's
disease is twice that in the same age control group (38). In electrophysiology, LTP is a
synaptic plasticity mechanism underlying learning and memory
(39–40). In this study, LTP in the CA1 region
of rat hippocampus was recorded using slice electrophysiological
techniques. It was found that CMB caused a decrease in LTP, which
was consistent with the behavioral test results.
CysC is an important endogenous inhibitor of
cysteine protease activity (41).
Its function is still unclear in the brain, but it is associated
with neuronal degeneration and nervous system repair. CysC is
highly expressed in patients with epilepsy and neurodegenerative
diseases. According to literature, high expression was also found
in facial nerve transection, perforation pathway transection,
pituitary resection, and animal models of transient cerebral
ischemia and epilepsy (42). There
is an opinion that high CysC expression in injury or disease may
imply an intrinsic neuroprotection that counteracts disease
progression. It was also reported that CysC exerts a protective
effect when neurons are under attack by inducing autophagy
(31,43). Several studies have demonstrated that
upregulated expression of CysC is positively correlated with
cerebrovascular diseases (44,45). In
this study, overexpression of CysC in rat serum and hippocampus was
achieved by injection of external CysC aimed at studying its impact
on cognitive functions in CMB model rats. It was found that
overexpression of CysC aggravated learning and memory dysfunction
and LTP reduction in CMB model rats. Effects of CysC on neurons
have been reported inconsistently in different studies. The
inconsistency may be due to several reasons. The first may be
related to disease progression. CysC may play different roles in
different disease states. The second may be related to the way an
animal model was created, and different CysC concentrations were
used. The third is that more likely CysC has a different effect on
neurons in different diseases.
Extracellular signal-regulated kinase 1/2 (ERK1/2)
is a signal transduction protein belonging to the mitogen-activated
protein kinase (MAPK) family. It is ubiquitous in various tissues
and participates in cell proliferation and differentiation.
Activation of ERK can mediate physiological functions of
nutrient-related factor receptors and multiple growth factor
receptors. There are two ERK isoforms, i.e. ERK1 and ERK2 (46,47).
MAPKs are serine/threonine protein kinases in cells, which also
include two signal transduction pathways, i.e. the c-Jun N-terminal
kinase (JNK) and p38 MAPK, in addition to ERK. The MAPK/ERK pathway
in the brain can communicate a signal from a receptor on the
surface of the cell to the DNA in the nucleus of the cell, thereby
participating in the regulation of neuronal apoptosis and
proliferation. This pathway is closely associated with learning and
memory functions (48). The ERK
signaling pathway is activated by synaptic activity in learning and
induction of LTP, but its activity is reduced in defected LTP and
learning (49–51). Delayed audiogenic seizure development
in a genetic rat model is associated with overactivation of ERK1/2
and disturbances in glutamatergic signaling (52). In the present study, it was found
that the level of p-ERK1/2 was decreased after CMB model creation,
and overexpression of CysC further decreased the level of p-ERK1/2,
indicating that ERK signaling was involved in the promoting process
of cognitive dysfunction in CMB rats due to CysC
overexpression.
Synapsin I is a key regulator of synaptic vesicle
dynamics in the presynaptic terminals, regulating synaptic
transmission by modulating the storage and mobilization of synaptic
vesicles (53). It can selectively
control synaptic maturation of long-range projections in the
lateral amygdala (54). Synapsin I
binds to a variety of upstream molecules, through which it
participates in LTP and learning and memory functions. Among these
upstream molecules, leucine-rich repeat kinase 2 (LRRK2) is a large
multi-domain scaffold protein. LRRK2 exhibits GTPase and kinase
activity, involving in synaptic kinetics. LRRK2 can modulate
glutamate release from presynaptic sites by interacting with
synapsin I via its WD40 domain (55). It was reported in literature that the
modulatory function of neurofibrin on learning is via its
regulation of the ERK-synapsin I pathway and GABA release (56). ERK phosphorylates mammalian synapsin
I at positions 4 and 5 of domain B and at position 6 of domain D
(57). In addition, PKA and CaMKII
can also act by phosphorylating synapsin I (58). In this study, a decrease was observed
in the level of p-synapsin Ia/b (Ser549) after CMB model creation.
According to literature, the Ser549 site is targeted by ERK and
Cdk1 (59). Overexpression of CysC
further decreased the level of p-synapsin Ia/b (Ser549), indicating
that synapsin I was involved in the promoting process of cognitive
dysfunction in CMB rats due to CysC overexpression.
Although this work demonstrated the promoting effect
of CysC on cognitive impairment and the involvement of the
ERK-synapsin I signal pathway, it does not rule out the possibility
of CysC having other mechanisms of promoting cognitive dysfunction
in the CMB model rats. Thus, further work is still necessary for
clarification.
In conclusion, this study preliminarily demonstrated
that CysC overexpression can promote cognitive dysfunction in rats
with cerebral microbleeds by inhibiting the ERK/synapsinIa/b
pathway. Based on these findings, it is postulated that reducing
the expression level of CysC in serum of CMB patients may help
alleviate the symptoms and slow down disease progression of
cognitive impairment.
Acknowledgements
Not applicable.
Funding
This study was supported by Qiqihar Science and
Technology Project (SFGG-201952).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GY wrote the manuscript. XS and LL conceived and
designed the study. LH and HL were responsible for the collection
and analysis of the experimental data. SW interpreted the data and
drafted the manuscript. ZR and YZ revised the manuscript critically
for important intellectual content. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Qiqihar Medical University
(Qiqihar, China). All subjects signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moulin S and Cordonnier C: Role of
cerebral microbleeds for intracerebral haemorrhage and dementia.
Curr Neurol Neurosci Rep. 19:512019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cordonnier C, Al-Shahi Salman R and
Wardlaw J: Spontaneous brain microbleeds: Systematic review,
subgroup analyses and standards for study design and reporting.
Brain. 130:1988–2003. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daugherty AM and Raz N: Incident risk and
progression of cerebral microbleeds in healthy adults: A
multi-occasion longitudinal study. Neurobiol Aging. 59:22–29. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vernooij MW, van der Lugt A, Ikram MA,
Wielopolski PA, Niessen WJ, Hofman A, Krestin GP and Breteler MM:
Prevalence and risk factors of cerebral microbleeds: The Rotterdam
Scan Study. Neurology. 70:1208–1214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Charidimou A, Imaizumi T, Moulin S, Biffi
A, Samarasekera N, Yakushiji Y, Peeters A, Vandermeeren Y, Laloux
P, Baron JC, et al: Brain hemorrhage recurrence, small vessel
disease type, and cerebral microbleeds: A meta-analysis. Neurology.
89:820–829. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Charidimou A, Kakar P, Fox Z and Werring
DJ: Cerebral microbleeds and recurrent stroke risk: Systematic
review and meta-analysis of prospective ischemic stroke and
transient ischemic attack cohorts. Stroke. 44:995–1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cordonnier C and van der Flier WM: Brain
microbleeds and Alzheimer's disease: Innocent observation or key
player? Brain. 134:335–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Staekenborg SS, Koedam EL, Henneman WJ,
Stokman P, Barkhof F, Scheltens P and van der Flier WM: Progression
of mild cognitive impairment to dementia: Contribution of
cerebrovascular disease compared with medial temporal lobe atrophy.
Stroke. 40:1269–1274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dierksen GA, Skehan ME, Khan MA, Jeng J,
Nandigam RN, Becker JA, Kumar A, Neal KL, Betensky RA, Frosch MP,
et al: Spatial relation between microbleeds and amyloid deposits in
amyloid angiopathy. Ann Neurol. 68:545–548. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gurol ME, Dierksen G, Betensky R, Gidicsin
C, Halpin A, Becker A, Carmasin J, Ayres A, Schwab K, Viswanathan
A, et al: Predicting sites of new hemorrhage with amyloid imaging
in cerebral amyloid angiopathy. Neurology. 79:320–326. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenberg SM, Vernooij MW, Cordonnier C,
Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem
MA and Breteler MM; Microbleed Study Group, : Cerebral microbleeds:
A guide to detection and interpretation. Lancet Neurol. 8:165–174.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lawrence AJ, Patel B, Morris RG, MacKinnon
AD, Rich PM, Barrick TR and Markus HS: Mechanisms of cognitive
impairment in cerebral small vessel disease: Multimodal MRI results
from the St. George's cognition and neuroimaging in stroke (SCANS)
study. PLoS One. 8:e610142013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lawrence AJ, Chung AW, Morris RG, Markus
HS and Barrick TR: Structural network efficiency is associated with
cognitive impairment in small-vessel disease. Neurology.
83:304–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poels MM, Ikram MA, van der Lugt A, Hofman
A, Niessen WJ, Krestin GP, Breteler MM and Vernooij MW: Cerebral
microbleeds are associated with worse cognitive function: The
Rotterdam Scan Study. Neurology. 78:326–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leem AY, Park MS, Park BH, Jung WJ, Chung
KS, Kim SY, Kim EY, Jung JY, Kang YA, Kim YS, et al: Value of serum
Cystatin C measurement in the diagnosis of sepsis-induced kidney
injury and prediction of renal function recovery. Yonsei Med J.
58:604–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benndorf RA: Renal Biomarker and
Angiostatic Mediator? Cystatin C as a negative regulator of
vascular endothelial cell homeostasis and angiogenesis. J Am Heart
Assoc. 7:e0109972018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ekström U, Wallin H, Lorenzo J, Holmqvist
B, Abrahamson M and Avilés FX: Internalization of cystatin C in
human cell lines. FEBS J. 275:4571–4582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mathews PM and Levy E: Cystatin C in aging
and in Alzheimer's disease. Ageing Res Rev. 32:38–50. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oh MY, Lee H, Kim JS, Ryu WS, Lee SH, Ko
SB, Kim C, Kim CH and Yoon BW: Cystatin C, a novel indicator of
renal function, reflects severity of cerebral microbleeds. BMC
Neurol. 14:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang S, Cai J, Lu R, Wu J, Zhang M and
Zhou X: Association between serum cystatin C level and total
magnetic resonance imaging burden of cerebral small vessel disease
in patients with acute lacunar stroke. J Stroke Cerebrovasc Dis.
26:186–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang JB, Jü XH, Wang J, Sun HR and Li F:
Serum cystatin C and cerebral microbleeds in patients with acute
cerebral stroke. J Clin Neurosci. 21:268–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang JB, Liu LF, Li ZG, Sun HR and Jü XH:
Associations between biomarkers of renal function with cerebral
microbleeds in hypertensive patients. Am J Hypertens. 28:739–745.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang Z, Deng J, Wu Z, Dong B, Wang S, Chen
X, Nie H, Dong H, Xiong L and Cystatin C: Cystatin C is a crucial
endogenous protective determinant against stroke. Stroke.
48:436–444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou J, Chen Z, Wei X, Chen Z, Fu Y, Yang
X, Chen D, Wang R, Jenner P, Lu JH, et al: Cystatin C as a
potential therapeutic mediator against Parkinson's disease via
VEGF-induced angiogenesis and enhanced neuronal autophagy in
neurovascular units. Cell Death Dis. 8:e28542017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kono S, Adachi H, Enomoto M, Fukami A,
Kumagai E, Nakamura S, Nohara Y, Morikawa N, Nakao E, Sakaue A, et
al: Impact of cystatin C and microalbuminuria on cognitive
impairment in the population of community-dwelling Japanese.
Atherosclerosis. 265:71–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin Z, Yan Z, Liang Y, Jiang H, Cai C,
Song A, Feng L and Qiu C: Interactive effects of diabetes and
impaired kidney function on cognitive performance in old age: A
population-based study. BMC Geriatr. 16:72016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stephan Y, Sutin AR and Terracciano A:
Subjective age and cystatin C among older adults. J Gerontol B
Psychol Sci Soc Sci. 74:382–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei Y, Wei YK and Zhu J: Early markers of
kidney dysfunction and cognitive impairment among older adults. J
Neurol Sci. 375:209–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park YS, Chung MS and Choi BS: MRI
assessment of cerebral small vessel disease in patients with
spontaneous intracerebral hemorrhage. Yonsei Med J. 60:774–781.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tóth A, Berente Z, Bogner P, Környei B,
Balogh B, Czeiter E, Amrein K, Dóczi T, Büki A and Schwarcz A:
Cerebral microbleeds temporarily become less visible or invisible
in acute susceptibility weighted magnetic resonance imaging: A Rat
Study. J Neurotrauma. 36:1670–1677. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Li J, Wang Z, Yu Z and Chen G:
Attenuation of early brain injury and learning deficits following
experimental subarachnoid hemorrhage secondary to cystatin C:
Possible involvement of the autophagy pathway. Mol Neurobiol.
49:1043–1054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li XH, Matsuura T, Liu RH, Xue M and Zhuo
M: Calcitonin gene-related peptide potentiated the excitatory
transmission and network propagation in the anterior cingulate
cortex of adult mice. Mol Pain. Feb 28–2019.(Epub ahead of print).
doi: 10.1177/1744806919832718, 2019. View Article : Google Scholar
|
|
33
|
Pantoni L: Cerebral small vessel disease:
From pathogenesis and clinical characteristics to therapeutic
challenges. Lancet Neurol. 9:689–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dey AK, Stamenova V, Turner G, Black SE
and Levine B: Pathoconnectomics of cognitive impairment in small
vessel disease: A systematic review. Alzheimers Dement. 12:831–845.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roseborough A, Ramirez J, Black SE and
Edwards JD: Associations between amyloid β and white matter
hyperintensities: A systematic review. Alzheimers Dement.
13:1154–1167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akoudad S, Wolters FJ, Viswanathan A, de
Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA and
Vernooij MW: Association of cerebral microbleeds with cognitive
decline and dementia. JAMA Neurol. 73:934–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai Z, Wang C, He W, Tu H, Tang Z, Xiao M
and Yan LJ: Cerebral small vessel disease and Alzheimer's disease.
Clin Interv Aging. 10:1695–1704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Love S and Miners JS: Small vessel
disease, neurovascular regulation and cognitive impairment:
Post-mortem studies reveal a complex relationship, still poorly
understood. Clin Sci (Lond). 131:1579–1589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cobar LF, Yuan L and Tashiro A: Place
cells and long-term potentiation in the hippocampus. Neurobiol
Learn Mem. 138:206–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lømo T: Discovering long-term potentiation
(LTP) - recollections and reflections on what came after. Acta
Physiol (Oxf). 222:2222018. View Article : Google Scholar
|
|
41
|
Abrahamson M, Alvarez-Fernandez M and
Nathanson CM: Cystatins. Biochem Soc Symp. 70:179–199. 2003.
View Article : Google Scholar
|
|
42
|
Levy E, Jaskolski M and Grubb A: The role
of cystatin C in cerebral amyloid angiopathy and stroke: Cell
biology and animal models. Brain Pathol. 16:60–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tizon B, Sahoo S, Yu H, Gauthier S, Kumar
AR, Mohan P, Figliola M, Pawlik M, Grubb A, Uchiyama Y, et al:
Induction of autophagy by cystatin C: A mechanism that protects
murine primary cortical neurons and neuronal cell lines. PLoS One.
5:e98192010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Y and Sun L: Cystatin C in
cerebrovascular disorders. Curr Neurovasc Res. 14:406–414. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Osk Snorradottir A, Isaksson HJ, Kaeser
SA, Skodras AA, Olafsson E, Palsdottir A and Thor Bragason B:
Parenchymal cystatin C focal deposits and glial scar formation
around brain arteries in hereditary cystatin C amyloid angiopathy.
Brain Res. 1622:149–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li J, Zhang M and Ma J: Myricitrin
inhibits PDGF-BB-stimulated vascular smooth muscle cell
proliferation and migration through suppressing PDGFRβ/Akt/Erk
signaling. Int J Clin Exp Med. 8:21715–21723. 2015.PubMed/NCBI
|
|
47
|
Frost EE, Zhou Z, Krasnesky K and
Armstrong RC: Initiation of oligodendrocyte progenitor cell
migration by a PDGF-A activated extracellular regulated kinase
(ERK) signaling pathway. Neurochem Res. 34:169–181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Horikawa J and Ojima H: Cortical
activation patterns evoked by temporally asymmetric sounds and
their modulation by learning. eNeuro. 4:42017. View Article : Google Scholar
|
|
49
|
Atkins CM, Selcher JC, Petraitis JJ,
Trzaskos JM and Sweatt JD: The MAPK cascade is required for
mammalian associative learning. Nat Neurosci. 1:602–609. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
English JD and Sweatt JD: A requirement
for the mitogen-activated protein kinase cascade in hippocampal
long term potentiation. J Biol Chem. 272:19103–19106. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Selcher JC, Atkins CM, Trzaskos JM, Paylor
R and Sweatt JD: A necessity for MAP kinase activation in mammalian
spatial learning. Learn Mem. 6:478–490. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chernigovskaya EV, Korotkov AA, Dorofeeva
NA, Gorbacheva EL, Kulikov AA and Glazova MV: Delayed audiogenic
seizure development in a genetic rat model is associated with
overactivation of ERK1/2 and disturbances in glutamatergic
signaling. Epilepsy Behav. 99:1064942019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Song SH and Augustine GJ: Synapsin
isoforms and synaptic vesicle trafficking. Mol Cells. 38:936–940.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lugarà E, De Fusco A, Lignani G, Benfenati
F, Humeau Y and Synapsin I: Synapsin I controls synaptic maturation
of long-range projections in the lateral amygdala in a targeted
selective fashion. Front Cell Neurosci. 13:2202019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Marte A, Russo I, Rebosio C, Valente P,
Belluzzi E, Pischedda F, Montani C, Lavarello C, Petretto A, Fedele
E, et al: Leucine-rich repeat kinase 2 phosphorylation on synapsin
I regulates glutamate release at pre-synaptic sites. J Neurochem.
150:264–281. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cui Y, Costa RM, Murphy GG, Elgersma Y,
Zhu Y, Gutmann DH, Parada LF, Mody I and Silva AJ: Neurofibromin
regulation of ERK signaling modulates GABA release and learning.
Cell. 135:549–560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Giachello CN, Fiumara F, Giacomini C,
Corradi A, Milanese C, Ghirardi M, Benfenati F and Montarolo PG:
MAPK/Erk-dependent phosphorylation of synapsin mediates formation
of functional synapses and short-term homosynaptic plasticity. J
Cell Sci. 123:881–893. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen X, Wang X, Yang Y, Li Z, Zhang Y, Gao
W, Xiao J and Li B: Schwann cells protect against CaMKII- and
PKA-dependent acrylamide-induced synapsin I phosphorylation. Brain
Res. 1701:18–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Verstegen AM, Tagliatti E, Lignani G,
Marte A, Stolero T, Atias M, Corradi A, Valtorta F, Gitler D,
Onofri F, et al: Phosphorylation of synapsin I by cyclin-dependent
kinase-5 sets the ratio between the resting and recycling pools of
synaptic vesicles at hippocampal synapses. J Neurosci.
34:7266–7280. 2014. View Article : Google Scholar : PubMed/NCBI
|