Introduction
Sepsis is a serious syndrome that is induced by
infections and characterized by a systemic inflammatory response
(1). Neonatal sepsis (NS) is sepsis
that occurs in infants within 28 days of age and is mainly caused
by bacterial infection (2).
Approximately 1 million infants die from NS every year worldwide,
which therefore represents a global health burden (3). However, current strategies for the
diagnosis of NS remain limited. Bacterial culture is considered the
gold standard for the diagnosis of NS, but it takes 1 to 2 days to
obtain the examination results with low sensitivity (4). In addition, several biological markers
with high sensitivity have been identified, including interleukins
(ILs), C-reactive protein (CRP), micro-erythrocyte sedimentation
rate and procalcitonin (PCT) (5–7).
However, the clinical application of these indicators is limited
due to their poor specificity. Thus, novel diagnostic biomarkers
with high sensitivity and specificity are urgently required for the
early diagnosis of NS.
MicroRNAs (miRNAs) are small non-coding RNAs that
may be easily detected from blood samples (8). It is generally accepted that miRNAs
regulate gene expression by directly binding to the 3′-untranslated
region (3′-UTR) of target messenger RNA (mRNA), leading to mRNA
degradation or suppression of subsequent translation (9). Furthermore, pivotal roles of miRNAs
have been demonstrated in a number of biological processes,
including cell proliferation, differentiation, cell cycle and cell
apoptosis (10). Emerging studies
have reported differentially expressed miRNAs in different types of
human diseases (11–13). The clinical significance of the
aberrant expression of miRNAs has attracted increasing attention
due to their high diagnostic and prognostic values (14,15).
Downregulated expression of microRNA-181a (miR-181a) has been
identified in NS patients by Chen et al (16). A study by He et al (17) demonstrated that miR-181a improved
immune thrombocytopenia by regulating Toll-like receptor 4 (TLR4),
a key molecule in the innate immune system and the development of
NS (18). However, the clinical and
biological roles of miR-181a in NS have remained to be fully
elucidated.
To improve the diagnosis of NS, the present study
sought to compare the serum levels of miR-181a between NS patients
and healthy newborns and explore the diagnostic value of miR-181a.
Additionally, the effect of miR-181a on the lipopolysaccharide
(LPS)-induced inflammatory response was further analyzed in primary
monocytes.
Materials and methods
Patients and blood sample
collection
The experimental protocols were approved by the
Ethics Committee of Yidu Central Hospital of Weifang Hospital
(Weifang, China) and written informed consent was obtained from the
families of the patients. Blood samples were collected from 102
patients with NS at the time of initial laboratory evaluation at
the Yidu Central Hospital of Weifang (Weifang, China) between May
2014 and April 2018, and stored at −80°C for further analysis.
Furthermore, 50 neonates without any symptoms and signs of sepsis,
who underwent routine consultation or vaccination at an outpatient
neonatal clinic and were diagnosed with respiratory infection or
pneumonia were included in the present study as a control group.
The diagnosis of NS was determined based on the criteria
established at the 2003 Kunming Neonatal Sepsis Definitions
Conference (19); it mainly relies
on the clinical manifestations and the detection of blood
pathogens. Staphylococcus and Escherichia coli were
the most common types among all of the detected bacteria. The
clinicopathological characteristics of the participants are listed
in Table I.
 | Table I.Clinicopathological characteristics of
the NS patients and the controls. |
Table I.
Clinicopathological characteristics of
the NS patients and the controls.
| Characteristic | Control (n=50) | NS (n=102) | P-value |
|---|
| Age (days) | 11.74±4.41 | 11.52±4.01 | 0.758 |
| Sex
(male/female) | 28/22 | 53/49 | 0.730 |
| Body weight
(g) | 3485.18±308.79 | 3472.77±299.91 | 0.813 |
| CRP (mg/l) | 10.89±4.74 | 12.46±5.86 | 0.099 |
| WBC
(×109/l) | 10.62±4.92 | 11.59±5.77 | 0.307 |
| PCT (ng/ml) | 1.60±0.75 | 4.76±2.72 | 0.001 |
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The collected blood was centrifuged to isolate the
serum samples. Total RNA from the serum, including miRNAs, was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A
NanoDrop 2000 (Thermo Fisher Scientific, Inc.) was used to evaluate
the purity and concentration of the RNA. Single-stranded
complementary DNA was synthesized from the RNA using the
PrimeScript RT reagent kit (Takara Bio, Inc.) and stored at −20°C
for subsequent qPCR. The serum levels of miR-181a and mRNA of TLR4
were determined using qPCR, which was performed using a SYBR Green
I Master Mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) and a
7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The reaction conditions are as follows: miR-181a
95°C for 10 min, 40 cycles of 95es of m0 sec, 60°C for 20 sec, 72°C
for 15 sec; TLR4 95°C for 10 min, 40 cycles of 95°C for 30 sec,
58°C for 30 sec, 72°C for 20 sec. U6 and GAPDH were respectively
used as the internal control gene for miR-181a and TLR4. The final
expression value was calculated using the 2−ΔΔCq method
(20) and normalized to U6 or GAPDH.
The sequences of the primers used in the present study were as
follows: miR-181a forward, 5′-GCCGAGAACAUUCAACGCUGU-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′; TLR4 forward,
5′-CAGAGTTGCTTTCAATGGCATC-3′ and reverse,
5′-AGACTGTAATCAAGAACCTGGAGG-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′.
Cell culture and stimulation
conditions
Blood samples collected from the patients with NS
were settled by addition of 4.5% dextran 500 (1:5; Amersham
Biosciences) and the leukocytes were separated from the red blood
cells. Monocytes were isolated using density gradient
centrifugation with FicollPaque (Amersham Pharmacia, Biotech AB) as
previously described (21), and the
purity of the cells was confirmed to be >95% by flow cytometry
based on detection of the specific cell markers CD14 and CD45. The
extracted monocytes were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) in a humidified
atmosphere with 5% CO2 at 37°C. To explore the effects
of miR-181a on LPS-induced inflammation, the monocytes were
stimulated using 100 ng/ml LPS (Sigma-Aldrich; Merck KGaA) for 4
h.
Cell transfection
Monocytes were seeded into 48-well plates and
transfected with miR-181a mimics, miR-181a inhibitor and
miR-negative control (miR-NC) (GenePharma) using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The sequences of the vectors were as follows: miR-181a
mimics, 5′-AACAUUCAACGCUGUCGGUGAGU-3′; miR-181a inhibitor,
5′-ACUCACCGACAGCGUUGAAUGUU-3′; miR-NC,
5′-CAGUACUUUUGUGUAGUACAA-3′.
Luciferase reporter assay
In a bioinformatics analysis using TargetScan
(http://www.targetscan.org/vert_72/),
a complementary sequence of miR-181a was identified in the 3′-UTR
of TLR4. To verify whether there was a direct interaction between
miR-181a and TLR4, a luciferase reporter assay was performed in the
present study. The wild-type (WT) or mutant-type (MT) 3′-UTR was
cloned into the pGL3 basic vector (Promega Corp.) to obtain
pLUC-WT-TLR4 or pLUC-MT-TLR4, respectively. miR-181a mimics,
miR-181a inhibitor or miR-NC were co-transfected into the isolated
monocytes with pLUC-WT-TLR4 or pLUC-MT-TLR4 using Lipofectamine
2000 (Thermo Fisher Scientific, Inc.). A Dual-Luciferase Reporter
Assay System (Promega Corp.) was used to measure the luciferase
activity in the different groups.
ELISA
The concentration of the inflammatory cytokines
tumor necrosis factor (TNF)-α and IL-8 in the culture supernatant
of the monocytes was estimated using ELISA, which was performed
using a TNF-α ELISA kit (cat. no. 550610; BD Biosciences) and an
IL-8 ELISA kit (cat. no. 550999; BD Biosciences) according to the
manufacturer's protocol. The optical density at 450 nm was read by
using a microplate reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses were performed by using
SPSS 18.0 software (SPSS Inc.) and GraphPad Prism 5.0 software
(GraphPad Software, Inc., USA). Values are expressed as the mean ±
standard deviation and compared with Student's t-test, the
χ2 test or one-way analysis of variance followed by
Tukey's multiple-comparisons test. A receiver operating
characteristic (ROC) curve was drawn to evaluate the diagnostic
value of miR-181a regarding NS. P<0.05 was considered to
indicate statistical significance.
Results
Clinicopathological characteristics of
the patients with NS and the controls
A total of 102 patients with NS and 50 controls were
included in the present study. The clinicopathological
characteristics, including age, sex, body weight, concentration of
CRP and PCT, as well as white blood cell (WBC) count, were
summarized in Table I. The control
group included 28 males and 22 females with the age of 11.74±4.41
days, and 53 males and 49 females were included in the NS patients
with an average of 11.52±4.01 days. There were no significant
differences between the NS cases and controls in terms of age, sex,
body weight, CRP and WBC count (all P>0.05), while a higher PCT
was observed in the patients with NS compared with that in the
controls (P=0.001).
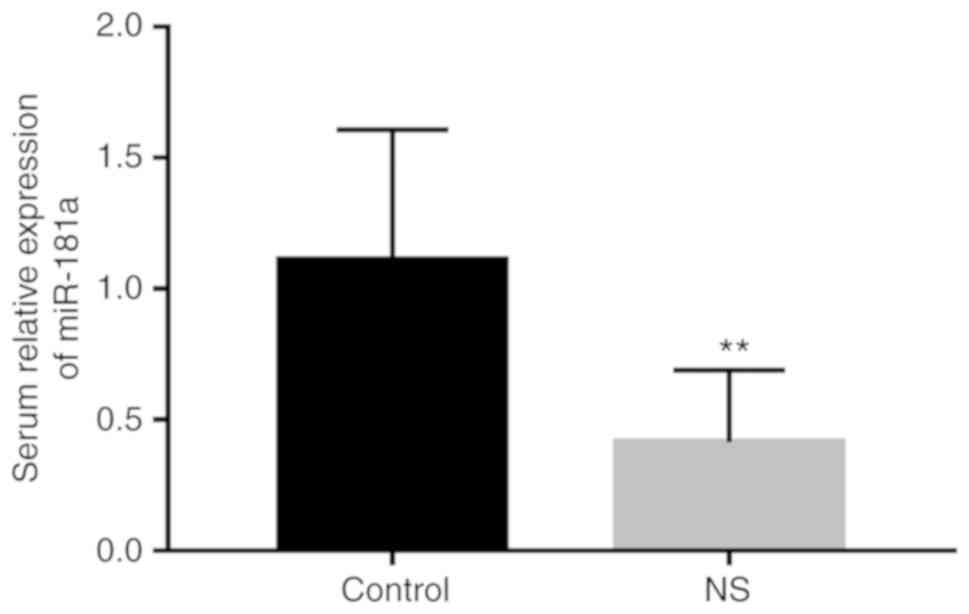
Serum miR-181a is downregulated in
patients with NS
To investigate the role of miR-181a in NS, the serum
levels of miR-181a in the NS patients were measured using RT-qPCR.
As presented in Fig. 1, the relative
serum levels of miR-181a were significantly downregulated in
patients with NS compared with those in the controls
(P<0.01).
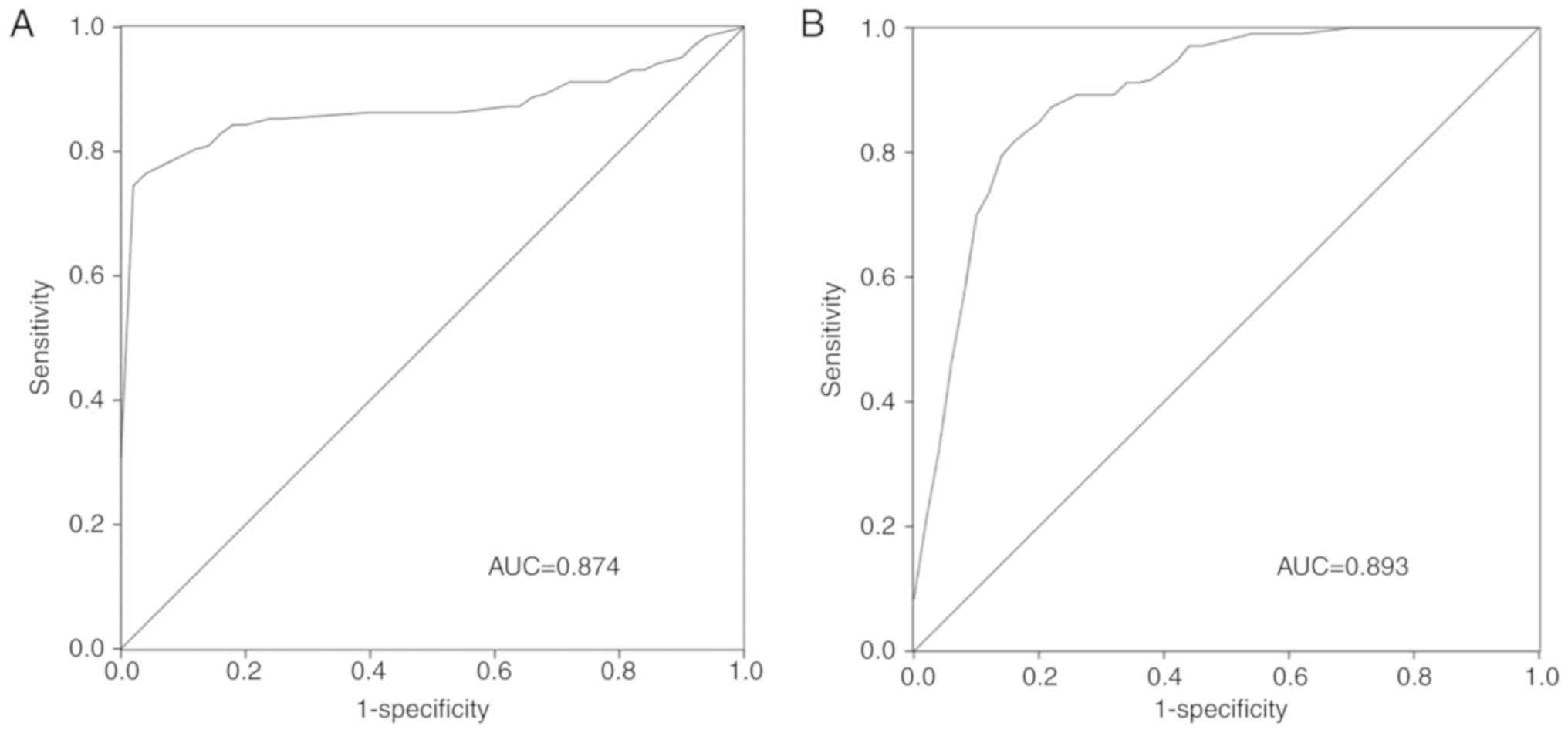
Diagnostic value of miR-181a in
patients with NS
Given the dysregulation of miR-181a in the serum of
patients with NS, its clinical significance in the diagnosis of NS
was assessed in the present study. A ROC curve for PCT was first
constructed based on the established diagnostic value of PCT in NS
and its significantly different concentration in the present NS
group compared with that in the controls. As presented in Fig. 2A, the area under the curve (AUC) for
PCT was 0.874 with a sensitivity of 75.5% and a specificity of
98.0% at a cutoff value of 0.980. The ROC curve for the levels of
miR-181a is presented in Fig. 2B,
with an AUC of 0.893, and a sensitivity and specificity of 83.3 and
84.0%, respectively, at a cutoff value of 0.625.
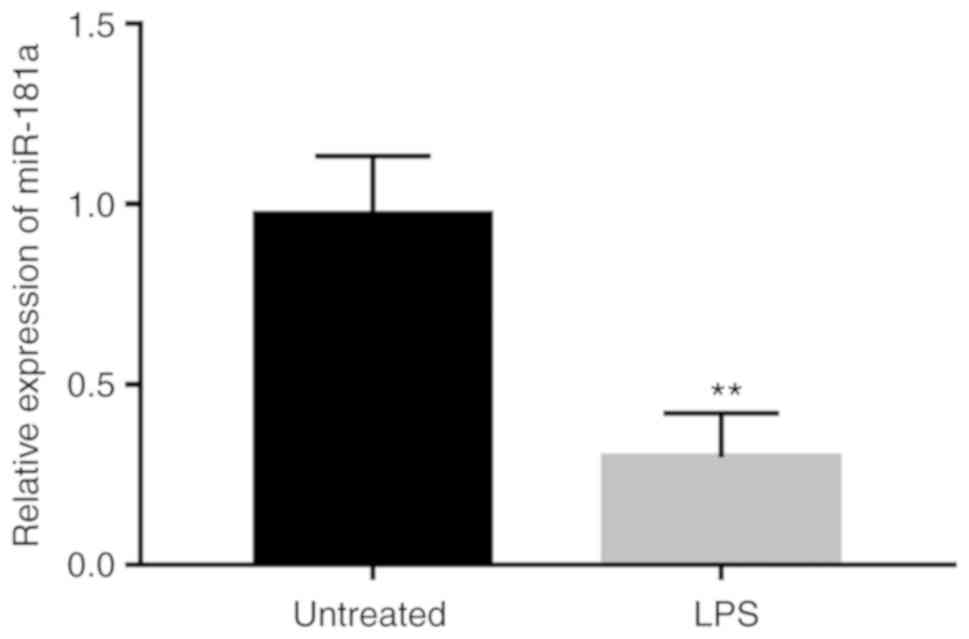
Expression of miR-181a in LPS-treated
monocytes
To investigate the functional role of miR-181a in
LPS-induced inflammation, the expression of miR-181a in monocytes
treated with LPS was measured. As presented in Fig. 3, the expression of miR-181a was
obviously decreased in the monocytes after LPS stimulation compared
with that in the untreated cells (P<0.01).
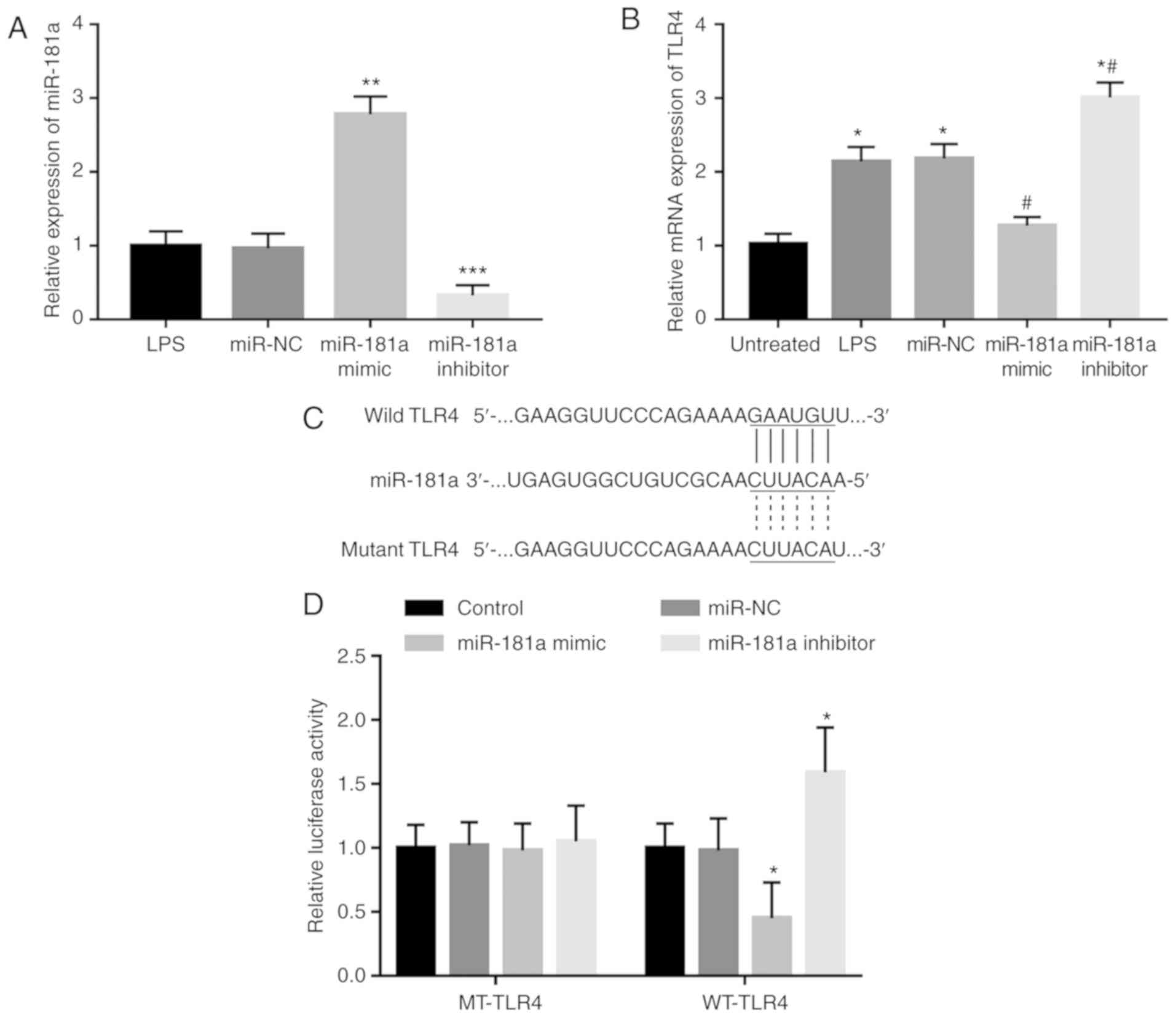
miR-181a directly regulates the
expression of TLR4
TLR4 is a key molecule in the innate immune system
and the development of NS. TLR4 has been reported to be a direct
target of miR-181a in immune thrombocytopenia (17,18). The
present study focused on the association between these two
molecules in the monocytes. Following transfection with miR-181a
mimics or miR-181a inhibitor, the expression of miR-181a was
significantly increased or decreased, respectively, as confirmed by
RT-qPCR (all P<0.01, Fig. 4A).
After the in vitro modification of miR-181a levels, the
LPS-induced elevated expression of TLR4 was indicated to be
significantly suppressed in the cells with overexpression of
miR-181a, whereas it was significantly enhanced in the cells with
knockdown of miR-181a (all P<0.05, Fig. 4B), indicating that miR-181a in
LPS-treated monocytes led to inhibition of TLR4. In order to
further confirm the direct interaction between miR-181a and TLR4, a
luciferase reporter assay was performed. A complementary sequence
of miR-181a was identified in the 3′-UTR of TLR4 (Fig. 4C). After co-transfection of the
reporter vector containing the 3′-UTR sequence of TLR4 and miR-181a
mimics or inhibitor, it was observed that the relative luciferase
activity in the WT-TLR4 group was markedly decreased in the
presence of miR-181a mimics but was increased in the presence of
miR-181a inhibitor (all P<0.05, Fig.
4D). However, no significant changes in luciferase activity
were observed in the MT-TLR4 groups.
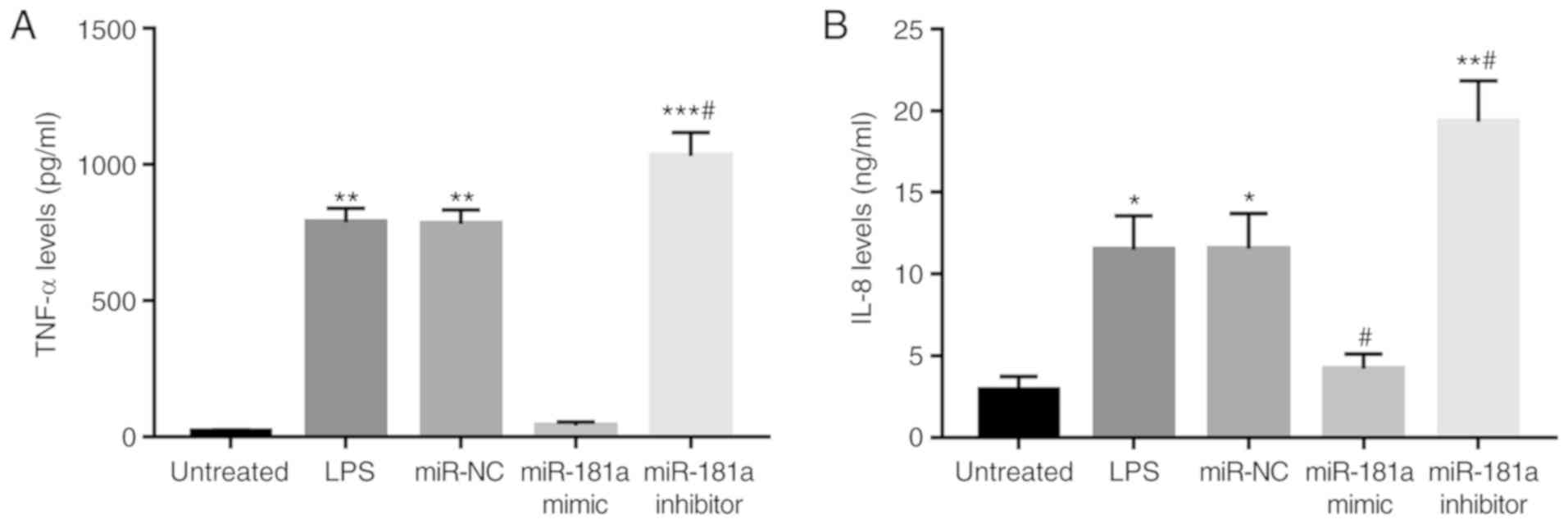
Effects of miR-181a on the levels of
pro-inflammatory cytokines in monocytes
The effects of miR-181a on inflammatory cytokines
were then investigated to demonstrate the regulatory role of
miR-181a on inflammation in monocytes. As presented in Fig. 5, the concentration of TNF-α and IL-8
was increased after LPS stimulation (all P<0.05). Following
modification of miR-181a levels in the monocytes, it was observed
that overexpression of miR-181a resulted in decreased levels of
TNF-α and IL-8, while inhibition of miR-181a led to increased
concentrations of these two cytokines in the presence of LPS (all
P<0.05).
Discussion
The present study focused on the expression and
clinical significance of miR-181a in patients with NS and explored
the effects of miR-181a on LPS-induced inflammation in monocytes.
RT-qPCR indicated that the serum levels of miR-181a were
significantly downregulated in patients with NS compared with those
in the controls, which may be of diagnostic value with considerable
sensitivity and specificity. In the monocytes extracted from the
serum of patients with NS, the expression of miR-181a was also
downregulated after LPS stimulation. TLR4 has been previously
reported to be a target gene of miR-181a in immune thrombocytopenia
(17), and in the present study, it
was demonstrated that miR-181a directly inhibits the expression of
TLR4 in monocytes. Furthermore, overexpression of miR-181a led to
inhibition of LPS-induced inflammation, as evidenced by the
decreased TNF-α and IL-8 concentrations.
Numerous studies have indicated the pivotal roles of
miRNAs in the initiation and development of various human diseases,
including malignancies (22),
metabolic diseases (23) and
cardiovascular diseases (24). In
sepsis, there are also functional miRNAs that are linked to the
progression of the disease by the regulation of inflammatory
response, such as miR-150 (25) and
miR-27a (26), e.g. miR-375
(25) and miR-25 (26). In addition, certain miRNAs with
ectopic expression patterns have critical roles in the pathogenesis
of NS by regulating the inflammatory response. For instance,
miR-15a/16 has been reported to be upregulated in serum samples of
patients with NS and may be involved in the inflammatory response
in this disease (18). The
expression of miR-132 and miR-223 was demonstrated to be
downregulated in patients with NS compared with that in healthy
infants and was associated with the expression of immune-associated
genes involved in the TLR signaling pathway (27). In the present study, NS patients were
recruited to estimate the expression of miR-181a. This study
enrolled neonates with respiratory infection or pneumonia as
controls but did not include healthy neonates. Firstly, blood
samples were difficult to obtain from healthy individuals for
ethical considerations; however, neonates with infections, but not
sepsis, already underwent blood collection and examination. Thus,
their blood samples were available with the approval from the
families. Additionally, infections in neonates with
pneumonia/respiratory tract infection contribute to the occurrence
of NS (28). Thus, our study data
may provide a diagnostic biomarker to screen the NS cases from the
infection cohort. The expression analysis data shown a significant
decrease in the expression of miR-181a in the serum specimens of
patients with NS compared with the controls. In a study by Chen
et al (16), downregulated
expression of circulating miR-181a was also reported in patients
with NS. Thus, miR-181a may have a pivotal role in the progression
of NS.
Accurate diagnosis is the first and most important
prerequisite of the efficient treatment of diseases. To improve the
treatment of infectious diseases in neonates, it is of value to
screen NS cases from the neonates with pneumonia/respiratory tract
infection. CRP, WBC and PCT are the established and widely used
diagnostic biomarkers for NS, but their application has limited
specificity (27). Previous studies
indicated that infection with bacteria, fungi or parasites may lead
to increases in WBC and the levels of CRP and PCT (29). Thus, elevated WBC, CRP or PCT may be
detected in certain infectious and inflammatory diseases other than
NS, including pneumonia (30) and
respiratory infection (31). In the
present study, patients with NS and neonates with respiratory
infection or pneumonia as the control group were enrolled. No
significant differences were observed in age, sex and body weight
between the two groups, but the cases with NS had significantly
higher PCT levels than the controls. Regarding the CRP and WBC
count, the difference did not reach statistical significance,
although their values were increased in the NS group compared with
that in the controls, which may be due to the increased
inflammatory responses induced by respiratory infection in the
control neonates. Thus, in addition to the detection of biological
biomarkers, the clinical manifestations, as well as further
verification from bacterial cultures, is essential for the
diagnosis of NS.
miRNAs are considered ideal diagnostic tools for
various human diseases, which is mainly due to their specific
expression patterns and stability in blood samples (32). For instance, downregulated expression
of miR-124 in the serum was previously described as a diagnostic
biomarker for patients with osteosarcoma (33). Increased serum levels of miR-155-5p
and miR-133a-3p were proven to serve as diagnostic and prognostic
biomarkers in patients with sepsis (34). In patients with NS, upregulated
expression of miR-15a/16 was also determined to be a diagnostic
biomarker (18). Given the markedly
decreased expression of miR-181a in the serum samples of patients
with NS, an ROC analysis was performed in the present study,
demonstrating that aberrant expression of miR-181a may serve as a
diagnostic biomarker to screen NS patients from the neonates with
pneumonia/respiratory tract infection, with relatively high
sensitivity and specificity. At present, the available diagnostic
methods for NS are limited by their poor sensitivity or
specificity. The present study may provide novel and efficient
diagnostic biomarkers for patients with NS.
NS is characterized by inflammatory responses. TLR4
is a protein that has a key role in the innate immune system
(35). TLR4 is sensitive to external
signals and is an important mediator for inflammatory events
(36). He et al (17) reported that TLR4 is a target gene of
miR-181a in immune thrombocytopenia. Thus, the present study also
focused on the effect of miR-181a on the expression of TLR4 in
monocytes collected from the blood samples of NS patients. The
results indicated that TLR4 was a target gene of miR-181a and was
downregulated by overexpression of miR-181a. Furthermore, the role
of miR-181a in the regulation of inflammatory cytokines was
explored in monocytes treated with LPS. The LPS-induced increases
in the levels of inflammatory cytokines were all suppressed
following overexpression of miR-181a, indicating the suppressive
role of miR-181a in the regulation of LPS-induced inflammation.
Collectively, it may be indicated that miR-181a may inhibit the
LPS-induced inflammatory response by downregulation of TLR4.
Although the present study provided evidence for the regulatory
effect of miR-181a on the expression of TLR4, further research is
required to confirm this interaction and investigate the effects of
miR-181a on TLR4-associated signaling. Autophagy, which can be
regulated by TLR4, has been reported to be involved in the
progression of sepsis (37).
Interestingly, previous studies have found a regulatory role for
miR-181a on autophagy in the pathogenesis of some diseases, such as
myocardial hypertrophy (38) and
gastric cancer (39). Thus, it was
deduced that the miR-181a/TLR4 axis might also be involved in the
regulation of autophagy in NS development. However, this hypothesis
was not investigated in the present study, which is one of the
limitations of this present study. Additionally, the accuracy of
the clinical research data may be limited by the small sample size
and further investigations with larger research cohorts are
required.
In conclusion, the present study revealed that the
serum expression of miR-181a is downregulated in patients with NS
and the dysregulation of miR-181a serves as a candidate diagnostic
biomarker for NS. Overexpression of miR-181a in monocytes was able
to improve the LPS-induced inflammatory reaction by targeting TLR4,
which may further uncover the pathologic mechanisms of action
underlying the development of NS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL made substantial contributions to the conception
and design of the study, analysis and interpretation of data and
revision of the manuscript. WL and JG were involved in the
acquisition of data and drafting of the manuscript. All authors
gave final approval of the version to be published.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Ethics Committee of Yidu Central Hospital of Weifang (Weifang,
China) and written informed consent was obtained from the families
of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gotts JE and Matthay MA: Sepsis:
Pathophysiology and clinical management. BMJ. 353:i15852016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shane AL, Sanchez PJ and Stoll BJ:
Neonatal sepsis. Lancet. 390:1770–1780. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma D, Farahbakhsh N, Shastri S and
Sharma P: Biomarkers for diagnosis of neonatal sepsis: A literature
review. J Matern Fetal Neonatal Med. 31:1646–1659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Prost N, Razazi K and Brun-Buisson C:
Unrevealing culture-negative severe sepsis. Crit Care. 17:10012013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boskabadi H and Zakerihamidi M: Evaluate
the diagnosis of neonatal sepsis by measuring interleukins: A
systematic review. Pediatr Neonatol. 59:329–338. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Omran A, Maaroof A, Saleh MH and
Abdelwahab A: Salivary C-reactive protein, mean platelet volume and
neutrophil lymphocyte ratio as diagnostic markers for neonatal
sepsis. J Pediatr (Rio J). 94:82–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iroh Tam PY and Bendel CM: Diagnostics for
neonatal sepsis: Current approaches and future directions. Pediatr
Res. 82:574–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi GL, Chen Y, Sun Y, Yin YJ and Song CX:
Significance of serum MicroRNAs in the auxiliary diagnosis of
non-small cell lung cancer. Clin Lab. 63:133–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui S, Liu L, Wan T, Jiang L, Shi Y and
Luo L: MiR-520b inhibits the development of glioma by directly
targeting MBD2. Am J Cancer Res. 7:1528–1539. 2017.PubMed/NCBI
|
|
10
|
Shen DW, Li YL, Hou YJ, Xu ZD, Li YZ and
Chang JY: MicroRNA-543 promotes cell invasion and impedes apoptosis
in pituitary adenoma via activating the Wnt/β-catenin pathway by
negative regulation of Smad7. Biosci Biotechnol Biochem.
83:1034–1044. 2019. View Article : Google Scholar
|
|
11
|
Liu CH, Wang Z, Huang S, Sun Y and Chen J:
MicroRNA-145 regulates pathological retinal angiogenesis by
suppression of TMOD3. Mol Ther Nucleic Acids. 16:335–347. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JK, Wang Z and Li G: MicroRNA-125 in
immunity and cancer. Cancer Lett. 10:134–145. 2019. View Article : Google Scholar
|
|
13
|
Karam RA and Abd Elrahman DM: Differential
expression of miR-155 and Let-7a in the plasma of childhood asthma:
Potential biomarkers for diagnosis and severity. Clin Biochem.
68:30–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu Z, Li H, Wang J and Sun C: miR-146a
and miR-146b in the diagnosis and prognosis of papillary thyroid
carcinoma. Oncol Rep. 38:2735–2740. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin C, Huang RY and Wang ZX: Potential
role of miR-100 in cancer diagnosis, prognosis, and therapy. Tumour
Biol. 36:1403–1409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Jiang S, Cao Y and Yang Y: Altered
miRNAs expression profiles and modulation of immune response genes
and proteins during neonatal sepsis. J Clin Immunol. 34:340–348.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He YZ, Lu RF, Zhu C and Hua JY: Qian five
rhinoceros gindeng (QFRG) protects against development of immune
thrombocytopenia via miR-181a inhibition of TLR-4 expression. Int J
Clin Exp Med. 8:6986–6993. 2015.PubMed/NCBI
|
|
18
|
Wang X, Wang X, Liu X, Wang X, Xu J, Hou
S, Zhang X and Ding Y: miR-15a/16 are upreuglated in the serum of
neonatal sepsis patients and inhibit the LPS-induced inflammatory
pathway. Int J Clin Exp Med. 8:5683–5690. 2015.PubMed/NCBI
|
|
19
|
Subspecialty Group of Neonatology
Pediatric Society Chinese Medical Association: Editorial Board
Chinese Journal of Pediatrics: Protocol for diagnosis and treatment
of neonatal septicemia. Zhonghua Er Ke Za Zhi. 41:897–899. 2003.(In
Chinese). PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu HR, Chen RF, Hong KC, Bong CN, Lee WI,
Kuo HC and Yang KD: IL-12-independent Th1 polarization in human
mononuclear cells infected with varicella-zoster virus. Eur J
Immunol. 35:3664–3672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang G, Lou T, Pan J, Ye Z, Yin Z, Li L,
Cheng W and Cao Z: MiR-204 reduces cisplatin resistance in
non-small cell lung cancer through suppression of the
caveolin-1/AKT/Bad pathway. Aging (Albany NY). 11:2138–2150. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh YS, Bae GD, Park EY and Jun HS:
MicroRNA-181c inhibits interleukin-6-mediated beta cell apoptosis
by targeting TNF-α expression. Molecules. 24:E14102019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang D, Li M, Yu Y, Shi H and Chen R:
MicroRNA-34a aggravates coxsackievirus B3-induced apoptosis of
cardiomyocytes through the SIRT1-p53 pathway. J Med Virol.
91:1643–1651. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Y, Liu Y, Hou H, Yao Y and Meng H:
MiR-150 predicts survival in patients with sepsis and inhibits
LPS-induced inflammatory factors and apoptosis by targeting NF-κB1
in human umbilical vein endothelial cells. Biochem Biophys Res
Commun. 500:828–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y,
Yin N and Jiang L: miR-27a is up regulated and promotes
inflammatory response in sepsis. Cell Immunol. 290:190–195. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhas BB, Dirisala VR and Bhat BV:
Expression levels of candidate circulating microRNAs in early-onset
neonatal sepsis compared with healthy newborns. Genomics Insights.
11:11786310187970792018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cerone JB, Santos RP, Tristram D, Lamson
DM, Stellrecht KA, St George K, Horgan MJ and Rios A: Incidence of
respiratory viral infection in infants with respiratory symptoms
evaluated for late-onset sepsis. J Perinatol. 37:922–926. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Z, Zhang B, Xu Y, Hao Y, Tang J, Yu W
and Gu Q: Analysis of clinical characteristics of bloodstream
infection in patients with immune function inhibition. Zhonghua Wei
Zhong Bing Ji Jiu Yi Xue. 30:1087–1090. 2018.(In Chinese).
PubMed/NCBI
|
|
30
|
Guo S, Mao X and Liang M: The moderate
predictive value of serial serum CRP and PCT levels for the
prognosis of hospitalized community-acquired pneumonia. Respir Res.
19:1932018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim HS, Won S, Lee EK, Chun YH, Yoon JS,
Kim HH and Kim JT: Pentraxin 3 as a clinical marker in children
with lower respiratory tract infection. Pediatr Pulmonol. 51:42–48.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bertoli G, Cava C and Castiglioni I: The
potential of miRNAs for diagnosis, treatment and monitoring of
breast cancer. Scand J Clin Lab Invest Suppl. 245:S34–S39. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cong C, Wang W, Tian J, Gao T, Zheng W and
Zhou C: Identification of serum miR-124 as a biomarker for
diagnosis and prognosis in osteosarcoma. Cancer Biomark.
21:449–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lan C, Shi X, Guo N, Pei H and Zhang H:
Value of serum miR-155-5p and miR-133a-3p expression for the
diagnosis and prognosis evaluation of sepsis. Zhonghua Wei Zhong
Bing Ji Jiu Yi Xue. 28:694–698. 2016.(In Chinese). PubMed/NCBI
|
|
35
|
Wang J, Wang R, Yang J, Yang X, Hu S, Wang
H, Zhou C, Xiong W, Wen Q and Ma L: Glucocorticoids differentially
regulate the innate immune responses of TLR4 and the cytosolic DNA
sensing pathway. Int Immunopharmacol. 47:190–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Alexander CM, Xiong KN, Velmurugan K,
Xiong J, Osgood RS and Bauer AK: Differential innate immune cell
signatures and effects regulated by toll-like receptor 4 during
murine lung tumor promotion. Exp Lung Res. 42:154–173. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carchman EH, Whelan S, Loughran P, Mollen
K, Stratamirovic S, Shiva S, Rosengart MR and Zuckerbraun BS:
Experimental sepsis-induced mitochondrial biogenesis is dependent
on autophagy, TLR4, and TLR9 signaling in liver. FASEB J.
27:4703–4711. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li AL, Lv JB and Gao L: MiR-181a mediates
Ang II-induced myocardial hypertrophy by mediating autophagy. Eur
Rev Med Pharmacol Sci. 21:5462–5470. 2017.PubMed/NCBI
|
|
39
|
Zhao J, Nie Y, Wang H and Lin Y: MiR-181a
suppresses autophagy and sensitizes gastric cancer cells to
cisplatin. Gene. 576:828–833. 2016. View Article : Google Scholar : PubMed/NCBI
|