Introduction
Tibial fracture is a clinically common type of long
tubular bone fracture accounting for 13.7% of whole body fractures
(1). The tibial fracture healing is
a very complex process of injury repair, which can be affected by
many factors. Hyperhomocysteinemia (hHcys) is an important risk
factor for cardiovascular disease and thrombotic disease (2). The clinical manifestations of hHcys
include developmental retardation and skeletal anomalies, and
high-level homocysteine (Hcy) will damage the function of
osteocytes regulating bone remodeling, thereby resulting in
fractures. Hcy, through increasing osteoclast activity, can
regulate bone remodeling (3), induce
apoptosis of bone marrow stromal cells (4), osteocytes and osteoblasts (5,6), and
inhibit osteoblast differentiation (7).
The phosphatidylinositol 3-hydroxy kinase
(PI3K)/protein kinase B (AKT) signal transduction pathway plays an
important role in post-traumatic repair and healing, which is a
regulatory pathway for cell growth. Studies have proved that this
pathway is essential for the differentiation of osteoprogenitor
cells (8,9). Tetrahydroxystilbene glycoside (TSG) can
facilitate the differentiation of MC3T3-E1 cells through activating
the PI3K/AKT signal transduction. The downregulation of PTEN, a
negative feedback regulator of PI3K/AKT in osteoblasts, can
activate the PI3K/AKT signaling pathway, thus affecting osteocyte
differentiation (10).
In this study, therefore, the effect of hHcys on the
tibial fracture healing in rats was observed, and the role of
PI3K/AKT signaling pathway in tibial fracture was explored, hoping
to provide a theoretical basis for the diagnosis and treatment of
tibial fracture.
Materials and methods
Animal experiments and grouping
A total of 36 specific pathogen-free male
Sprague-Dawley rats weighing 200–240 g were adaptively fed in an
environment in line with animal ethical requirements for 1 week
before surgery, and they had free access to food and water. They
were deprived of food, but not water, for 12 h before surgery, and
divided into sham group (n=12), tibial fracture group (n=12) and
hHcys + fracture group (n=12) using a random number table. This
study was approved by the Animal Ethics Committee of Soochow
University Animal Center (Suzhou, China).
Modeling
i) Establishment of tibial fracture model (11): After intraperitoneal anesthesia with
pentobarbital sodium, the left lower limb was depilated, and the
fascia and muscle were separated to expose the tibia. Then the
fracture model was established in the middle tibia, and fixed
intramedullaryly using the Kirschner wire (1 mm in diameter). ii)
Establishment of hHcys model (12):
After establishment of tibial fracture model, the rats in hHcys +
fracture group were fed with L-methionine for 4 weeks. iii) In sham
group, the tibia was exposed only without establishing the tibial
fracture model, and the rats were fed with normal diet.
Main instruments and equipment
AG-1S electronic universal mechanical testing
machine (Shimadzu), PI3K, p-AKT, Bax, caspase-3 and tumor necrosis
factor-α (TNF-α) primary antibodies (Abcam), terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) assay kit (Shanghai Beyotime Biotechnology), quantitative
polymerase chain reaction (qPCR) kit (Vazyme), immunohistochemistry
kit (Maxim), bicinchoninic acid (BCA) protein quantification kit
(Shanghai Beyotime Biotechnology), fluorescence qPCR instrument
(ABI 7500; Applied Biosystems; Thermo Fisher Scientific, Inc.),
Image-Lab image analysis system (Bio-Rad Laboratories), and Leica
DM4000B LED microscope (Leica Microsystems GmbH).
Detection of plasma Hcy
concentration
The venous blood was drawn, placed in the test tube
containing ethylenediaminetetraacetic acid (EDTA) and coagulant,
and centrifuged at 2,000 × g at 4°C for 5 min to separate the
plasma and serum. Then the plasma Hcy concentration was measured
using a full-automatic biochemical analyzer. Plasma (100–150 µl)
was used for each measurement, and each measurement was repeated
for 3 times.
Biomechanical measurement of fracture
stress
After the rats were sacrificed, the tibial specimens
were taken for the biomechanical three-point bending test at a
loading rate of 0.01 mm/sec and span of 15 mm. The mechanical
properties of fractured tibia were analyzed using a clinical
biomechanical three-point bending strain detector, and the
load-displacement relation curve and stress-strain relation curve
were plotted, based on which the ultimate bending strength and
torque were read and calculated.
Determination of protein expressions
of P13K and p-AKT in tibial tissues via western blotting
The tibial tissues were taken, fully lysed with cell
lysis buffer in an ultrasonic homogenizer and centrifuged at 3,500
× g at 4°C for 10 min. After the supernatant was discarded, the
protein samples were obtained, and the total protein concentration
was determined using the BCA protein concentration kit. The protein
was separated via gel electrophoresis, transferred onto a
polyvinylidene fluoride (PVDF) membranes (Roche Diagnostics),
sealed at room temperature and incubated with PI3K and p-AKT
primary antibodies at 37°C for 1 h, with β-actin antibody as an
internal reference. After the membrane was washed, the protein was
incubated again with horse radish peroxidase (HRP)-labeled
secondary antibodies, and the membrane was washed again. Finally,
the image was developed using the electrochemiluminescence (ECL)
kit in a darkroom.
Determination of messenger ribonucleic
acid (mRNA) levels of Bax and caspase-3 via qPCR
The total RNA was extracted from tibial tissues
using the TRIzol method (Invitrogen; Thermo Fisher Scientific,
Inc.), and its concentration was measured using a
spectrophotometer. Then the total RNA was reversely transcribed
into complementary deoxyribose nucleic acid (cDNA) and
quantitatively amplified using the reverse transcription kit and
qPCR kit. The amplification conditions are as follows:
pre-denaturation at 95°C for 1 min, denaturation at 95°C for 5 sec,
and annealing/extension at 58°C for 15 sec, a total of 40 cycles.
Primer sequences of glyceraldheyde 3-phosphate dehydrogenase
(GAPDH) (internal reference gene), forward,
5′-GGTGCTGAGTATGTCGTGGA-3′ and reverse, 5′-TGCTGACAATCTTGAGGGAG-3′.
Primer sequences of caspase-3, forward, 5′-GACCCGGTGCCTCAGGATGC-3′
and reverse, 5′-GTGGCATGAGCTCTTGATAATG-3′. Primer sequences of Bax
forward, 5′-CAGAGGCGGGGGATGATTG-3′ and reverse,
5′-TGTCCAGCCCATGATGGTTC-3′. The relative mRNA expression level was
calculated using the 2−ΔCt formula.
Detection of apoptosis using TUNEL
staining
The tissue sections were deparaffinized with xylene,
dehydrated with gradient alcohol, and subjected to citrate antigen
retrieval, followed by staining using the TUNEL staining kit. Then
the sections were sealed with anti-fluorescence quenching blocking
buffer and observed under a fluorescence microscope, and the number
of positive cells and apoptosis rate were calculated based on
apoptosis rate = number of positive cells/total number of cells
×100%.
Detection of expressions of
inflammatory factors through immunohistochemistry
The tibial tissues were taken in each group, washed
with phosphate-buffered saline (PBS), and decalcified with 10% EDTA
decalcifying solution until softening of tissues, followed by
paraffin embedding into 5 µm-thick coronal sections. Then the
sections were deparaffinized with xylene, dehydrated with gradient
alcohol, and subjected to citrate antigen retrieval, followed by
staining using the immunohistochemistry kit. The sections were
incubated with 3% H2O2 at room temperature
for 10 min, washed, sealed with 5% goat serum at room temperature
for 10 min, and dropwise added with TNF-α primary antibody in a wet
box at 4°C overnight. After rewarming at 37°C, the sections were
incubated again with biotinylated secondary antibody at room
temperature for 10 min, followed by color development using the
diaminobenzidine (DAB) developer and counterstaining with
hematoxylin. Finally, the absorbance was analyzed using the Motic
Med 6.0 pathologic image analysis system.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
24.0 (IBM Corp.) software was used for the data processing. The
t-test was used for analyzing measurement data. Differences between
two groups were analyzed by using the Student's t-test. Comparison
between multiple groups was done using One-way ANOVA test followed
by Post Hoc Test (Least Significant Difference). P<0.05
suggested that the difference was statistically significant.
Results
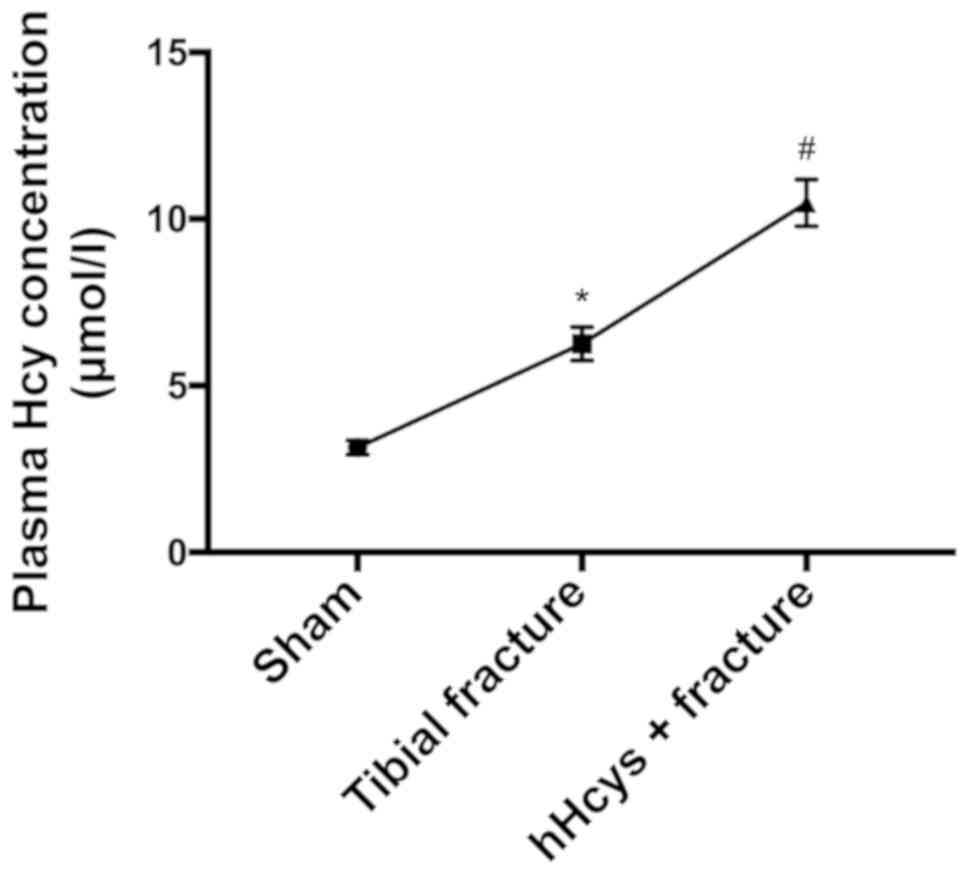
Comparison of plasma Hcy concentration
in each group
The plasma Hcy concentration was significantly
increased in tibial fracture group and hHcys + fracture group
compared with that in sham group (P<0.05), while it was also
significantly increased in hHcys + fracture group compared with
that in tibial fracture group (P<0.05), showing statistically
significant differences (Fig.
1).
Comparison of fracture biomechanics in
each group
The ultimate bending strength and torque were
obviously decreased in tibial fracture group and hHcys + fracture
group compared with those in sham group (P<0.05), while they
declined in hHcys + fracture group compared with those in tibial
fracture group (P<0.05), showing statistically significant
differences (Table I).
 | Table I.Comparison of fracture biomechanics in
each group (mean ± SD). |
Table I.
Comparison of fracture biomechanics in
each group (mean ± SD).
| Group | n | Ultimate bending
strength (N) | Torque (N·mm) |
|---|
| Sham group | 6 | 116.78±12.65 | 357.49±7.65 |
| Tibial fracture
group | 6 |
77.23±6.77a |
216.82±17.71a |
| hHcys + fracture
group | 6 |
45.84±4.32a,b |
124.15±11.84a,b |
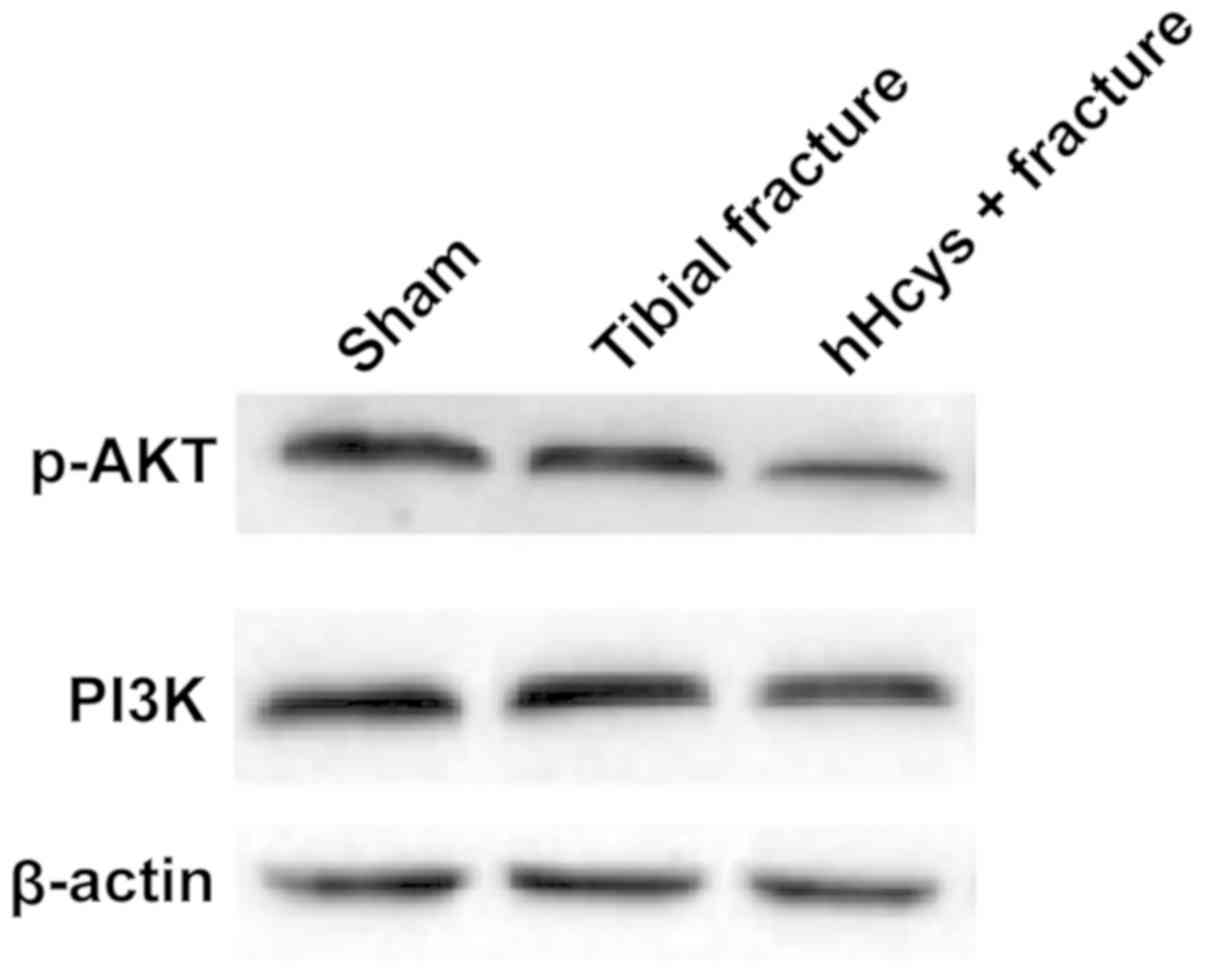
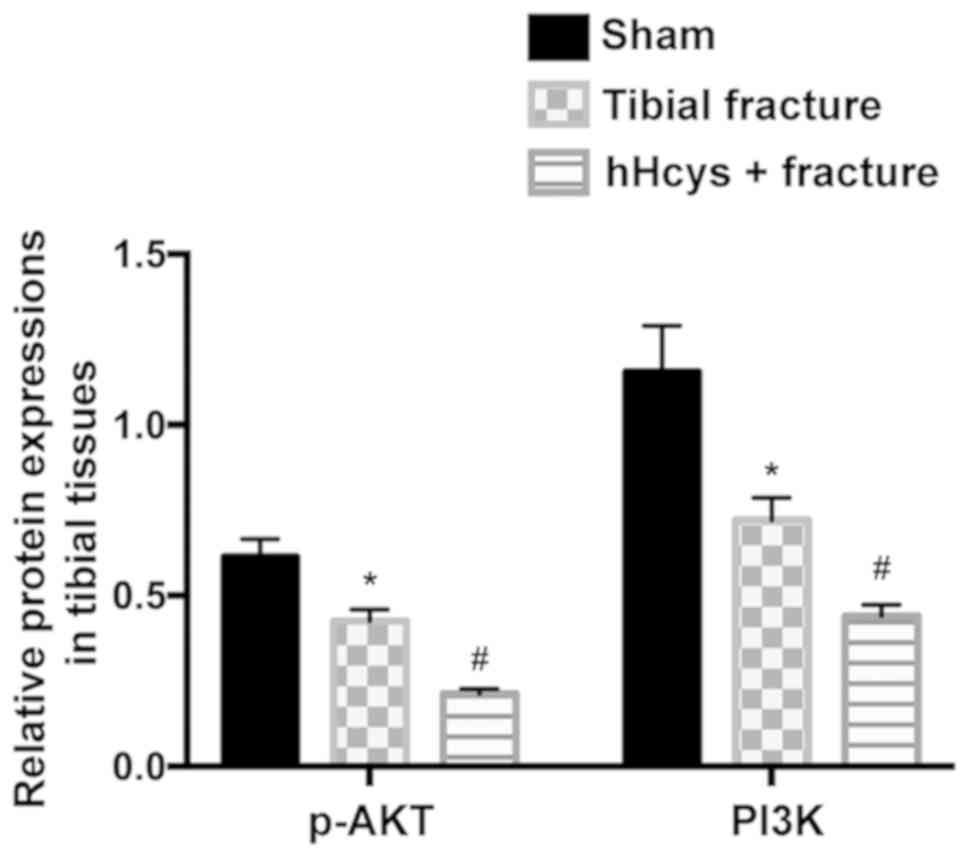
Protein expressions of PI3K and p-AKT
in tibial tissues detected via western blotting
The relative protein expressions of PI3K and p-AKT
evidently declined in tibial fracture group and hHcys + fracture
group compared with those in sham group (P<0.05), while they
also declined in hHcys + fracture group compared with those in
tibial fracture group (P<0.05), displaying statistically
significant differences (Figs. 2 and
3).
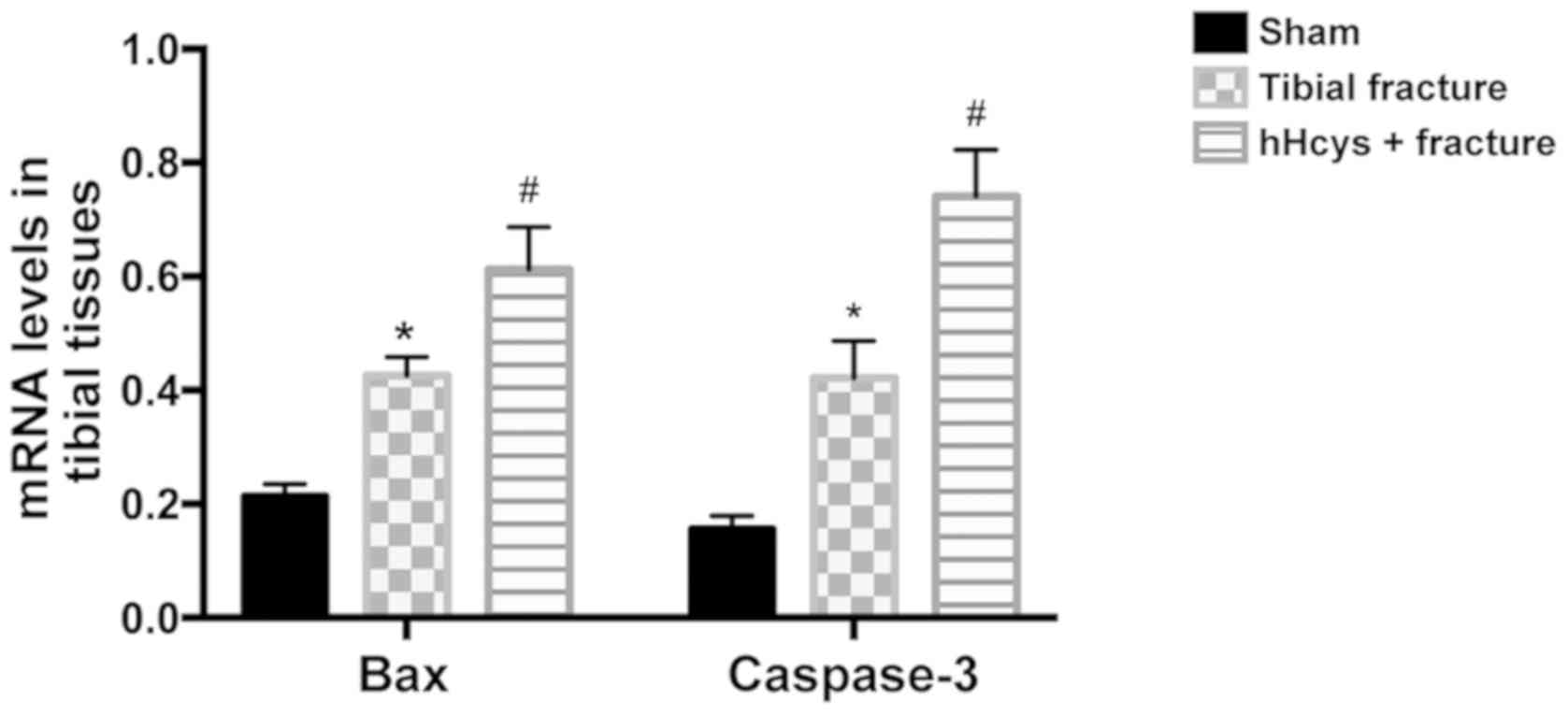
Comparison of mRNA levels of Bax and
caspase-3
Compared with sham group, tibial fracture group and
hHcys + fracture group had increased mRNA levels of Bax and
caspase-3 (P<0.05). The mRNA levels of Bax and caspase-3 were
higher in hHcys + fracture group than those in hHcys + fracture
group (P<0.05), and the differences were statistically
significant (Fig. 4).
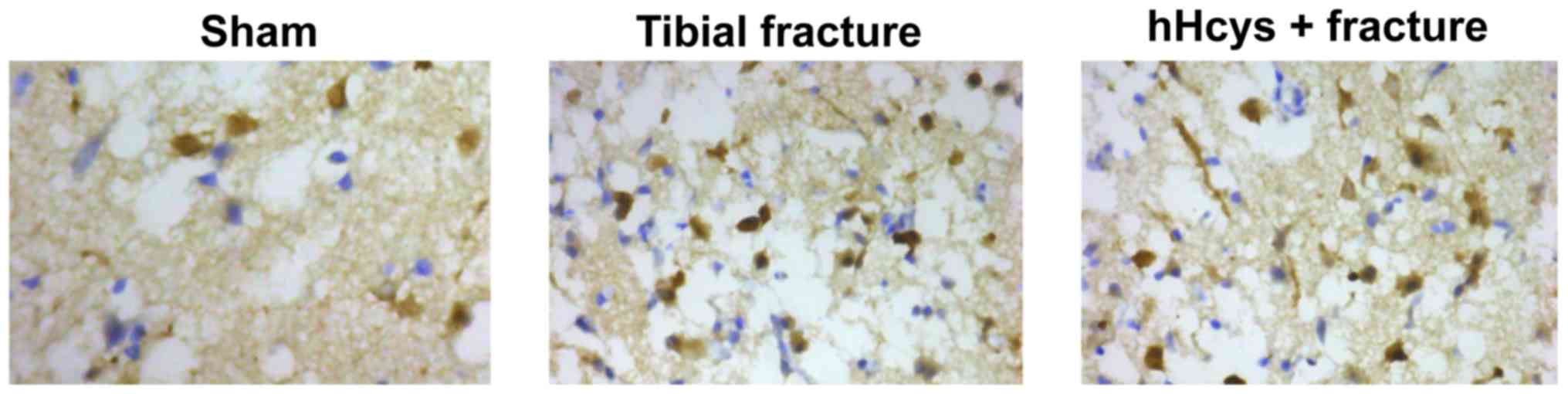
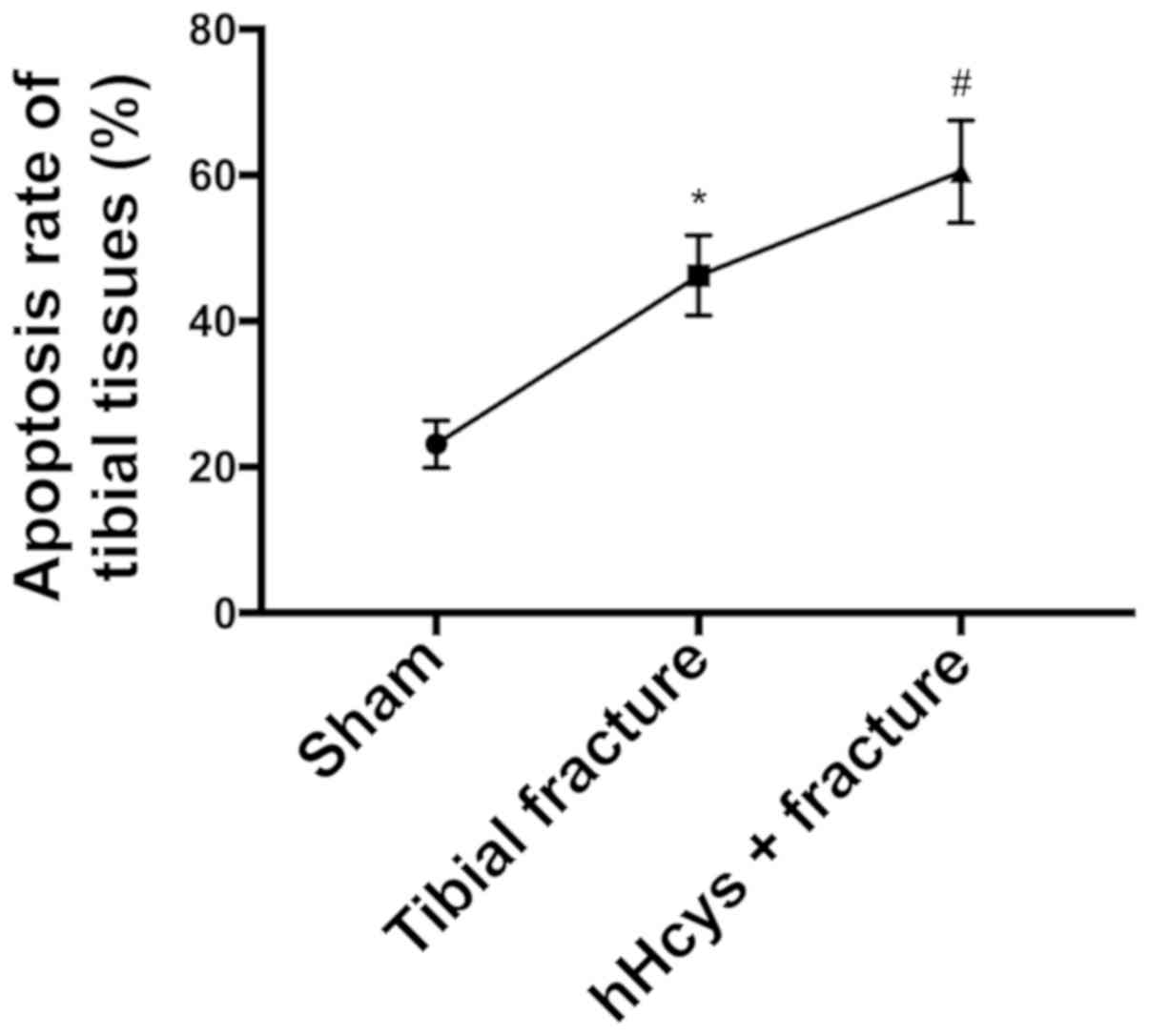
Comparison of apoptosis rate
Tibial fracture group and hHcys + fracture group had
a higher apoptosis rate than sham group (P<0.05), while hHcys +
fracture group also had a higher apoptosis rate than tibial
fracture group (P<0.05), and there were statistically
significant differences (Figs. 5 and
6).
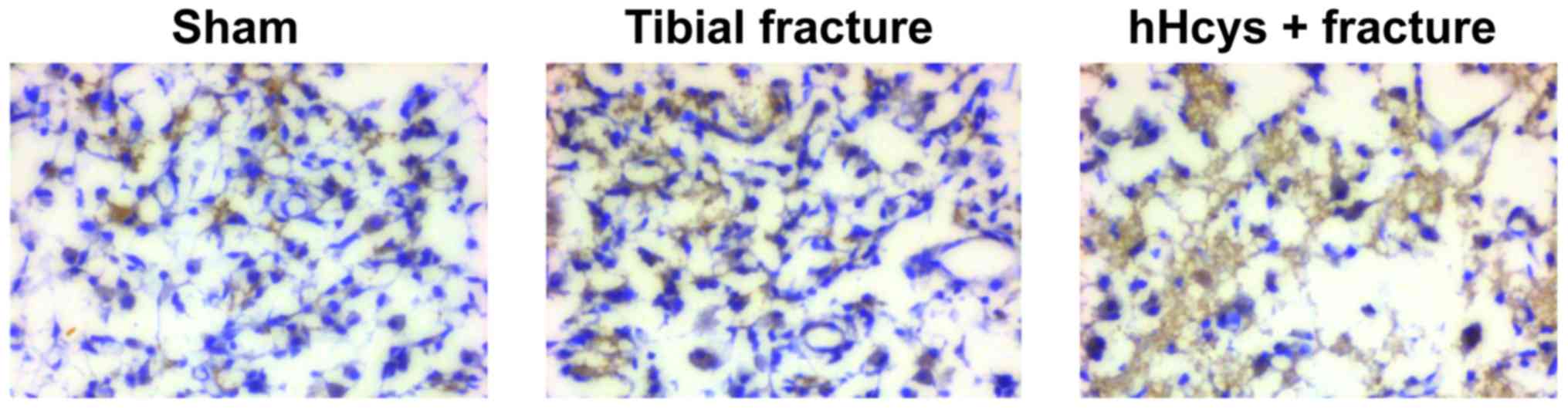
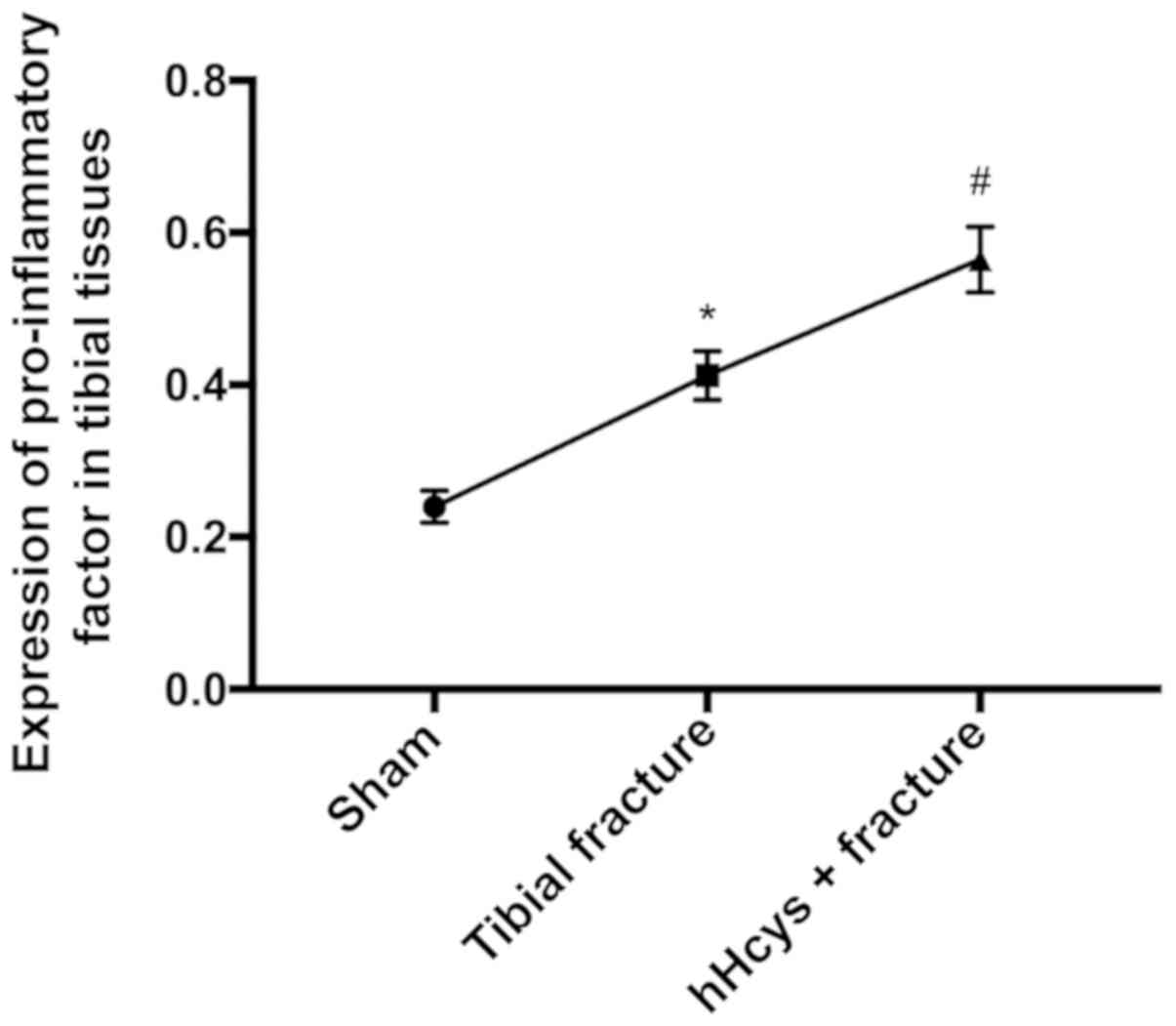
Comparison of expression of
pro-inflammatory factor
The expression of TNF-α was increased in tibial
fracture group and hHcys + fracture group compared with that in
sham group (P<0.05), while it was also increased in hHcys +
fracture group compared with that in tibial fracture group
(P<0.05), and the differences were statistically significant
(Figs. 7 and 8).
Discussion
The bone possesses a strong self-repairing ability,
but ~5–10% of fracture patients suffer from inadequate union,
delayed union or nonunion. In this study, the plasma Hcy level rose
after tibial fracture. Hcy is a kind of non-protein amino acid
synthesized by methionine and recycled into methionine or converted
into cysteine with the help of B vitamins. The increased level of
plasma Hcy is associated with the increase in incidence rate of
fracture, and the high-level plasma Hcy may affect bone health,
which will lead to bone resorption disorders through stimulating
osteoclast activity, and interfere with collagen cross-linking. The
in vitro studies have shown that the elevated concentration of Hcy
has an inhibitory effect on bone formation, which induces apoptosis
through ROS-mediated mitochondrial pathway and NF-κB activation in
human bone marrow mesenchymal stem cells (hBMSCs), and Hcy can
promote the development of osteoporosis by reducing bone formation
(13). Moreover, high-concentration
Hcy inhibits the activity of lysyl oxidase (an enzyme involved in
collagen cross-linking), and the interference with cross-linking
will alter the bone matrix and result in fragile bones. Excessive
Hcy also leads to loss of bone substance. Type I collagen, the main
organic component in bone, is composed of two non-helical
telopeptides and a triple helix region at the amino terminal (N)
and carboxy terminal (C) of the molecule, which stabilizes the
newly secreted collagen molecules through forming cross-linking
between adjacent collagen molecules, thus affecting the tensile
strength and toughness of bone (14,15). In
this study, it was found via biomechanical measurement that both
ultimate bending strength and torque declined in hHcys + fracture
group compared with those in tibial fracture group, indicating that
excessive Hcy affects the bone quality in tibial fracture.
The bone is a kind of dynamic tissue that constantly
renews the osteoclast activity through muscles, and absorbs
mineralized bones and osteoblasts, forming the new bone matrix.
During the whole process, some osteoblasts are embedded in new
bones and differentiate into osteoblasts (16). The PI3K/AKT signaling pathway is an
important pathway in stress fracture repair, and it is activated in
stress fracture callus tissues, which is important for osteoblast
differentiation and function (17,18). The
AKT signal regulates the osteogenesis after injury through
producing osteoprotegerin, and inhibiting the AKT signal
transduction in the rat model of fracture nonunion can reduce the
transplantation and differentiation of MSCs into fracture callus
tissues (19). In addition, studies
have demonstrated that PI3K can regulate periosteal thickening in
the early stage of fracture repair (20). Therefore, PI3K/AKT is an important
signal transduction pathway for bone regeneration. In this study,
the PI3K/AKT signaling pathway was damaged in rats after tibial
fracture, and the expressions of PI3K and p-AKT obviously declined
in hHcys + fracture group compared with those in tibial fracture
group. Besides, the PI3K/AKT signaling pathway mediated apoptosis
and inflammatory response, and affected osteocyte
differentiation.
In conclusion, in the present study, the role of the
PI3K/AKT signaling pathway in fracture repair was explored using
the rat model of hHcys and tibial fracture. The results demonstrate
that hHcys blocks the downstream apoptotic signal transduction,
promotes apoptosis and inflammatory response, and affects fracture
healing through affecting the PI3K/AKT signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL and JG designed the study and performed the
experiments, SL and YH established the animal models, JG and ST
collected the data, WZ and YX analyzed the data, SL and JG prepared
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Soochow University Animal Center (Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu ZC, Xu YL, Jiang Y, Liu Y, Wei ZC, Liu
SG and Yang SJ: Low-expression of lncRNA-ANCR promotes tibial
fracture healing via targeting RUNX2. Eur Rev Med Pharmacol Sci. 23
(Suppl 3):60–66. 2019.PubMed/NCBI
|
|
2
|
Stepanova TV, Ivanov AN, Tereshkina NE,
Popyhova EB and Lagutina DD: Markers of endothelial dysfunction:
Pathogenetic role and diagnostic significance. Klin Lab Diagn.
64:34–41. 2019.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Behera J, George AK, Voor MJ, Tyagi SC and
Tyagi N: Hydrogen sulfide epigenetically mitigates bone loss
through OPG/RANKL regulation during hyperhomocysteinemia in mice.
Bone. 114:90–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai B, Li X, Wang Y, Liu Y, Yang F, Chen
H, Yin K, Tan X, Zhu J, Pan Z, et al: Apoptosis of bone marrow
mesenchymal stem cells caused by homocysteine via activating JNK
signal. PLoS One. 8:e635612013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeno A, Kanazawa I, Tanaka K, Notsu M,
Yokomoto M, Yamaguchi T and Sugimoto T: Activation of AMP-activated
protein kinase protects against homocysteine-induced apoptosis of
osteocytic MLO-Y4 cells by regulating the expressions of NADPH
oxidase 1 (Nox1) and Nox2. Bone. 77:135–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanazawa I, Tomita T, Miyazaki S, Ozawa E,
Yamamoto LA and Sugimoto T: Bazedoxifene ameliorates
homocysteine-induced apoptosis and accumulation of advanced
glycation end products by reducing oxidative stress in MC3T3-E1
cells. Calcif Tissue Int. 100:286–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thaler R, Zwerina J, Rumpler M, Spitzer S,
Gamsjaeger S, Paschalis EP, Klaushofer K and Varga F: Homocysteine
induces serum amyloid A3 in osteoblasts via unlocking RGD-motifs in
collagen. FASEB J. 27:446–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan YS, Li Q, Hamdan N, Bian YF, Zhuang S,
Fan K and Liu ZJ: Tetrahydroxystilbene glucoside regulates
proliferation, differentiation, and OPG/RANKL/M-CSF expression in
MC3T3-E1 cells via the PI3K/Akt pathway. Molecules. 23:23062018.
View Article : Google Scholar
|
|
9
|
Chai C, Song LJ, Han SY, Li XQ and Li M:
MicroRNA-21 promotes glioma cell proliferation and inhibits
senescence and apoptosis by targeting SPRY1 via the PTEN/PI3K/AKT
signaling pathway. CNS Neurosci Ther. 24:369–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jing X, Cheng W, Wang S, Li P and He L:
Resveratrol induces cell cycle arrest in human gastric cancer
MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway.
Oncol Rep. 35:472–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Handool KO, Ibrahim SM, Kaka U, Omar MA,
Abu J, Yusoff MS and Yusof LM: Optimization of a closed rat tibial
fracture model. J Exp Orthop. 5:132018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaouad B, Moudilou EN, Ghoul A, Zerrouk
F, Moulahoum A, Othmani-Mecif K, Cherifi MEH, Exbrayat JM and
Benazzoug Y: Hyperhomocysteinemia and myocardial remodeling in the
sand rat, Psammomys obesus. Acta Histochem. 121:823–832. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim DJ, Koh JM, Lee O, Kim NJ, Lee YS, Kim
YS, Park JY, Lee KU and Kim GS: Homocysteine enhances apoptosis in
human bone marrow stromal cells. Bone. 39:582–590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thaler R, Agsten M, Spitzer S, Paschalis
EP, Karlic H, Klaushofer K and Varga F: Homocysteine suppresses the
expression of the collagen cross-linker lysyl oxidase involving
IL-6, Fli1, and epigenetic DNA methylation. J Biol Chem.
286:5578–5588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito M, Fujii K and Marumo K: Degree of
mineralization-related collagen crosslinking in the femoral neck
cancellous bone in cases of hip fracture and controls. Calcif
Tissue Int. 79:160–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boyce BF, Li J, Xing L and Yao Z: Bone
Remodeling and the Role of TRAF3 in Osteoclastic Bone Resorption.
Front Immunol. 9:22632018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang A, Lu Y, Xing J, Li Z, Yin X, Dou C,
Dong S, Luo F, Xie Z, Hou T, et al: IL-8 enhances therapeutic
effects of BMSCs on bone regeneration via CXCR2-mediated PI3k/Akt
signaling pathway. Cell Physiol Biochem. 48:361–370. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ayala-Peña VB, Scolaro LA and Santillán
GE: ATP and UTP stimulate bone morphogenetic protein-2,-4 and −5
gene expression and mineralization by rat primary osteoblasts
involving PI3K/AKT pathway. Exp Cell Res. 319:2028–2036. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qu Z, Guo S, Fang G, Cui Z and Liu Y: AKT
pathway affects bone regeneration in nonunion treated with
umbilical cord-derived mesenchymal stem cells. Cell Biochem
Biophys. 71:1543–1551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scanlon V, Walia B, Yu J, Hansen M, Drissi
H, Maye P and Sanjay A: Loss of Cbl-PI3K interaction modulates the
periosteal response to fracture by enhancing osteogenic commitment
and differentiation. Bone. 95:124–135. 2017. View Article : Google Scholar : PubMed/NCBI
|