Introduction
Stroke is one of the diseases seriously endangering
human life health. According to the epidemiological survey of the
World Health Organization, the number of deaths due to stroke ranks
2nd among all major diseases, and stroke is characterized by acute
onset, a high mortality rate and many complications. China has
gradually stepped into the stage of population aging, and stroke
has become the first major cause of deaths (1,2). Stroke
has high mortality and disability rates, and edema will occur
rapidly with space occupation after cerebral infarction (CI),
leading to intracranial hypertension and cerebral hernia, and
resulting in death (3). Currently,
imaging is an important technique for diagnosing and evaluating the
severity of CI, and there is a large amount of research evidence
that the volume of cerebral edema after CI is closely related to
the treatment and prognosis of CI patients (4–6).
However, the volume of edema cannot be detected by imaging until
the disease occurs, causing a lag effect, so it is vitally
important to search for new indexes for the diagnosis and
prediction of the therapeutic effect and prognosis of CI
patients.
A large amount of research evidence has confirmed
that micro ribonucleic acids (miRNAs) are involved in regulating
various pathophysiological processes, which are closely related to
cell apoptosis, proliferation and individual development (7,8). Tan
et al (9) analyzed the tissue
samples of stroke patients using bioinformatics, and found that
stroke will cause significant changes in the expression of miRNAs.
Ding et al (10) found that
the expression level of miR-130a significantly increases after
ischemia-reperfusion, and it is closely related to the therapeutic
effect and prognosis of patients. The role of phosphatase and
tensin homolog deleted on chromosome ten (PTEN), as a cancer
suppressor gene with phosphatase activity, in brain injury has
gradually received attention, and strong research evidence exists
to support the close correlation of PTEN with neuronal
proliferation and apoptosis. Furthermore, PTEN can also activate
the downstream phosphatidylinositol 3-hydroxy kinase (PI3K)/protein
kinase B (Akt) signaling pathway, and participate in the stress
induced by cerebral ischemia-reperfusion (11). However, there is scarce literature on
the effects of miR-130a on cerebral ischemia and PTEN/PI3K/Akt
signaling pathway at present. Therefore, in this study, the rat
model of CI was established to evaluate the effect of miR-130a on
neuronal injury in CI rats and whether the PTEN/PI3K/Akt signaling
pathway is involved in the regulation of this process.
Materials and methods
Laboratory animals and grouping
Thirty-six male Sprague-Dawley (SD) rats (250–280 g)
were adaptively fed in a specific pathogen-free environment for 1
week under the temperature of 23±2°C, humidity of 45±5% and regular
circadian rhythm and had free access to food and water. At 12 h
before the experiment, the rats were deprived of food, not
water.
The above rats were randomly divided into blank
control group (n=12), model group (n=12) and miR-130a
low-expression group (n=12). No treatment was performed in blank
control group, and the CI model was established in the model group
and miR-130a low-expression group. miR-130a inhibitor negative
control was injected into the lateral ventricle of rats before
modeling in the model group, while miR-130a inhibitors were
injected into the lateral ventricle of rats before modeling in the
miR-130a low-expression group. This research was approved by the
Animal Ethics Committee of the Animal Center of the Third People's
Hospital of Wuxi (Wuxi, China).
Injection of miR-130a inhibitors into
lateral ventricle
The miR-130a inhibitors and miR-130a inhibitor
negative control were purchased from Shanghai GenePharma Co., Ltd.
The lyophilized powder was diluted with RNase-free H2O
to a concentration of 200 µmol/l and incubated at room temperature
for 5 min. Then it was gently mixed with Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) diluted with
serum-free Dulbecco's modified Eagles medium (DMEM) (Gibco; Thermo
Fisher Scientific, Inc.) and incubated at room temperature for 5
min. Lipofectamine™ 2000 was mixed with miR-130a inhibitors and
miR-130a inhibitor negative control, respectively, incubated at
room temperature for 20 min and stored for later use. After the
rats were anesthetized with 10% chloral hydrate via intraperitoneal
injection at a dose of 300–350 mg/kg, the head skin was cut to
expose the anterior fontanel, and the rats were fixed on a
stereotaxic apparatus. No rat exhibited signs of peritonitis after
the administration of 10% chloral hydrate. With the anterior
fontanel as the original point, the parameters of the stereotaxic
apparatus were adjusted to: 0.8 mm (P), 1.5 mm (R), and 4.5 mm (V).
Then the skull was drilled, from which the miR-130a inhibitors and
miR-130a inhibitor negative control were injected. After 60 min,
the rat model of CI was established.
Establishment of CI model
After anesthesia via 10% chloral hydrate by
intraperitoneal injection at a dose of 300–350 mg/kg, the rats were
fixed on a plate in a supine position, and the neck hair was shaved
off. No rat exhibited signs of peritonitis after the administration
of 10% chloral hydrate. A longitudinal incision was made in the
middle-right line of the neck, and the subcutaneous tissues were
separated using surgical instruments. The distal external carotid
artery was ligated with the thread, and the common carotid artery
and internal carotid artery were clamped with artery clamps. A
small incision was made in the external carotid artery, from which
the suture was inserted. Then the artery clamps in the internal
carotid artery were released, and the suture was reversely inserted
into the internal carotid artery to block the blood supply of the
middle cerebral artery. After that, the rats were fed in separate
cages until resuscitation, and the water drinking status and wounds
were observed. In blank control group, the suture was not inserted,
and the remaining operations were the same as those in the model
group.
Evaluation of behavioral changes in
rats via behavioral experiments
At 3 days after modeling, the behavioral changes of
rats in each group were evaluated using the following three
behavioral experiments: i) Rotation response: 0 points, no
rotation; 2 points, rotation towards the affected side; and 4
points, spontaneous rotation or death. ii) Forepaw grip: The rats
in each group were suspended using the wire rope at 60 cm above the
ground, with the hind limbs away from the ground. The score was
given based on the suspension time: 0 point, >20 sec; 2 points,
<10 sec; 3 points, the rats fell immediately after catching the
rope; and 4 points, the rats could not catch the rope. iii)
Crossbar walking: The rats were put on a crossbar (2 cm wide and 60
cm long), and their passage was recorded. 0 point: The rats went
across the crossbar, 1 point, the rats fell from the crossbar after
30 cm; 2 points, the rats fell from the crossbar before 30 cm; 3
points, the rats lay on the crossbar and did not walk; 4 points,
the rats fell immediately. After behavioral experiments, the rats
in each group were sacrificed immediately, and the whole brain was
taken out. The tissues around the hemorrhagic hemisphere were
isolated and stored at −80°C for later use.
Detection of miR-130a expression level
in brain tissues using quantitative polymerase chain reaction
(qPCR)
At 3 days after modeling, the rats in each group
were sacrificed immediately, and the tissues around the hemorrhagic
hemisphere were isolated and weighed. Then the tissues were added
with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at
a mass/volume ratio of 100 mg/ml, homogenized using a homogenizer
and placed at room temperature for 10 min. Chloroform (200 µl) was
added and the mixture was placed at room temperature for 5 min,
followed by centrifugation at 10,500 × g and 4°C for 10 min. Then
the supernatant was taken into new centrifuge tubes, and the total
RNA was extracted according to instructions of the RNA extraction
kit (Vazyme). The absorbance (A)260/A280 and
optical density (OD) value of total RNA were determined, the
quality and concentration of RNA were evaluated, and RNA was stored
at −30°C for later use. The reverse transcription system was
prepared in strict accordance with the instructions of the reverse
transcription kit (Vazyme), and the PCR conditions were: at 37°C
for 15 min and at 85°C for 5 min. Moreover, the qPCR system was
prepared for amplification, as follows: pre-denaturation at 95°C
for 30 sec; PCR: at 95°C for 5 sec, at 60°C for 30 sec, for a total
of 40 cycles. With β-actin as an internal reference, the primers
were synthesized by Invitrogen; Thermo Fisher Scientific, Inc.
(Table I). The relative expression
level of miR-130a was calculated using 2−ΔΔCt, and
expressed as miR-130a/β-actin.
 | Table I.Primers used in this study. |
Table I.
Primers used in this study.
| Gene | Forward primer | Reverse primer |
|---|
| miR-130a |
5′-CAGTGCAATGTTAAAAGGGCAT-3′ |
5′-CTCGCTTCGGCAGCACA-3′ |
| β-actin |
5′-AACCGTCGGGGACGGAT-3′ |
5′-TGGCGATCAGACGCAGGTC-3′ |
Determination of content of
inflammatory factors
At 3 days after modeling, the rats in each group
were sacrificed immediately, and the tissues around the hemorrhagic
hemisphere were isolated. Then inflammatory factors in brain
tissues were measured using the enzyme-linked immunosorbent assay
(ELISA) kits of tumor necrosis factor-α (TNF-α), interleukin-6
(IL-6) and IL-10 (Wuhan Boster Biological Technology Co., Ltd.) in
strict accordance with the instructions, and recorded in
detail.
Determination of the number of
apoptotic cells using terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) staining
At 3 days after modeling, the rats in each group
were sacrificed immediately, and the tissues around the hemorrhagic
hemisphere were isolated and prepared into paraffin sections. The
neuronal apoptosis level of brain tissues in each group was
detected through TUNEL staining: After deparaffinization, the
paraffin sections were treated with 3% hydrogen peroxide for 10
min, rinsed with phosphate-buffered saline (PBS) 3 times, dropwise
added with proteinase K solution, and incubated in a wet box at
37°C for 10 min. After sections were rinsed with PBS 3 times, they
were dropwise added with mixed solution of TdT and DIG-d-UTP, and
incubated in the wet box at 4°C for 2 h. After sections were rinsed
with PBS 3 times, they were sealed with 40 µl of blocking buffer at
room temperature for 30 min, dropwise added with antibodies (1:100)
and incubated in a wet box at 37°C for 30 min. After sections were
rinsed with PBS 3 times, they were dropwise added with the
SABC-FITC secondary antibody (1:100) for incubation in the wet box
at 37°C for 30 min, and rinsed again with PBS 3 times. Finally, the
sections were added dropwise with anti-fluorescence quenching
blocking buffer, and observed and photographed under a confocal
fluorescence microscope. The number of TUNEL-positive cells in the
hemorrhagic hemisphere was recorded to evaluate the neuronal
apoptosis level (the TUNEL-positive cells displayed yellow green
fluorescence).
Detection of related protein
expression through western blotting
At 3 days after modeling, the rats in each group
were sacrificed immediately, and the tissues around the hemorrhagic
hemisphere were isolated, weighed and added with
radioimmunoprecipitation assay (RIPA) lysis buffer (Beijing TDY
Biotech Co., Ltd.) at a mass/volume ratio of 100 mg/ml, as well as
1% phosphatase inhibitor and 1% protease inhibitor, followed by
homogenization using the homogenizer, standing at room temperature
for 10 min and centrifugation at 10,000 × g and 4°C for 10 min.
Then the supernatant was taken into new centrifuge tubes as the
total protein, and the total protein was quantified using the
bicinchoninic acid (BCA) protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.).
The total protein in each group was diluted with
loading buffer until the same concentration, and boiled for 10 min
for inactivation. After the sodium dodecyl sulphate-polyacrylamide
gel electrophoresis (SDS-PAGE) gel was prepared, an equal volume of
protein samples was added for electrophoresis under 80 V until the
blue band reached the end of separation gel. After that, the
protein was transferred onto a polyvinylidene fluoride (PVDF)
membranes (Millipore) using the wet method under the constant
pressure of 100 V for 90 min. Then the protein band was sealed with
freshly-prepared 5% skim milk powder for 1 h, and the target band
was cut and incubated with the primary antibodies of caspase-3
(1:1,000), B-cell lymphoma-2 (Bcl-2) associated X protein (Bax)
(1:1,000), Bcl-2 (1:1,000), phosphorylated (p)-PTEN (1:1,000), PTEN
(1:1,000), PI3K (1:1,000), p-Akt (1:1,000), Akt (1:1,000) and GAPDH
(internal reference antibody, 1:1,000) (all from Cell Signaling
Technology, Inc.) at 4°C overnight. The band was washed with
Tris-buffered saline with Tween-20 (TBST) 3 times (5 min/time),
incubated again with the horseradish peroxidase-labeled goat
anti-rabbit secondary antibody (1:5,000; Shanghai Yihyson
Biological Co., Ltd.) at room temperature for 1 h and washed again
with TBST 3 times (5 min/time). Then an appropriate amount of
electrochemiluminescence (ECL) solution was added to each band,
followed by color development using the developing instrument. The
results were processed using ImageJ, and the expression level of
each protein was calculated.
Statistical analysis
The data in this study were expressed as mean ±
standard deviation. Statistical Product and Service Solutions
(SPSS) 22.0 software (IBM, Corp.) was used for the data processing.
The t-test was used for analyzing measurement data. Differences
between two groups were analyzed by using the Student's t-test.
Comparison between multiple groups was done using One-way ANOVA
test followed by Post Hoc Test (Least Significant Difference).
P<0.05 indicates that the difference was statistically
significant.
Results
Neurological function of rats
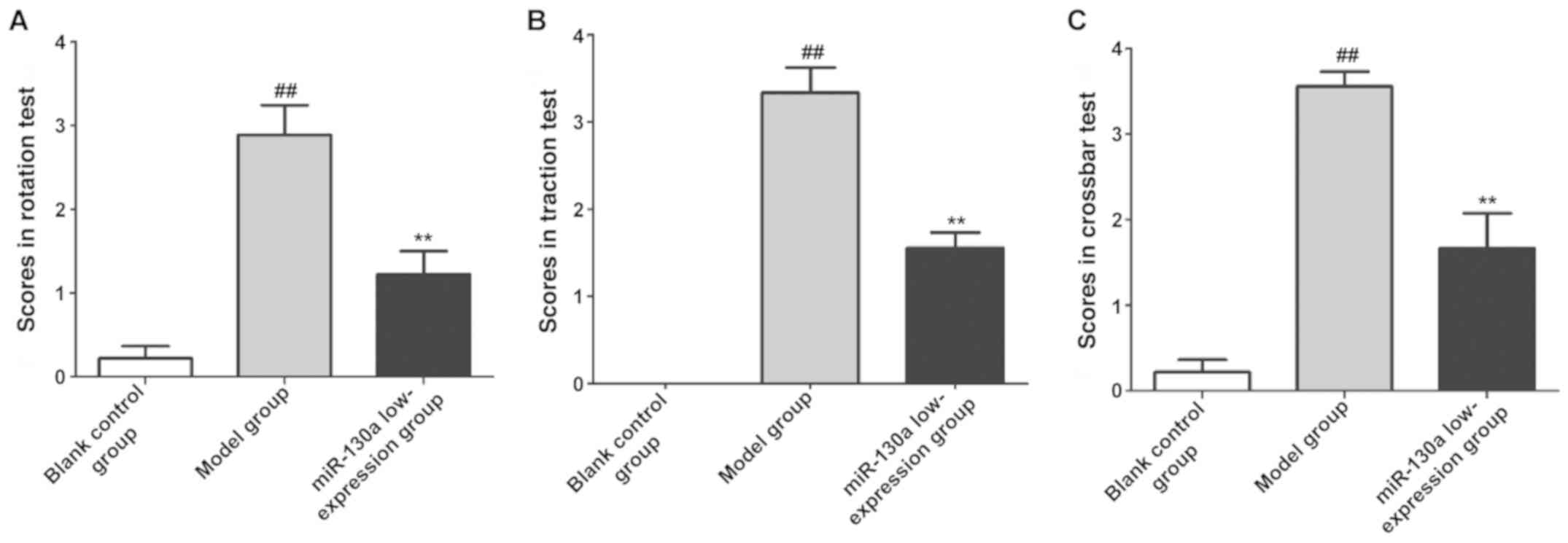
The neurological function of rats in each group was
evaluated using behavioral experiments. As shown in Fig. 1, the scores in rotation test,
traction test and crossbar test were significantly higher in the
model group than those in the blank control group (P<0.01,
P<0.01, P<0.01), while they significantly declined after
intervention with miR-130a inhibitors (P<0.01, P<0.01,
P<0.01, respectively).
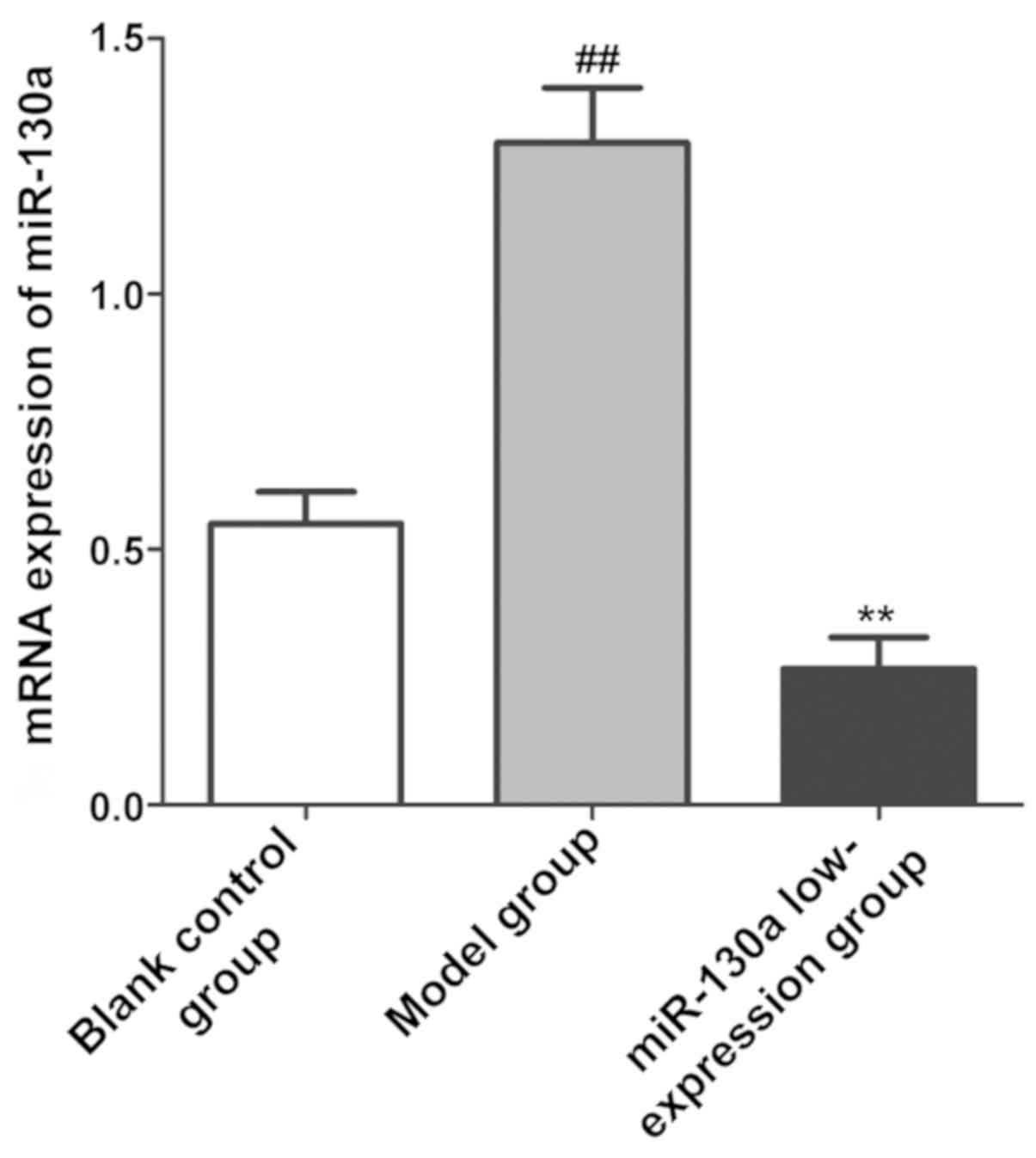
Expression of miR-130a in brain
tissues
The relative expression level of miR-130a in CI
tissues in each group was detected via qPCR. As shown in Fig. 2, the expression level of miR-130a in
CI tissues was obviously increased in model group (P<0.01),
while it obviously declined in miR-130a low-expression group after
intervention with miR-130a inhibitors (P<0.01).
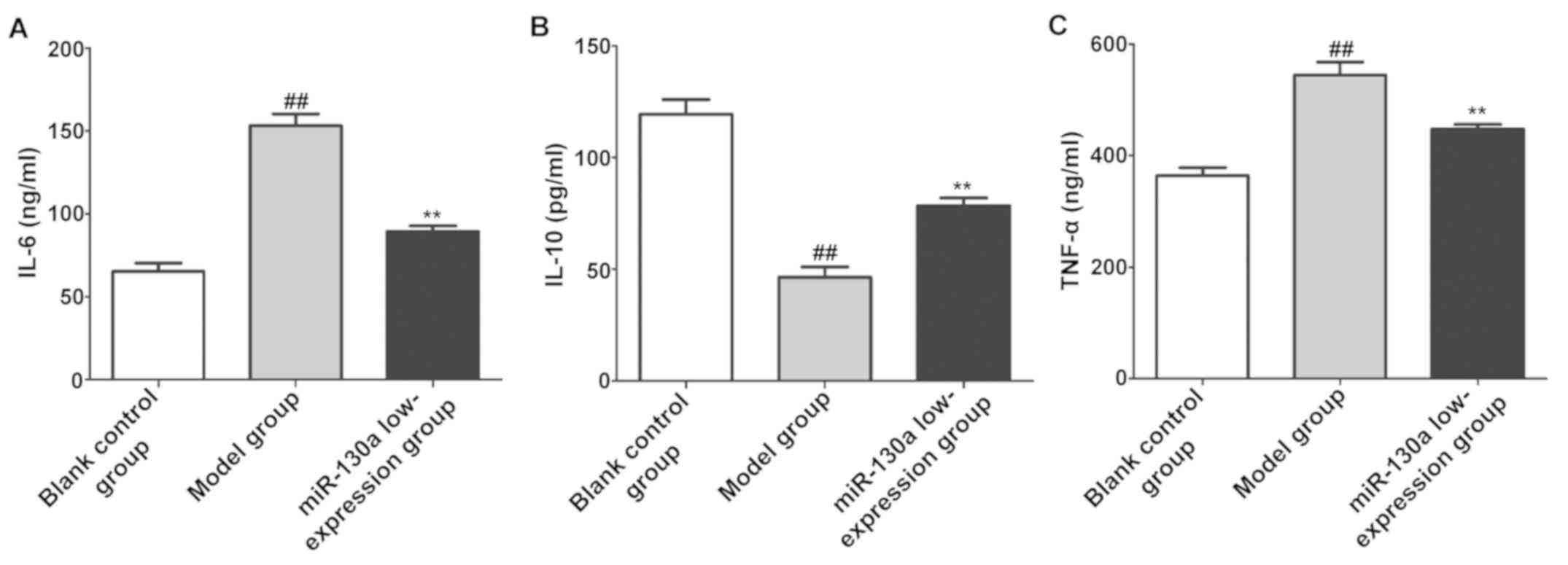
Content of inflammatory factors in
brain tissues
The content of inflammatory factors in CI tissues in
each group was determined using the ELISA kits. The results showed
that compared with blank control group, model group had evidently
increased content of IL-6 and TNF-α in brain tissues (P<0.01)
and evidently decreased content of IL-10 (P<0.01). After
intervention with miR-130a inhibitors, the content of IL-6 and
TNF-α in brain tissues evidently declined (P<0.01) and that of
IL-10 was evidently increased (P<0.01) in miR-130a
low-expression group (Fig. 3).
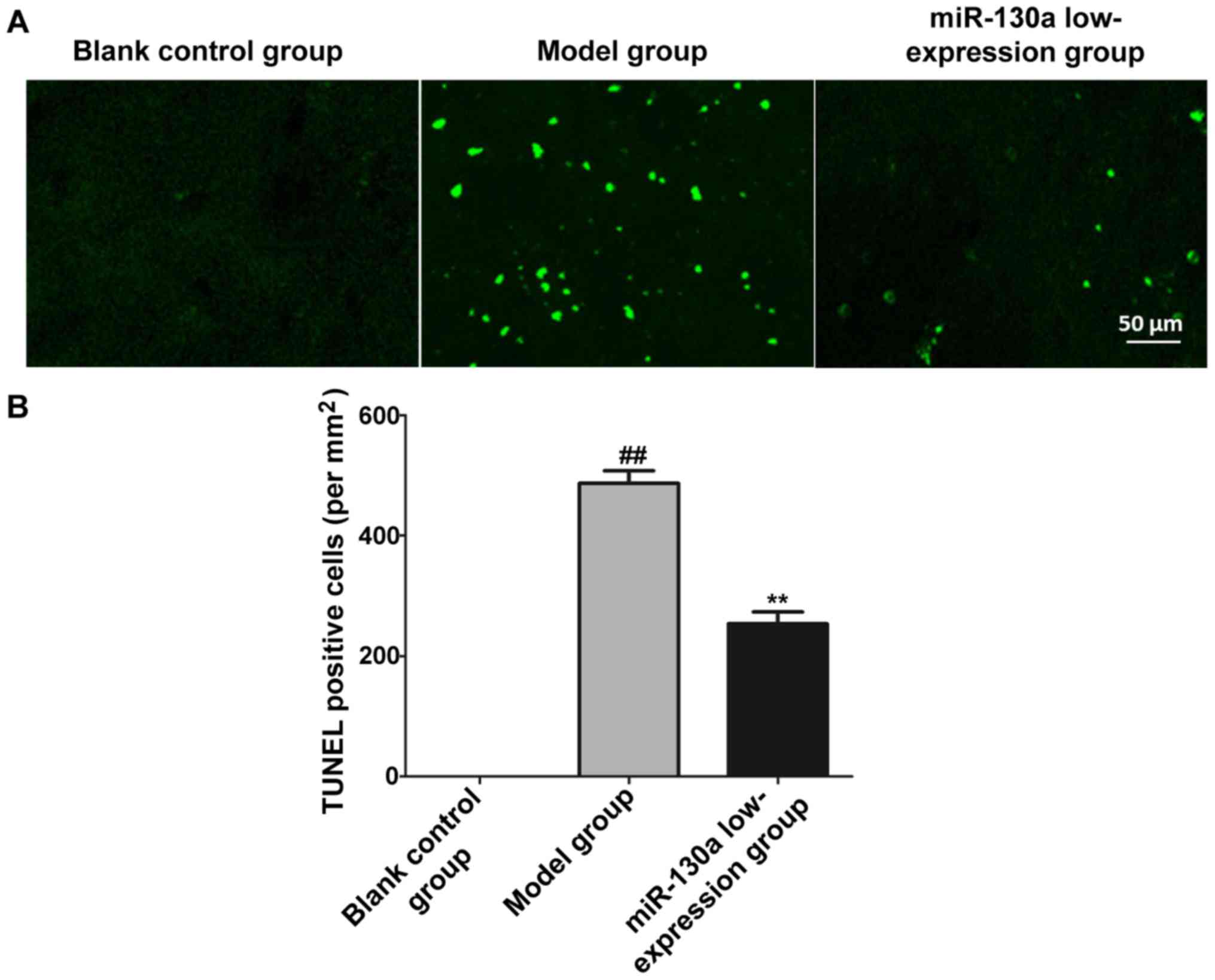
Neuronal injury in brain tissues
The neuronal apoptosis level of CI tissues in each
group was detected through TUNEL staining, and the number of
TUNEL-positive cells was counted to indicate the number of
apoptotic neurons. It was found that the number of TUNEL-positive
cells was obviously larger in model group than that in blank
control group (P<0.01), while it was obviously smaller in
miR-130a low-expression group than that in model group (P<0.01)
(Fig. 4).
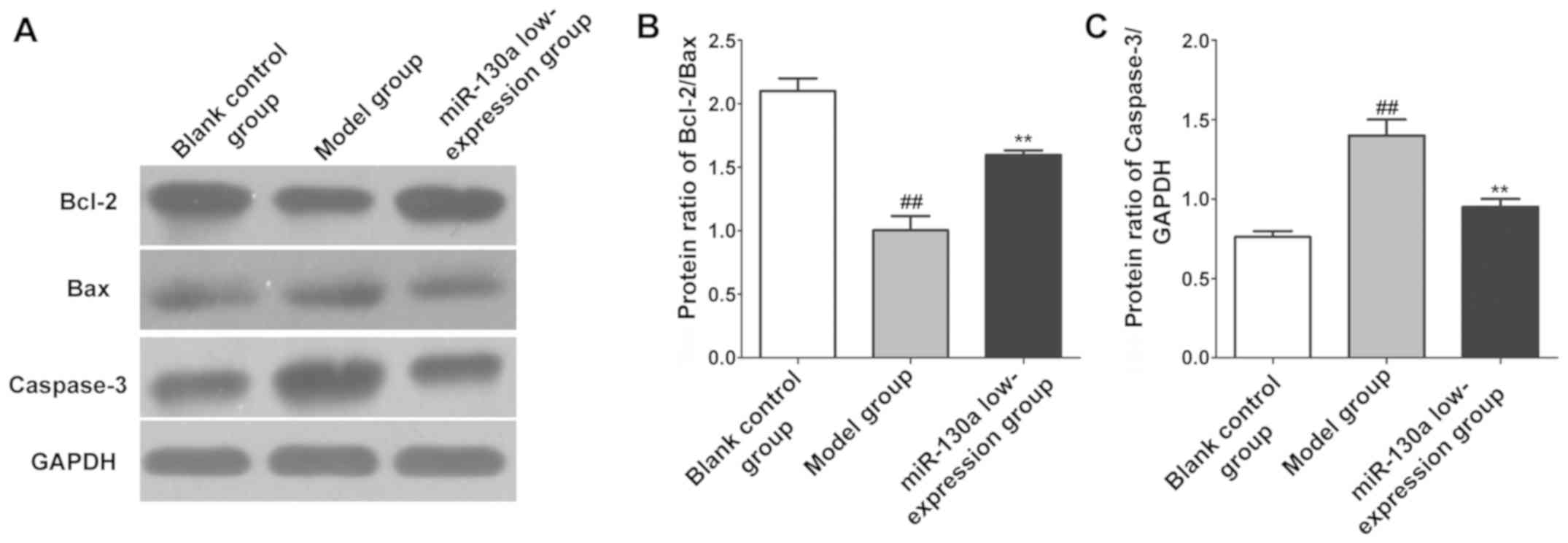
Expression levels of apoptosis
proteins in brain tissues
The expression levels of apoptosis-related proteins
in CI tissues in each group were detected using western blotting.
The results revealed that compared with blank control group, model
group had a remarkably lower expression of Bcl-2/Bax (P<0.01)
and a remarkably higher expression of caspase-3 in brain tissues
(P<0.01). Compared with model group, miR-130a low-expression
group had a remarkably higher expression of Bcl-2/Bax (P<0.01)
and a remarkably lower expression of caspase-3 in brain tissues
(P<0.01) (Fig. 5).
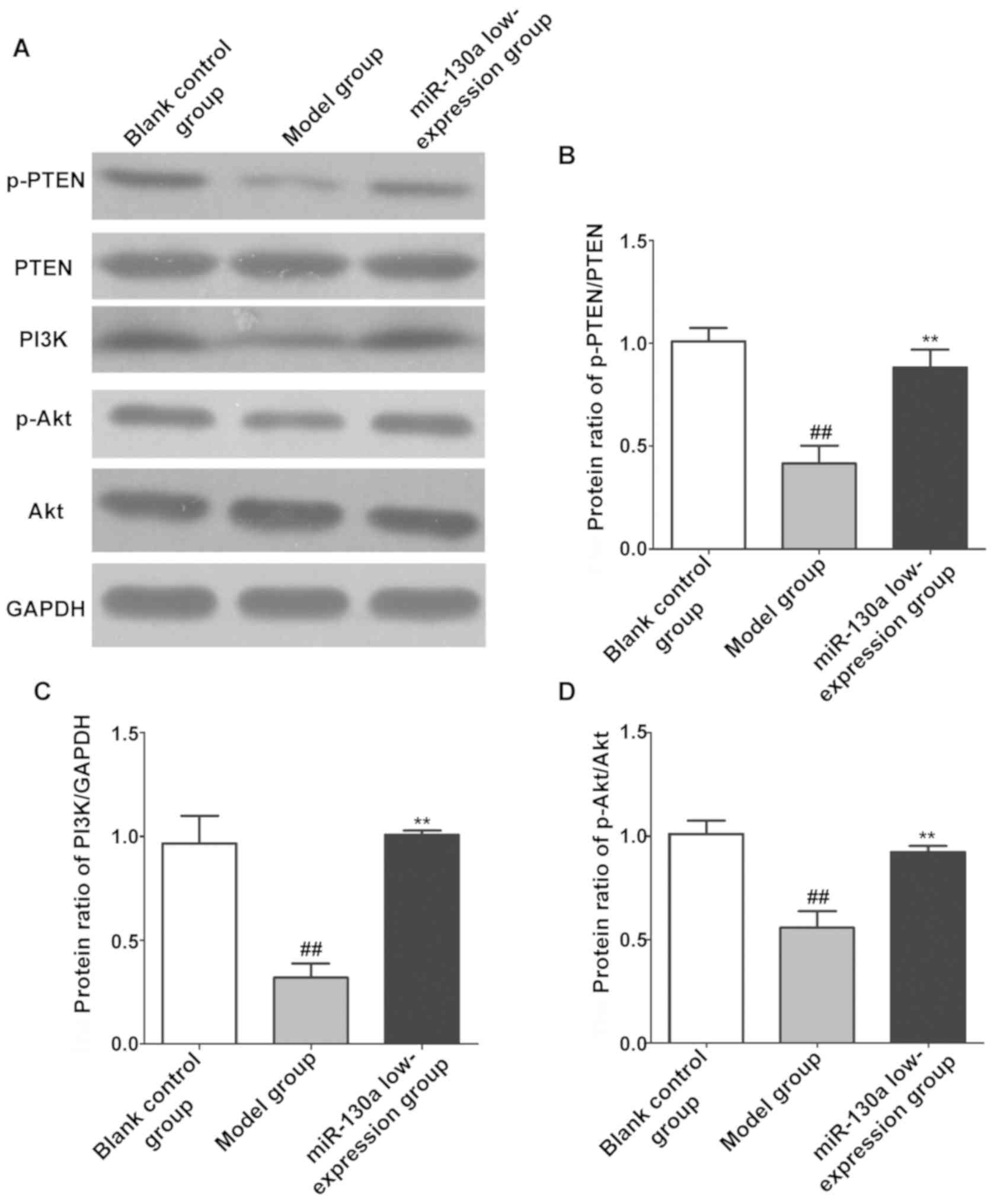
Expression levels of PTEN/PI3K/Akt
pathway-related proteins in brain tissues
The expression levels of PTEN/PI3K/Akt signaling
pathway-related proteins in CI tissues in each group were also
detected using western blotting. As shown in Fig. 6, the expression of p-PTEN, PI3K and
p-Akt in brain tissues was remarkably lower in model group than
those in blank control group (P<0.01), while inhibiting the
expression of miR-130a remarkably increased the expression of
p-PTEN, PI3K and p-Akt in brain tissues in the model group
(P<0.01).
Discussion
Hemorrhagic stroke is a serious disease leading to
death, and a large amount of research evidence demonstrates that
the volume of hematoma in CI and the complications of patients are
important factors affecting prognosis, which can be used to
evaluate the prognosis of CI patients (12). MiRNAs are a group of non-coding RNAs
that play regulatory roles in vivo, and they widely exist in
many eukaryotic organisms, which are closely related to the
individual development, energy metabolism, cell proliferation and
apoptosis (13). For example,
Selvamani et al (14) found
that such miRNAs as miR-21 and miR-181b are involved in the
formation of cerebral edema, and they are important factors
affecting the prognosis of stroke patients. miR-130a is an
anti-angiogenic transcription factor. The study of Jiang et
al (15) showed that the
expression of miR-130a is positively proportional to the cerebral
water content in rats with cerebral edema, suggesting that miR-130a
may be associated with the formation of cerebral edema (16). Furthermore, Altintas et al
(17) found that miR-130a also plays
an important regulatory role in diabetic rats with transient CI. In
the present study, the rat model of CI was established. It was
found that the expression level of miR-130a in CI tissues in model
group was significantly increased, and the results of behavioral
experiments revealed that there was severe neurological injury in
model group. After intervention in the expression of miR-130a in
brain tissues, the neurological function was effectively restored.
The above findings indicate that the high expression of miR-130a
may be closely related to brain injury in CI rats.
PTEN is a cancer suppressor gene with phosphatase
activity, and it has been proven to be involved in cell invasion,
migration and apoptosis (18). PI3K
is the most important substrate for PTEN and an important messenger
for cell proliferation, and PTEN can specifically act on PI3K to
regulate the phosphorylation of Akt. p-Akt can directly or
indirectly regulate the activity of apoptosis proteins, thereby
disturbing cell proliferation and arresting cell cycle, and
ultimately leading to apoptosis (19). After phosphorylation of PTEN,
structural instability will be caused and phosphatase activity will
be lost (20). In this study, it was
found that after CI for 3 days, the expression level of p-PTEN in
brain tissues in each group was obviously decreased, and the
expression levels of PI3K and p-Akt in brain tissues of CI rats
also obviously declined, suggesting that CI will reduce the
phosphorylation level of PTEN in brain tissues, thus inhibiting
PI3K/Akt and resulting in neuronal injury in rats. Moreover, in
this study, the neuronal apoptosis level in brain tissues of CI
rats was obviously increased, and the levels of pro-inflammatory
factors IL-6 and TNF-α significantly increased, while the level of
anti-inflammatory factor IL-10 significantly declined. In addition,
obvious changes were observed in the expression of
apoptosis-related proteins Bcl-2/Bax and caspase-3. The above
results highly demonstrate that the mechanism of CI may be that the
phosphorylation of PTEN is suppressed, thereby inhibiting the
activity of the PI3K/Akt signaling pathway, and causing neuronal
injury and neuronal apoptosis. Besides, inflammatory factors are
the basic substances causing inflammation, and their content
evidently increases in brain tissues after CI, which can lead to
the activation and infiltration of inflammatory cells in brain
tissues, thereby producing a direct toxic effect on neurons in
brain tissues and inducing cerebral edema (21).
In conclusion, CI can significantly raise the
expression level of miR-130a in brain tissues in rats, thereby
promoting the release of inflammatory factors and enhancing the
neuronal apoptosis through inhibiting the PTEN/PI3K/Akt signaling
pathway, ultimately causing typical symptoms of neurological injury
in rats. The expression level of miR-130a may serve as an important
index for evaluating the brain injury and prognosis of CI
patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW, JG and SG designed the study and performed the
experiments; LH, CL and LK established the animal models; JG and LK
collected the data; TW and MD analyzed the data; YW, JG and CL
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This research was approved by the Animal Ethics
Committee of the Animal Center of the Third People's Hospital of
Wuxi (Wuxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Lackland DT, Roccella EJ, Deutsch AF,
Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman
JH, Lisabeth LD, et al: American Heart Association Stroke Council;
Council on Cardiovascular and Stroke Nursing: Council on Quality of
Care and Outcomes Research; Council on Functional Genomics and
Translational Biology: Factors influencing the decline in stroke
mortality: A statement from the American Heart Association/American
Stroke Association. Stroke. 45:315–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhaskar S, Stanwell P, Cordato D, Attia J
and Levi C: Reperfusion therapy in acute ischemic stroke: Dawn of a
new era? BMC Neurol. 18:82018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu D, Tang ZY, Hu ZJ, Li WW and Yuan WN:
MiR-940 regulates angiogenesis after cerebral infarction through
VEGF. Eur Rev Med Pharmacol Sci. 22:7899–7907. 2018.PubMed/NCBI
|
|
4
|
Lin MP, Sanossian N and Liebeskind DS:
Imaging of prehospital stroke therapeutics. Expert Rev Cardiovasc
Ther. 13:1001–1015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCulloch L, Smith CJ and McColl BW:
Adrenergic-mediated loss of splenic marginal zone B cells
contributes to infection susceptibility after stroke. Nat Commun.
8:150512017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gulli G, Rutten-Jacobs LC, Kalra L, Rudd
AG, Wolfe CD and Markus HS: Differences in the distribution of
stroke subtypes in a UK black stroke population - final results
from the South London Ethnicity and Stroke Study. BMC Med.
14:772016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weiss CN and Ito K: A Macro View of
MicroRNAs: The discovery of microRNAs and their role in
hematopoiesis and hematologic disease. Int Rev Cell Mol Biol.
334:99–175. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soares RJ, Cagnin S, Chemello F,
Silvestrin M, Musaro A, De Pitta C, Lanfranchi G and Sandri M:
Involvement of microRNAs in the regulation of muscle wasting during
catabolic conditions. J Biol Chem. 289:21909–21925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan JR, Tan KS, Yong FL, Armugam A, Wang
CW, Jeyaseelan K and Wong PT: MicroRNAs regulating cluster of
differentiation 46 (CD46) in cardioembolic and non-cardioembolic
stroke. PLoS One. 12:e01721312017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding C, Chen SN, Macleod RAF, Drexler HG,
Nagel S, Wu DP, Sun AN and Dai HP: MiR-130a is aberrantly
overexpressed in adult acute myeloid leukemia with t(8;21) and its
suppression induces AML cell death. Ups J Med Sci. 123:19–27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu G, Zhang W, Liu Y and Wang S: miR 371b
5p inhibits endothelial cell apoptosis in monocrotaline induced
pulmonary arterial hypertension via PTEN/PI3K/Akt signaling
pathways. Mol Med Rep. 18:5489–5501. 2018.PubMed/NCBI
|
|
12
|
Rothwell PM, Algra A, Chen Z, Diener HC,
Norrving B and Mehta Z: Effects of aspirin on risk and severity of
early recurrent stroke after transient ischaemic attack and
ischaemic stroke: Time-course analysis of randomised trials.
Lancet. 388:365–375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lamontagne J, Steel LF and Bouchard MJ:
Hepatitis B virus and microRNAs: Complex interactions affecting
hepatitis B virus replication and hepatitis B virus-associated
diseases. World J Gastroenterol. 21:7375–7399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Selvamani A, Williams MH, Miranda RC and
Sohrabji F: Circulating miRNA profiles provide a biomarker for
severity of stroke outcomes associated with age and sex in a rat
model. Clin Sci (Lond). 127:77–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang H, Yu WW, Wang LL and Peng Y:
miR-130a acts as a potential diagnostic biomarker and promotes
gastric cancer migration, invasion and proliferation by targeting
RUNX3. Oncol Rep. 34:1153–1161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Chen M, Qiu Z, Hu K, McGee W,
Chen X, Liu J, Zhu L and Wu JY: MiR-130a regulates neurite
outgrowth and dendritic spine density by targeting MeCP2. Protein
Cell. 7:489–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altintas O, Ozgen Altintas M, Kumas M and
Asil T: Neuroprotective effect of ischemic preconditioning via
modulating the expression of cerebral miRNAs against transient
cerebral ischemia in diabetic rats. Neurol Res. 38:1003–1011. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Qiu S, Qian L, Tian Y, Chen Y, Bi
L and Chen W: OCF can repress tumor metastasis by inhibiting
epithelial-mesenchymal transition involved in PTEN/PI3K/AKT pathway
in lung cancer cells. PLoS One. 12:e01740212017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han M, Chen L and Wang Y: miR-218
overexpression suppresses tumorigenesis of papillary thyroid cancer
via inactivation of PTEN/PI3K/AKT pathway by targeting Runx2.
OncoTargets Ther. 11:6305–6316. 2018. View Article : Google Scholar
|
|
20
|
Chang M, Wu M and Li H: Curcumin combined
with glycyrrhetinic acid inhibits the development of hepatocellular
carcinoma cells by down-regulating the PTEN/PI3K/AKT signalling
pathway. Am J Transl Res. 9:5567–5575. 2017.PubMed/NCBI
|
|
21
|
Acosta SA, Tajiri N, Hoover J, Kaneko Y
and Borlongan CV: Intravenous bone marrow stem cell grafts
preferentially migrate to spleen and abrogate chronic inflammation
in stroke. Stroke. 46:2616–2627. 2015. View Article : Google Scholar : PubMed/NCBI
|