Introduction
Small ubiquitin-like modifier (SUMO) modification
(SUMOylation) is a highly dynamic and reversible post-translational
modification of proteins that has been implicated in various
biological functions, including gene transcriptional regulation,
protein expression and interaction, cell proliferation and
apoptosis (1,2). The SUMO family consists of five
isoforms and in vertebrates, there are three genes encoding
distinct SUMO proteins, SUMO1, SUMO2 and SUMO3 (3). The SUMO2 and SUMO3 proteins share a
high degree of homology and are usually considered together as
SUMO2/3 (2). SUMOylation involves a
series of different proteins and enzymes (4), including SUMO activating enzyme
subunits 1 and 2, SUMO-conjugating enzyme UBC9 (UBC9) and SUMO
protein ligases, including the E3 protein ligase PIAS family
ligases, E3 SUMO-protein ligase RANBP2 and human polycomb group
proteins (5,6). The process of dissociating SUMO
subunits from target proteins is referred to as de-SUMOlyation, and
is carried out by sentrin specific proteases (SENPs). Six SENP
family proteins have been identified in mammals (SENP1, SENP2,
SENP3, SENP5, SENP6 and SENP7) and each has a different subcellular
localization and substrate specificity and participates in
different physiological and pathological processes (7,8). All of
these SUMO-associated proteins play a key role in reversible SUMO
regulation in cells.
The human menstrual cycle lasts approximately 28
days and is under the strict control of estrogen and progesterone,
the release of which is regulated by the
hypothalamic-pituitary-ovarian axis (9). During the menstrual cycle, the uterus
endometrium undergoes a series of morphological and functional
changes, cyclically preparing for implantation of a fertilized egg
(10). Uterine expression of SUMO
isoforms and their targets has been described in several
endometrial diseases (11) and has
been linked with hormone-related endometrial cancers. SUMOylation
may play a role in cancer cell proliferation, DNA damage responses,
metastasis and apoptosis (1,12). Research has shown that reducing SUMO1
gene expression may inhibit the proliferation and induce apoptosis
of Ishikawa cells (13).
Estrogen receptor (ER)-β has been identified as a
SUMO1 target (14). ER-α has also
been reported to undergo SUMO modification, which led to
transcriptional activation of the receptor (15). A number of uterine-related diseases,
such as endometrial adenocarcinoma (16), endometriosis (17) and cervical high-grade squamous
intraepithelial lesion (17), are
closely related to the expression of estrogen progesterone and
their receptors and it is hypothesized that SUMO regulation is
involved in the development and progression of these diseases.
However, sex hormone levels are not stable throughout the
physiological estrous cycle, so the expression and localization of
SUMO-associated proteins in the uterus at various stages of the
physiological estrous cycle is of interest.
Rodent estrous cycles are similar to human menstrual
cycles (18). The mouse estrous
cycle typically lasts 4–5 days (19). Vaginal smear cytology can be used to
identify the five stages of the estrous cycle: Proestrus, estrus,
metestrus I, metestrus II and diestrus (20). In the present study, the stages of
the estrous cycles of female C57BL/6 mice were determined based on
the morphology of exfoliated vaginal cells, and the levels of
estrogen and progesterone were measured at different periods of the
estrous cycle. Changes in the endometrium at the different periods
were observed using hematoxylin and eosin (H&E) staining.
Immunohistochemistry (IHC) was used to determine the localization
and expression of SUMO-associated proteins in the mouse endometrium
throughout the estrous cycle. Protein expression was also analyzed
by western blotting.
The purpose of the present study was to assess
changes in SUMO-associated protein expression and localization
throughout the endometrium during different stages of the estrous
cycle, which exhibit different ovarian hormone levels.
Investigating the role of SUMO-associated proteins in the
maintenance of the normal estrous cycle may elucidate their role in
hormone-dependent endometrial disease.
Materials and methods
Experimental animals
A total of 40 C57BL/6 female mice (age, 6–8 weeks,
weight, 20±2 g) were purchased from the Animal Center of the
Institute of Cancer Research, Chinese Academy of Medical Sciences.
Animals were maintained under specific pathogen-free conditions at
the Animal Experimental Center of the Fifth Central Hospital of
Tianjin (Tianjin, China) at 20–25°C with 50±5% humidity and on a 12
h light/dark cycle. Animals had free access to food and water. All
surgical procedures were performed as approved by the Principles of
Laboratory Animal Care (21) and
according to the Ethics Committee of The Fifth Central Hospital of
Tianjin.
Papanicolaou (Pap) stain
The tip of a sterile cotton swab moistened with
saline was placed in the mouse vagina, gently rotated and the
collected vaginal mucus then smeared onto a slide and dried
naturally. Pap Stain (Beijing Solarbio Science & Technology
Co., Ltd.) was used for staining according to the manufacturer's
instructions. The smears were evaluated using a light microscope
(DP73; Olympus Corporation) under ×10 and ×40 objective lenses.
Vaginal smear staining was used to predict estrous cycle stage
using the ratio of the three cell types observed: Round and
nucleated cells were epithelial cells, irregular cells without
nuclei were cornified cells and the small round cells were
leukocytes. The mouse complete estrus cycle lasts for 4–5 days and
can be divided into five phases. The proestrus stage has a
predominance of anterior keratinocyte cells. The estrus stage is
characterized by a large number of non-nucleated keratinized
squamous cells, which appear in clusters. The metestrus I stage has
reduced squamous epithelial cells compared to other stages with
nuclear epithelium and/or keratinization and the appearance of
leucocytes. The metestrus II stage has a predominance of
leucocytes. The diestrus stage consists predominantly of nuclear
epithelial cells, and occasional leucocytes and keratinocytes
(20).
Sample collection
Mice were anesthetized by isoflurane inhalation, and
blood samples were collected from the ophthalmic vein at different
estrous cycle stages (n=3/group). Blood samples were incubated for
1 h at 4°C and then centrifuged at 1,000 × g for 10 min at 4°C.
Serum was collected and stored at −80°C until further analysis.
Anesthetized mice were sacrificed by cervical dislocation and
uterine tissues were collected, with one half thoroughly washed
with phosphate buffered saline and frozen at −80°C, and the other
half placed in an embedding box and fixed with 4% paraformaldehyde
for 24 h at 4°C. Fixed tissues were dehydrated and immersed in
xylene and then embedded in paraffin wax and sectioned into a thin
ribbon of 6-µm thickness for H&E and IHC staining.
Enzyme-linked immunosorbent assay
(ELISA)
The serum of each mouse was divided according to the
estrous cycle stage, and concentrations of serum pituitary hormones
[luteinizing hormone (LH; cat. no. XYA441Mu; BioLegend, Inc.) and
follicle stimulating hormone (FSH; cat. no. EF013606; LSM Bio)] and
ovarian hormones [estradiol (E2; cat. no. KGE014; R&D Systems,
Inc.) and progesterone (Pr; cat. no. ab108670; Abcam)] were
quantified by ELISA kits according to the manufacturer's
instructions.
H&E stain and IHC staining
Mouse uterus paraffin sections from different
estrous cycle stages were stained with H&E according to the kit
instructions (cat. no. G1120; Beijing Solarbio Science &
Technology Co., Ltd.). IHC staining was performed using a universal
IHC kit (mouse/rabbit polymer assay system; cat. no. PV-6000;
OriGene Technologies, Inc.) according to the manufacturer's
instructions. The antibodies used were supplied by Abcam and
dilutions were as follows: SUMO1 (cat. no. ab133352; 1:200),
SUMO2/3 (cat. no. ab3742; 1:300), UBC9 (cat. no. ab7585; 1:500),
SENP1 (cat. no. ab108981, 1:300), SENP2 (cat. no. ab58418, 1:500),
SENP3 (cat. no. ab71677, 1:500) and SENP5 (cat. no. ab583477,
1:200). Stained images were obtained using light microscopy (DP73
microscope; Olympus Corporation). Yellow or brown-yellow granules
in the cytoplasm and/or the nucleus indicated positive staining.
Two pathologists, blind to the origin of the slides, observed
images independently. Ten high power microscopic fields (×400) were
selected in a homogeneous staining area and the level of positive
staining was determined using two methods. The first method
evaluated the numbers of stained cells. If no stained cells were
seen the field of view was rated 0, if <25% of cells were
stained the field of view was rated 1, if 26–50% of cells were
stained the field of view was rated 2 and if ≥51% of cells in the
field of view were stained it was rated 3. The second method
evaluated the staining intensity. No staining was rated 0, pale
yellow staining was rated 1, dark yellow staining was rated 2 and
brown was rated 3. The scores obtained from both methods were then
added to produce the final staining evaluation. If a field of view
was rated 0–1 it was considered not to express the protein of
interest (−), if it was rated 2 there was considered to be a low
level of protein expression (+), a rating of 3–4 was considered
moderate expression (++) and a rating of 5–6 was considered a high
level of protein expression (+++). The uterine basal lamina could
be distinguished from the functional uterine layers. The
endometrium basal layer made up the inner third of the uterus,
close to the myometrium and was not affected by hormones so
underwent no cyclical changes. The functional layer made up the
outer two thirds, close to the uterine cavity and was periodically
affected by hormones, this could be divided into the dense layer
and the sponge layer.
Western blot analysis
To avoid de-SUMOylation of proteins, the uterine
tissues were homogenized in pre-chilled RIPA lysis buffer (EMD
Millipore) containing 20 mM N-ethylmaleimide and 1 mM PSMF (Beijing
Solarbio Science & Technology Co., Ltd.). Tissue lysates were
centrifuged at 10,778 × g for 15 min at 4°C (1730 GENESPEED).
Supernatants were collected and protein concentrations measured.
Samples were mixed with sample buffer (Invitrogen; Thermo Fisher
Scientific, Inc.) and incubated at 70°C for 10 min. Samples (30 µg)
were then resolved by SDS-PAGE (4–15% gels; Invitrogen; Thermo
Fisher Scientific, Inc.), and then transferred onto a PVDF membrane
(EMD Millipore). The membrane was blocked in a Tris-HCl-buffered
salt solution containing 0.1% Tween-20 (TBST) and 5% skimmed milk
powder at room temperature for 1 h, then incubated with primary
antibodies provided by Abcam overnight at 4°C in PBS with 0.1%
Tween-20 and 5% skimmed milk powder (Thermo Fisher Scientific,
Inc.), at the following dilutions: Anti-SUMO1 (cat. no. ab11672;
1:1,000), anti-SUMO2/3 (cat. no. ab3742; 1:1,000), anti-UBC9 (cat.
no. ab7585; 1:1,000), anti-SENP1 (cat. no. ab108981; 1:1,000),
anti-SENP2 (cat. no. ab58418; 1:1,000), anti-SENP3 (cat. no.
ab71677; 1:800), anti-SENP5 (cat. no. ab583477; 1:500). Membranes
were then incubated with secondary peroxidase-conjugated goat
anti-rabbit IgG (cat. no. 111-035-003; 1:2,000; Jackson
ImmunoResearch Laboratories, Inc.) at room temperature for 2 h.
Membranes were incubated with Pierce™ Fast Western Blot kit ECL
reagent (cat. no. 35055; Thermo Fisher Scientific, Inc.) for 5 min
and then protein signals were detected using a C-Digit Blot Scanner
(LI-COR Biosciences), ImageJ (version 1.48, National Institutes of
Health) was used to quantify protein bands on X-ray film.
Statistical analysis
All data are presented as the mean ± SD of three
independent experiments. Western blots were quantified using
GraphPad Prism version 5.0 (GraphPad Software, Inc.), and
statistical analysis was performed using SPSS 17.0 (SPSS, Inc.).
Student's t-test or one-way analysis of variance followed by
Dunnett's test was performed. P<0.05 was considered a
statistically significant difference.
Results
Vaginal smear cytology
Typical vaginal smear cytology was seen in 35 mice
in the current study. These mice were classified into five stages
of the estrous cycle according to cytological criteria, as
described in Table I. The largest
proportion (43%) of mice were in diestrus, while only 9% of mice
were in estrus (Fig. 1A; Table I).
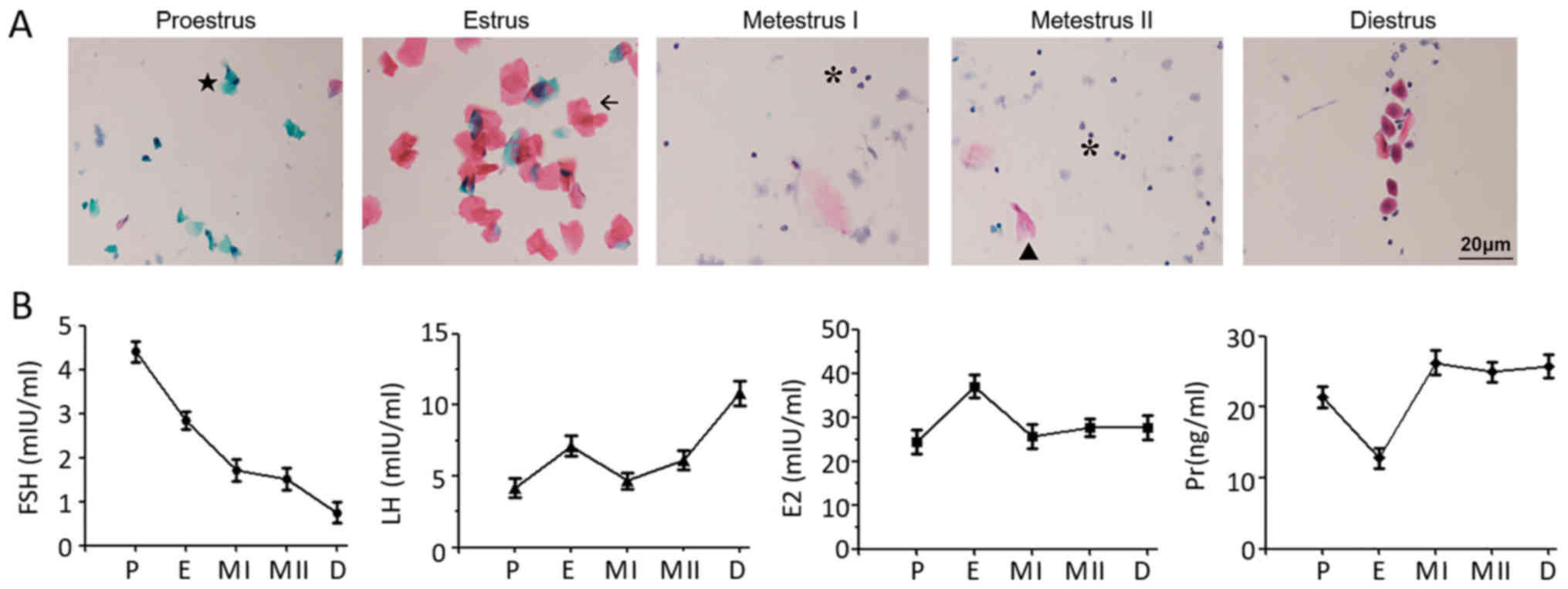 | Figure 1.PAP staining of vaginal exfoliated
cells and serum hormone levels during different stages of the mouse
estrous cycle. (A) Images of Pap-stained vaginal exfoliated cells:
Proestrus-anterior keratinocyte cells (n=7); estrus-non-nucleated
keratinized squamous cells (n=3); metestrus I-reduced non-nucleated
keratinized squamous cells and appearance of leucocytes (n=6);
metestrus II-predominance of leucocytes (n=4);
diestrus-predominantly nuclear epithelial cells, occasional
leucocytes and keratinocytes (n=15). (B) Serum FSH, LH, E2 and Pr
levels in female mice at different stages of the estrous cycle. E2,
estradiol; FSH, follicle stimulating hormone; LH, luteinizing
hormone; Pr, progesterone; ★, anterior keratinocyte cell; ←,
non-nucleated keratinized squamous cell; #, nuclear epithelial
cell; *, leucocytes; P, proestrus; E, estrus; MI, metestrus I; MII,
metestrus II; D, diestrus. |
 | Table I.Proportion and characteristics of
vaginal smears of 35 mice at different stages of the estrous cycle
based on Papanicolaou stain. |
Table I.
Proportion and characteristics of
vaginal smears of 35 mice at different stages of the estrous cycle
based on Papanicolaou stain.
| Estrous cycle
stage | Number of mice | Proportion out of
total number of mice (%) | Main features of
vaginal smear |
|---|
| Proestrus | 7 | 20 | Epithelial and
stromal cell proliferation. Predominance of anterior keratinocyte
cells |
| Estrus | 3 | 9 | Endometrial
hyperplasia, congestion and edema. Non-nucleated keratinized
squamous cells appear in clusters |
| Metestrus I | 6 | 17 | Luteal formation.
Predominance of non-nucleated and keratinized squamous cells |
| Metestrus II | 4 | 11 | Luteal
degeneration. A predominance of leucocytes |
| Diestrus | 15 | 43 | Follicles begin to
mature. Most cells are nuclear epithelium there are occasional
keratinocytes and leucocytes |
Expression levels of serum hormones
during the estrous cycle
Serum FSH levels were 4.41±0.40 mIU/ml at proestrus,
2.84±0.35 mIU/ml at estrus, 1.72±0.44 mIU/ml at metestrus I,
1.51±0.42 mIU/ml at metestrus II and 0.75±0.41 mIU/ml at diestrus,
with a peak in proestrus compared with other periods (P<0.05).
Serum LH was 4.45±0.51 mIU/ml at proestrus, 7.10±0.41 mIU/ml at
estrus, 4.67±0.35 mIU/ml at metestrus I, 6.72±0.37 mIU/ml at
metestrus II and 10.82±0.49 mIU/ml at diestrus, showing a +
significant peak at diestrus compared with other periods
(P<0.05). Serum E2 levels were significantly higher (P<0.05)
in estrus (37.02±2.53 mIU/ml) than in proestrus (24.41±2.73),
metestrus I (25.67±2.86) and metestrus II (27.63±2.04 mIU/ml) but
there was no significant difference compared with diestrus
(27.71±2.73 mIU/ml). Serum Pr levels were significantly lower
(P<0.05) in estrus (12.80±1.44 ng/m) than in proestrus
(21.36±1.50 ng/ml), metestrus I (26.23±1.73 ng/ml), metestrus II
(24.96±1.45 ng/ml) and diestrus (25.73±1.63 ng/ml), but there were
no significant differences between other stages of the estrus cycle
(Fig. 1B).
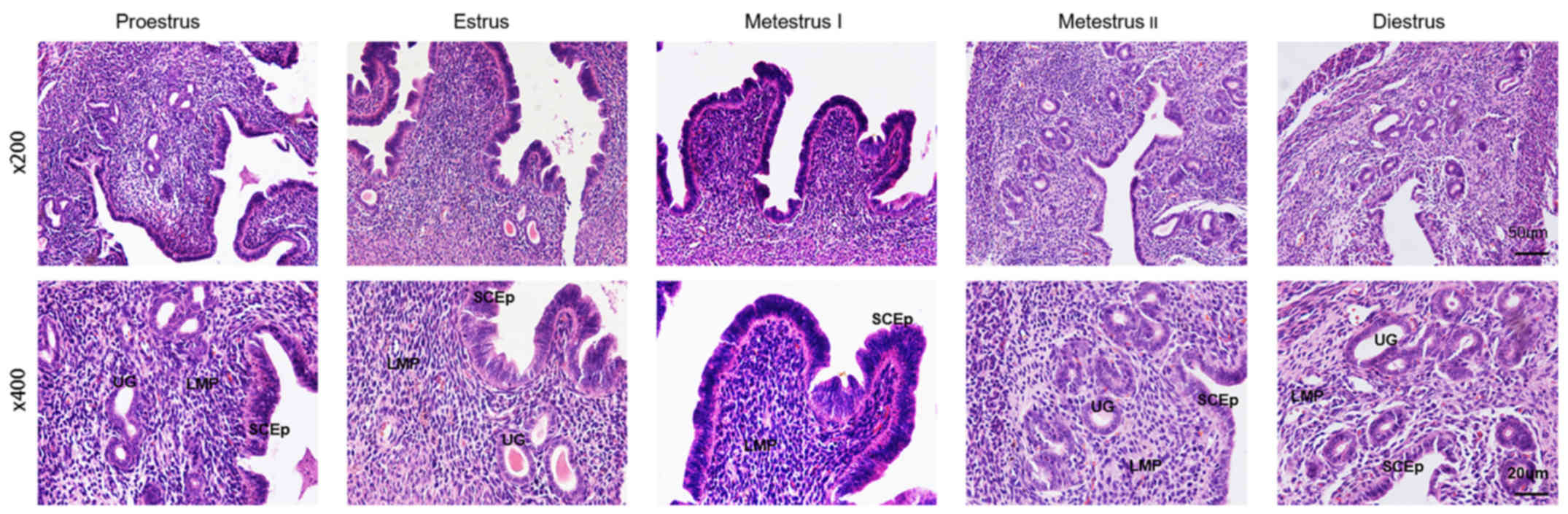
H&E staining
The results of H&E staining showed that in
proestrus the endometrial columnar epithelial cells and stromal
cells had proliferated, a thickened intimal connective tissue
displayed cellular proliferation and the number of blood vessels
had increased in comparison with diestrus (Fig. 2). Estrus exhibited endometrial
hyperplasia and hyperemia, endometrial edema and bleeding with
erythrocyte exudation. Metestrus I displayed degenerated
endometrium and capillary micro-bleeding. Metestrus II had further
degeneration of endometrial columnar epithelium cells and
endometrial atrophy. Until diestrus, the endometrium was very thin
with atrophied endometrial matrix, but glands gradually increased
with the start of a new estrous cycle.
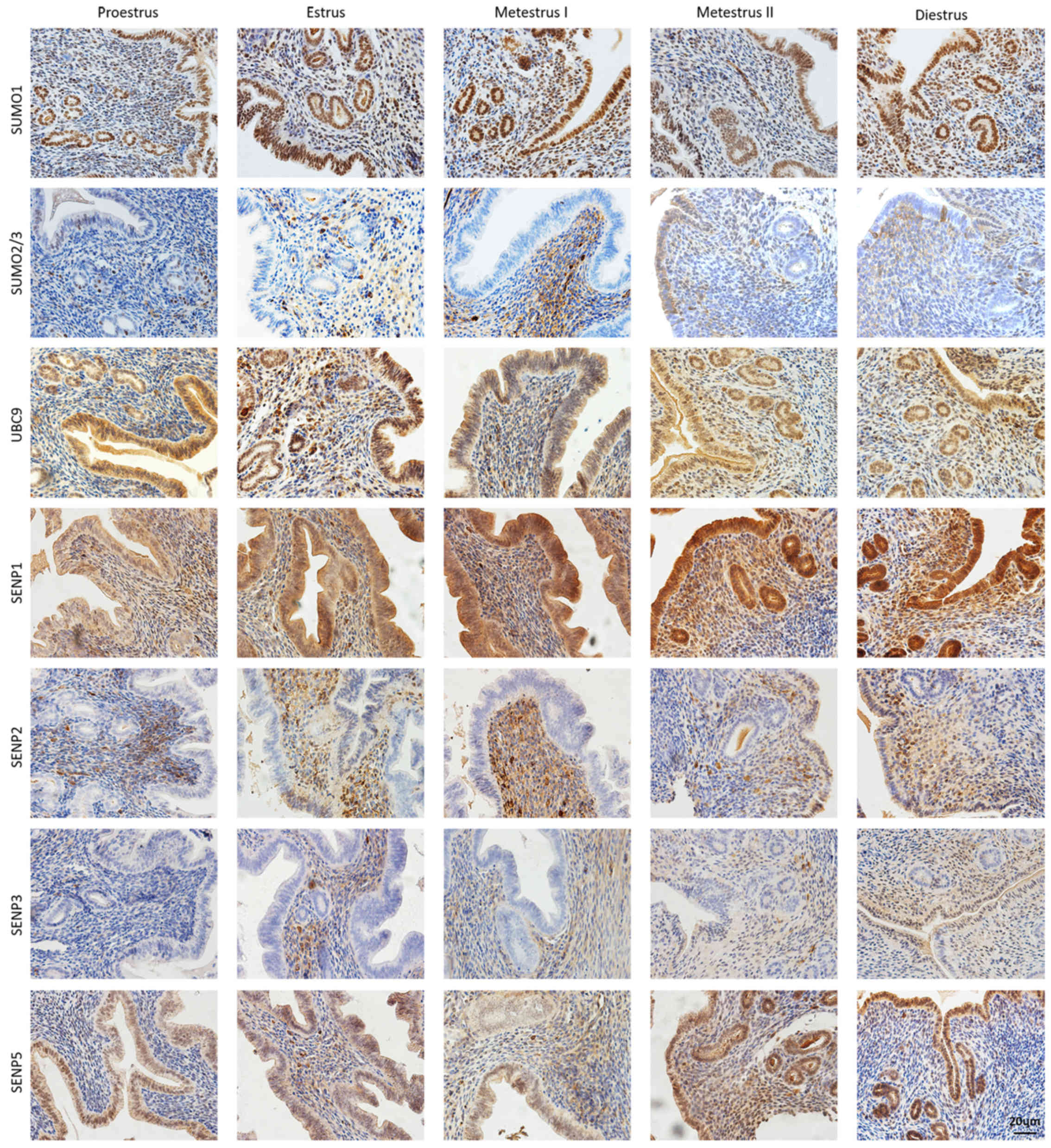
Murine endometrial expression and
localization of SUMO pathway members during the estrous cycle
Uterine IHC was performed to investigate the
expression and localization of SUMO-associated proteins in the
endometrium during the estrous cycle (Fig. 3). SUMO1 was mainly detected in
uterine luminal and glandular epithelial cells of the endometrium,
during the ovulation period, with a high level of staining
associated with increased columnar epithelium on the intima
surface. Unlike SUMO1, SUMO2/3 was mainly detected in the
endometrial stroma, with low level staining in columnar epithelium
and glands. The expression of SUMO2/3 showed no marked changes
during the estrous cycle. UBC9, the only E2 binding enzyme,
exhibited extensive uterine expression throughout the estrous
cycle. Subsequently, four important SUMO-specific proteases, SENP1,
SENP2, SENP3 and SENP5, were detected and found to display marked
differences in distribution and expression. SENP1 displayed the
strongest and most widely distributed expression, with a high level
of staining in the columnar epithelium and endometrial stroma
before and after estrus, and gradually decreasing levels in the
endometrial stroma during metestrus and diestrus. SENP2 and SENP3
were mainly distributed in endometrial stroma, and showed no
significant changes throughout the estrous cycle. The expression of
SENP5 was high in the endometrial epidermis, and increased during
estrus and metestrus. Characterization of IHC staining for the
SUMO-associated proteins during the estrous cycle is shown in
Table II.
 | Table II.Classification of
immunohistochemistry staining for SUMO pathway members in uterine
tissue during the estrous cycle. |
Table II.
Classification of
immunohistochemistry staining for SUMO pathway members in uterine
tissue during the estrous cycle.
| A, Proestrus |
|---|
|
|---|
|
| Protein |
|---|
|
|
|
|---|
| Area of tissue | SUMO1 | SUMO 2/3 | UBC9 | SENP1 | SENP2 | SENP3 | SENP5 |
|---|
| Basal lamina | ++ | ± | +++ | ++ | − | − | ++ |
| Functional
layers | ++ | − | + | ++ | ++ | − | − |
|
| B,
Estrus |
|
|
| Protein |
|
|
|
| Area of
tissue | SUMO1 | SUMO
2/3 | UBC9 | SENP1 | SENP2 | SENP3 | SENP5 |
|
| Basal lamina | +++ | − | +++ | +++ | + | − | +++ |
| Functional
layers | +++ | ++ | +++ | ++ | ++ | ++ | +++ |
|
| C, Metestrus
I |
|
| Protein |
|
|
|
| Area of
tissue | SUMO1 | SUMO
2/3 | UBC9 | SENP1 | SENP2 | SENP3 | SENP5 |
|
| Basal lamina | +++ | − | +++ | +++ | − | − | +++ |
| Functional
layers | +++ | + | ++ | +++ | +++ | + | ++ |
|
| D, Metestrus
II |
|
|
| Protein |
|
|
|
| Area of
tissue | SUMO1 | SUMO
2/3 | UBC9 | SENP1 | SENP2 | SENP3 | SENP5 |
|
| Basal lamina | ++ | ± | ++ | +++ | + | ± | +++ |
| Functional
layers | ++ | + | + | +++ | + | + | +++ |
|
| E,
Diestrus |
|
|
| Protein |
|
|
|
| Area of
tissue | SUMO1 | SUMO
2/3 | UBC9 | SENP1 | SENP2 | SENP3 | SENP5 |
|
| Basal lamina | +++ | − | ++ | +++ | + | ± | +++ |
| Functional
layers | +++ | + | + | ++ | ++ | + | ± |
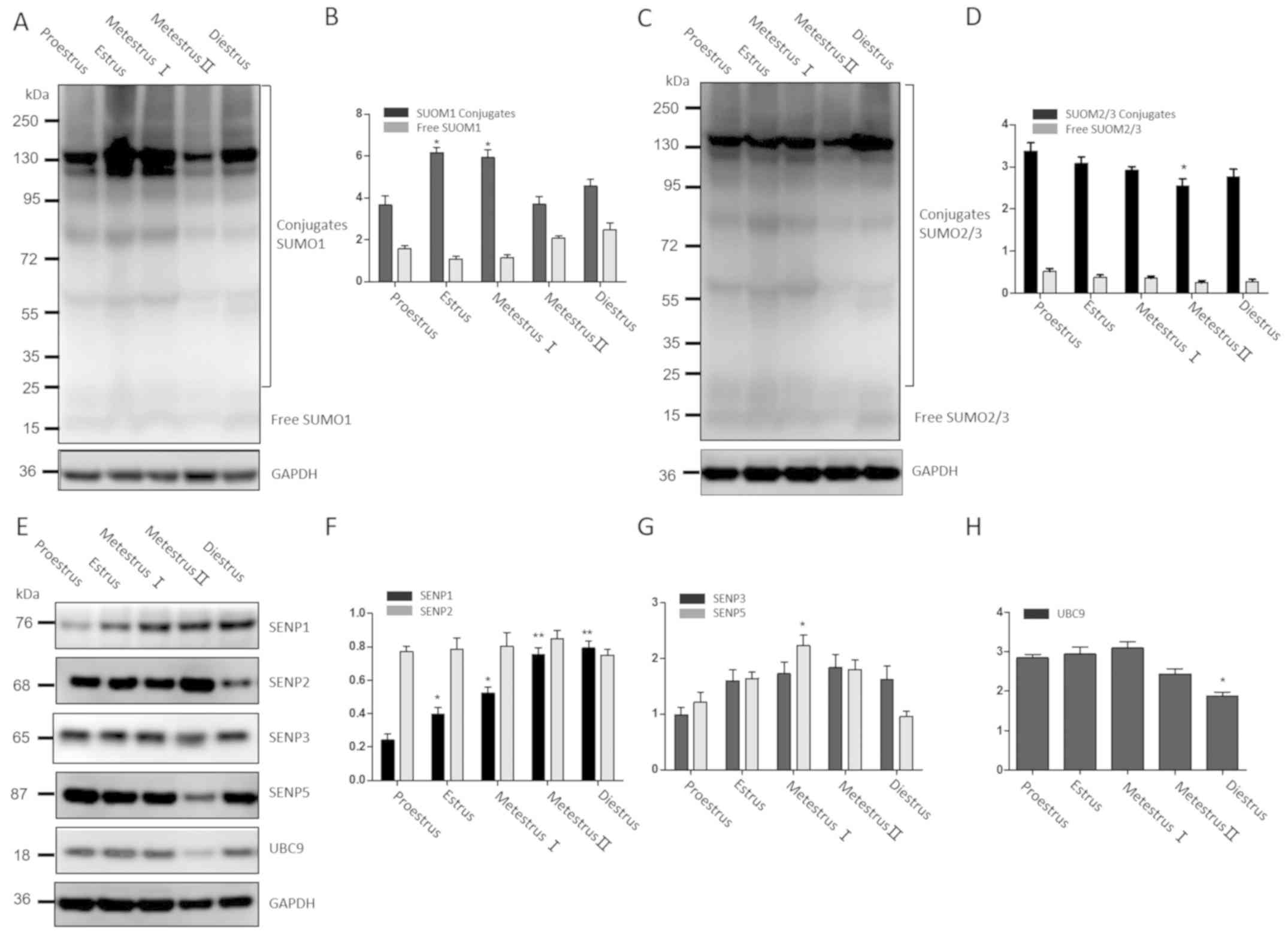
Uterine expression levels of SUMO
pathway members
Western blot analysis was used to further examine
whether periodic changes in the endometrium during the mouse
estrous cycle were related to de-SUMOylation. The protein
expression levels of SUMO1, SUMO2/3, UBC9, SENP1, SENP2, SENP3 and
SENP5 were examined in the mouse uterus at different stages of the
estrous cycle.
In the mouse estrous cycle, large molecular weight
(>100 kDa) SUMO1-conjugated proteins were abundant during estrus
and metestrus I, with no significant difference between the two
stages, as shown in Fig. 4A and B.
The levels of SUMO2/3-conjugated proteins in metestrus II were
decreased compared with estrus and metestrus I, but levels were
similar to those at diestrus and proestrus (Fig. 4C and D). UBC9 had a similar
expression pattern to SUMO2/3. The expression of SENP1 gradually
increased from proestrus to diestrus (P<0.05), but SENP2
remained stable throughout the estrus cycle. The expression of
SENP5 was significantly higher in metestrus I compared with
proestrus (P<0.05). Unlike the other SENP members, SENP3 was
expressed at low levels during the estrous cycle and showed no
significant variation in expression between the five stages
(Fig. 4E-H).
Discussion
In mammalian ovaries, pituitary gonadotrophins cause
follicles to produce estrogen, and the luteal body to produce
estrogen and progesterone (22,23).
Secondary sexual characteristics of women, such as breast
development, fat distribution, monthly menstruation, regular
ovulation and fetal growth and development in utero after
conception, are closely related to the regular ovarian secretion of
steroid hormones (24). The uterus
is a primary target of estrogen and progesterone and the
endometrium, cervix and vagina are all important target organs of
estrogen and progesterone, the levels of which change regularly
during the menstrual cycle. Studies have shown the high expression
of estrogen and progesterone receptors in endometrial tissue
(25,26). In the present study, the vaginal
smears of mature female mice were examined and typical cytology
patterns were used to characterize different stages in the estrous
cycle of the experimental animals (27). Changes in FSH, LH, E2 and Pr levels
were detected in mouse serum during the estrous cycle, and
endometrium histopathology changes induced by these hormones were
observed using H&E staining. These changes included the regular
proliferation and exfoliation of endometrial columnar epithelium
and the periodic changes in glands. The above results indicated
that changes of serum sex hormones and periodic changes to the
endometrium in mice were highly consistent with the equivalent
changes of the human menstrual cycle (28).
The endometrium undergoes continual changes in
features including cell proliferation, secretion and exfoliation
throughout the estrous cycle and these changes are regulated by
ovarian hormones (29). A number of
studies support a relationship between endogenous hormones and the
increased risk of several female cancers. In epidemiologic studies,
consistent associations have been observed between risk of breast,
ovarian and endometrial cancers and hormonal risk factors caused by
high postmenopausal body mass index and postmenopausal hormone use,
which suggest the relationship between hormones and gynecological
tumors (26,30). Additional studies have confirmed the
abnormal expression of SUMO-associated proteins in
hormone-dependent gynecological tumors. SUMO1 has an important role
in the occurrence and development of certain malignancies, and its
expression was shown to be significantly elevated in malignant
tumors (31,32). In addition, SUMO1 and ER-α expression
were found to be correlated in endometrial carcinoma (13). However, the specific relationship
between SUMOs and endometrial cancer is unclear and requires
further exploration. To the best of our knowledge, there are few
reports on the expression of SUMO isoforms throughout the estrous
cycle and the role of SUMO in maintaining the endometrial cycle is
unknown. The present study determined the expression and
localization of SUMO-associated proteins in the mouse uterus at
different stages of the estrous cycle.
Post-translational modifications are a fast and
efficient cellular mechanism to react to pathophysiological stimuli
(33,34). SUMO-related proteins were largely
expressed in a conjugated form. The results of western blot
analysis were consistent with IHC data, and both showed cyclical
changes in expression patterns and levels of SUMO-related proteins
in the endometrium throughout the estrous cycle. It can be
hypothesized that SUMO modification is related to hormonal changes
and plays an important role in maintaining endometrial function
during normal female estrous cycles.
The E2 ligase UCB9 is responsible for the
conjugation of SUMO to target molecules (35). The present study found that UBC9 was
highly expressed throughout the endometrium, with no obvious
changes throughout the estrous cycle.
SENPs play a vital role in de-SUMOylation of
proteins, and SENP1 was reported to mediate de-SUMOylation during
the regulation of cellular proliferation, and involved in the
regulation of angiogenesis (36).
Increased expression of SENP1 in prostate cancer enhanced
AR-dependent transcription, resulting in increased cell
proliferation (37). SENP2
expression was reported in bladder cancer, and its overexpression
could inhibit the migration and invasion of cultured bladder cancer
cells (38). Overexpression of SENP5
in human idiopathic heart failure leads to cardiac dysfunction,
accompanied by decreased proliferation of cardiomyocyte and
increased apoptosis (39). The
present study showed that SENP1, SENP2 and SENP5 were highly
expressed throughout the mouse estrous cycle. IHC staining showed
that SENP1 was widely distributed and highly expressed in the
uterus during the estrous cycle. Western blot analysis showed that
SENP5 was highly expressed in the endometrium during estrus. These
data suggest that SENP1 and SENP5 are likely to play an important
role in regulating deSUMOylation in endometrial tissues, thereby
further participating in the proliferation, secretion and
exfoliation of uterine cells during the estrous cycle. The results
of the present study suggest that SUMO-associated proteins
participate in the maintenance of the physiological function of
endometrium under the precise regulation of hormones during the
estrous cycle. It is hypothesized that hormone level changes,
hormone receptor sensitivity increases or any abnormality in
SUMO-associated proteins may induce endometrial diseases.
Defining the role of SUMO isoforms in the course of
uterine endometrial cyclical changes is likely to be crucial to
understand how they function under pathological conditions.
However, the mechanism of hormone regulation of target molecules by
SUMOylation and de-SUMOylation needs further study. This research
is a preliminary study into the relationship between endogenous
hormone actions and SUMO expression and how this is associated with
uterine tissue remodeling during the menstrual/estrous cycle, and
provides the theoretical foundation for pathological studies to
screen for potential therapeutic targets of endometrial
lesions.
Acknowledgments
The authors would like to thank Dr Charles Allan for
editing the English text of this manuscript.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81471175 and grant no.
81602363) and the Tianjin Binhai New District Health and Family
Planning Commission Science and Technology Projects (grant no.
2016BWKY005 and grant no. 2015BWKY005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and YB participated in the design of the study.
YL wrote the manuscript. YL, LY, XC, LL and JC collected the tissue
samples and participated in the study. YL and XM analyzed and
interpreted data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All surgical procedures were performed as approved
by the Principles of Laboratory Animal Care and according to the
Ethics Committee of The Fifth Central Hospital of Tianjin.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han ZJ, Feng YH, Gu BH, Li YM and Chen H:
The post-translational modification, SUMOylation, and cancer
(Review). Int J Oncol. 52:1081–1094. 2018.PubMed/NCBI
|
|
2
|
Wang YG and Dasso M: SUMOylation and
deSUMOylation at a glance. J Cell Sci. 122:4249–4252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeh ET: SUMOylation and De-SUMOylation:
Wrestling with life's processes. J Biol Chem. 284:8223–8227. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hickey CM, Wilson NR and Hochstrasser M:
Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol.
13:755–766. 2013. View
Article : Google Scholar
|
|
5
|
Hochstrasser M: Origin and function of
ubiquitin-like proteins. Nature. 458:422–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hay RT: Decoding the SUMO signal. Biochem
Soc Trans. 41:463–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilkinson KA and Henley JM: Mechanisms,
regulation and consequences of protein SUMOylation. Biochem J.
428:133–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cimarosti H, Ashikaga E, Jaafari N,
Dearden L, Rubin P, Wilkinson KA and Henley JM: Enhanced
SUMOylation and SENP-1 protein levels following oxygen and glucose
deprivation in neurones. J Cereb Blood Flow Metab. 32:17–22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whitcomb BW, Purdue-Smithe A, Hankinson
SE, Manson JE, Rosner BA and Bertone-Johnson ER: Menstrual cycle
characteristics in adolescence and early adulthood are associated
with risk of early natural menopause. J Clin Endocrinol Metab.
103:3909–3918. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yip KS, Suvorov A, Connerney J, Lodato NJ
and Waxman DJ: Changes in mouse uterine transcriptome in estrus and
proestrus. Biol Reprod. 89:132013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flotho A and Melchior F: Sumoylation: A
regulatory protein modification in health and disease. Annu Rev
Biochem. 82:357–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng JD, Liu LL, Wang SF and Huang X:
SUMO-1 promotes ishikawa cell proliferation and apoptosis in
endometrial cancer by increasing sumoylation of histone H4. Int J
Gynecol Cancer. 25:1364–1368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su S, Zhang Y and Liu P: Roles of
ubiquitination and SUMOylation in DNA damage response. Curr Issues
Mol Biol. 35:59–84. 2019.PubMed/NCBI
|
|
14
|
Picard N, Caron V, Bilodeau S, Sanchez M,
Mascle X, Aubry M and Tremblay A: Identification of estrogen
receptor β as a SUMO-1 target reveals a novel phosphorylated
sumoylation motif and regulation by glycogen synthase kinase 3beta.
Mol Cell Biol. 32:2709–2721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ying SB, Dünnebier T, Si J and Hamann U:
Estrogen receptor alpha and nuclear factor Y coordinately regulate
the transcription of the SUMO-conjugating UBC9 gene in MCF-7 breast
cancer cells. PLoS One. 8:e756952013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guan J, Xie L, Luo X, Yang B, Zhang H, Zhu
Q and Chen X: The prognostic significance of estrogen and
progesterone receptors in grade I and II endometrioid endometrial
adenocarcinoma: Hormone receptors in risk stratification. J Gynecol
Oncol. 30:e132019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vercellini P, Buggio L, Berlanda N,
Barbara G, Somigliana E and Bosari S: Estrogen-progestins and
progestins for the management of endometriosis. Fertil Steril.
106:1552–1571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chaffin CL and Vandevoort CA: Follicle
growth, ovulation, and luteal formation in primates and rodents: A
comparative perspective. Exp Biol Med (Maywood). 238:539–548. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caligioni C: Assessing reproductive
status/stages in mice. Curr Protoc Neurosci Appendix.
4:Appendix–4I. 2009.
|
|
20
|
Cora MC, Kooistra L and Travlos G: Vaginal
cytology of the laboratory rat and mouse: Review and criteria for
the staging of the estrous cycle using stained vaginal smears.
Toxicol Pathol. 43:776–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trappe K, Thomas D, Bikou O, Kelemen K,
Lugenbiel P, Voss F, Becker R, Katus HA and Bauer A: Suppression of
persistent atrial fibrillation by genetic knockdown of caspase 3: A
pre-clinical pilot study. Eur Heart J. 34:147–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wood GA, Fata JE, Watson KL and Khokha R:
Circulating hormones and estrous stage predict cellular and stromal
remodeling in murine uterus. Reproduction. 133:1035–1044. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chuffa LG, Lupi-Júnior LA, Costa AB,
Amorim JP and Seiva FR: The role of sex hormones and steroid
receptors on female reproductive cancers. Steroids. 118:93–108.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Annie L, Gurusubramanian G and Roy VK:
Estrogen and progesterone dependent expression of visfatin/NAMPT
regulates proliferation and apoptosis in mice uterus during estrous
cycle. J Steroid Biochem Mol Biol. 185:225–236. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Draper CF, Duisters K, Weger B,
Chakrabarti A, Harms AC, Brennan L, Hankemeier T, Goulet L, Konz T,
Martin FP, et al: Menstrual cycle rhythmicity: Metabolic patterns
in healthy women. Sci Rep. 8:145682018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao M, Cao C, Zhang X, Tang F, Zhao L, Luo
S and Li L: Abnormal expression of estrogen receptor is associated
with thin endometrium. Gynecol Endocrinol. 35:544–547. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cladel NM, Budgeon LR, Balogh KK, Cooper
TK, Hu JF and Christensen ND: A novel pre-clinical murine model to
study the life cycle and progression of cervical and anal
papillomavirus infections. PLoS One. 10:e01201282015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahrens K, Mumford SL, Schliep KC, Kissell
KA, Perkins NJ, Wactawski-Wende J and Schisterman EF: Serum leptin
levels and reproductive function during the menstrual cycle. Am J
Obstet Gynecol. 210:248.e1–9. 2013. View Article : Google Scholar
|
|
29
|
Kalyne B and Bruce DM: Reproductive tract
changes during the mouse estrous cycle. Laboratory Equipment.
2014.
|
|
30
|
Brown SB and Hankinson SE: Endogenous
estrogens and the risk of breast, endometrial, and ovarian cancers.
Steroids. 99:8–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li R, Wei J, Jiang C, Liu D, Deng L, Zhang
K and Wang P: Akt SUMOylation regulates cell proliferation and
tumorigenesis. Cancer Res. 73:5742–5753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu F, Li L, Li Y, Ma X, Bian X, Liu X,
Wang G and Zhang D: Overexpression of SENP1 reduces the stemness
capacity of osteosarcoma stem cells and increases their sensitivity
to HSVtk/GCV. Int J Oncol. 53:2010–2020. 2018.PubMed/NCBI
|
|
33
|
Rodriguez AC, Blanchard Z, Maurer KA and
Gertz J: Estrogen signaling in endometrial cancer: A key oncogenic
pathway with several open questions. Horm Cancer. 10:51–63. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu A, Zhang D, Yang X and Song Y:
Estrogen receptor alpha activates MAPK signaling pathway to promote
the development of endometrial cancer. J Cell Biochem.
120:17593–17601. 2019.PubMed/NCBI
|
|
35
|
Hewitt WM, Lountos GT, Zlotkowski K,
Dahlhauser SD, Saunders LB, Needle D, Tropea JE, Zhan C, Wei GH, Ma
BY, et al: Insights into the allosteric inhibition of the SUMO E2
enzyme Ubc9. Angew Chem Int Ed Engl. 5:5703–5707. 2016. View Article : Google Scholar
|
|
36
|
Zhou HJ, Xu Z, Wang ZR, Zhang HF, Simons M
and Min W: SUMOylation of VEGFR2 regulates its intracellular
trafficking and pathological angiogenesis. Nat Commun. 9:33032018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng JK, Bawa T, Lee P, Gong LM and Yeh
E: Role of desumoylation in the development of prostate cancer.
Neoplasia. 8:667–676. 2016. View Article : Google Scholar
|
|
38
|
Tan MY, Gong H, Wang J, Tao L, Xu DL, Bao
E, Liu ZH and Qiu JX: SENP2 regulates MMP13 expression in a bladder
cancer cell line through SUMOylation of TBL1/TBLR1. Sci Rep.
5:139962015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim EY, Zhang Y, Beketaev I, Segura AM, Yu
W, Xi YT, Chang J and Wang J: SENP5, a SUMO isopeptidase, induces
apoptosis and cardiomyopathy. J Mol Cell Cardiol. 78:154–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|