Introduction
Most patients with heart failure have a preserved
ejection fraction (1). In cases of
heart failure, population with preserved ejection fraction is
associated with higher mortality rates compared with that of
healthy hearts (2). Ethnicity has a
major impact on the mortality and morbidity of patients with heart
failure and preserved ejection fraction (2,3). Asian
patients with heart failure and preserved ejection fractions have
poorer outcomes compared with Caucasian patients (2,4,5).
The pathophysiology behind heart failure with
preserved ejection fraction is complicated and therefore difficult
to diagnose (6). However, diastolic
stiffness (which presents myocardial relaxation and filing
pressure) of the left ventricle serves a crucial role in the
pathophysiology (7). Diastolic
stiffness is measured using M-mode echocardiography. According to
the linear elastic theory, the diastolic epicardial movement is a
noninvasive measurement of the left ventricular wall distensibility
(8). Echocardiography is able to
provide a comprehensive assessment of the anatomy and physiology of
the heart (9). Echocardiographic
parameters of anatomical and physiological changes of the heart
provide a framework for providing effective prognoses and outcomes
in the management of patients with heart failure and preserved
ejection fraction (10).
One of the echocardiographic parameters, the
diastolic wall strain (DWS), is a physical property of the
myocardial tissue. There is less myocardial thinning and less wall
movement in patients with heart failure and preserved ejection
fraction compared with cardiac healthy patients (8). DWS predicts the presence of heart
failure with preserved diastolic function (7). An experimental study demonstrated that
the epicardial movement during diastole is inversely proportional
to the myocardial stiffness of heart failure in rats with preserved
ejection fraction (8). Until
recently, DWS has not been examined in heart failure patients with
preserved ejection fraction.
The primary aim of the present study was to
investigate the relationship between M-mode echocardiographic
parameters and the structure and function of the heart. The
secondary endpoint of the workup was to test the hypothesis that a
correlation is present between M-mode echocardiography parameters
and poor outcomes of patients with heart failure and preserved
ejection fraction. The tertiary aim of the present study was to
identify an independent parameter associated with mortality of
patients with heart failure and preserved ejection fraction in
Chinese populations.
Materials and methods
Inclusion criteria
Patients with heart failure and symptoms including
left ventricular dysfunction, palm edema, plasma brain natriuretic
peptide (BNP) ≥100 pg/ml, pulmonary capillary wedge pressure ≥15
mmHg and/or left ventricular end-diastolic pressure at rest ≥12
mmHg, with an ejection fraction (measured by echocardiography)
>50% and no pericardial disease, hypertrophic/infiltrative
cardiomyopathy or significant left-sided valvular disease, were
included in the test populations of the current study. Patients
without known cardiovascular disease, hypertension, obesity
(according to body mass index) or diabetes (according to blood
glucose levels) were included in the control population of the
current study (Table I).
 | Table I.Demographics and social
characteristics of enrolled patients. |
Table I.
Demographics and social
characteristics of enrolled patients.
|
| Group |
|
|---|
|
|
|
|
|---|
| Characteristics | Test population | Control
population | Comparison between
groups, P-value |
|---|
| Number of patients
enrolled | 1,244 | 1,952 | N/A |
| Age, years |
|
Minimum | 45 | 65 |
|
|
Maximum | 90 | 90 |
|
| Mean ±
SD | 61.12±4.12 | 71.75±11.15 | <0.0001 |
| Sex |
| Male | 807 (65) | 1,326 (68) |
|
|
Female | 437 (35) | 626 (32) | 0.079 |
| Height, m | 1.56±0.09 | 1.55±0.17 | 0.056 |
|
Smokers | 187 (15) | 343 (18) | 0.066 |
| Blood
serum creatinine | 1.20±0.2 | 1.21±0.05 | 0.035 |
| Waist
circumferencea | 95.12±5.27 | 89.56±7.51 | <0.0001 |
| Routine
exercise | Tai Chi | No exercise except
occasional walking | N/A |
| Alcohol intake | 115 (9) | 85 (4) | <0.0001 |
| Live
steatosisb | 21 (2) | 0 (0) | <0.0001 |
| Types of heart
failure |
|
Right-sided | 545 (43) | 0 (0) |
|
|
Left-sided | 699 (56) | 0 (0) | N/A |
|
Acute | 312 (25) | 0 (0) |
|
|
Chronic | 932 (75) | 0 (0) |
|
|
Stroke | 32 (3) | 1 (0.05) | N/A |
| Family history |
|
Coronary artery disease | 21 (2) | 1 (0.05) |
|
|
Peripheral artery disease | 7 (1) | 0 (0) | 0.835 |
| Present
medications |
|
β-blockers | 247 (20) | 0 (0) |
|
| Calcium
channel blockers | 152 (12) | 0 (0) |
|
|
Hematinic | 41 (3) | 42 (2) |
|
| Immune
Tonic | 31 (3) | 35 (2) | <0.0001 |
Exclusion criteria
Patients aged <45 years for the test population,
<65 years for the control population and those that had not
signed an informed consent form were excluded from the present
study. Patients with heart failure and an ejection fraction
<50%, posterior wall motion abnormalities, hypertrophic
cardiomyopathy, pericardial effusion, infiltrative cardiomyopathy
and significant left-sided valvular disease were excluded from the
test population of the current study. Patients who displayed heart
failure risk factors (such as cardiovascular disease, hypertension,
obesity and diabetes) were excluded from the control population of
the current study. Patients who presented ambiguous images that
were difficult to interpret and draw conclusions from during
diagnosis were excluded from analysis.
Cohorts
Patients with known cardiovascular disease were
included in the test group (n=1,244) and patients without known
cardiovascular disease were included in the control group
(n=1,952).
Routine measurements
Blood pressure was measured using a sphygmomanometer
(Omron HEM-8712; Omron Healthcare, Inc.). Hypertension was defined
as systolic blood pressure and diastolic blood pressure >140 and
>90 mmHg, respectively (11).
Blood samples were collected from all enrolled patients and samples
were evaluated for total cholesterol, low-density lipoprotein
(LDL), high-density lipoprotein (HDL), blood serum creatinine,
random blood plasma glucose levels, hemoglobin, and % glycated
hemoglobin (% HbA1c, normal value: ≤7%) (12). Patients were considered diabetic if
random plasma glucose was >140 mg/dl. Hyperlipidemia was defined
as a total cholesterol level >240 mg/dl, LDL >160 mg/dl
or/and HDL <40 mg/dl (11). ELISA
kits (NPPB/BNP Human ELISA Kit; cat. no. EHNPPB; Thermo Fisher
Scientific, Inc.) were used to determine BNP (13).
M-mode echocardiography
Ultrasound equipment (iE33; Philips Medical Systems,
Inc.) was used to perform M-mode echocardiography using cardiac
ultrasonographers. The dimensions, shortening fraction (SF) and the
left ventricle's wall thickness was measured from the long-axis
view using a 2–3 MHz transducer (14).
DWS was calculated using the following equation
(8): DWS=(left ventricle posterior
wall thickness at end systole-left ventricle posterior wall
thickness at end diastole)/left ventricle posterior wall thickness
at end diastole. Echo machine data were printed out, magnified to
300%, and measurements were performed manually.
A four-chambered view was taken for the mitral flow
velocity and the myocardial wall motion velocity measurements. The
sample was placed at the mitral leaflet's tip. Peak atrial and the
peak early diastolic flow velocities were determined. The mitral
flow E/A was calculated using the following equation (8): Mitral flow (E/A)=(peak early diastolic
flow velocity)/(peak atrial flow velocity). The sample was kept at
the lateral site of an atrial-ventricular valve annulus. The peak
systolic velocity and the peak early diastolic wall velocity was
measured. E/e' ratio was measured using the following equation
(8): E/e' ratio=peak early diastolic
flow velocity/peak early diastolic wall velocity.
The Tei index was calculated using the following
equation: Tei index=(X-Y)/Y. Where X represents the time between
the mitral annular late diastolic wave trailing edge and the
subsequent mitral annular early diastolic wave leading edge. Y
represents the time between the leading edge of the mitral annular
systolic wave and the trailing edge of the mitral annular systolic
wave (15).
Mortality records were collected from Digital
Imaging and Communications in Medicine (DICOM®).
Statistical analysis
For all parameters of M-mode echocardiography, the
mean value of five individual heartbeats were considered for
statistical analysis. Individual heartbeat was measured at the time
interval of 5 min of the rest period. Categorical data were
analyzed using the Mann-Whitney U test (7) and continuous data were analyzed using
unpaired t-test (16). Bland-Altman
analysis was used to check intra-observer and inter-observer
reliabilities (14). Multiple
regression analysis was used to analyze survival rates. Statistical
analysis was performed using InStat (Windows version 3.01; GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic characters and medical
history
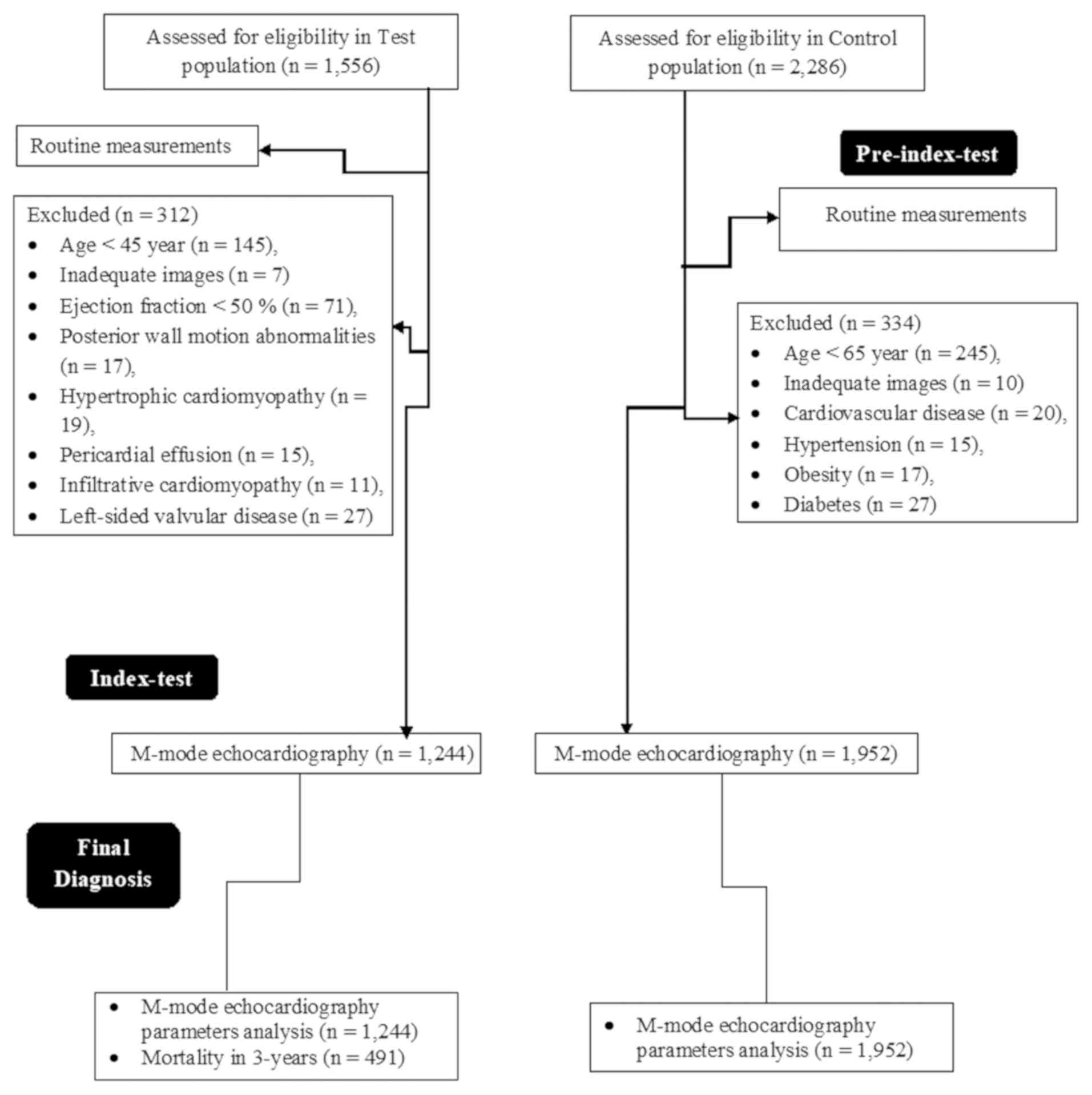
A total of 1,556 patients with heart failure were
admitted to the Affiliated Dongfeng Hospital, China between 15th
August 2012 and 1st August 2015. Among these, there were a total of
145 patients <45 years, images of 7 patients were indicated to
be inadequate, 71 patients had ejection fraction <50%, 17
patients had posterior wall motion abnormalities, 19 patients had
hypertrophic cardiomyopathy, 15 patients had pericardial effusion,
11 patients had infiltrative cardiomyopathy and 27 patients had
left-sided valvular disease; therefore, these patients were
excluded from the test population. From the Affiliated Dongfeng
Hospital, China and the same referring hospitals of China during
the same time period, a total of 2,286 patients were available at
an outpatient setting without known cardiovascular disease. Among
these patients, 245 patients <65 years, images of 10 patients
were indicated to be inadequate, 20 patients had cardiovascular
diseases with 15 of whom had reported hypertension during
measurements, 17 patients were obese and 27 patients had diabetes;
therefore, these patients were excluded from the control population
of the current study. A flowchart of the study is presented in
Fig. 1.
A total of 1,244 patients were included in the test
population. A total of 1,952 patients were included in the control
population. There were no significant differences in height
(P=0.056), sex (P=0.079), and family history for cardiac disease
(P=0.835) but significant differences in blood serum creatinine
(P=0.035) between the control and the test groups were observed.
Both groups had the same numbers of smokers (P=0.066). The control
population was younger than the test population (P<0.0001).
Waist circumference (P<0.0001), patients who had consumed
alcohol, (P<0.0001) and liver steatosis (P<0.0001) were
higher in the test population compared with the control population.
Unlike the control group, test group patients received a treatment
of β-blockers or calcium channel blockers. Further demographic
characteristics of enrolled patients are presented in Table I.
Routine measurements
Laboratory tests confirmed that the test population
exhibited higher rates of obesity (P<0.0001), hypertension
(P<0.0001), diabetes (P<0.0001), high heart rate
(P<0.0001) and high BNP levels (P<0.0001) compared with the
control population (Table II).
 | Table II.Routine measurements. |
Table II.
Routine measurements.
|
|
| Group |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Normal
valuea | Test
population | Control
population | Comparison between
groups, P-value |
|---|
| Numbers of patients
enrolled | N/A | 1,244 | 1,952 | N/A |
| Weight, kg | 60–65 | 75.15±7.12 | 63.16±9.12 | <0.0001 |
| BMI,
kg/m2 | ≤30 | 30.87±3.15 | 25.12±3.85 | <0.0001 |
| Diastolic blood
pressure, mmHg | 90 | 91±3 | 85±4 | <0.0001 |
| Systolic blood
pressure, mmHg | 140 | 146±4 | 131±8 | <0.0001 |
| Heart rate,
bpm | 60–100 | 93±8 | 86±4 | <0.0001 |
| Random glucose,
mg/dl | ≤140 | 171±9 | 132±7 | <0.0001 |
| The total
cholesterol | ≤240 | 261±9 | 215±7 | <0.0001 |
|
LDL | ≤160 | 171±8 | 145±3 | <0.0001 |
|
HDL | ≥40 | 33±3 | 55±7 | <0.0001 |
| HbA1c, % | ≤7 | 8.2±0.5 | 6.5±0.25 | <0.0001 |
| BNP, pg/ml | ≤100 | 115±8 | 32±2 | <0.0001 |
M-mode echocardiography
evaluation
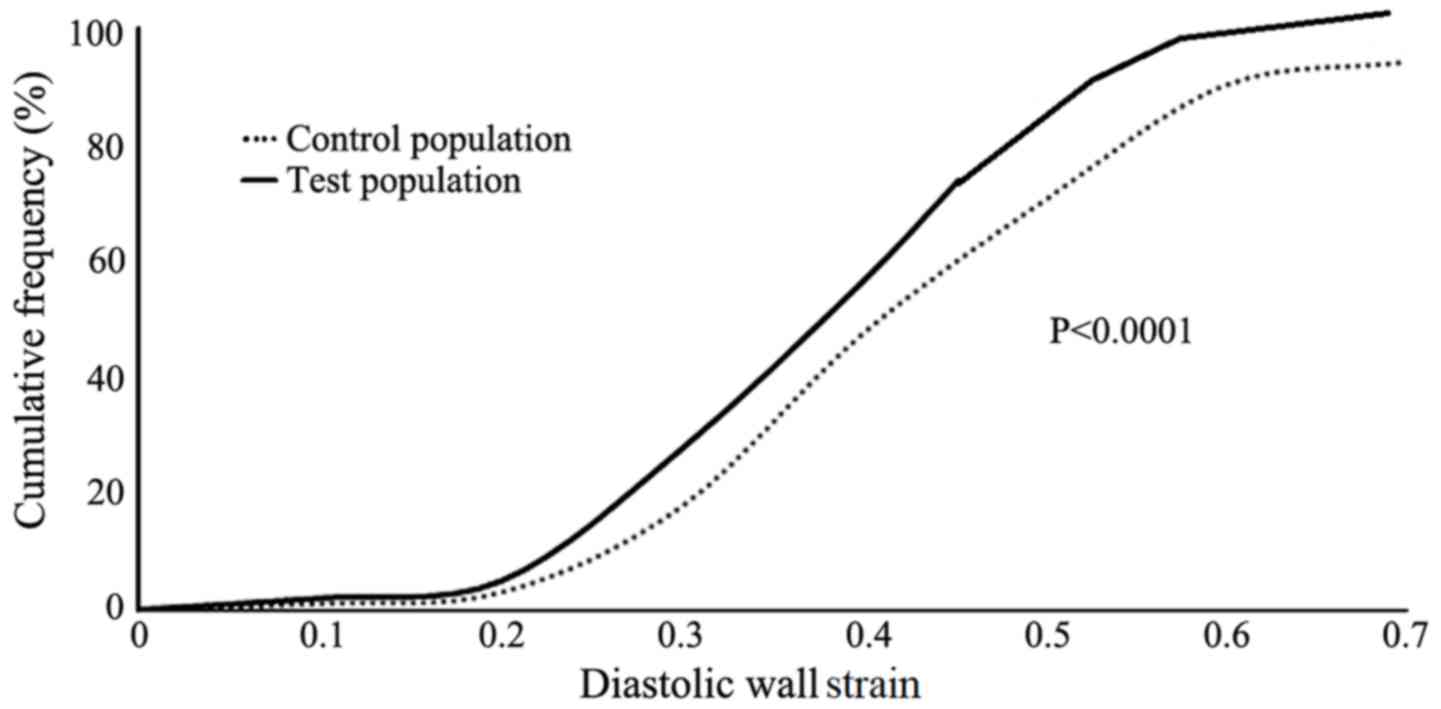
The median value of the 25th to 75th percentile DWS
for the control population was 0.45 (range, 0.35–0.55) and that of
the test populations was 0.38 (range, 0.28–0.48). The distribution
of DWS in the test populations of the current study was less
compared with the control population (Fig. 2; P<0.0001). Intra-observer and
interobserver reliabilities were 0.0021±0.00051 and 0.0031±0.00061,
respectively.
With respect to control population, the test
population had the same left ventricular end-diastolic dimension
(P=0.060) and the left ventricular end-diastolic volume (P=0.054).
However, the test population had a higher left ventricular
end-systolic dimension (P<0.0001), reduced SF (P<0.0001),
reduced posterior wall thickness at end-systole (P=0.007), reduced
posterior wall thickness at end-diastole (P<0.0001), less DWS
(P<0.0001), reduced mitral flow velocity (P<0.05), reduced
myocardial wall motion velocity (P<0.05), increased Tei index
(P<0.0001), increased left ventricular end-systolic volume
(P<0.0001), and reduced ejection fraction (P<0.0001) compared
with the control population (Table
III).
 | Table III.Left ventricle structure and M-mode
echocardiography parameters. |
Table III.
Left ventricle structure and M-mode
echocardiography parameters.
|
| Group |
|
|---|
|
|
|
|
|---|
|
Characteristics | Test
population | Control
population | Comparison between
groups, P-value |
|---|
| Numbers of patients
enrolled | 1,244 | 1,952 | N/A |
| LVIDs, mm |
30.81±5.45a | 29.92±4.51 | <0.0001 |
| LVIDd, mm | 46.92±5.45 | 47.46±9.15 | 0.060 |
| Shortening
fraction, % |
31.23±3.41a | 35.45±5.41 | <0.0001 |
| Posterior wall
thickness at end-systole |
10.01±2.01a | 10.21±2.11 | 0.007 |
| Posterior wall
thickness at end-diastole |
14.01±1.91a | 15.12±2.92 | <0.0001 |
| DWS |
0.30±0.07a | 0.34±0.05 | <0.0001 |
| Mitral flow
velocity |
1.78±2.45a | 2.15±6.21 | 0.040 |
| Myocardial wall
motion velocity |
6.09±1.95a | 6.28±2.11 | 0.004 |
| Tei index |
0.39±0.016a | 0.38±0.015 | <0.0001 |
| EDV, ml | 162.25±5.47 | 161.51±12.81 | 0.053 |
| ESV, ml |
81.15±4.47a | 80.12±4.14 | <0.0001 |
| % EF |
59.12±2.91a | 63.35±3.12 | <0.0001 |
Analysis of mortality
Patients from the test population who demonstrated
the most severe M-mode echocardiography parameters, which were
determined according to DWS, left ventricular end-diastolic volume,
left ventricular end-systolic dimension, posterior wall thickness
at end-systole and mitral flow velocity, died during a follow-up
period of 3-years. Demographic characteristics and medical history
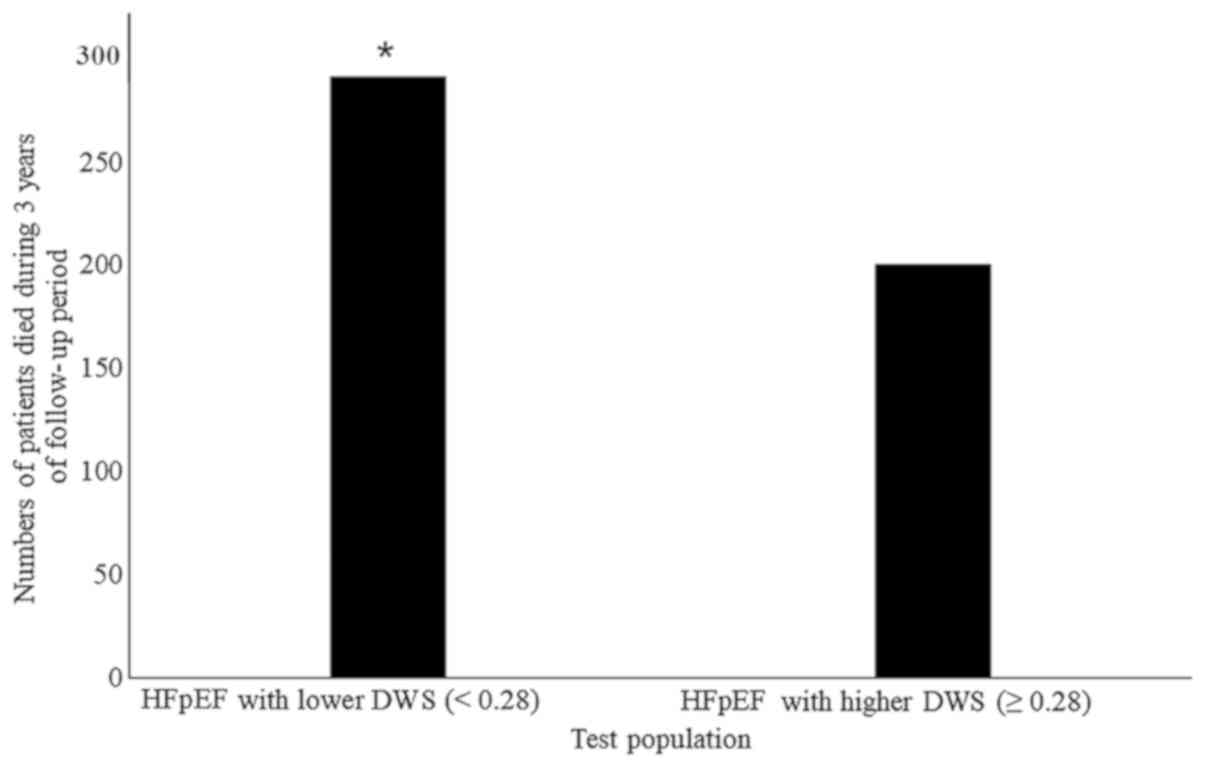
did not appear to be associated with mortality (Table IV). A total of 491 patients from the
test population died during the 3-year follow-up period. In the
test population, the numbers of patients with lower DWS (<0.28)
died within 3-years of the follow-up and mortality rate was higher
compared with the numbers of patients with higher (≥0.28) DWS
(P<0.0001; Fig. 3).
 | Table IV.Multiple regression analysis for
mortality in the test population during follow-up of 3-years. |
Table IV.
Multiple regression analysis for
mortality in the test population during follow-up of 3-years.
|
Characteristics | P-value for the
analysis of parameter |
|---|
| LVIDs, mm | 0.001a |
| LVIDd, mm | 0.002a |
| Shortening
fraction, % | 0.015a |
| Posterior wall
thickness at end-systole | 0.025a |
| Posterior wall
thickness at end-diastole | 0.026a |
| DWS | 0.001a |
| The mitral flow
velocity | 0.002a |
| The myocardial wall
motion velocity | 0.021a |
| Tei index | 0.031a |
| EDV, ml | 0.041a |
| ESV, ml | 0.032a |
| % EF | 0.021a |
| Age, years | 0.12 |
| Sex | 0.15 |
| Body mass index,
kg/m2 | 0.17 |
| Smoking | 0.55 |
| Family history | 0.69 |
| Brain natriuretic
peptide, pg/ml | 0.052 |
| Diabetes | 0.062 |
| Hypertension | 0.075 |
Discussion
In a large comparative M-mode echocardiography study
on patients with heart failure with preserved ejection fraction vs.
normal (cardiac healthy) research subjects, DWS was indicated to be
lower (P<0.0001) in the test population than in the cardiac
healthy patients. Decreased DWS measurements were associated with
worse outcomes, and within the test populations, patients with
reduced DWS had a higher mortality rate (P<0.0001). DWS is
associated with left ventricle stiffness (17). Patients with heart failure and
preserved ejection fraction exhibiting high diastolic stiffness,
and lower DWS has been previously associated with higher mortality
(7). DWS is easy to calculate and
cost-effective (7). The DWS
measurements of the present study were in line with previous
studies (7,17). In consideration of the results of the
present study, DWS may be important M-mode echocardiography
parameter for evaluating the risk of diastolic stiffness in
patients with heart failure and preserved ejection fraction.
The present study demonstrated that the test
population had high BNP, impaired left ventricular relaxation and
lower myocardial wall motion velocity compared with the control
population. Increased impaired left ventricular relaxation, raised
BNP (18) and lower myocardial wall
motion velocity results in the increased filling pressure of the
left ventricle, in turn increasing its stiffness and poorer
outcomes such as myocardial infraction (7). The results of the present study were
consistent with previous studies (7,19,20).
However, these parameters are load dependent and intrinsic passive
myocardial stiffness is not possible to predict (21). Furthermore, BNP results are also
unreliable for predicting whether patients will have poor outcomes
as BNP is also affected by renal function, obesity and pulmonary
dysfunction (7,22). With respect to the diagnostic method
adopted in the present study, M-mode echocardiography has provided
valuable information, including DWS, E/e' ratio and mitral flow
velocity, for the diagnosis of patients with heart failure and
myocardial infarction.
Patients with heart failure and preserved ejection
fraction (45–90 years) were younger than the control populations
(45–90 years compared with 65–90 years, respectively; P<0.0001)
but the left ventricular end-diastolic dimension (P=0.062) and the
left ventricular end-diastolic volume (P=0.054) were the same
between the groups. The present study aimed to assess if left
ventricular end-diastolic dimension and the left ventricular
end-diastolic volume were successfully predicted by M-mode
echocardiography. If the present study had enrolled patients of
equal age for both groups, it is possible that the results could be
different for left ventricular end-diastolic dimension and the left
ventricular end-diastolic volume between for both groups. In this
condition, the study should perform multivariate analysis to
evaluate the effect of age on left ventricular end-diastolic
dimension and the left ventricular end-diastolic volume considering
the control group as the reference standard. However, in the
present study, logistic regression analysis was not possible, using
the control group as a reference standard because height, waist
circumference, present medication and other demographic parameters
varied between the control and test populations. Therefore, the
present study enrolled patients (age range, 45–90 years) with heart
failure and preserved ejection fraction in the test group, and
65–90 years old patients without known cardiovascular disease in
the control population. Additionally, the left ventricular
end-diastolic dimension and volume declines with age (14,23).
Left ventricular end-diastolic dimension and left ventricular
end-diastolic volume are successfully evaluated by the present
M-mode echocardiographic study. There is a requirement for further
tailored made studies investigating the relationship between M-mode
echocardiography and demographical characteristics of patients with
heart failure and preserved ejection fraction, on adverse
outcomes.
The present study used comparative M-mode
echocardiography to investigate the outcome in patients with heart
failure and preserved ejection fraction. Unlike M-mode
echocardiography, cardiac magnetic resonance (CMR) can acquire
images in any plane and/or any long axis, and diastolic stiffness
parameters have been found to be similar to those of M-mode
echocardiography (1,24). However, CMR has limited facilities in
China and T1 CMR mapping data in patients with heart failure and
preserved ejection fraction are still limited (25). Therefore, the present study adopted
traditional M-mode echocardiography to perform the study.
During the present study, following a 3-year
follow-up period, ~40% of patients died. This is higher than
demonstrated in previous studies (2,7,19,20). It
is well-established that systolic regional thickening abnormalities
in patients with heart failure patients and preserved ejection
fraction accompany significant ischemia, but systolic regional
thickening cannot precisely identify ischemic territories (26). Therefore, the finding that patients
with lower systolic regional thickening have a higher mortality
rate may be an important finding in itself.
In the present study, the DWS values were 0.30±0.007
in the test population, but all test population patients were
hypertensive, diabetic and possibly under medication. The
echocardiogram profile appeared similar to the normal values for
the test population. However, since significant differences were
observed in all echocardiogram parameters in the test population
compared with the control group (P<0.05). Therefore, there is
the possibility of the false positive errors (α-errors) for
echocardiogram parameters of the present study. The present study
performed multiple regression analysis to predict mortality.
Multiple regression analysis showed that the left ventricular
end-systolic and end-diastolic dimension, SF, posterior wall
thickness at end-systole and at end-diastole, DWS, the mitral flow
velocity, the myocardial wall motion velocity and the other
abnormal echocardiogram parameters of the test population were
associated with a higher mortality.
There are a number of limitations of the present
study. DWS was found to be significantly lower in control patients.
The reason behind these false low values of DWS in the normal
subjects is not clear. The effect of the different types of heart
failure on M-mode echocardiography parameters were not evaluated.
M-mode echocardiography parameters were not compared with CMR T1
images. Although none of the patients in the present study
exhibited abnormalities of regional wall motion, M-mode
echocardiography parameters do not provide reliable results in such
cases (27), instead, speckle
tracking echocardiography should be performed in such patients.
In conclusion, the majority of echo parameters
between the two groups were statistically different. Each of these
parameters were found to be sufficient to indicate a higher
mortality rate among patients with heart failure and preserved
ejection fraction, similar to that of DWS. Poor outcomes in Chinese
patients with heart failure patients and preserved ejection
fraction can be predicted using DWS measurements and further M-mode
echocardiography parameters. Echocardiography is a useful tool for
diagnosing the clinically important dilemma of heart failure with
preserved ejection fraction.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors had read and approved submission for
publication. XL was a project administrator who contributed to the
conceptualization and literature review of the study. XM
contributed to the formal analysis, data curation, software
analysis, and drafted and edited the manuscript for intellectual
content. The authors agree to be accountable for all aspects of
work ensuring integrity and accuracy.
Ethics approval and consent to
participate
The study had been registered in the Research
registry (www.researchregistry.com), UID No.
researchregistry4465 (https://www.researchregistry.com/register-now#home/registrationdetails/5bc043c4e8a7130a3e730a64/)
dated 1 August 2012. The protocol (ADH/HUM/CL/5/15 dated 25 July
2012) had been granted by the Affiliated Dongfeng Hospital review
board. The study adhered to the laws of China, standards for the
reporting of diagnostic accuracy studies (http://www.equator-network.org/reporting-guidelines/stard/)
guidelines, the strengthening the reporting of observational
studies in epidemiology (STROBE) statement, and the Declaration of
Helsinki (V2008). All patients had signed an informed consent form
regarding radiology and pathology.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DWS
|
diastolic wall strain
|
|
STARD
|
standards for the reporting of
diagnostic accuracy studies
|
|
STROBE
|
the strengthening the reporting of
observational studies in epidemiology
|
|
LDL
|
low-density lipoprotein
|
|
HDL
|
high-density lipoprotein
|
|
BNP
|
brain natriuretic peptide
|
|
SF
|
shortening fraction
|
|
DICOM
|
digital imaging and communications in
medicine
|
|
CMR
|
cardiac magnetic resonance
|
|
HbA1c
|
glycated hemoglobin
|
References
|
1
|
Paulus WJ, Tschope C, Sanderson JE,
Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De
Keulenaer G, Leite-Moreira AF, et al: How to diagnose diastolic
heart failure: A consensus statement on the diagnosis of heart
failure with normal left ventricular ejection fraction by the Heart
Failure and Echocardiography Associations of the European Society
of Cardiology. Eur Heart J. 28:2539–2550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yap J, Sim D, Lim CP, Chia SY, Go YY,
Jaufeerally FR, Sim LL, Liew R and Ching CK: Predictors of two-year
mortality in Asian patients with heart failure and preserved
ejection fraction. Int J Cardiol. 183:33–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee R, Chan SP, Chan YH, Wong J, Lau D and
Ng K: Impact of race on morbidity and mortality in patients with
congestive heart failure: A study of the multiracial population in
Singapore. Int J Cardiol. 134:422–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
West R, Liang L, Fonarow GC, Kociol R,
Mills RM, O'Connor CM and Hernandez AF: Characterization of heart
failure patients with preserved ejection fraction: A comparison
between ADHERE-US registry and ADHERE-International registry. Eur J
Heart Fail. 13:945–952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
King A: Heart failure: Registry data
highlight global differences in care for HF-PEF. Nat Rev Cardiol.
8:4822011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrari R, Bohm M, Cleland JG, Paulus WJ,
Pieske B, Rapezzi C and Tavazzi L: Heart failure with preserved
ejection fraction: Uncertainties and dilemmas. Eur J Heart Fail.
17:665–671. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohtani T, Mohammed SF, Yamamoto K, Dunlay
SM, Weston SA, Sakata Y, Rodeheffer RJ, Roger VL and Redfield MM:
Diastolic stiffness as assessed by diastolic wall strain is
associated with adverse remodelling and poor outcomes in heart
failure with preserved ejection fraction. Eur Heart J.
33:1742–1749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeda Y, Sakata Y, Higashimori M, Mano T,
Nishio M, Ohtani T, Hori M, Masuyama T, Kaneko M and Yamamoto K:
Noninvasive assessment of wall distensibility with the evaluation
of diastolic epicardial movement. J Card Fail. 15:68–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Omar AM, Bansal M and Sengupta PP:
Advances in Echocardiographic imaging in heart failure with reduced
and preserved ejection fraction. Circ Res. 119:357–374. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tribouilloy C, Rusinaru D, Mahjoub H,
Goissen T, Lévy F and Peltier M: Impact of echocardiography in
patients hospitalized for heart failure: A prospective
observational study. Arch Cardiovasc Dis. 101:465–473. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanaya AM, Kandula NR, Ewing SK,
Herrington D, Liu K, Blaha MJ, Srivastava S, Dave SS and Budoff MJ:
Comparing coronary artery calcium among U.S. South Asians with four
racial/ethnic groups: The MASALA and MESA studies. Atherosclerosis.
234:102–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Newby DE, Williams MC, Flapan AD, Forbes
JF, Hargreaves AD, Leslie SJ, Lewis SC, McKillop G, McLean S, Reid
JH, et al: Role of multidetector computed tomography in the
diagnosis and management of patients attending the rapid access
chest pain clinic, The Scottish computed tomography of the heart
(SCOT-HEART) trial: Study protocol for randomized controlled trial.
Trials. 13:1842012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hulsmann M, Berger R, Mortl D, Gore O,
Meyer B and Pacher R: Incidence of normal values of natriuretic
peptides in patients with chronic heart failure and impact on
survival: A direct comparison of N-terminal atrial natriuretic
peptide, N-terminal brain natriuretic peptide and brain natriuretic
peptide. Eur J Heart Fail. 7:552–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzue M, Mori K, Inoue M, Hayabuchi Y,
Nakagawa R and Kagami S: Developmental changes in the left
ventricular diastolic wall strain on M-mode echocardiography. J
Echocardiogr. 12:98–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blanchard DG, Malouf PJ, Gurudevan SV,
Auger WR, Madani MM, Thistlethwaite P, Waltman TJ, Daniels LB,
Raisinghani AB and DeMaria AN: Utility of right ventricular Tei
index in the noninvasive evaluation of chronic thromboembolic
pulmonary hypertension before and after pulmonary
thromboendarterectomy. JACC Cardiovasc Imaging. 2:143–169. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lurz P, Eitel I, Adam J, Steiner J,
Grothoff M, Desch S, Fuernau G, de Waha S, Sareban M, Luecke C, et
al: Diagnostic performance of CMR imaging compared with EMB in
patients with suspected myocarditis. JACC Cardiovasc Imaging.
5:513–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hay I, Rich J, Ferber P, Burkhoff D and
Maurer MS: Role of impaired myocardial relaxation in the production
of elevated left ventricular filling pressure. Am J Physiol Heart
Circ Physiol. 288:H1203–H1208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grewal J, McKelvie R, Lonn E, Tait P,
Carlsson J, Gianni M, Jarnert C and Persson H: BNP and NT-proBNP
predict echocardiographic severity of diastolic dysfunction. Eur J
Heart Fail. 10:252–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zile MR, Gaasch WH, Anand IS, Haass M,
Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M,
McMurray JJ, et al: Mode of death in patients with heart failure
and a preserved ejection fraction: Results from the Irbesartan in
Heart Failure with Preserved Ejection Fraction Study (I-Preserve)
trial. Circulation. 121:1393–1405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lam CS, Roger VL, Rodeheffer RJ, Borlaug
BA, Enders FT and Redfield MM: Pulmonary hypertension in heart
failure with preserved ejection fraction: A community-based study.
J Am Coll Cardiol. 53:1119–1126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tschope C and Paulus WJ: Is
echocardiographic evaluation of diastolic function useful in
determining clinical care? Doppler echocardiography yields dubious
estimates of left ventricular diastolic pressures. Circulation.
120:810–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Borlaug BA and Paulus WJ: Heart failure
with preserved ejection fraction: Pathophysiology, diagnosis and
treatment. Eur Heart J. 32:670–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cain PA, Ahl R, Hedstrom E, Ugander M,
Allansdotter-Johnsson A, Friberg P and Arheden H: Age and
sex-specific normal values of left ventricular mass, volume and
function for gradient echo magnetic resonance imaging: A
cross-sectional study. BMC Med Imaging. 9:22009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang XS and Sun JP: Advances in diastolic
heart failure. World J Cardiol. 2:58–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rommel KP, Lucke C and Lurz P: Diagnostic
and prognostic value of CMR T1-mapping in patients with heart
failure and preserved ejection fraction. Rev Esp Cardiol (Engl Ed).
70:848–855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsaftaris SA, Zhou X, Tang R, Li D and
Dharmakumar R: Detecting myocardial ischemia at rest with cardiac
phase-resolved blood oxygen level-dependent cardiovascular magnetic
resonance. Circ Cardiovasc Imaging. 6:311–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Omar AM, Vallabhajosyula S and Sengupta
PP: Left ventricular twist and torsion: Research observations and
clinical applications. Circ Cardiovasc Imaging. 8:e0030292015.
View Article : Google Scholar : PubMed/NCBI
|