Introduction
Acute myocardial infarction refers to myocardial
necrosis caused by acute, persistent ischemia and hypoxia (coronary
artery dysfunction) (1). This
disease has a rapid onset and a high mortality rate causing great
harm to the health of patients (2).
There have been many advances in the treatment of myocardial
infarction. Rapid thrombus aspiration or PCI is the first choice in
patients with acute myocardial infarction, and the routine
treatment is anticoagulation and thrombolysis (3,4).
However, it has been found in clinic that even if
infarction-related blood vessels were opened at first occurence,
not all cardiomyocytes could obtain effective blood perfusion
(5), which led to poor prognosis.
When acute myocardial infarction occurs, how to reduce thrombosis
and increase myocardial blood supply is a hot research topic at
present (6).
Atorvastatin is a new generation of HMG-CoA
reductase inhibitor, which reduces the content of blood lipid in
blood vessels by inhibiting cholesterol synthesis in hepatocytes.
It has pharmacological effects of anti-inflammation, lipid
regulation and improvement of ventricular function (7), and can improve ventricular ejection
fraction and weaken myocardial remodeling in patients with
ischemia-induced heart failure (8).
Recently, some studies have reported that high-dose of atorvastatin
(40 mg) pretreatment can improve the clinical outcome of patients
with myocardial infarction undergoing emergency percutaneous
coronary intervention (9,10).
Hypoxia inducible factor (HIF) is a transcription
factor that responds to the reduction or hypoxia of available
oxygen in the cell environment (11). The HIF-1 signal cascade reaction
mediates the effects of hypoxia on the cells, which typically allow
the cells to differentiate continuously, promote the formation of
blood vessels, and are important for the formation of a vascular
system in an embryo and a tumor. Vascular endothelial growth factor
(VEGF) is a signal protein produced by cells that stimulate blood
vessels and is part of the system of restoring tissue oxygen
supply. The normal function of VEGF is to produce new blood vessels
and collateral circulation during embryonic development, which
plays an indirect role in improving neuronal vascular perfusion
(12). At present, it has been
reported that the concentrations of VEGF and HIF-1α may be related
to myocardial remodeling and angiogenesis (13). It also has been reported that HIF-1
may be an ischemic response medium and has the potential diagnostic
effect of HIF-1 tissue table as a marker of ischemia (14), which can effectively reflect the
process of acute myocardial infarction to a certain extent. In this
study, the effect of atorvastatin combined with routine therapy on
the concentrations of HIF-1 and VEGF in rats with acute myocardial
infarction was explored, and the therapeutic effect of atorvastatin
combined with routine therapy was investigated, so as to provide
reference for clinical diagnosis and treatment of acute myocardial
infarction.
Materials and methods
General materials
Fifty male Wistar rats were purchased from the
Experimental Animal Center of Zhejiang Academy of Medical Sciences
(Hangzhou, China), and were randomly divided into 5 groups (n=10).
The rats were 2 months old and weighed 200–220 g. Four groups were
modeled for myocardial infarction, while one group (the control
group) was not treated. From the four successful modeled groups,
one group of rats was randomly selected as the routine therapy
group to receive intravenous infusion of nitroglycerin (0.3 mg/kg)
(Shandong Miyoshi Medicine Co., Ltd.; H13022503) and aspirin (20
mg/kg/d) (CR Double-Crane; H11022441). Another group of rats (the
study group) was treated with intravenous nitroglycerin and aspirin
combined with atorvastatin (20 mg/kg/d) (Pfizer; H20051408). One
group (the single drug group) was only treated with atorvastatin
(20 mg/kg/d). The last group (the model group) did not receive any
treatment. Rats were kept in cages with controlled temperature,
light cycles and humidity (23°C, 12/12 light cycles, 55±10%) and
with free access to water.
Empirical method
Establishment of rat model of acute
myocardial infarction
The rat model of acute myocardial infarction (AMI)
was established. The rats were anesthetized by intraperitoneal
injection, and then endotracheal intubation connected with
ventilator was performed to assist respiration. Multi-channel
physiological signal acquisition and processing system were
connected to record the electrocardiogram (ECG). The chest was
opened, and the heart was exposed. The pericardium was cut, and the
left anterior descending branch of the coronary artery was ligated
by non-invasive suture. The thoracic cavity was closed layer by
layer, and routinely disinfected to prevent infection. The rats
were fed in a single cage. The standard lead II on the
multi-channel physiological recorder was observed. If the
ST-segment elevation and/or T-wave elevation or decrease was
observed, and the muscle tissue around the rat appeared pale or
dark, the modeling was successful.
Medication
In the control group and the model group, the mice
were given intragastric administration of the same amount of saline
once a day for 7 days. Twenty-four hours after modeling,
nitroglycerin (0.3 mg/kg) (Shandong Miyoshi Medicine Co., Ltd.;
H13022503) and aspirin (20 mg/kg/d) (CR Double-Crane; H11022441)
were given intravenously to the routine therapy group. The study
group was given atovastatin (20 mg/kg/d) (Pfizer; H20051408) on the
basis of routine treatment, once a day, intragastrically for 7
days. The single drug group was only treated with atorvastatin. All
experimental steps were approved by the Animal Ethics Committee of
Zibo Central Hospital (Zibo, China).
Determination of HIF-1, VEGF content
in serum of rats in each group
The venous blood of the rats was taken before
treatment (T0), and 3 days (T1), 5 days (T2), and 7 days (T3) after
treatment. The blood sample was centrifuged. The upper serum was
obtained after centrifugation (2,000 × g, at 4°C for 15 min). The
levels of HIF-1 (Shanghai Guangrui Biotechnology Co., Ltd.; rat
elisa389) and VEGF (Shanghai Guangrui Biotechnology Co., Ltd.; rat
elisa179) in the serum of the rats were detected by ELISA. The
above steps were performed strictly according to the instructions
of the kit.
Examination of left ventricular
function in each group
After anesthesia with 2% xylazine (5 mg/kg) and 5%
ketamine (35 mg/kg) (15) residual
hair in the left chest was removed by hair removal cream and skin
preparation knife after anaesthesia administration.
Echocardiography was used to determine cardiac function. The left
ventricular internal diameter at systole (LVIDs), left ventricular
diastolic diameter (LVIDd), left ventricular ejection fraction
(LVEF) and left ventricular fractional shortening (LVFS) were
measured.
Observation index
The concentrations of HIF-1 and VEGF in the serum of
rats in each group; comparison of the effects of left ventricular
function in each group of rats; correlation between left
ventricular function and HIF-1 and VEGF in rats.
Statistical analysis
The SPSS 24.0 (IBM Corp., Armonk, NY, USA) software
was used to statistically calculate all experimental results. All
graphics were plotted by GraphPad 8 (Soft Ηead Inc.) and the
results were checked twice. The measurement data are presented as
the mean ± standard deviation. One factor variance analysis and LSD
(Least-Significant Difference) post test were used for the
comparison among groups. Variance analysis of repeated measurement
and bonferroni post-test were used for the comparison of data among
multiple time-points. Pearson's correlation coefficient was used
for the correlation analysis. Statistical significance was set at
P<0.05.
Results
Modeling results
Forty rats were successfully modeled with acute
myocardial infarction (AMI). The success rate of modeling was
100%.
Concentration of HIF-1 and VEGF in rat
serum at T0, T1, T2 and T3
There were significant differences in HIF-1 at T0,
T1, T2 and T3 among the groups (P<0.001), and the control group
was the lowest at each time-point. There was a significant
difference in HIF-1 among multiple time-points within the group
(P<0.001). There was no significant difference in HIF-1 at T0,
T1, T2 and T3 in the control group (P>0.05). In the model group,
routine therapy group, single drug group and study group, HIF-1 at
T0 was the lowest, T1 was higher than T0, and T3 was the highest.
There were significant differences in VEGF at T0, T1, T2 and T3
among the groups (P<0.001); the control group was the lowest,
and the study group was the highest at each time-point. There was a
significant difference in VEGF among multiple time-points within
the group (P<0.001) (Table I).
There was no significant difference in VEGF at T0, T1, T2 and T3 in
the control group (P>0.05). In the model group, routine therapy
group, single drug group and study group, VEGF at T0 was the
lowest, T1 was higher than T0 and T3 was the highest (Table II).
 | Table I.Comparison of HIF-1 concentrations at
different time-points in the four groups (ng/ml, n=10). |
Table I.
Comparison of HIF-1 concentrations at
different time-points in the four groups (ng/ml, n=10).
| Time-point | Control group | Model group | Routine therapy
group | Single drug
group | Study group | F value | P-value |
|---|
| T0 | 0.28±0.09 |
1.12±0.07a |
1.11±0.06a |
1.12±0.05a,e |
1.13±0.11a | 226.21 | <0.001 |
| T1 | 0.26±0.17 |
1.36±0.11a,e |
1.66±0.12a,b,e |
1.67±0.15a,b,e |
1.76±0.13a–e | 205.165 | <0.001 |
| T2 | 0.27±0.32 |
1.54±0.14a,e,f |
1.79±0.15a,b,e,f |
1.76±0.16a,b,e,f |
1.96±0.16a–f | 119.513 | <0.001 |
| T3 | 0.30±0.17 |
1.73±0.09a,e–g |
2.25±0.24a,b,e–g |
2.23±0.26a,b,e–g |
2.69±0.23a–g | 199.124 | <0.001 |
| F value | 0.069 | 60.51 | 89.74 | 99.79 | 153.7 |
|
|
| P-value | 0.976 | <0.001 | <0.001 | <0.001 | <0.001 |
|
|
 | Table II.Comparison of VEGF concentrations at
different time-points in the four groups (ng/ml, n=10). |
Table II.
Comparison of VEGF concentrations at
different time-points in the four groups (ng/ml, n=10).
| Time-point | Control group | Model group | Routine therapy
group | Single drug
group | Study group | F value | P-value |
|---|
| T0 | 0.09±0.10 |
0.31±0.12a |
0.26±0.05a,b |
0.25±0.06a,e |
0.27±0.12a–c | 7.996 | <0.001 |
| T1 | 0.09±0.16 |
0.37±0.07a,e |
0.54±0.14a,b,e |
0.55±0.12a,b,e |
0.66±0.12a–e | 74.153 | <0.001 |
| T2 | 0.09±0.17 |
0.51±0.08a,e,f |
0.69±0.09a,b,e,f |
0.68±0.08a,b,e,f |
0.97±0.13a–f | 100.314 | <0.001 |
| T3 | 0.09±0.12 |
0.62±0.14a,e–g |
0.96±0.27a,b,e–g |
0.95±0.28a,b,e–g |
1.25±0.23a–g | 41.134 | <0.001 |
| F value | 1.000 | 17.218 | 33.142 | 32.912 | 71.843 |
|
|
| P-value | 0.404 | <0.001 | <0.001 | <0.001 | <0.001 |
|
|
Comparison of left ventricular
function in each group one week after administration
One week after administration, there was significant
difference in left ventricular function among the five groups
(P<0.001). The LVIDs and LVIDd of the model group, the routine
therapy group, single drug group and the study group were higher
than those of the control group, and the LVIDs and LVIDd were
lowest among them. The LVEF and LVFS of the model group, the
routine therapy group, single drug group and the study group were
lower than those of the control group (P<0.001), and the LVEF
and LVFS were the highest among them (P<0.001) (Table III).
 | Table III.Comparison of left ventricular
function in each group of rats. |
Table III.
Comparison of left ventricular
function in each group of rats.
| Index | Control group | Model group | Routine therapy
group | Single drug
group | Study group | F value | P-value |
|---|
| LVIDs (mm) | 4.47±0.52 |
8.21±2.18a |
6.59±2.01a,b |
6.58±2.14a,b |
5.28±1.38a–d | 9.745 | <0.001 |
| LVIDd (mm) | 6.07±0.47 |
10.37±1.72a |
9.82±1.38a,b |
9.85±1.29a,b |
8.26±1.48a–d | 20.413 | <0.001 |
| LVEF% | 70.50±4.61 |
25.62±3.73a |
55.82±3.04a,b |
54.96±3.14a,b |
63.18±1.02a–d | 348.1 | <0.001 |
| LVFS% | 44.93±4.08 |
23.63±4.02a |
31.38±3.92a,b |
31.54±4.03a,b |
39.74±3.07a–d | 60.982 | <0.001 |
Analysis of the correlation between
HIF-1, VEGF and left ventricular function
The level of HIF-1 in the serum of rats with
myocardial infarction was negatively correlated with LVIDs
(r=−0.580, P=0.001) and LVIDd (r=−0.548, P=0.001), but was
positively correlated with LVEF (r=0.822, P=0.001) and LVFS
(r=0.853, P=0.001) (Fig. 1). VEGF
level was negatively correlated with LVIDs (r=−0.555, P=0.044) and
LVIDd (r=−0.613, P=0.001), but was positively correlated with LVEF
(r=0.791, P=0.001) and LVFS (r=0.783, P=0.001) (Fig. 2).
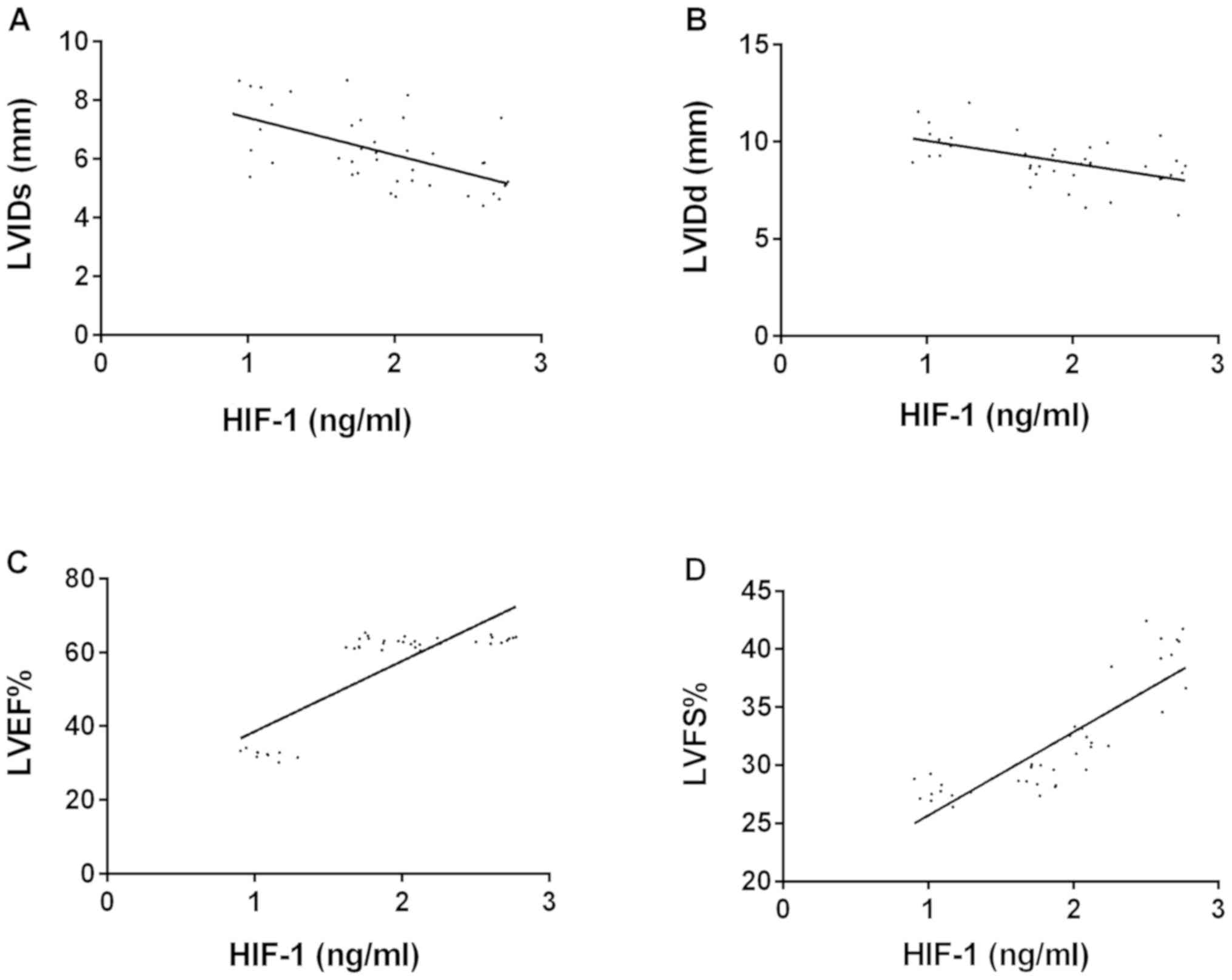 | Figure 1.Correlation analysis between LVIDs,
LVIDd, LVEF, LVFS and HIF-1 levels. (A) Pearson's correlation
coefficient analysis showed that HIF-1 level was negatively
correlated with LVIDs (r=−0.580, P=0.001). (B) Pearson's
correlation coefficient analysis showed that HIF-1 level was
negatively correlated with LVIDd (r=−0.548, P=0.001). (C) Pearson's
correlation coefficient analysis showed that HIF-1 level was
positively correlated with LVEF (r=0.822, P=0.001). (D) Pearson's
correlation coefficient analysis showed that HIF-1 level was
positively correlated with LVFS (r=0.853, P=0.001). HIF-1, hypoxia
inducible factor-1; VEGF, vascular endothelial growth factor;
LVIDs, left ventricular internal diameter at systole; LVIDd, left
ventricular diastolic diameter; LVEF, left ventricular ejection
fraction; LVFS, left ventricular fractional shortening. |
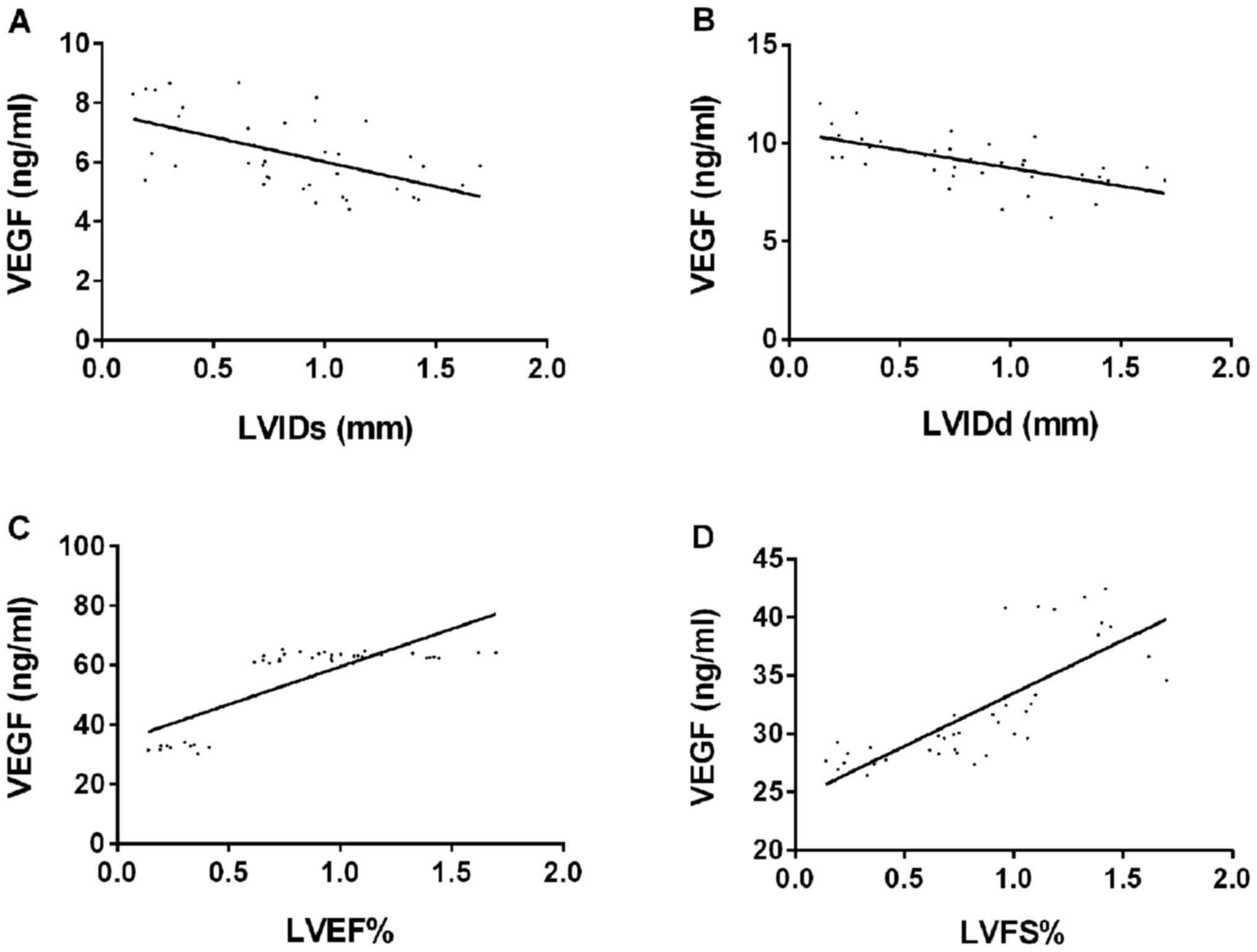 | Figure 2.Correlation analysis between LVIDs,
LVIDd, LVEF, LVFS and VEGF levels. (A) Pearson's correlation
coefficient analysis showed that VEGF level was negatively
correlated with LVIDs (r=−0.555, P=0.044). (B) Pearson's
correlation coefficient analysis showed that VEGF level was
negatively correlated with LVIDd (r=−0.613, P=0.001). (C) Pearson's
correlation coefficient analysis showed that VEGF level was
positively correlated with LVED (r=0.791, P=0.001). (D) Pearson's
correlation coefficient analysis showed that VEGF level was
positively correlated with LVFS (r=0.783, P=0.001). VEGF, vascular
endothelial growth factor; LVIDs, left ventricular internal
diameter at systole; LVIDd, left ventricular diastolic diameter;
LVEF, left ventricular ejection fraction; LVFS, left ventricular
fractional shortening. |
Discussion
Acute myocardial infarction, one of the diseases
threatening human life and health, not only has a very rapid onset,
but also a very poor prognosis (16). In recent years, the incidence of
myocardial infarction has shown an upward trend. The number of
deaths caused by cardiovascular disease is higher than that caused
by cancer or any other disease (17). At present, the focus of the treatment
of acute myocardial infarction is how to improve the survival rate
of patients (18), and atorvastatin
has been proved to have a good effect in the treatment of acute
myocardial infarction (19). The
effects of atorvastatin combined with conventional treatment drugs
(nitroglycerin and aspirin) for acute myocardial infarction on rats
were explored in this study, which provide reference for clinical
practice.
The results of this study showed that the
concentrations of HIF-1 and VEGF in the serum of rats with acute
myocardial infarction were significantly higher than that of normal
rats, suggesting that HIF-1 and VEGF may be involved in the
occurrence of myocardial infarction. This was consistent with the
study by Du et al on the concentration of HIF-1 α in rats
with early acute myocardial ischemia (20). Further comparison of HIF-1 and VEGF
levels between the routine therapy group and the study group showed
that the content in the study group was higher than that in the
routine therapy group, suggesting that the combination of
atorvastatin and routine therapy increased the concentrations of
HIF-1 and VEGF. HIF-1 is the key factor of cell regulation in
hypoxia (21). HIF-1 can stimulate
the release of VEGF-A when cells are anoxic. When cells are
hypoxic, they produce HIF-1, which stimulates the release of
VEGF-A. VEGF is the main downstream target gene of HIF-1α, which
can promote neovascularization and make cells adapt to hypoxic
environment. Therefore, we speculate that the activation of
HIF-1α/VEGF signaling pathway after myocardial infarction
stimulates angiogenesis, which plays a key role in the
proliferation of myocardial tissue in patients with myocardial
infarction. In the study on the effects of triple mutation HIF-1α
on angiogenesis and cardiac function in rats with myocardial
infarction, Li et al (22)
found that triple mutation HIF-1α could improve angiogenesis and
cardiac function, which was consistent with our conjecture. At the
same time, we believe that the protective effect of atorvastatin on
patients with acute coronary syndrome may enhance the stability of
coronary atherosclerotic plaques (23) by reducing inflammatory cascade
(24) and antithrombus activity
(25), and may alleviate the
possibility of thrombus embolism in the distal arteriole and
alleviate myocardial remodeling and left ventricular function
damage to a certain extent. We further studied the cardiac function
of rats in each group. The results showed that there was no
difference in the ventricular function between the study group and
the model group before treatment, but the ventricular function in
the study group was significantly higher than that in the model
group and routine therapy group after treatment, suggesting that
the combined treatment has better effect. Atorvastatin is a statin
lipid-regulating drug (26), mainly
acting on the liver, which can reduce the synthesis of cholesterol
and has the effect of lowering blood lipid (27,28).
Statins lipid-lowering drugs can improve the cardiac function of
patients with acute myocardial infarction (29), and can be used for the treatment of
the disorder of lipid metabolism in patients caused by acute
myocardial infarction, which is beneficial to the treatment of
acute myocardial infarction. Ielasi et al (30) found that atorvastatin combined with
aspirin has a synergistic effect in the treatment of ischemic
stroke, which can be a testament to our experimental results. The
levels of HIF-1 and VEGF in rats of each group were further
analyzed, and the levels of HIF-1 and VEGF in serum of rats with
acute myocardial infarction were positively correlated with the
time of treatment, negatively correlated with LVIDs and LVIDd, and
positively correlated with LVEF and LVFS, suggesting that the
recovery of myocardial function can be judged by measuring the
concentration of HIF-1 and VEGF in peripheral blood in the
future.
In this investigation, the concentrations and
changes of HIF-1 and VEGF in rats with acute myocardial infarction
were compared and analyzed after treatment of routine therapy alone
and atorvastatin combined with routine therapy, and the therapeutic
effect of atorvastatin combined with routine therapy on acute
myocardial infarction was explored. However, due to the limited
experimental conditions, there are certain limitations in this
study. It was not proven that HIF-1 and VEGF are involved in the
occurrence and progression of myocardial infarction. This can be a
key direction of future research.
In conclusion, the concentration of HIF-1 and VEGF
in serum of rats with acute myocardial infarction was significantly
increased, and the concentration of HIF-1 and VEGF in the study
group was significantly lower than that in the routine therapy
group and the single drug group. Compared with routine therapy
alone and the single drug, atorvastatin combined with routine
therapy has more advantages in the treatment of myocardial
infarction.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY and HZ conceived and designed the study, and
drafted the manuscript. JY, LZ and HZ collected, analyzed and
interpreted the experimental data, and revised the manuscript
critically for important intellectual content. JY wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zibo Central Hospital (Zibo, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu C, Tkebuchava T and Hu D: Managing
acute myocardial infarction in China. Eur Heart J. 40:1179–1181.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang CJ, Chen PC, Lin CS, Tsai CL and Tsai
SH: Thrombolytic therapy-associated acute myocardial infarction in
patients with acute ischemic stroke: A treatment dilemma. Am J
Emerg Med. 35:804.e1–804.e3. 2017. View Article : Google Scholar
|
|
4
|
Kassimis G and Picard F: Resorbable
magnesium scaffolds in acute myocardial infarction patients: ‘To be
or not to be’? Cardiology. 142:97–99. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoeke G, Wang Y, van Dam AD, Mol IM, Gart
E, Klop HG, van den Berg SM, Pieterman EH, Princen HMG, Groen AK,
et al: Atorvastatin accelerates clearance of lipoprotein remnants
generated by activated brown fat to further reduce
hypercholesterolemia and atherosclerosis. Atherosclerosis.
267:116–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCarthy CP, Vaduganathan M, McCarthy KJ,
Januzzi JL Jr, Bhatt DL and McEvoy JW: Left ventricular thrombus
after acute myocardial infarction: Screening, prevention, and
treatment. JAMA Cardiol. 3:642–649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma Y, Kong L, Qi S and Wang D:
Atorvastatin blocks increased l-type Ca2+ current and
cell injury elicited by angiotensin II via inhibiting oxide stress.
Acta Biochim Biophys Sin (Shanghai). 48:378–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gavazzoni M, Gorga E, Derosa G, Maffioli
P, Metra M and Raddino R: High-dose atorvastatin versus moderate
dose on early vascular protection after ST-elevation myocardial
infarction. Drug Des Devel Ther. 11:3425–3434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Q, Zhao YG, Wang Z, Jiang HP, Liu WB
and Cao BF: Effects of first high-dose atorvastatin loading in
patients with ST-segment elevation myocardial infarction undergoing
percutaneous coronary intervention. Am J Ther. 25:e291–e298. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu XR, Li KB, Wang P, Xu L, Liu Y, Yang ZS
and Yang XC: The impact of different doses of atorvastatin on
plasma endothelin and platelet function in acute ST-segment
elevation myocardial infarction after emergency percutaneous
coronary intervention. Zhonghua Nei Ke Za Zhi. 55:932–936. 2016.(In
Chinese). PubMed/NCBI
|
|
11
|
Koyasu S, Kobayashi M, Goto Y, Hiraoka M
and Harada H: Regulatory mechanisms of hypoxia-inducible factor 1
activity: Two decades of knowledge. Cancer Sci. 109:560–571. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang N, Chen J, Ferraro GB, Wu L, Datta
M, Jain RK, Plotkin SR, Stemmer-Rachamimov A and Xu L: Anti-VEGF
treatment improves neurological function in tumors of the nervous
system. Exp Neurol. 299:326–333. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng C, Li P, Wang YG, Bi MH and Wu PS:
Study on the expression of VEGF and HIF-1α in infarct area of rats
with AMI. Eur Rev Med Pharmacol Sci. 20:115–119. 2016.PubMed/NCBI
|
|
14
|
Parisi Q, Biondi-Zoccai GG, Abbate A,
Santini D, Vasaturo F, Scarpa S, Bussani R, Leone AM, Petrolini A,
Silvestri F, et al: Hypoxia inducible factor-1 expression mediates
myocardial response to ischemia late after acute myocardial
infarction. Int J Cardiol. 99:337–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eyarefe OD, Ologunagba FM and Emikpe BO:
Wound healing potential of natural honey in diabetic and
non-diabetic wistar rats. Afr J Biomed Res. 17:15–21. 2014.
|
|
16
|
Helft G, Georges JL, Mouranche X, Loyeau
A, Spaulding C, Caussin C, Benamer H, Garot P, Livarek B, Teiger E,
et al e-MUST and CARDIO-ARSIF Registries, : Outcomes of primary
percutaneous coronary interventions in nonagenarians with acute
myocardial infarction. Int J Cardiol. 192:24–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen WW, Gao RL, Liu LS, Zhu ML, Wang W,
Wang YJ, Wu ZS, Li HJ, Gu DF, Yang YJ, et al: China cardiovascular
diseases report 2015: A summary. J Geriatr Cardiol. 14:1–10.
2017.PubMed/NCBI
|
|
18
|
Yamamoto K, Sakakura K, Akashi N, Watanabe
Y, Noguchi M, Seguchi M, Taniguchi Y, Ugata Y, Wada H, Momomura SI,
et al: Novel acute myocardial infarction risk stratification (nARS)
system reduces the length of hospitalization for acute myocardial
infarction. Circ J. 83:1039–1046. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mensah K, Mocanu MM and Yellon DM: Failure
to protect the myocardium against ischemia/reperfusion injury after
chronic atorvastatin treatment is recaptured by acute atorvastatin
treatment: A potential role for phosphatase and tensin homolog
deleted on chromosome ten? J Am Coll Cardiol. 45:1287–1291. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du ZB, Mao RM, Gao WM, Mi L, Cao ZP and
Zhu BL: Early expression of hypoxia-inducible factor-1 alpha after
acute myocardial ischemia in rats. Fa Yi Xue Za Zhi. 28:327–332.
2012.(In Chinese). PubMed/NCBI
|
|
21
|
Chen Y, Tao Y, Zhang L, Xu W and Zhou X:
Diagnostic and prognostic value of biomarkers in acute myocardial
infarction. Postgrad Med J. 95:210–216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Cui Y, He W, Deng X, Wang Y, Cai M,
Wang Y, Pei J, Mei X and Wu P: Effects of triple-mutated
hypoxia-inducible factor-1α on angiogenesis and cardiac function
improvement in rats with myocardial infarction. Cell Physiol
Biochem. 50:2329–2340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Yang J, Zheng J and Gu X: Acute
myocardial infarction in pregnancy: Spasm caused by
hyperthyroidism? J Int Med Res. 47:2269–2273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sivri N, Tekin G, Yalta K, Aksoy Y, Senen
K and Yetkin E: Statins decrease mean platelet volume irrespective
of cholesterol lowering effect. Kardiol Pol. 71:1042–1047. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nordøy A, Svensson B and Hansen JB:
Atorvastatin and omega-3 fatty acids protect against activation of
the coagulation system in patients with combined hyperlipemia. J
Thromb Haemost. 1:690–697. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Otto CM: Heartbeat: Improving acute
myocardial infarction outcomes in women. Heart. 105:501–502. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang M, Vaez M, Dorner TE, Rahman S,
Helgesson M, Ivert T and Mittendorfer-Rutz E: Risk factors for
subsequent work disability in patients with acute myocardial
infarction. Eur J Public Health. 29:531–540. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar M, Rehan HS, Puri R, Yadav M and
Gupta LK: Randomized controlled trial comparing the efficacy of
daily and every other day atorvastatin therapy and its correlation
with serum hydroxymethylglutaryl-CoA reductase enzyme levels in
naïve dyslipidemic patients. Indian Heart J. 70 (Suppl 3):S64–S67.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Komarova IS, Karova LB, Andreeva NV,
Cherkasova NA and Zhelnov VV: Effect of myocardial reperfusion on
ischemic mitral regurgitation in patients with acute myocardial
infarction. Kardiologiia. 59:18–25. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ielasi A, Cerrato E, Geraci S, Campo G,
Garro N, Leoncini M, Sganzerla P, Granata F, Ruggiero R, Varbella
F, et al: Sirolimus-eluting magnesium resorbable scaffold
implantation in patients with acute myocardial infarction.
Cardiology. 142:93–96. 2019. View Article : Google Scholar : PubMed/NCBI
|
















