Introduction
Acute myeloid leukemia (AML) is a common
hematological malignancy characterized by an increase in the number
of undifferentiated cells in the bone marrow and peripheral blood
(1). AML-M5, an AML subtype, has a
mortality rate of nearly 90% (1–3). One of
the main clinical techniques used to treat AML is conventional
chemotherapy. Although allogeneic hemopoietic stem cell
transplantation has emerged as an alternative (4), it is costly, and finding suitable
donors for transplantation is difficult. Both treatment methods
face the challenge of strong drug resistance (5). While many researchers have attempted to
address this issue, there is still a lack of effective drugs for
the treatment of AML; therefore, it is necessary to develop
strategies to overcome drug resistance in AML therapy.
CD40 ligand (CD40L), otherwise known as CD154, is a
33-kDa type II transmembrane protein expressed on the membranes of
T lymphocytes, and belongs to the tumor necrosis factor superfamily
(6). CD40 is the CD40L receptor, and
is expressed on the membranes of B lymphocytes and macrophages
(7). CD40 has been observed not only
in normal healthy cells, but also in cancer cells, such as those
from melanoma, prostate cancer, lung cancer, bladder cancer,
cervical cancer, ovarian cancer, lymphocytic leukemia, lymphoma,
multiple myeloma and AML (7). The
CD40/CD40L axis plays important roles in immunity and inflammation,
and exhibits both pro- and anti-neoplastic activity in many
diseases and cancer types (8). It
has also been reported that CD40L improves the sensitivity to drugs
used in the treatment of stomach cancer (9); however, to the best of our knowledge no
studies have been conducted into the role of CD40L in AML-M5
treatment.
In this study, the involvement of CD40L in the drug
resistance mechanisms of AML-M5 cells was explored. THP-1 cells
were rendered resistant to Adriamycin (ADM), a common
chemotherapeutic agent, and treated with daunorubicin (DNR), a drug
with a highly similar structure and the same pharmacological
effects as ADM. The effects of CD40L on drug treatment and DNR
resistance were evaluated by inducing raised CD40L expression in
THP-1 cells, and assessing apoptosis and the expression of drug
resistance-related genes.
Materials and methods
Preparation of ADM-resistant THP-1/A
cells
The human monocytic THP-1 cell line was obtained
from The Cell Bank of Type Culture Collection of the Chinese
Academy of Sciences, and cultured in RPMI 1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) in an atmosphere containing 5%
CO2 and 95% air at 37°C. To obtain ADM-resistant cells
(denoted THP-1/A cells), normal THP-1 cells were treated with an
ADM concentration gradient (0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 µg/ml)
as previously described (10–13).
When the ADM concentration was 1.2 µg/ml, most of the cells grew
stably. When the ADM concentration was further increased, THP-1
cell apoptosis increased significantly and cell morphology changed.
Therefore, 1.2 µg/ml was chosen as the intervention concentration
(preliminary study; data not shown). The THP-1/A cells were then
cultured in an ADM-free medium for 2 weeks before being used in the
subsequent experiments.
Vector construction and
transfection
The CD40L sequence (accession no. NM_000074.2, CD40L
sequence:
5′-ATGATCGAAACATACAACCAAACTTCTCCCCGATCTGCGGCCACTGGACTGCCCATCAGCATGAAAATTTTTATGTATTTACTTACTGTTTTTCTTATCACCCAGATGATTGGGTCAGCACTTTTTGCTGTGTATCTTCATAGAAGGTTGGACAAGATAGAAGATGAAAGGAATCTTCATGAAGATTTTGTATTCATGAAAACGATACAGAGATGCAACACAGGAGAAAGATCCTTATCCTTACTGAACTGTGAGGAGATTAAAAGCCAGTTTGAAGGCTTTGTGAAGGATATAATGTTAAACAAAGAGGAGACGAAGAAAGAAAACAGCTTTGAAATGCAAAAAGGTGATCAGAATCCTCAAATTGCGGCACATGTCATAAGTGAGGCCAGCAGTAAAACAACATCTGTGTTACAGTGGGCTGAAAAAGGATACTACACCATGAGCAACAACTTGGTAACCCTGGAAAATGGGAAACAGCTGACCGTTAAAAGACAAGGACTCTATTATATCTATGCCCAAGTCACCTTCTGTTCCAATCGGGAAGCTTCGAGTCAAGCTCCATTTATAGCCAGCCTCTGCCTAAAGTCCCCCGGTAGATTCGAGAGAATCTTACTCAGAGCTGCAAATACCCACAGTTCCGCCAAACCTTGCGGGCAACAATCCATTCACTTGGGAGGAGTATTTGAATTGCAACCAGGTGCTTCGGTGTTTGTCAATGTGACTGATCCAAGCCAAGTGAGCCATGGCACTGGCTTCACGTCCTTTGGCTTACTCAAACTCTGA-3′)
was obtained from the National Center for Biotechnology Information
database (https://www.ncbi.nlm.nih.gov/nuccore). The human CD40L
gene cDNA was inserted into the pHBLV-CMVIE-Zs Green-T2A-Puro
lentiviral transfer vector (Hanbio Biotechnology Co., Ltd.), which
emits green fluorescence at the restriction sites for EcoRI
and BamHI. Vector stocks were produced via the
calcium-phosphate transient transfection of 293T cells
(2–5×104 cells) at 70–80% confluence. Cells were
transfected with vectors at 50 TU/ml by using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Stably transduced THP-1/A cells
were maintained under G418 selection (200 µg/ml) for 12 days at
37°C, 5% CO2 incubator, and the transfection rate was
observed by western blotting and fluorescence microscopy (DMIL LED;
Leica Microsystems GmBH).
Cell Counting Kit-8 (CCK-8) assay
To confirm the successful preparation of
ADM-resistant THP-1/A cells, the degree to which THP-1 and THP-1/A
cell proliferation was inhibited after ADM treatment was evaluated
using the CCK-8 assay (Beyotime Institute of Biotechnology). ADM
was administered to THP-1 and THP-1/A cells at concentrations of
0.5, 2, 4, 8 and 16 µg/ml for 24 h at 37°C. Subsequently, 10 µl
CCK-8 solution was added and the optical density was measured at a
wavelength of 450 nm. Inhibition of cell proliferation (%) was
calculated using the following formula:
[1-(ODexperimental-ODblank/ODcontrol-ODblank)]
×100%.
The effect of CD40L on THP-1/A cells was explored by
transfecting with negative control (NC) lentiviral vectors (LV) or
CD40L-LV. The CCK-8 assay was performed to determine the viability
of THP-1/A, NC-LV THP-1/A and CD40L-LV THP-1/A cells between days 0
and 7. Additionally, whether CD40L affected the resistance of
THP-1/A cells to chemotherapeutic agents was examined, using DNR as
a model drug. Cells were cultured in 24-well plates at a density of
1×105 cells/ml, and treated with DNR at 0, 0.5, 2, 4, 8,
16, 32 or 64 µg/ml for 24 h at 37°C. The proliferation of the
DNR-treated THP-1/A, NC-LV THP-1/A and CD40L-LV THP-1/A cells was
then determined using the CCK-8 assay, with results presented as
the degree of cell proliferation inhibition (%).
Cell apoptosis
To investigate the effect of DNR on THP-1/A cells
transfected with CD40L vectors, apoptosis was measured using an
Annexin V/propidium iodide (PI) kit (Beyotime Institute of
Biotechnology). Cells were divided into six groups: THP-1/A, NC-LV
THP-1/A, CD40L-LV THP-1/A, THP-1/A + DNR, NC-LV THP-1/A + DNR and
CD40L-LV THP-1/A + DNR. Cells (1×105 cells/ml) were
seeded into 24-well plates and treated with normal saline or 4
µg/ml DNR once they had reached 70% confluence. After 24 h, 5 µl
Annexin V and 5 µl PI were added to each well, the cells were
incubated for 20 min in the dark at 4°C and apoptosis was
determined by flow cytometry (FC500 MCL flow cytometer with, CXP
Analysis Software Version 2.2; Beckman Coulter, Inc.).
Western blotting
Total protein was extracted using a total protein
extraction kit (ProteoPrep; Sigma-Aldrich; Merck KGaA), and the
protein concentration was measured using a 3,3′-diaminobenzidine
tetrahydrochloride kit (Beyotime Institute of Biotechnology).
Proteins were separated by 12% SDS-PAGE and transferred onto PVDF
membranes. The membranes were blocked with 5% non-fat milk at 4°C
for 2 h, and incubated with primary and secondary antibodies for 2
h each at room temperature. The following primary and secondary
antibodies were used: Anti-CD40L (Abcam; ab65854; 1:500),
anti-multidrug resistance (MDR)-associated protein (MRP) 1 (Abcam;
ab84320; 1:1,000), anti-permeability glycoprotein (P-gp; Abcam;
ab129450; 1:5,000) and horseradish peroxidase-conjugated goat
anti-rabbit IgG H&L (Abcam; ab6721; 1:20,000). Images of
protein bands were captured using an ECL color detection kit (cat.
no. WBKLS0010; EMD Millipore) and the Tanon-5200 Chemiluminescent
Imaging System (Tanon Science and Technology Co., Ltd.).
Caspase-3 activity
The caspase-3 activity of the six aforementioned
experimental groups was determined using a caspase-3 colorimetric
assay kit (Nanjing Keygen Biotech Co., Ltd.), according to the
manufacturer's instructions.
Trypan blue staining
Trypan blue dye (Beyotime Institute of
Biotechnology) was used to evaluate cell death in the six
aforementioned experimental groups. A total of 106
cells/well were pretreated with normal saline or 4 µg/ml DNR at
room temperature for 24 h, in 24-well plates, harvested, mixed with
0.4 % trypan blue, and counted using a hemocytometer under an
optical microscope (×200).
Statistical analysis
All data are from three replicates and presented as
the mean ± SD. Statistical analysis was performed by one-way ANOVA
followed by Duncan's multiple comparisons test using SPSS 19.0 (IBM
Corp.). All figures were prepared using GraphPad Prism 5.0 software
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
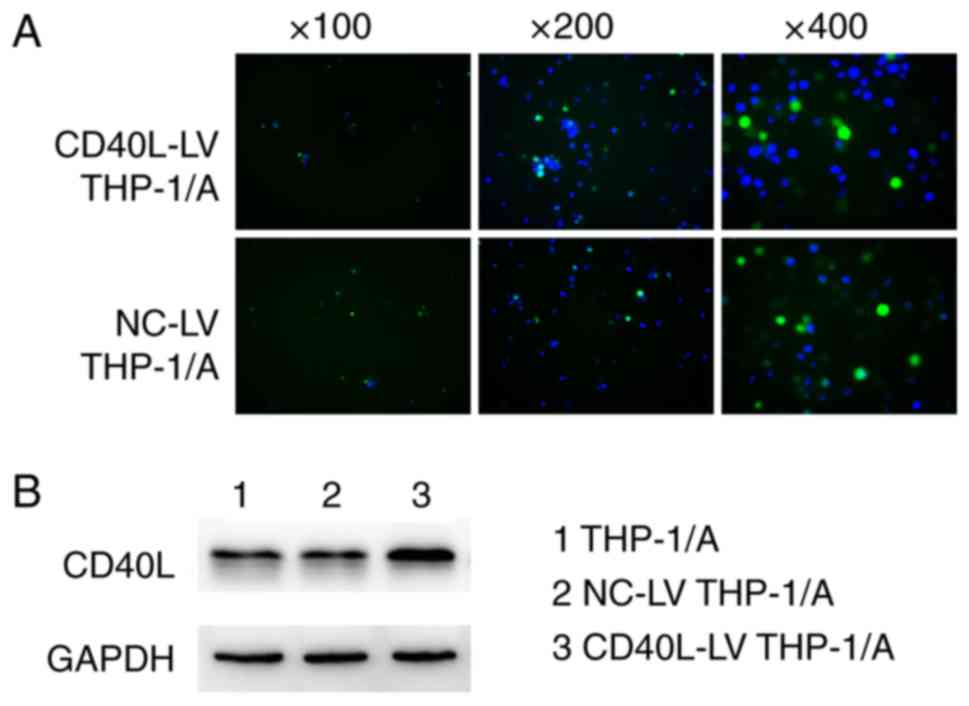
Vector transfection efficiency
To verify the transfection efficiency of the CD40L
expression vector, the green fluorescence from the lentiviral
vectors was visualized under a fluorescence microscope (Fig. 1A). Green fluorescence was observed in
both the NC-LV and CD40L-LV THP-1/A cells, indicating that both of
the fluorescent lentiviral vectors had successfully transfected a
number of the THP-1A cells. Fig. 1B
shows that CD40L protein expression in the CD40L-LV THP-1/A cells
appeared higher than that in the non-transfected cells or those
transfected with NC vectors, suggesting that raised CD40L
expression was achieved via transfection.
CD40L suppresses THP-1/A cell
proliferation
The CCK-8 assay (Fig.
2A) revealed that cell proliferation was inhibited to a
significantly lower level in THP-1/A cells than that in THP-1 cells
(P<0.05) when treated with various ADM concentrations. This
result suggested that when THP-1 cells were rendered resistant to
ADM (THP-1/A cells), the effect of the ADM treatment was attenuated
due to resistance having been developed. This, in turn, led to
greater proliferation of the cells. CD40L vectors were transfected
into the ADM-resistant cells, and it was observed that the
viability of the CD40L-LV THP-1/A cells was lower than that of the
untransfected and NC-LV-transfected THP-1/A cells over a 7 day
period (P<0.05; Fig. 2B). There
was no difference between the viability of THP-1/A and NC-LV
THP-1/A cells during this period.
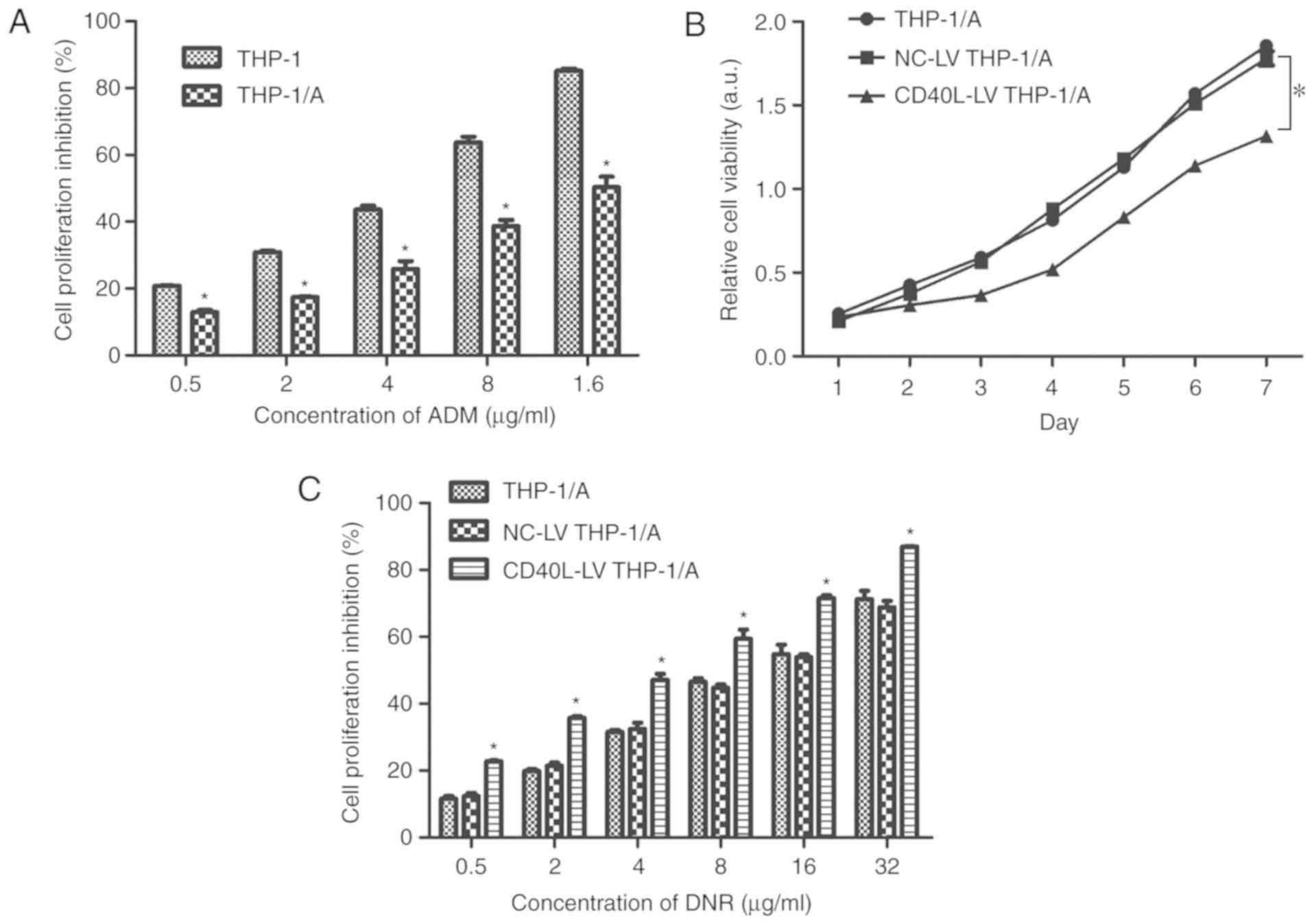 | Figure 2.Proliferation of THP-1 cells subjected
to ADM resistance, CD40L vector transfection and DNR treatment. (A)
The extent of cell proliferation inhibition (%) in THP-1 and
THP-1/A cells was detected using a Cell Counting Kit-8 assay (n=3).
*P<0.05 vs. THP-1. (B) The viability of THP-1/A, NC-LV THP-1/A,
and CD40L-LV THP-1/A cells was evaluated once per day for 7 days.
(C) The extent of cell proliferation inhibition (%) in THP-1/A,
NC-LV THP-1/A and CD40L-LV THP-1/A cells, treated with DNR (0–32
µg/ml), was evaluated using a Cell Counting Kit-8 assay. *P<0.05
vs. NC-LV THP-1/A cells (n=3). ADM, Adriamycin; CD40L, CD40 ligand;
DNR, daunorubicin; LV, lentivirus; NC, negative control. |
CD40L enhances the inhibitory effects
of DNR on THP-1/A cells
To explore whether CD40L contributes to the
inhibitory effect of DNR on THP-1/A cell growth, the proliferation
of THP-1/A, NC-LV THP-1/A and CD40L-THP-1/A cells was measured
after treatment with various concentrations of DNR. The results
shown in Fig. 2C suggest that, upon
DNR treatment, the proliferation of THP-1/A cells transfected with
CD40L vectors was inhibited to a significantly greater extent than
that of both the non-transfected or NC-LV-transfected cells
(P<0.05). These results indicated that DNR exerted a stronger
effect when CD40L expression was raised, suggesting that the
drug-resistant properties of THP-1/A cells were suppressed by
CD40L.
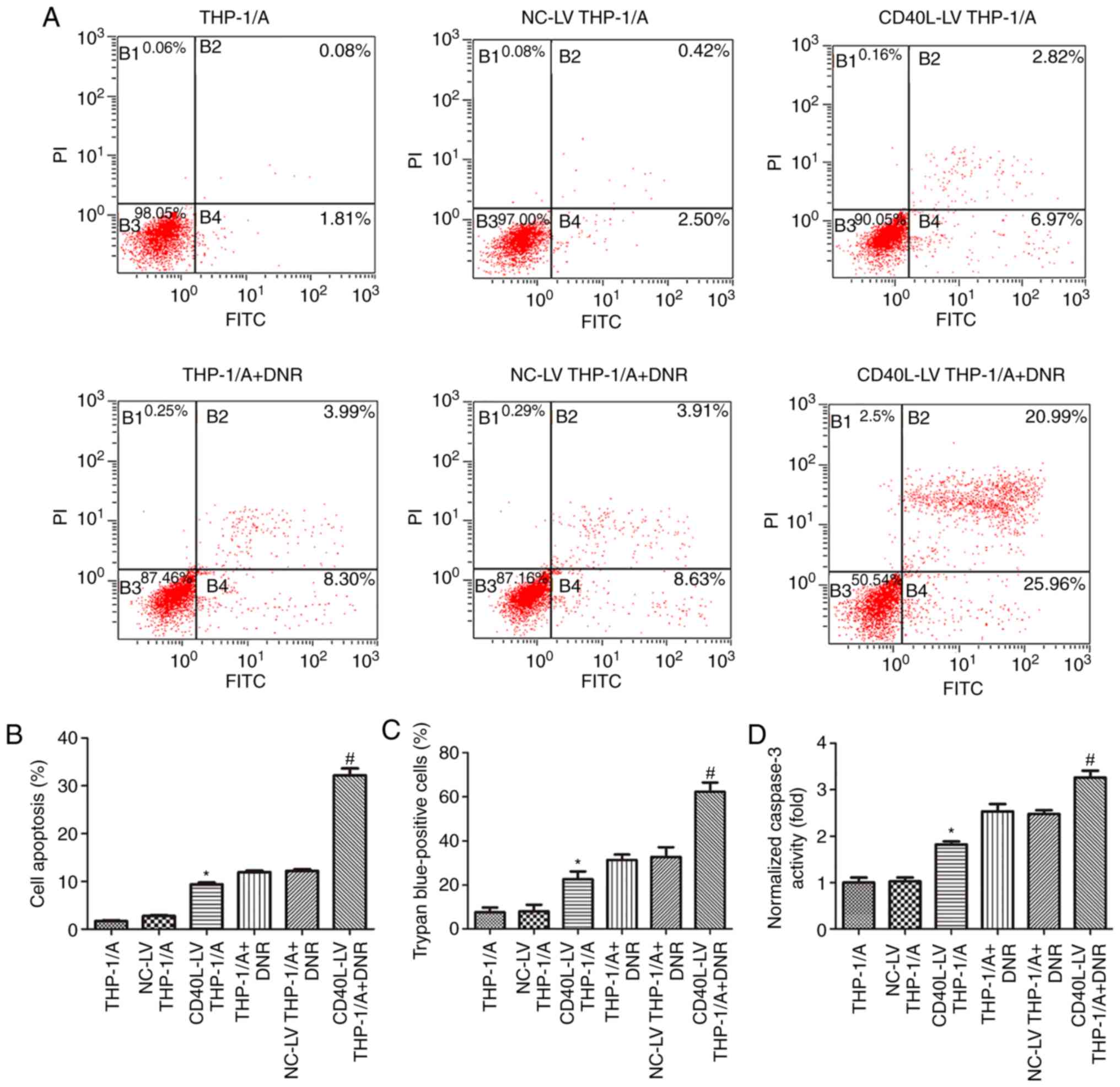
Raised CD40L expression induces
THP-1/A cell apoptosis
The apoptosis of THP-1/A cells with or without
transfection of CD40L vectors and DNR treatment was assessed by
flow cytometry, caspase-3 activity colorimetric assays and trypan
blue staining. In this study, CD40L transfection increased the
apoptosis levels in cells that had not been treated with DNR. The
flow cytometry results demonstrated that THP-1/A cells had a
certain degree of resistance to DNR, but transfection with CD40L
vectors (CD40L-LV THP-1/A + DNR) significantly promoted apoptosis
(P<0.05), indicating that DNR resistance was attenuated by CD40L
(Fig. 3A and B). Similar results
were obtained by trypan blue staining (Fig. 3C), in which positive staining
represents dead cells. The percentage of trypan blue-positive cells
transfected with CD40L-LV and treated with DNR was higher than for
DNR-treated cells that were untransfected or were transfected with
NC-LV (P<0.05). Furthermore, the colorimetry assay revealed that
the activity of caspase-3, a prominent pro-apoptotic protein, was
enhanced in DNR-treated THP-1 cells transfected with the CD40L
vector (Fig. 3D; P<0.05). These
results collectively suggested that CD40L may suppress DNR
resistance in THP-1/A cells.
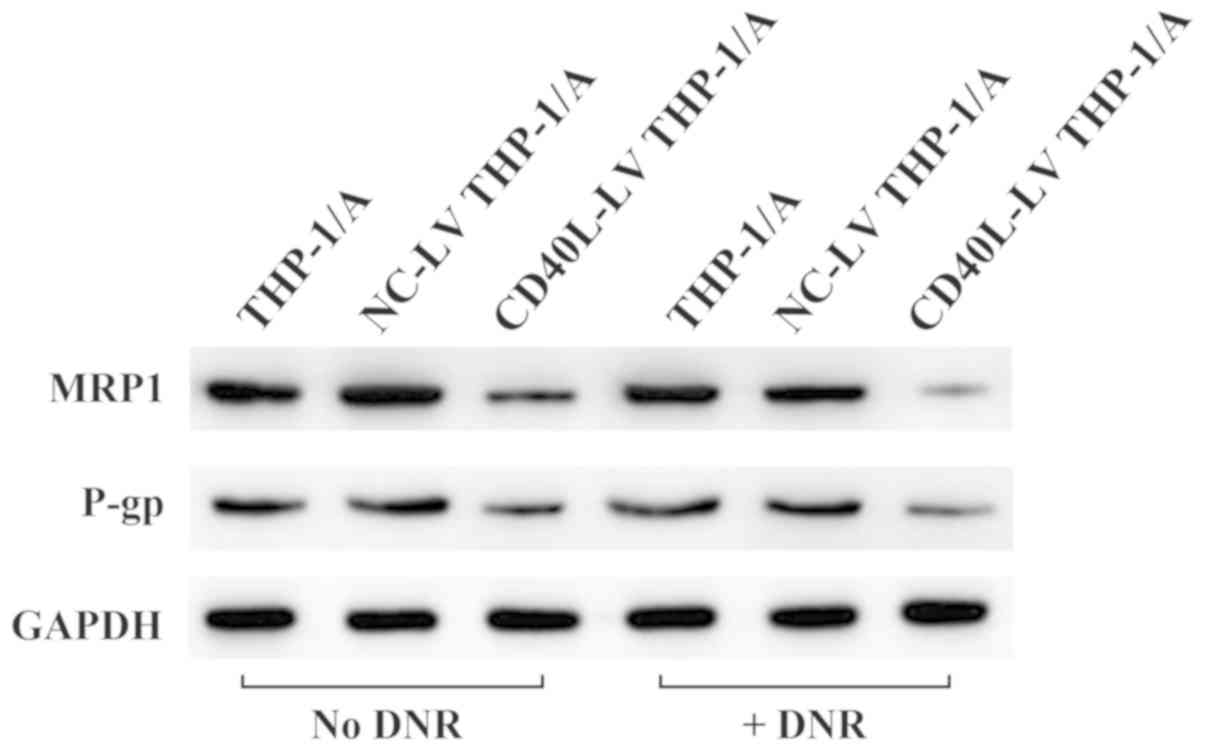
CD40L downregulates the expression of
drug resistance-related genes
To further explore drug resistance in THP-1/A cells,
the protein expression levels of MRP1 and P-pg were measured
(Fig. 4), two proteins which are
associated with MDR. Fig. 4 shows
that the protein expression levels of MRP1 and P-pg appear
attenuated in THP-1/A cells after CD40L-LV transfection. The
reduction appeared even more prominent following treatment with
DNR, indicating that raised CD40L expression effectively
downregulated the expression levels of MRP and P-gp.
Discussion
After long-term exposure to chemotherapeutic drugs,
tumors can become resistant and lose sensitivity to other
antineoplastic drugs with different structures and mechanisms in a
process known as MDR. Although progress has been made in AML
therapy, appropriate and effective treatment methods for the
disease are lacking, with the issue of drug resistance being a
major concern. MDR is a major obstacle to the treatment of leukemia
and the main cause of its recurrence (14). It has been previously found that the
drug resistance index of leukemia cells to doxorubicin was as high
as 182 (15). Styczyński et
al (16) tested the
anti-leukemia activity of five glucocorticoids in 25 AML cell
samples, and found that there was significant cross-resistance
against all of the glucocorticoids in all samples. The ATP-binding
cassette transmembrane protein family is the largest family of
transmembrane proteins (17) and
includes P-gp, MDR-related proteins and lung resistance proteins.
Raised expression of ATP-binding cassette transmembrane proteins is
recognized as the main cause of MDR in leukemia cells (18). P-gp can be detected in almost all
drug-resistant leukemia strains and has a very broad range of
substrates, including ADM, epirubicin, docetaxel and other
anti-cancer drugs (19). Leukemia
cell MDR is also closely related to the activity of the apoptotic
gene p53 and NF-κB.
Both CD40L and soluble CD40L generated from
surface-expressed CD40L interact with the CD40 receptor, resulting
in the activation of various pro-inflammatory responses (20). Qin et al (21) reported that soluble CD40L enhanced
the drug sensitivity of ovarian cancer cells, whilst during bladder
cancer treatment in a mouse model, simultaneous CD40L and
5-fluorouracil treatment resulted in an enhanced therapeutic effect
compared to 5-fluorouracil treatment alone (22). However, few studies have examined the
effect of CD40L on MDR in AML-M5.
ADM (23) and DNR
(24) are important and
commonly-used drugs in the clinical treatment of AML. In this
study, THP-1 cells were rendered resistant to ADM (THP-1/A cells)
and served as a cellular model of drug-resistant AML-M5 (3,25). The
cells underwent transfection to raise CD40L expression, and the
resulting changes in drug resistance were elucidated via DNR
administration. The CCK-8 assay and flow cytometry were used to
measure cell growth and apoptosis. It was revealed that CD40L
inhibited THP-1/A cell growth and enhanced DNR-induced cell death.
Furthermore, since the drug resistance-related proteins MRP1 and
P-pg are highly expressed in drug-resistant cells (26,27), the
effect of CD40L and DNR on the activity of these proteins in THP-1
cells was investigated. Following DNR treatment, the expression
levels of MRP1 and P-pg in THP-1/A cells with raised CD40L
expression were lower than those in normal non-transfected cells,
suggesting that CD40L successfully suppressed the DNR resistance of
THP-1/A cells. Due to the pivotal role of P-gp in MDR, current
research on reversing drug resistance in leukemia has mainly
focused on inhibiting P-gp expression. Thus far, studies have been
carried out on four generations of P-gp inhibitors. The first
generation consists of verapamil (a calcium channel blocker) and
cyclosporin A (an immunosuppressant); the second generation
consists of dextral verapamil and valspodar (28,29); the
third generation consists of tariquidar and laniquidar (30); and the fourth generation consists of
alkanol compounds (31). Despite
this research, little has been achieved due to the considerable
side effects and low inhibition ability of these P-gp inhibitors.
Reversing MDR by altering drug accumulation in drug-resistant cells
is also a focus of current research. Increased expression of
ATP-binding cassette-associated proteins can reduce the
concentration of therapeutic drugs within cells, thereby resulting
in failure to achieve the therapeutic effects and leading to drug
resistance (32). Styczynski et
al (33) found that the
concentrations of the drugs idarubicin (IDA) and DNR were lower in
drug-resistant AML cells, and that raised P-gp expression decreases
the IDA and DNR concentrations. When multiple drugs are used in
combination, the sensitivity of leukemia drug-resistant cells to
chemotherapeutic drugs can be increased (34). In this study, the protein expression
of MRP1 and P-gp in the DNR group (without CD40L) did not change
significantly; however, the protein expression of MRP1 and P-pg in
the raised CD40L expression group (without DNR) and CD40L + DNR
group appeared to decrease, particularly in the CD40L + DNR group.
These results suggested that raised CD40L expression not only
inhibits MRP1 and P-gp expression, but also promotes the DNR
sensitivity of AML drug-resistant cells. Raised CD40L may inhibit
the drug-resistance of AML cells by inhibiting P-gp expression.
Whether raised CD40L expression can promote the accumulation of DNR
in drug-resistant AML cells and alter their drug release system
remains to be elucidated.
In the present study, the effect of CD40L on drug
resistance in ADM-resistant THP-1 cells was observed, however the
use of multiple cell lines was beyond the scope of the present
study. The use of a greater number of cell lines would allow for
further verification that raised expression of CD40L attenuates
drug resistance in AML-5M. In conclusion, CD40L may enhance the
anti-tumor properties of DNR and may be a possible solution for
treating drug resistance in AML by reducing P-gp expression and
promoting DNR-induced cell apoptosis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Guizhou Social
Development Research Project [Qian SY (2014); grant no. 3025].
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contribution
ZF conducted the experiments, analyzed the data, and
partially wrote the manuscript. QC conducted the experiments and
partially wrote the manuscript. Both were responsible for the
design of this study and ensured the accuracy and completeness of
the results.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferrara F and Schiffer CA: Acute myeloid
leukaemia in adults. Lancet. 381:484–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walter RB, Othus M, Burnett AK, Löwenberg
B, Kantarjian HM, Ossenkoppele GJ, Hills RK, van Montfort KG,
Ravandi F, Evans A, et al: Significance of FAB subclassification of
‘acute myeloid leukemia, NOS’ in the 2008 WHO classification:
Analysis of 5848 newly diagnosed patients. Blood. 121:2424–2431.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pession A, Martino V, Tonelli R,
Beltramini C, Locatelli F, Biserni G, Franzoni M, Freccero F,
Montemurro L, Pattacini L and Paolucci G: MLL-AF9 oncogene
expression affects cell growth but not terminal differentiation and
is downregulated during monocyte-macrophage maturation in AML-M5
THP-1 cells. Oncogene. 22:8671–9676. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basara N, Schulze A, Wedding U, Mohren M,
Gerhardt A, Junghanss C, Peter N, Dölken G, Becker C, Heyn S, et
al: Early related or unrelated haematopoietic cell transplantation
results in higher overall survival and leukaemia-free survival
compared with conventional chemotherapy in high-risk acute myeloid
leukaemia patients in first complete remission. Leukemia.
23:635–640. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Magenau J and Couriel DR: Hematopoietic
stem cell transplantation for acute myeloid leukemia: To whom,
when, and how. Curr Oncol Rep. 15:436–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hassan GS, Stagg J and Mourad W: Role of
CD154 in cancer pathogenesis and immunotherapy. Cancer Treat Rev.
41:431–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elgueta R, Benson MJ, de Vries VC, Wasiuk
A, Guo Y and Noelle RJ: Molecular mechanism and function of
CD40/CD40L engagement in the immune system. Immunol Rev.
229:152–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korniluk A, Kemona H and Dymicka-Piekarska
V: Multifunctional CD40L: Pro- and anti-neoplastic activity. Tumour
Biol. 35:9447–9457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li R, Chen WC, Wang WP, Tian WY and Zhang
XG: CD40 signaling activated by agonistic anti-CD40 monoclonal
antibody 5C11 has different effects on biological behavior of
gastric carcinoma cells. Immunol Lett. 131:120–125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Ling XL, Li SW, Li XQ and Yan B:
Establishment of a human hepantoma multidrug resistant cell line in
vitro. World J Gastroenterol. 16:2291–2297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng TT, Pan ZH, Zheng XS, et al:
Establishment and drug resistance of adriamycin-resistant breast
cancer MCF-7/Adm cell line. J Zhejiang Sci Tech Univ. 31:216–219.
2014.(In Chinese).
|
|
12
|
Li DY: Effects of overexpression of CD40L
gene on proliferation, apoptosis and drug resistance of
Kasumi-1/ADM cell line. Zunyi Med Coll. 2016.(In Chinese).
|
|
13
|
Aldinucci D, Poletto D, Nanni P, Degan M,
Rupolo M, Pinto A and Gattei V: CD40L induces proliferation,
self-renewal, rescue from apoptosis, and production of cytokines by
CD40-expressing AML blasts. Exp Hematol. 30:1283–1292. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marie JP, Huet S, Faussat AM, Perrot JY,
Chevillard S, Barbu V, Bayle C, Boutonnat J, Calvo F,
Campos-Guyotat L, et al: Multicentric evaluation of the MDR
phenotype in leukemia French network of the drug resistance
intergroup, and drug resistance network of assistance
publique-hôpitaux de Paris. Leukemia. 11:1086–1094. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun Y, Wang C, Meng Q, Liu Z, Huo X, Sun
P, Sun H, Ma X, Peng J and Liu K: Targeting P-glycoprotein and
SORCIN: Dihydromyricetin strengthens anti-proliferative efficiency
of Adriamycin via MAPK/ERK and Ca 2+ -mediated apoptosis pathways
in MCF-7/ADR and K562/ADR. J Cell Physiol. 233:3066–3079. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Styczyński J, Wysocki M, Debski R,
Balwierz W, Rokicka-Milewska R, Matysiak M, Balcerska A, Kowalczyk
J, Wachowiak J, Sońta-Jakimczyk D and Chybicka A: Cross-resistance
to five glucocorticoids in childhood acute lymphoblastic and
non-lymphoblastic leukemia samples tested by the MTT assay:
Preliminary report. Acta Biochim Pol. 49:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rees DC, Johnson E and Lewinson O: ABC
transporters: The power to change. Nat Rev Mol Cell Biol.
10:218–227. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benabbou N, Mirshahi P, Bordu C, Faussat
AM, Tang R, Therwath A, Soria J, Marie JP and Mirshahi M: A subset
of bone marrow stromal cells regulate ATP-binding cassette gene
expression via insulin-like growth factor-I in a leukemia cell
line. Int J Oncol. 45:1372–1380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fletcher JI, Williams RT, Henderson MJ,
Norris MD and Haber M: ABC transporters as mediators of drug
resistance and contributors to cancer cell biology. Drug Resist
Updat. 26:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davidson JD, Ma L, Flagella M, Geeganage
S, Gelbert LM and Slapak CA: An increase in the expression of
ribonucleotide teductase large subunit 1 is associated with
gemcitabine resistance in non-small cell lung cancer cell lines.
Cancer Res. 64:3761–3766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin L, Qiu H, Zhang M, Zhang F, Yang H,
Yang L, Jia L, Qin K, Jia L, Dou X, et al: Soluble CD40 ligands
sensitize the epithelial ovarian cancer cells to cisplatin
treatment. Biomed Pharmacother. 79:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liljenfeldt L, Gkirtzimanaki K, Vyrla D,
Svensson E, Loskog AS and Eliopoulos AG: Enhanced therapeutic
anti-tumor immunity induced by co-administration of 5-fluorouracil
and adenovirus expressing CD40 ligand. Cancer Immunol Immunother.
63:273–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen C, Xu W and Wang CM: Combination of
celecoxib and doxorubicin increases growth inhibition and apoptosis
in acute myeloid leukemia cells. Leuk Lymphoma. 54:2517–2522. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pophali P and Litzow M: What is the best
daunorubicin dose and schedule for acute myeloid leukemia
induction? Curr Treat Options Oncol. 18:32017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chanput W, Mes JJ and Wichers HJ: THP-1
cell line: An in vitro cell model for immune modulation approach.
Int Immunopharmacol. 23:37–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YK, Wang YJ, Gupta P and Chen ZS:
Multidrug resistance proteins (MRPs) and cancer therapy. AAPS J.
17:802–812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Binkhathlan Z and Lavasanifar A:
P-glycoprotein inhibition as a therapeutic approach for overcoming
multidrug resistance in cancer: Current status and future
perspectives. Curr Cancer Drug Targets. 13:326–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuruo T, Iida H, Tsukagoshi S and Sakurai
Y: Overcoming of vincristine resistance in P388 leukemia in vivo
and in vitro through enhanced cytotoxicity of vincristine and
vinblastine by verapamil. Cancer Res. 41:1967–1972. 1981.PubMed/NCBI
|
|
29
|
Mickisch GH, Noordzij MA, vd Gaast A,
Gebreamlack P, Köhrmann KU, Mogler-Drautz E, Kupper H and Schröder
FH: Dexverapamil to modulate vinblastine resistance in metastatic
renal cell carcinoma. J Cancer Res Clin Oncol. 121 (Suppl
3):R11–R16. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomas H and Coley HM: Overcoming
multidrug resistance in cancer: An update on the clinical strategy
of inhibiting p-glycoprotein. Cancer Control. 10:159–165. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martelli C, Coronnello M, Dei S, Manetti
D, Orlandi F, Scapecchi S, Novella Romanelli M, Salerno M, Mini E
and Teodori E: Structure-activity relationships studies in a series
of N,N-bis(alkanol)amine aryl esters as P-glycoprotein (Pgp)
dependent multidrug resistance (MDR) inhibitors. J Med Chem.
53:1755–1762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dei S, Romanelli MN, Manetti D,
Chiaramonte N, Coronnello M, Salerno M and Teodori E: Design and
synthesis of aminoester heterodimers containing flavone or chromone
moieties as modulators of P-glycoprotein-based multidrug resistance
(MDR). Bioorg Med Chem. 26:50–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Styczynski J, Wysocki M, Debski R, Kurylak
A, Balwierz W, Rokicka-Milewska R, Matysiak M, Balcerska A,
Kowalczyk J, Wachowiak J, et al: The influence of intracellular
Idarubicin and daunorubicin levels on drug cytotoxicity in
childhood acute leukemia. Acta Biochim Pol. 49:99–107. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Styczynski J, Toporski J, Wysocki M,
Debski R, Chybicka A, Boruczkowski D, Wachowiak J, Wojcik B,
Kowalczyk J, Gil L, et al: Fludarabine, treosulfan and etoposide
sensitivity and the outcome of hematopoietic stem cell
transplantation in childhood acute myeloid leukemia. Anticancer
Res. 27:1547–1551. 2007.PubMed/NCBI
|