Introduction
Insomnia is a serious clinical problem, affecting
~30% of the general worldwide population (1). Chronic insomnia causes impairments in
cognitive, physical and daytime functions (1,2). People
suffering from insomnia are more prone to accidents and psychiatric
disorders (1). Though current
well-established pharmacotherapy greatly improves insomnia, it is
limited by unwanted negative side effects, including cognitive and
memory impairment, tolerance, withdrawal and dependency (3). Modern therapeutic research aims to find
safer drugs that act on specific targets.
Phenotypic screening in zebrafish combined with
classical pharmacology and in vivo models provides a
successful strategy for drug discovery (4–6).
Zebrafish are a rapidly developing and high-throughput diurnal
vertebrate model that exhibits high conservation of the structure
and function of the central nervous system (CNS) compared with mice
and humans (7). These attractive
features make zebrafish well suited to study sleep and neurological
disorders. Various behavioural phenotypes in zebrafish have been
generated, including sleep, memory, learning, stress and anxiety
(8–11). Furthermore, a study performed by
Rihel et al (12) assessed
the effects of thousands of psychotropic drugs or compounds on
sleep parameters in zebrafish, revealing that there is
pharmacological conservation in zebrafish and that the zebrafish
model provides an appropriate platform for efficient sleep drug
screening.
The advantages of herbal medicine or traditional
Chinese treatments for the management of insomnia are being
increasingly recognized, particularly for their stable curative
effects and limited side effects (13,14). A
variety of herbal medicines are available for insomnia treatment
(14). Radix Polygala (the dried
root of Polygala tenuifolia Willd) is a common single herb
prescribed for patients with insomnia (15). Previous in vivo studies have
revealed that the extract of Radix Polygala can improve cognitive
deficits and has antipsychotic, antidepressive and
anti-inflammatory activities (16,17).
Additionally, exposing mice to Radix Polygala extract resulted in
the attenuation of cocaine-induced hyperlocomotion and prolonged
pentobarbital-induced sleep duration, suggesting that Radix
Polygala has a sedative effect (18–20).
Tenuifolin is one of the constitutions of Radix Polygala, which has
blood-brain barrier permeability and can be quickly distributed in
the brain to perform pharmacological actions (21). Cao et al (15) revealed that tenuifolin enhanced the
hypnotic effects of pentobarbital by increasing total sleep time
and decreasing sleep latency. In wild-type mice, tenuifolin
promoted sleep without influencing the power density of a sleep
electroencephalogram, and this effect may have been mediated by the
activation of the gamma-aminobutyric acid (GABA)ergic systems
and/or the inhibition of the noradrenergic systems (15). However, studies on the
sleep-promoting effects of tenuifolin are still limited and there
is a lack of verification in other animal species. Considering the
advantages of zebrafish in sleep research, the present study
investigated the neuroactive effects of tenuifolin on sleep
behaviour in zebrafish and the neural signalling pathways that are
potentially involved, based on well-established behavioural
profiles. The present research aims to better understand the
biological effects of tenuifolin and its underlying mechanisms,
thus facilitating the development of novel therapeutics for
insomnia.
Materials and methods
Animals
Adult zebrafish of the AB strain (obtained from the
Laboratory of Neurology, The First Affiliated Hospital, Sun Yat-sen
University) were maintained in a 14/10 h light/dark (L/D) cycle at
28.5°C with a recirculating water system. Fertilized eggs were
collected from the natural spawning of breeding pairs (male and
female ratio of 1:2). Embryos and larvae were kept in standard
embryo water (0.05 g KCl, 0.025 g NaHCO3, 3.5 g NaCl,
0.1 g CaCl2 and 0.1% methylene blue in 1 l distilled
water) under a consistent temperature of 28.5°C and a photoperiod
cycle matching that of the adults in an incubator. The embryo water
was changed twice a day and the dead or abnormal embryos were
discarded. There is a certain mortality rate in the process of
natural hatching of fertilized eggs. All the larvae were disposed
of according to local regulation (Regulation on the Administration
of Experimental Animals; http://www.labagd.com/index.aspx) following the
completion of behavioural testing. All experiments were performed
following internationally recognized guidelines on animal welfare
(2013 AVMA Guidelines for the Euthanasia of Animals) (22), as well as the Guide for the Care and
Use of Laboratory Animals. The present study was approved by the
Animal Studies Committee of The Guangzhou University of Chinese
Medicine.
Chemical treatments
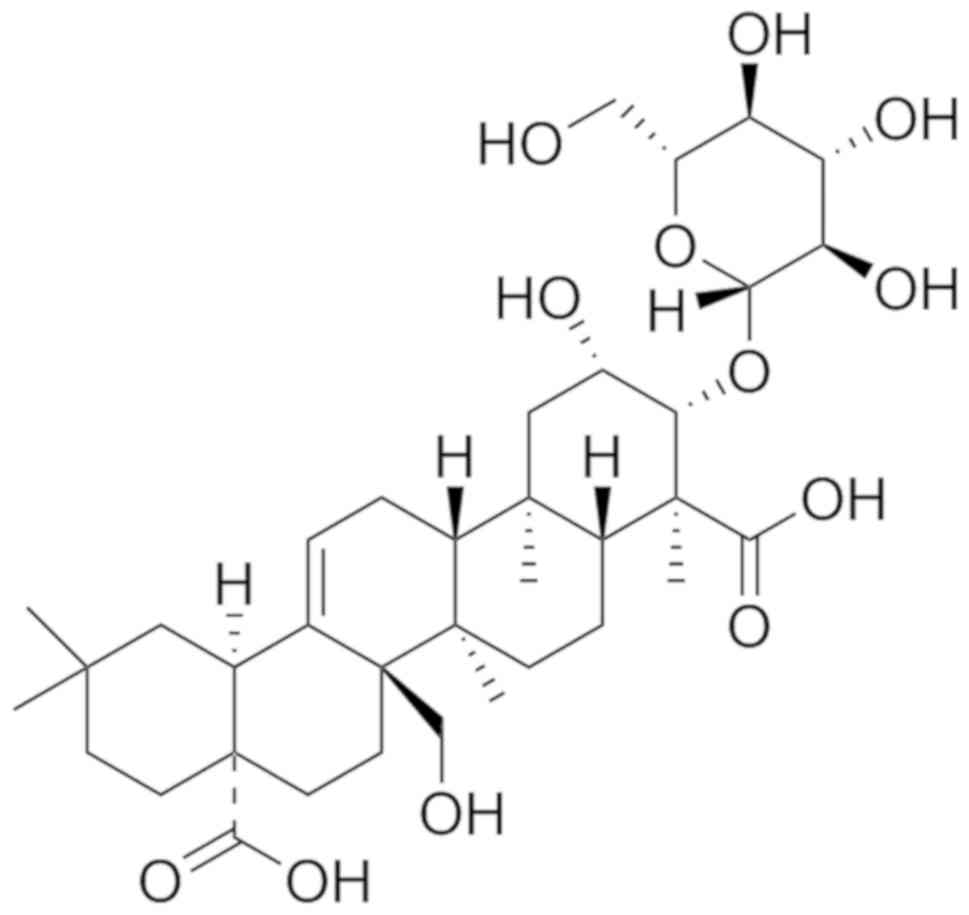
Tenuifolin (Meilun Bio Co., Ltd.; purity, >98%;
20 mg; Fig. 1) and melatonin
(Shanghai Aladdin Bio-Chem Technology Co., Ltd.; purity, 98%; 250
mg) were dissolved in DMSO to make stock solutions. Larvae at 4
days post-fertilization (dpf) were each transferred to a well of a
96-well plate (one larvae per well) with 300 µl standard embryo
water. A total of 60 µl 6X tenuifolin/melatonin solution was added
for dilution to the final treatment concentration. Larvae were
exposed to drugs beginning at 96 h post-fertilization (hpf). The
following tenuifolin concentrations were tested, 1, 10, 20 and 30
µM. Standard embryo water served as an untreated control and 10 µM
melatonin served as a positive control. In addition, the present
study used the GABAA antagonist, picrotoxin (Shanghai
Macklin Biochemical Co., Ltd.; purity, >98%; 25 mg). Cotreatment
with 30 µM tenuifolin and 20 µM picrotoxin was also performed. The
final DMSO exposure was <0.3%. All drug concentrations tested in
the current study did not cause animal death. Each treatment was
tested on 12 larvae and was repeated three times.
Behavioural testing
Behavioural testing was performed for 24 h beginning
at 11pm at 4 dpf (110 hpf) till 11pm at 5 dpf using a Viewpoint
video tracking system (Viewpoint Life Sciences, Inc.). The 96-well
plate was placed into a Zebrabox chamber (Viewpoint Life Sciences,
Inc.; http://www.viewpoint.fr) following drug
exposure and was immersed in a recirculating water bath that was
maintained at 28.5°C (24 h). The experimental parameters were set
as follows: Detection threshold, 40; burst, 25; freeze, 4; and bin
size, 60 sec (12). All recordings
were performed with the same 14/10 h L/D cycle.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed to investigate the effects of
tenuifolin on the transcription of genes associated with the
signalling pathways that may have been involved. Larvae were
exposed to 10 µM tenuifolin at 4 dpf for 24 h. They were rapidly
paralyzed (200 mg/l MS-222) before sacrifice and RNA extraction.
Total RNA was extracted from whole larvae using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA using a PrimeScript RT reagent kit with gDNA
Eraser (Takara Bio, Inc.) in accordance with the manufacturers
protocol (reaction conditions were 37°C for 15 min, 85°C for 5 sec;
4°C). qPCRs were run on a CFX96 touch instrument (Bio-Rad
Laboratories, Inc.) using SYBR Premix Ex Taq II (Takara Bio, Inc).
The thermocycling conditions were as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec. The
experiment was performed in triplicate with three samples (each
sample was pooled from 30 larvae) per group. The sequences of the
primers were as follows: Serotonin transporter (sert) protein
forward, 5′-CATCTATGCTGAGGCTATTG-3′ and reverse,
5′-AAGAATATGATGGCGAAGA-3′; serotonin 1A (5-HT1A)
receptor forward, 5′-ATGAGGATGAGCGGGATGTAG-3′ and reverse,
5′-CAATCAGCCAGGACCACG-3′; glutamate decarboxylase 1 (gad1) forward,
5′-AACTCAGGCGATTGTTGCAT-3′ and reverse, 5′-TGAGGACATTTCCAGCCTTC-3′;
GABAA receptor α1 (gabra1) forward, 5′-TCAGGCAGAGCTGGAAGGAT-3′ and
reverse, 5′-TGCCGTTGTGGAAGAACGT-3′; GABA transporter 1 (gat1)
forward, 5′-ATGCTGTTTATCCTGTTCATCCG-3′ and reverse,
5′-TGTTGAAGGGGTTGTAGCTCC-3′; and β-actin (β-act) forward,
5′-CATCCATCGTTCACAGGAAGTG-3′ and reverse,
5′-TGGTCGTTCGTTTGAATCTCAT-3′. The transcript levels of different
genes were normalized to that of β-act and expressed as the
relative expression, which was calculated using the
2−ΔΔCq method (23).
Data analysis
Data were analysed with R software (version 3.5.0;
http://www.r-project.org/). Detectable
movement of <0.1 sec over the course of 1 min was considered to
be 1 min of rest and a continuous string of rest was considered a
‘rest bout’. In total, six rest/wake parameters, including i) total
rest (total rest in min); ii) number of rest bouts; iii) rest bout
length (average length of rest bouts); iv) rest latency (the length
of time between the start of a light transition [on or off] and the
appearance of the first rest bout); v) total activity (average time
of detected activity in sec, including all rest bouts); and vi)
waking activity (total time of detected activity, excluding all 1
min periods of rest), were calculated for day and night. Each
parameter was averaged from three repeated experiments and
standardized to the matched untreated control to generate a
fingerprint. Time series analyses for total rest and waking
activity were conducted by averaging these two parameters in 10 min
intervals.
Since drugs with shared targets induced similar
phenotypes in zebrafish larvae, the comparison of well-known and
uncharacterized drugs based on behavioural phenotypes may predict
the targets of drugs whose mode of action is poorly understood
(12). Distance correlation analysis
was therefore performed by calculating Pearson's correlation
coefficient to identify known compounds that were similar to
tenuifolin in behavioural phenotypes. These compounds were obtained
from a well-established behavioural profiling database (12). Each compound was assigned a
behavioural fingerprint and in each, different colours indicated
the value of different behavioural parameters. By extracting the
colour value (red, green and blue), the corresponding behavioural
parameters were calculated (24).
Among >500 compounds with significantly altered behaviour
compared with controls, ~100 well-characterized compounds were
selected for correlation analysis in the present study. Statistical
comparisons between treatment groups and untreated group were
performed by one-way ANOVA followed by Tukey test. Student's t-test
was performed for comparisons between the transcript levels of
different genes between the 10 µM tenuifolin-treated group and the
untreated group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Behavioural fingerprint induced by
tenuifolin in zebrafish larvae
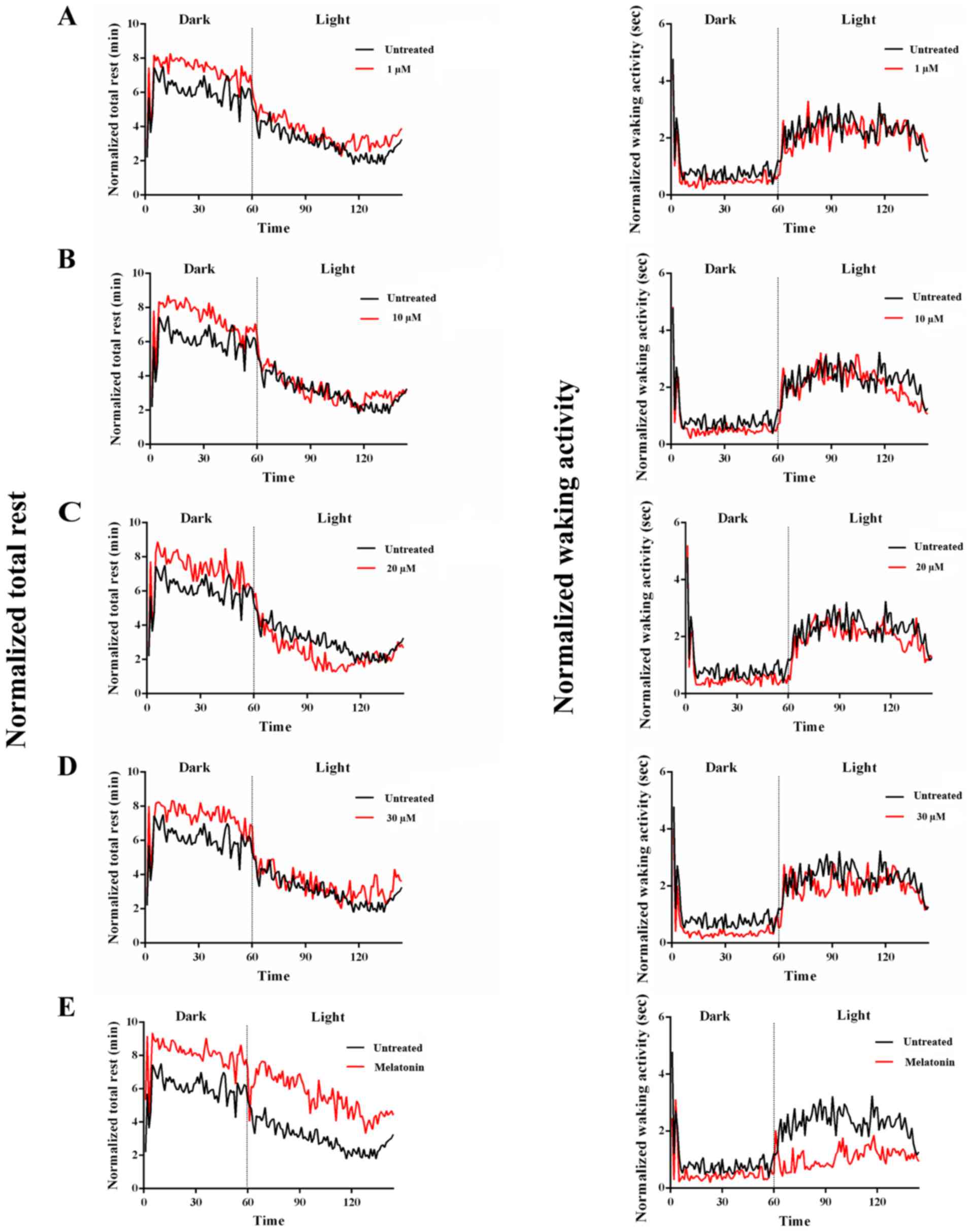
Consecutive 24 h behavioural monitoring revealed
that at all tested concentrations, tenuifolin increased total rest
and rest bout lengths during the dark phase, and caused a reduction
in total activity and waking activity when compared with the
untreated control. The light-phase total rest was increased at all
tested concentration except for 20 µM. The light-phase rest bout
lengths was increased at low concentrations (1 and 10 µM), and was
decreased in high concentrations (20 and 30 µM). The number of rest
bouts was decreased during the dark phase and increased during the
light phase in all tenuifolin-treated group except for 20 µM group
compared with the untreated control group. In addition, rest
latency was decreased during the light phase in response to
tenuifolin treatment. However, the responses to tenuifolin differed
during the dark phase, with low concentrations 1 and 10 µM
increasing the rest latency and high concentrations 20 and 30 µM
decreasing the rest latency (Fig.
2).
Time series analysis and quantitative
analysis of behavioural parameters
The present study performed time series analysis and
quantitative analysis for two principal parameters reflecting the
rest/wake behaviours; the total rest and waking activity. The
present results suggested that both the untreated control and
tenuifolin-treated larvae demonstrated apparent day-night rhythms,
with a longer duration of rest during the dark phase and higher
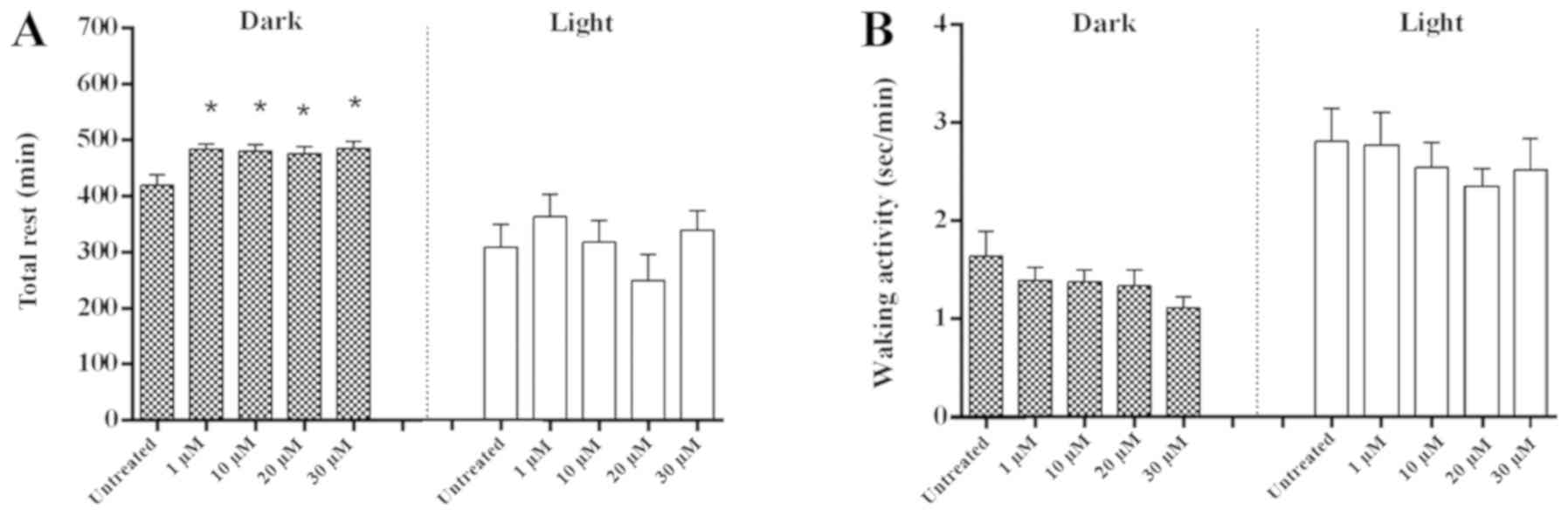
activity levels during the light phase (Fig. 3A-D). The total rest time of the dark
phase, but not of the light phase, was significantly increased in
larvae treated with tenuifolin compared with the untreated control
(Fig. 4A; P<0.05). No significant
difference in activity levels was demonstrated at any concentration
of tenuifolin tested during either the dark or light phase compared
with the untreated control (Fig.
4B).
Correlation analysis and target
prediction
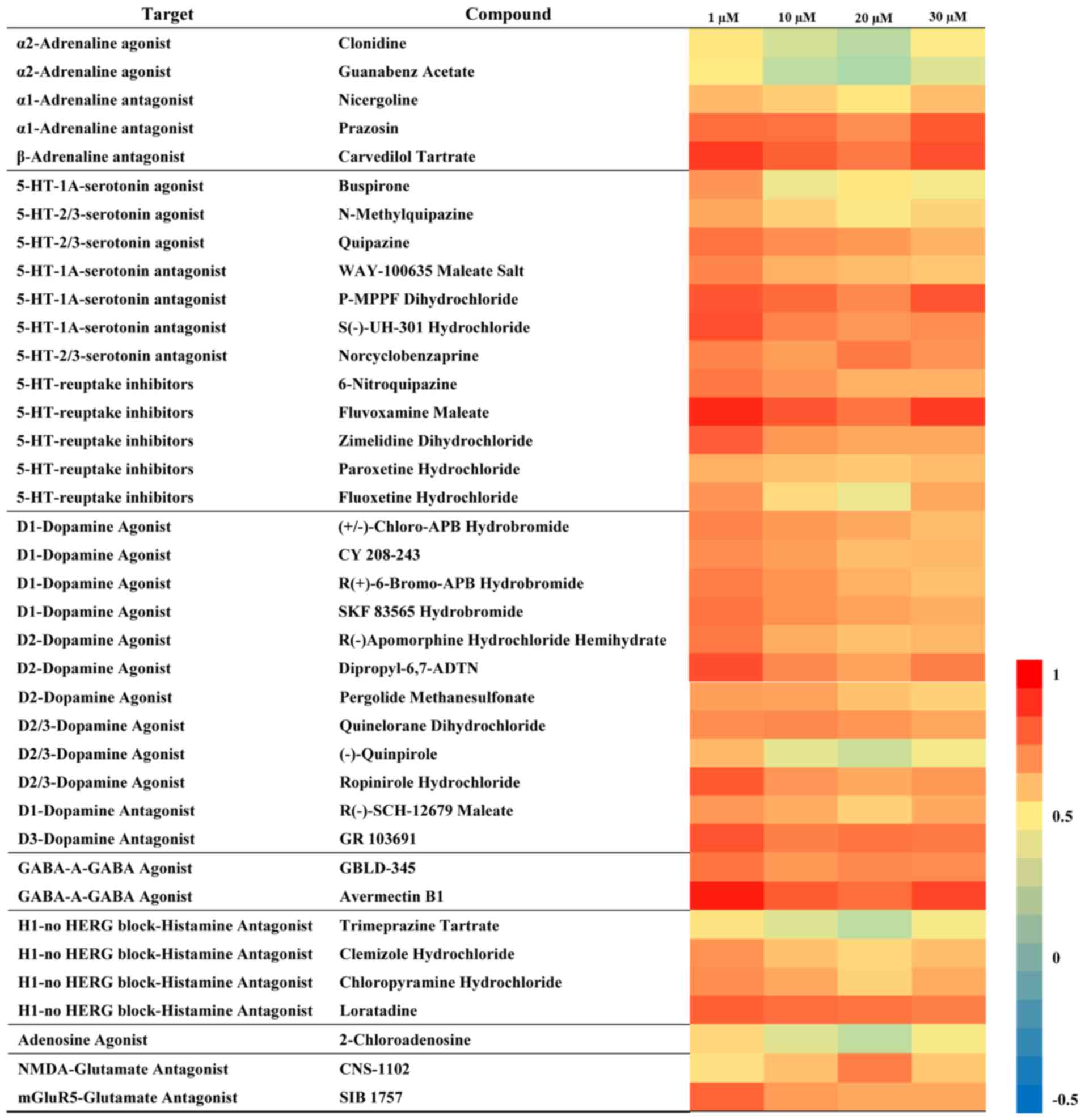
To identify the neural signalling pathways
associated with tenuifolin treatment, Pearson's correlation
coefficient between behavioural phenotypes elicited by tenuifolin
and by known neuroactive compounds was calculated (Fig. S1). The present results indicated the
effects of tenuifolin on larvae were similar to the behavioural
changes induced by selective serotonin reuptake inhibitors (SSRIs)
and GABA-A-GABA agonists (Fig. 5;
Pearson correlation coefficient, >0.75).
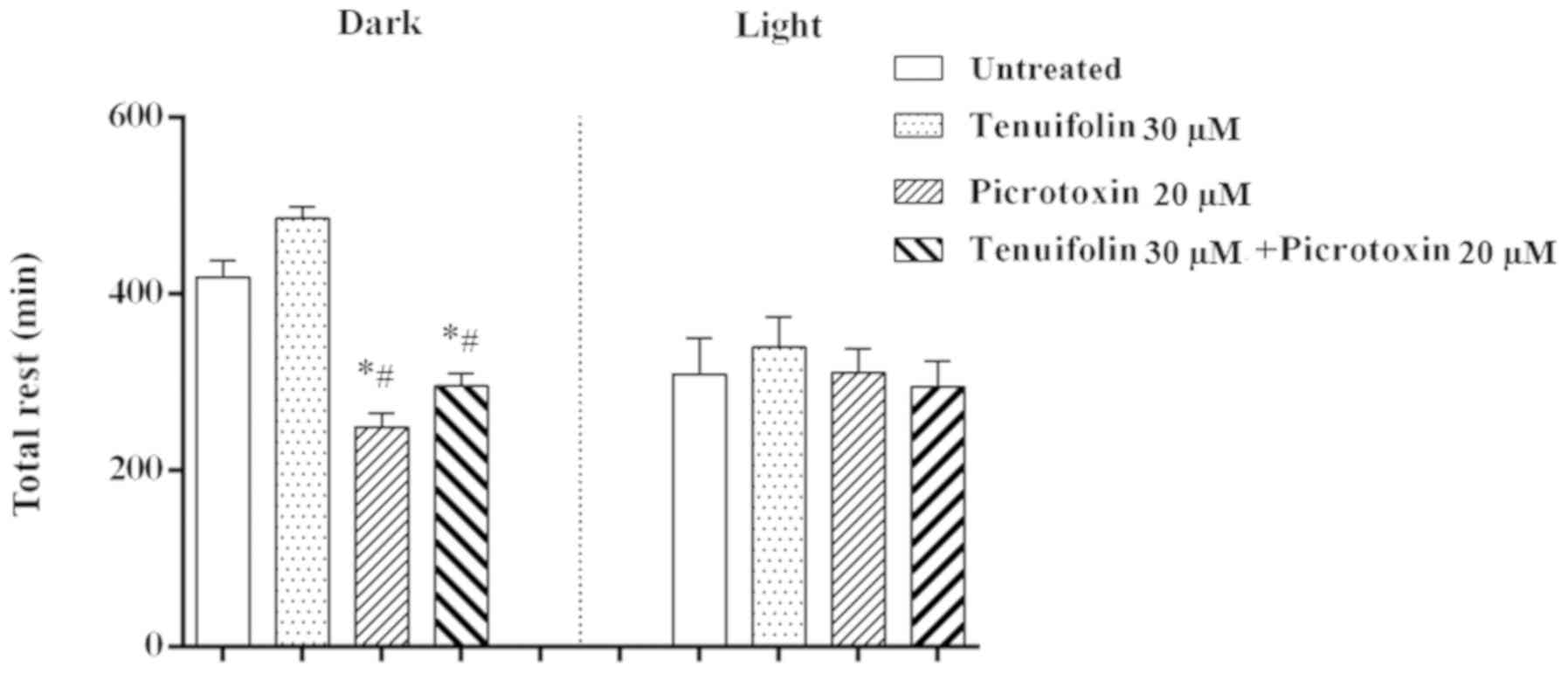
Cotreatment with tenuifolin and
picrotoxin
Since the effects induced by tenuifolin may be
associated with those of GABA-A-GABA agonists, cotreatment with
tenuifolin and the GABAA antagonist picrotoxin was
performed to determine whether the effects of the antagonist could
be counteracted by tenuifolin. The present results identified that
20 µM picrotoxin significantly decreased dark-time rest. However,
cotreatment with 30 µM tenuifolin and picrotoxin for 24 h slightly
counteracted the effect induced by picrotoxin (Fig. 6).
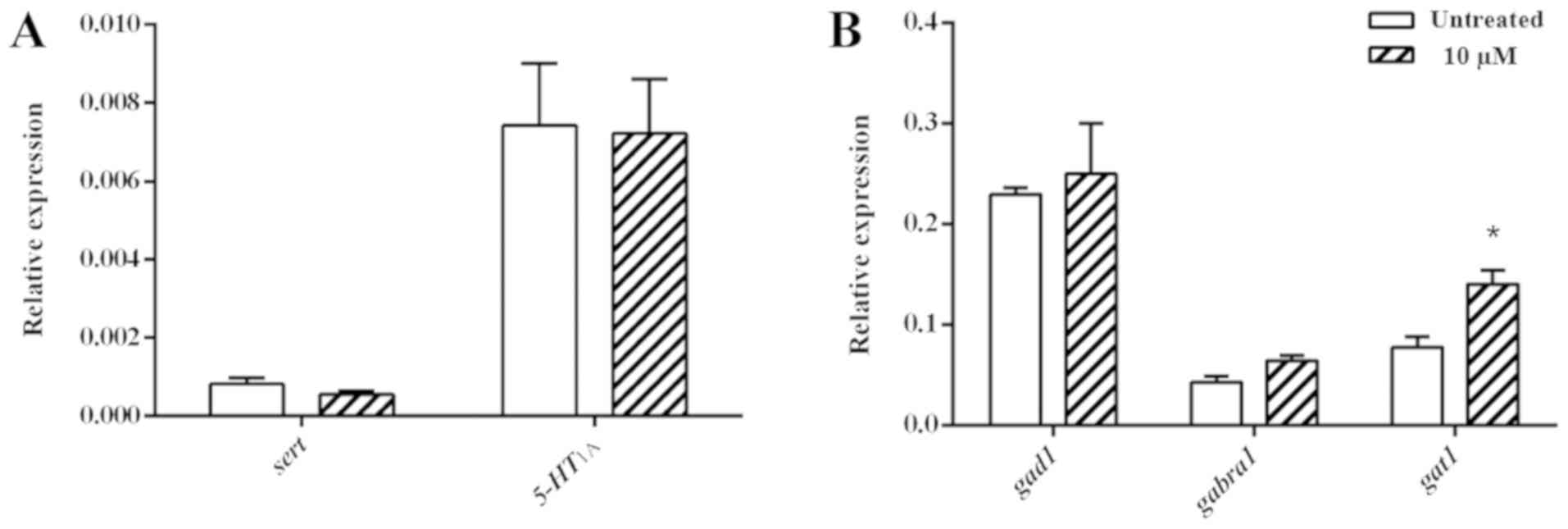
Transcription levels of serotonin- and
GABA-associated genes
The transcription levels of genes associated with
the serotoninergic and GABAergic signalling pathways were examined
after tenuifolin treatment. No significant difference was observed
in serotonin-associated genes in the untreated control and
tenuifolin-treated larvae (Fig. 7A).
The transcription of gad1 and gabra1 was slightly upregulated after
tenuifolin treatment compared with the untreated control. There was
a significant increase in gat1 transcript expression (Fig. 7B) in the tenuifolin-treated group
compared with the untreated control group.
Discussion
Zebrafish are important for sleep research and the
correlation of drugs with biological targets and rest/wake
regulation based on zebrafish behavioural profiling have been well
documented (8,12). To the best of our knowledge, the
present study was the first to characterize of the effects of
tenuifolin on the sleep behaviours of zebrafish and to predict the
biological signalling pathways targeted by tenuifolin using
quantitative behavioural profiling.
In the present study, tenuifolin was revealed to be
a hypnotic constituent of Radix Polygala that promotes sleep with a
pattern that is similar to the physiological pattern of mice
(15). Consistent with this
sleep-promoting effect in mice, the present behavioural monitoring
results suggested that zebrafish larvae exposed to tenuifolin
exhibited significantly increased dark-time rest, while maintaining
a normal day-night rhythm, with a slight effect on waking activity.
Therefore, tenuifolin may exhibit activity across different
species, supporting the biological effects of tenuifolin on sleep
regulation. In the present study tenuifolin significantly increased
total rest only in the dark phase, with little effect on light
phase rest or on waking activity. These selective effects for
distinct behavioural parameters and phases suggested that different
parameters may be regulated by distinct mechanisms during different
phases. In addition, the selective regulation of sleep also
suggested that the normal day-night rhythm and daytime activity
were unaffected. Therefore, comparing with the current
well-established pharmacotherapies to improve sleep, tenuifolin may
cause less daytime functional impairments, such as fatigue and
increased risk of accidents during work or while driving. In
addition, tenuifolin is one of the constitutions from Radix
Polygala (21). Radix Polygala has
long been used for in vivo study, which have demonstrated
that Radix Polygala extract improves cognitive deficits (16). Based on these, it is possible that
tenuifolin may exert effects on cognitive processing or memory.
However, to understand whether tenuifolin exerts these effects,
further study is required. Future study should focus on associated
behavioural changes such as memory and cognition, and genetically
manipulated zebrafish should be utilized.
The present results suggested that tenuifolin
induced a behavioural phenotype resembling that of 5-HT-reuptake
inhibitors and GABA agonists and it should be presumed that
serotoninergic systems and GABAergic systems may be involved in the
underlying mechanisms that contributed to the sleep-promoting
effects of tenuifolin. Serotonin has long been known to involved in
the regulation of sleep (25).
However, its role in sleep regulation remains controversial. It was
revealed that the destruction of the raphe nuclei, the areas
containing most of the serotoninergic neurons (~80%) of the brain,
caused complete insomnia (26).
However, the serotonin level of raphe nuclei and the firing rate of
brain stem motoneurons receiving serotoninergic signalling were
higher during the day-time (wake) than during the night-time
(sleep) (27). Generally, SSRIs
improve insomnia in patients with depression (12). However, in mammals, the effects of
SSRIs on sleep have demonstrated biphasic effects, with short-term
exposure promoting sleep and long-term exposure inducing the
opposite effect (12,28–30).
Similar to mammalian phenotypes, complicated phenotypes were
observed in zebrafish in response to drugs regulating serotonin
(12). These contradictory results
revealed the complexity of serotonin in sleep regulation, which may
be due to a direct effect. Serotonin could exert indirect effects
by modulating other non-serotoninergic systems (25). At present, there remains a lack of
studies assessing the correlations between serotoninergic systems
and tenuifolin. The aim of the present study was to identify the
potential molecular changes associated with serotoninergic systems
after tenuifolin exposure and to investigate the expression of
sert and 5-HT1A receptors. Sert is
responsible for transporting serotonin from the synaptic cleft and
regulating extracellular serotonin (31). The 5-HT1A receptor
is a 5-HT receptor. In the present study the expression of these
genes did not change after tenuifolin treatment. A similar result
was also revealed after exposure to the selective SSRI fluoxetine
in a previous study (31). However,
instead of using whole larvae, the same experiments were separately
performed in two brain regions (rostral and caudal segments) and
the spinal cord, which showed a specific fluoxetine-induced a
decrease in both genes in the spinal cord, but no change was
observed in the two brain regions (31). Accordingly, it could be presumed that
tenuifolin exerts its effects by targeting specific regions of the
CNS. Future study should focus on different regions of the CNS.
GABAergic neurons are active during sleep,
inhibiting activating and arousing systems, thereby promoting the
initiation and maintenance of non-rapid eye movement sleep at
multiple levels (32,33). The widely used hypnotic medications
in clinical settings, benzodiazepines and nonbenzodiazepines, are
modulators that target the GABAA receptor and act by
enhancing the inhibitory effects of GABA (34). In the present study, GABAergic
systems may represent a second pathway that tenuifolin affects. To
investigate whether the effects of a GABAA antagonist
could be counteracted by tenuifolin, cotreatment with tenuifolin
and the GABAA antagonist, picrotoxin, was also
performed. The present results indicated that treatment with
picrotoxin decreased dark-time rest, which was different from the
effects of tenuifolin. However, cotreatment of tenuifolin only
slightly reversed the effects of picrotoxin during dark-time rest.
This may be associated with the limitation of sample size in the
present study and therefore future studies should address this. In
addition, picrotoxin is one of many GABAA antagonists
and others should be tested. The ratio of picrotoxin and tenuifolin
may also exert effects on results, indicating that multiple ratios
of drug doses should be investigated.
Additionally, to correlate the behavioural changes
with molecular changes that mediated GABAergic signalling, the
expression of gad1, gabra1 and gat1 genes were investigated. Gad1
is a major marker associated with GABA-mediated synaptic function,
which catalyses GABA synthesis from glutamate (35–37).
Gabra1 is a subunit of the GABAA receptor involved in
GABA-mediated postsynaptic signalling (37). Gat1, the most abundant gat in the
brain, serves an important role in the regulation of GABA levels in
the synaptic cleft (38–40). Tenuifolin induced the expression of
gat1 in the present study, indicating a correlation between
increased transcript levels of gat1 and the sleep-promoting effects
of tenuifolin on zebrafish larvae. A previous study by Cao et
al (15) determined that
tenuifolin significantly increased GABA levels in the ventrolateral
preoptic area, locus coeruleus and perifornical area of mice. Thus,
the present results supported the association of GABAergic systems
with the hypnotic activity of tenuifolin at the molecular level.
However, there are certain limitations to the present study, as
many downstream gene products participate in the regulation of the
assessed signalling pathway. Further studies investigating multiple
molecular levels and using genetically manipulated zebrafish
deficient in genes associated with the GABAergic systems are
therefore required. In addition, the present study did not perform
a comparison of dark or light environments. A future study will aim
to detect the alternations in the gene expression patterns along
the L/D cycle after tenuifolin exposure.
Overall, the present results indicated that
tenuifolin treatment promoted dark-time sleep and elicited a
behavioural phenotype that is correlated with that of SSRIs and
GABA agonists. Changes in the gene expression of associated genes
further supported the involvement of GABAergic systems. In the
current study, a novel strategy based on zebrafish behavioural
profiling was applied to identify the potential biological targets
of tenuifolin. The present results identified that tenuifolin
exhibited promise for treating insomnia.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81974560
and 81503515), Natural Science Foundation of Guangdong Province
(grant no. 2015A030310255), Guangdong Provincial Key Laboratory for
Diagnosis and Treatment of Major Neurological Diseases (grant no.
2017B030314103) and The Southern China International Cooperation
Base for Early Intervention and Functional Rehabilitation of
Neurological Diseases (grant no. 2015B050501003).
Availability of data and materials
The data generated during the present study are
included in this published article.
Authors' contributions
ZMY and FPX conceived and designed the experiments.
ZWC, MRZ and TCY performed the experiments. ZWC, CBP and ZP
analysed the data. ZWC drafted the manuscript. ZMY and FPX revised
the manuscript. All authors have read and given final approval of
the manuscript before submission.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the Guide for the care and use of laboratory animals and approved
by the Animal Studies Committee of the Guangzhou University of
Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roth T: Insomnia: Definition, prevalence,
etiology, and consequences. J Clin Sleep Med. 3 (Suppl 5):S7–S10.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Léger D, Morin CM, Uchiyama M, Hakimi Z,
Cure S and Walsh JK: Chronic insomnia, quality-of-life, and utility
scores: Comparison with good sleepers in a cross-sectional
international survey. Sleep Med. 13:43–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asnis GM, Thomas M and Henderson MA:
Pharmacotherapy treatment options for insomnia: A primer for
clinicians. Int J Mol Sci. 17:E502015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swinney DC: The contribution of
mechanistic understanding to phenotypic screening for
first-in-class medicines. J Biomol Screen. 18:1186–1192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ek F, Malo M, Åberg Andersson M, Wedding
C, Kronborg J, Svensson P, Waters S, Petersson P and Olsson R:
Behavioral analysis of dopaminergic activation in zebrafish and
rats reveals similar phenotypes. ACS Chem Neurosci. 7:633–646.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellis LD and Soanes KH: A larval zebrafish
model of bipolar disorder as a screening platform for
neuro-therapeutics. Behav Brain Res. 233:450–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levitas-Djerbi T and Appelbaum L: Modeling
sleep and neuropsychiatric disorders in zebrafish. Curr Opin
Neurobiol. 44:89–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhdanova IV, Wang SY, Leclair OU and
Danilova NP: Melatonin promotes sleep-like state in zebrafish.
Brain Res. 903:263–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blank M, Guerim LD, Cordeiro RF and Vianna
MR: A one-trial inhibitory avoidance task to zebrafish: Rapid
acquisition of an NMDA-dependent long-term memory. Neurobiol Learn
Mem. 92:529–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pather S and Gerlai R: Shuttle box
learning in zebrafish (Danio rerio). Behav Brain Res. 196:323–327.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egan RJ, Bergner CL, Hart PC, Cachat JM,
Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien
DH, et al: Understanding behavioral and physiological phenotypes of
stress and anxiety in zebrafish. Behav Brain Res. 205:38–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rihel J, Prober DA, Arvanites A, Lam K,
Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT
and Schier AF: Zebrafish behavioral profiling links drugs to
biological targets and rest/wake regulation. Science. 327:348–351.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sánchez-Ortuño MM, Bélanger L, Ivers H,
LeBlanc M and Morin CM: The use of natural products for sleep: A
common practice? Sleep Med. 10:982–987. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wing YK: Herbal treatment of insomnia.
Hong Kong Med J. 7:392–402. 2001.PubMed/NCBI
|
|
15
|
Cao Q, Jiang Y, Cui SY, Tu PF, Chen YM, Ma
XL, Cui XY, Huang YL, Ding H, Song JZ, et al: Tenuifolin, a saponin
derived from Radix Polygalae, exhibits sleep-enhancing effects in
mice. Phytomedicine. 23:1797–1805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ling Y, Li Z, Chen M, Sun Z, Fan M and
Huang C: Analysis and detection of the chemical constituents of
Radix Polygalae and their metabolites in rats after oral
administration by ultra high-performance liquid chromatography
coupled with electrospray ionization quadrupole time-of-flight
tandem mass spectrometry. J Pharm Biomed Anal. 85:1–13. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng MC, Li CY, Ko HC, Ko FN, Lin YL and
Wu TS: Antidepressant principles of the roots of Polygala
tenuifolia. J Nat Prod. 69:1305–1309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shin EJ, Oh KW, Kim KW, Kwon YS, Jhoo JH,
Jhoo WK, Cha JY, Lim YK, Kim IS and Kim HC: Attenuation of
cocaine-induced conditioned place preference by Polygala
tenuifolia root extract. Life Sci. 75:2751–2764. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao Y, Jia M, Wu JG, Zhang H, Sun LN, Chen
WS and Rahman K: Anxiolytic and sedative-hypnotic activities of
polygalasaponins from Polygala tenuifolia in mice. Pharm
Biol. 48:801–807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee CI, Han JY, Hong JT and Oh KW:
3,4,5-Trimethoxycinnamic acid (TMCA), one of the constituents of
Polygalae Radix enhances pentobarbital-induced sleeping behaviors
via GABAAergic systems in mice. Arch Pharm Res. 36:1244–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma B, Li X, Li J, Zhang Q, Liu Y, Yang X,
Sun J, Yao D, Liu L, Liu X and Ying H: Quantitative analysis of
tenuifolin concentrations in rat plasma and tissue using LC-MS/MS:
application to pharmacokinetic and tissue distribution study. J
Pharm Biomed Anal. 88:191–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wallace CK, Bright LA, Marx JO, Andersen
RP, Mullins MC and Carty AJ: Effectiveness of rapid cooling as a
method of euthanasia for young zebrafish (Danio rerio). J Am Assoc
Lab Anim Sci. 57:58–63. 2018.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang YN, Hou YY, Sun MZ, Zhang CY, Bai G,
Zhao X and Feng XZ: Behavioural screening of zebrafish using
neuroactive traditional Chinese medicine prescriptions and
biological targets. Sci Rep. 4:53112014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Melancon MO, Lorrain D and Dionne IJ:
Exercise and sleep in aging: Emphasis on serotonin. Pathol Biol
(Paris). 62:276–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dugovic C: Role of serotonin in sleep
mechanisms. Rev Neurol (Paris). 157:S16–S19. 2001.PubMed/NCBI
|
|
27
|
Heym J, Steinfels GF and Jacobs BL:
Activity of serotonin-containing neurons in the nucleus raphe
pallidus of freely moving cats. Brain Res. 251:259–276. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pastel RH and Fernstrom JD: Short-term
effects of fluoxetine and trifluoromethylphenylpiperazine on
electroencephalographic sleep in the rat. Brain Res. 436:92–102.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sommerfelt L and Ursin R: Behavioral,
sleep-waking and EEG power spectral effects following the two
specific 5-HT uptake inhibitors zimeldine and alaproclate in cats.
Behav Brain Res. 45:105–115. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weber M, Talmon S, Schulze I, Boeddinghaus
C, Gross G, Schoemaker H and Wicke KM: Running wheel activity is
sensitive to acute treatment with selective inhibitors for either
serotonin or norepinephrine reuptake. Psychopharmacology (Berl).
203:753–762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Airhart MJ, Lee DH, Wilson TD, Miller BE,
Miller MN and Skalko RG: Movement disorders and neurochemical
changes in zebrafish larvae after bath exposure to fluoxetine
(PROZAC). Neurotoxicol Teratol. 29:652–664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jones BE: Neurobiology of waking and
sleeping. Handb Clin Neurol. 98:131–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Holst SC and Landolt HP: Sleep-Wake
Neurochemistry. Sleep Med Clin. 13:137–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Richey SM and Krystal AD: Pharmacological
advances in the treatment of insomnia. Curr Pharm Des.
17:1471–1475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Martin DL and Rimvall K: Regulation of
gamma-aminobutyric acid synthesis in the brain. J Neurochem.
60:395–407. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soghomonian JJ and Martin DL: Two isoforms
of glutamate decarboxylase: Why? Trends Pharmacol Sci. 19:500–505.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hortopan GA, Dinday MT and Baraban SC:
Spontaneous seizures and altered gene expression in GABA signaling
pathways in a mind bomb mutant zebrafish. The J Neurosci.
30:13718–13728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang TC, Kim HS, Seo MO, Park SK, Kwon HY,
Kang JH and Won MH: The changes in the expressions of
gamma-aminobutyric acid transporters in the gerbil hippocampal
complex following spontaneous seizure. Neurosci Lett. 310:29–32.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meldrum BS and Rogawski MA: Molecular
targets for antiepileptic drug development. Neurotherapeutics.
4:18–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen NH, Reith ME and Quick MW: Synaptic
uptake and beyond: The sodium- and chloride-dependent
neurotransmitter transporter family SLC6. Pflugers Arch.
447:519–531. 2004. View Article : Google Scholar : PubMed/NCBI
|